Abstract
In the submerged fermentation process, the introduction of novel nutrient media as sources of carbon and nitrogen can enhance antifungal activity. In this study, we used a submerged fermentation process to find the optimal media for the Bacillus velezensis BP-1 strain to grow in, and that would boost its antifungal activity against Peyronellaea arachidicola. By using a single-factor test and central composite design (CCD) in the response surface methodology, the optimal fermentation medium for the B. velezensis BP-1 strain was identified. The antagonistic potential of B. velezensis BP-1 was assayed against the P. arachidicola fungus to manage web blotch disease in peanut plant leaves. The LB medium was screened as the best medium, with a maximum antifungal activity of 90% in comparison to the other mediums. Semolina flour as the carbon source, peanut root extract as the nitrogen source, and magnesium sulfate as the inorganic salt were selected as the best nutrient components in comparison to the others. The response surface methodology was optimized by using 15 g/L of semolina flour as the carbon source, 13.68 g/L of peanut root extract as the nitrogen source, and 0.50 g/L of magnesium sulfate as the inorganic salt, achieving 90% inhibition of P. arachidicola. The in vitro bioassays showed that the optimized fermentation broth of B. velezensis BP-1 had significant antifungal activity, with an inhibition rate of 88.34% against P. arachidicola. In the pot experiments on disease control, the management effects revealed that the pre-inoculation spray of the B. velezensis BP-1 broth had significant efficiency (96%) when compared to the post-inoculation spray of the B. velezensis BP-1 broth. These findings suggest that the optimized fermentation broth of the B. velezensis BP-1 strain had strong antifungal activity. This could be a potent biocontrol tool for aiding in the early disease management of web blotch in peanut plants.
1. Introduction
China is the world’s largest producer of peanuts (Arachis hypogaea L.) [1], which constitute a major crop in Liaoning Province. About 370,000 hectares of peanuts have been planted in Liaoning Province on average, annually [2]. In contrast, fungus pathogens mainly affect plants’ leaves and may have an adverse effect on the whole plant [3]. In particular, peanut leaf has been adversely affected by fungal pathogens. “Web blotch” is a foliar disease in peanuts caused by the fungus Peyronellaea arachidicola. This illness is prevalent in several Chinese provinces, including Liaoning, Shandong, Shanxi, and Henan [4,5]. Agricultural yields can be devastatingly damaged by fungus diseases, posing a significant threat to food security [6,7]. Chemical fungicides are currently the most important strategy for controlling fungal diseases that grow in plants. Nonetheless, they are able to pollute the environment, pose risks to human health, and even induce resistance in pathogenic fungi [8,9]. Biocontrol methods that are safe, effective, and good for the environment can be used to limit the number of fungal pathogens and/or lessen their harmful effects [10,11]. In the past few years, bio-based products have become more and more popular. The Bacillus species have many uses in product development because they can synthesize bioactive compounds, and their endospores protect them from harsh environments [12]. It has been claimed that various Bacillus species are capable of controlling crop pathogens. The mechanism of action could be systemic resistance inducers or competitors with other pathogenic micro-organisms for space and nutrients. Bacillus can be a producer of antibiotics and enzymes, as well as bioactive compounds, and can even promote the growth of plants [13]. It has been found that a variety of biological control agents are potentially effective, but only a few are available commercially [14]. They contribute over 5% to the total amount of pesticides used in agriculture [15]. However, substantial investigations into the production and application of Bacillus spp. for biological control have been pursued in China and across the globe [16]. However, there are no reports on the biological control of peanut web blotch disease by Bacillus spp. Recently, we assessed a novel Bacillus velezensis BP-1 strain against P. arachidicola [17]. Producing Bacillus-based products for plant disease management is challenging due to their high manufacturing costs and poor yields [18]. As a result, it is critical to produce cost-effective and yield-effective culture media for biological control agents. It is possible that problems can occur when these substances are being fermented and formulated [14]. The physical characteristics and nutritional compositions of culture media are two such elements that can affect microbial fermentation processes [19]. More specifically, slight variations in the fermentation medium composition significantly affect microbial production and metabolic composition [20]. In this regard, the ratio of carbon to nitrogen (C/N) is widely established to be an important factor in many microbial processes [21,22]. However, nutritional improvements in fermentation medium formulation are significant as they address the chemical environment of the cells in a submerged system and the time factor in bioprocess synthesis [23]. In the fabrication of industrial applications of bacteria as biological control agents, efficient fermentation is required [24]. The fermentation medium must be optimized in order to overcome the limitations described above [25]. Optimization is a very crucial step because the fermentation process is complicated [15]. In the optimization process, conventional approaches, like the “one variable at a time” method, are inadequate for detecting the inter-relationship of multiple parameters. The response surface methodology (RSM) is a commonly used and efficient biotechnological optimization technique that employs a complete quadratic polynomial to demonstrate the correlations [26,27]. The response surface methodology (RSM) is a set of mathematical or statistical methods for planning experiments, visualizing models, and figuring out how the variables affect each other by setting up multivariable systems to get meaningful answers [28].
Central composite design (CCD), which is a statistical approach that appropriately includes component interaction and influence, is employed in RSM. It is frequently used for the optimization of fermentation nutrient conditions [29]. Basically, the CCD model is a fundamental component of the response surface methodology. This form of optimization model is more accurate and does not need a three-level factorial experiment to construct a second-order quadratic model [30]. In this design, the center points are supplemented with a collection of axial points known as star points. Using this architecture, it is possible to rapidly estimate first- and second-order terms. Many forms of central composite designs and their importance in various experimental designs have been explored in detail. Yet, computations based on the determination of alpha (α) and axial points have not been studied in detail [31].
In this work, we screened and optimized the medium nutrients in a submerged fermentation so that B. velezensis BP-1 could synthesize potent antifungal compounds. The optimized fermentation broth was tested in vitro and in vivo against P. arachidicola for the biological control of peanut web blotch.
2. Materials and Methods
2.1. Micro-Organisms
The antagonist (B. velezensis BP-1) and pathogen (P. arachidicola) were provided by the institute of plant protection, Liaoning Academy of Agricultural Sciences [17], and maintained on LB and PDA medium, respectively. Both micro-organisms were kept at 4 °C.
2.2. Inoculate Suspension and Mode of Fermentation
The cell suspension was made from two 5 mm spore cakes of the B. velezensis BP-1 strain in a 250 mL flask containing 40 mL of LB medium. A volume of 45 mL of fermentation medium in a 250 mL Erlenmeyer flask was utilized for the submerged fermentation process. Fermentation medium was composed of sodium chloride (2.5 g/L), tryptone (10 g/L), and yeast extract (7 g/L). Sterilization was performed at 121 °C and 15 lbs for 15 min after adjusting the pH from 6.6 to 6.8. To inoculate the submerged fermentation medium, 5 mL of spore suspension was used. The material was vigorously shaken for 96 h at 165 rpm at 30 °C. The broth was evaluated for antifungal activity against P. arachidicola.
2.3. Screening of Key Factors in Fermentation Medium of the B. velezensis BP-1 Strain by Single-Factor Experiment
We adopted the method of Sa, 2018 [32], with slight adjustments in fermentation base media, to screen the optimal medium. We screened the best medium using six media with LB medium as a control. The following recipes were used; No. 1 (soluble starch 20.0 g/L, MgSO4 1.0 g/L, NH4Cl 5.0 g/L, C6H12O6 20.0 g/L, and peptone 10.0 g/L), No. 2 (sucrose 30.0 g/L, yeast extract 3.0 g/L, (NH4)2SO4 10.0 g/L, MgSO4 5.0 g/L, and KH2PO4 0.3 g/L), No. 3 (corn flour 8.0 g/L, KH2PO4 2.0 g/L, MgSO4 0.5 g/L, semolina 16.0 g/L, and yeast extract 2.5 g/L), No. 4 (peanut root extract 3.0 g/L, C6H12O6 5.0 g/L, NaCl 10.0 g/L, and yeast extract 2.0 g/L), No. 5 (peptone 5.0 g/L, yeast extract 8.0 g/L, C₆H₁₂O₆ 10.0 g/L, and KCl 5.0 g/L), No. 6 (bean cake four 46.0 g/L, MnSO4 0.04 g/L, MgSO4 2.0 g/L, corn flour 14.0 g/L, and corn steep liquor 3.0 g/L), and control LB medium (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl). To assess the bioassay activity of the B. velezensis BP-1 strain, 200 μL of its fermentation broth was poured onto the 10 mL melted potato dextrose agar media. Later, the culture was inoculated with P. arachidicola and placed in an incubation chamber for fifteen days at a temperature of 28 °C. The inhibition zone of P. arachidicola was then measured. To screen the important nutritional components for the B. velezensis BP-1 strain fermentation, the following sources were used.
2.3.1. Carbon Sources
In the LB medium, the sole carbon sources (sucrose, soluble starch, lactose, and corn flour) were replaced one by one by yeast extract. Peanut root extract and semolina flour were introduced as novel carbon sources, as both were substituted with yeast extract. As a control group, LB with yeast extract was utilized. The bioassay activity of each combination against P. arachidicola was investigated.
2.3.2. Nitrogen Sources
In the LB medium, tryptone was replaced with beef extract, ammonia water, ammonia nitrate, ammonia sulfate, and peptone, one by one. Peanut root extract was introduced as a novel nitrogen source and substituted with tryptone. The control group used LB as a medium. Each combination was tested for bioassay activity against P. arachidecola.
2.3.3. Inorganic Salt Sources
NaCl was replaced one by one with KH2PO3, MnSO4, MgSO4, FeSO4, CaCl2, and KCl in the LB medium, which served as the study’s control. The control group used LB as a medium. Each combination was tested for bioassay activity against P. arachidicola.
2.4. Experimental Design for the Optimization of Nutrient Medium by Central Composites Design (CCD)
A central composite design (CCD) comprising three factors and three levels was adopted in the current study. The fractional factorial design consists of eight cube points, six center points, and six axial points with three parameters. The following variables and levels were set to optimize the fermentation process for the formation of secondary metabolites: a = peanut root extract (0–15 g/L), b = semolina (0–15 g/L), and c = MgSO4 (0–1.5 g/L). Table 1 contains the experimental central composite design. The contour plots show the interaction between the independent and dependent variables. The best jumble of factors can be shown by using canonical analysis and ridge maxima analysis. This can be proved by applying the optimization function of MINITAB 22. The software response optimizer tool was used to determine an ideal antifungal activity value.

Table 1.
Experiment design and results of optimization of nutrient medium for the production of secondary metabolites from the B. velezensis BP-1 Strain via central composite design.
2.5. Study of the Metabolism of the B. velezensis BP-1 Strain in a Submerged Fermentation
All of the fermentation batch analyses were conducted at the following times: 12, 24, 36, 48, 60, 72, 84, 96, 108, and 120 h. In the fermentation batch, Fehling’s reagent titration method was used to measure the total sugars [33]. To calculate amino nitrogen, a ninhydrin reagent method was adopted from “BMG LABTECH GmbH” [34]. The fermentation broth was diluted to 50 mL with distilled water, and 2 mL was transferred to 16 × 150 mm test tubes. We added 1 mL of Ninhydrin color reagent to the loosely covered tubes and heated them in a boiling water bath for 16 min. After 20 min in a cold water bath, 5 mL of dilution reagent was added and mixed in the tubes, and the absorbance at 575 nm was immediately recorded. The samples were taken at the above-mentioned time. The changes in the pH value of the fermentation broth during the fermentation process were measured with a pH meter. The dry mycelium weight was examined using the method described by (Wen et al., 2015) [35]. Take 5 mL of fermentation broth, centrifuge at 12,000 r/min for 15 min to obtain bacteria sediment, dry in a drying oven at 90 °C for 2 h, and weigh; then dry and weigh until weighing twice. The value remained unchanged.
Bacterial concentration (mg/mL) = mycelium dry weight/sample volume
2.6. Bioassay Activity of the B. velezensis BP-1 Strain against P. arachidicola In Vitro
A poisoned food assay was used to assess the in vitro antagonistic activity of B. velezensis BP-1 against P. arachidicola. A commercial fungicide (Tebuconazole) was utilized as the control, along with an optimized fermentation broth and a basic fermentation broth (Ck). A total of 20 mL of melted PDA was placed into the Petri dish, followed by 200 L of broth. The same method and concentration were also used for Ck. After that, we inoculated it with P. arachidicola and incubated it in a growth chamber at 28 °C for three weeks. A zone of inhibition was measured to assess the inhibition percentage of P. arachidicola. A total of three replications were conducted in this experiment.
2.7. Disease Control Effects of the B. velezensis BP-1 Strain on Peanut Plants in Pot Experiments
In the lab, pot experiments were carried out to evaluate the efficacy of the B. velezensis BP-1 strain to control web blotch disease on peanut plants. The plants had total access to air, water, and light at room temperature (20–24 °C). The seeds were planted on the same day. After the peanut plants had grown for 20 days, the treatments were given. The peanut plant received the treatments, including the control group (fungicide tebuconazole), at a concentration of 400 μL/plant. For peanut plant inoculation, a P. arachidicola spore suspension of 400 μL/plant was directly applied to the peanut leaf using an Eppendorf pipette. The above-mentioned optimized fermentation broth was used for bioassay activity. The following is the treatment approach:
- Inoculation with P. arachidicola + B. velezensis BP-1 broth treatment
- B. velezensis BP-1 broth treatment + Inoculation with P. arachidicola
The gap between the inoculations and treatments of B. velezensis BP-1 was 3 days. The same method was used for the control group (fungicide tebuconazole). An evaluation of the disease control efficiency was carried out after the fourteen days of treatments. The number of disease lesions was measured to assess the disease control efficacy of B. velezensis BP-1. A total of three replications were conducted in this experiment. The following formula was established:
2.8. Statistical Analysis
The experimental RSM findings were fitted using a regression approach of the response surface. The variables in the equation were denoted by the following coded values:
Xi = (Xi − Xi) /Xi = 1, 2, 3… K,
The coded value of an independent variable is Xi, but its real value, real mean, and step change are all X. We developed the second-order polynomial model by fitting the response curve using the following equation:
Y represents the response; b0 represents the intercept term, and bi, bij, and bii reflect the effects of the variables xi, xix, and xi, respectively. Xixj is a variable that represents the interaction of xi and xj. ANOVA was used to determine the goodness of fit of the regression by examining Fisher’s F-test, associated probability P(F), and determination coefficient R2. In addition to the estimated coefficients and related probabilities, the study includes P(t) and Student’s t-values. The quadratic models for each variable were represented using surface plots [36,37]. The statistical tool Minitab 18 was used for all statistical analyses (RSM) and regression analysis. Statistica 12 was used to create graphs (surface plots) as well as for ANOVA and Tukey’s tests.
Y = b0 + bixi + I j bij xi xj + bii xi,
3. Results
3.1. Single-Factor Screening of Fermentation Medium for B. velezensis BP−1
The results show that the B. velezensis BP-1 strain fermented in the LB medium showed a maximum inhibition rate of 90% against P. arachidicola, as shown in Figure 1A. For further medium adjustment, we used the LB medium with yeast extract as the primary carbon source (5.0 g/L), tryptone as the primary nitrogen source (10 g/L), and NaCl as the primary inorganic salt (5.0 g/L).
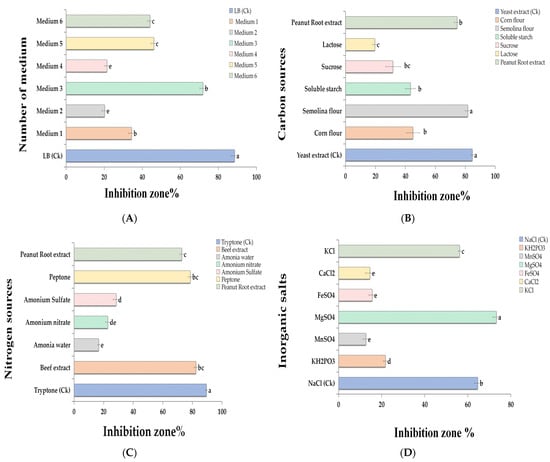
Figure 1.
One-factor one-time screening of the fermentation medium of B. velezensis BP-1 (the details of the medium are given in Material and Methods). Different basal mediums (A), carbon sources (B), nitrogen sources (C), and inorganic salts (D). Inhibition zones are measured as percentages. Ck represented the control group. All of the values are comparisons of mean ± SE. According to “Tukey’s HSD” (p > 0.05), means that share the same letter in a column are statistically insignificant. a, b, and c superscripts indicate pairwise comparisons, whether they are statistically different. To analyze the data, all treatments were repeated three times.
By substituting the yeast extract in LB medium with different carbon sources, the inhibition rate decreased to varying degrees (Figure 1B), with the greatest inhibition rate (87%) observed in the fermentation broth of the B. velezensis BP-1 strain cultivated with semolina as the carbon source (Figure 1B). When tryptone was taken out of the fermentation medium and replaced with a different nitrogen source, peanut root extract had the highest rate of inhibition at 83% and was not too dissimilar from tryptone (Figure 1C). Several inorganic salts were substituted for NaCl in the fermentation basal medium, resulting in a considerable increase in the inhibition rates of the fermentation broths of the B. velezensis BP-1 strains by more than 80% using magnesium sulfate as an inorganic salt (Figure 1D). The carbon, nitrogen, and inorganic salt sources were modified in the basic fermentation medium composition. Based on the preceding findings, in order to achieve the optimal upgraded fermentation product, semolina flour was employed as the carbon source, peanut root extract as the nitrogen source, and MgSO4 as the inorganic salt.
3.2. Fermentation Medium Optimization by Central Composite Design
A central composite design (CCD) based on response surface methods was utilized to enhance the antifungal effects of the B. velezensis BP-1 strain’s secondary metabolites. Over 15 trials, the concentrations of semolina flour, peanut root extract, and magnesium oxide were examined. In trial 11, the optimized fermentation broth had the lowest antagonistic activity against P. arachidicola (45.32 % inhibition zone), while in trial 7, it had the maximum activity (90.55 % inhibition zone), as shown in Table 1. For studies employing central composite designs, the response was calculated using multiple regression using second-order polynomial functions. It is stated in the following equation (Equation (3)) that Y represents the antifungal response (inhibition percentage), and a, b, and c represent the independent components of semolina flour, peanut root extract, and magnesium sulfate, respectively.
ANOVA was used to analyze the data collected from the experiments. The proposed model is significant, as indicated by a Fisher test value of 19.52 and a p-value of 0.002. In order to demonstrate that a model is adequate, its coefficient of determination (R2) must be sufficiently high. As well as determining the trail parameters and their interactions, the R2 values were also used to estimate the unpredictability. An R2 value of 0.9723 indicates that the model did not address approximately 2.77 percentage points of variance. Additionally, this model was significant, as shown by an adjusted determination coefficient R2 of 0.9225 (Table 2).

Table 2.
Analysis of variance of antifungal activity against P. arachidicola.
The antagonistic activity of fermented broth was significantly affected by all of the regression coefficients for the linear terms. The antagonistic activity of the fermentation broth was significantly impacted by the addition of semolina flour, peanut root extract, and magnesium sulfate, as shown by their respective p-values (pa = 0.003, pb = 0.010, and pc = 0.001). Significant increases were seen in the quadratic coefficients, i.e., a2a, b2b, and c2c. The parameter values in these quadrants were found to either boost or reduce the metabolites’ activity, respectively. Table 3 shows that the a2b, a2c, and b2c interaction coefficients of all samples had statistically significant values. The regression models were used to create surface plots to analyze the interactions of the independent variables and the relative importance of the individual ones. The surface plots of the independent variables convey the significance of the variables and the connections between them by processing the action for each paired variable while holding the other variables at their median values. With the optimal concentration of MgSO4 held constant, the antagonistic activity of semolina flour and peanut root extract is shown in Figure 2A. The graphs show that the antagonistic activity of the secondary metabolites is strongest in the middle range of semolina flour and peanut root extract concentrations, and it drops sharply at the lower and higher concentrations.

Table 3.
Coded coefficients for antifungal activity against P. arachidichola.
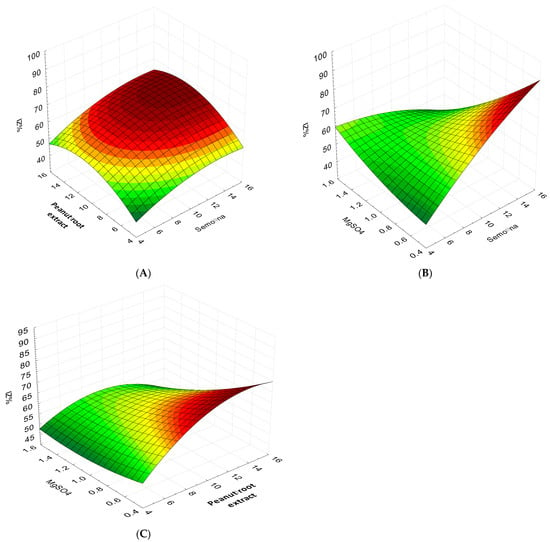
Figure 2.
Response surface plots of the effect of various factors on the inhibition rate of P. arachidicola growth by the fermented broth of the B. velezensis BP-1 strain and validation of the optimal formulation. (A) The response surface plot for the effects of semolina flour and peanut root extract on the inhibition rate at middle-level MgSO4 content. (B) The response surface plot for the effects of semolina flour and MgSO4 on the inhibition rate at middle-level peanut root extract content. (C) The response surface plot for the effects of peanut root extract and MgSO4 on the inhibition rate at middle-level semolina flour content.
The peanut root extract’s inhibitory activity, as a factor of semolina flour and MgSO4, is shown in Figure 2B, where the optimum value is maintained. The graph shows that the middle range of semolina flour + MgSO4 has the highest antagonistic activity in the fermented broth, while at lower and higher levels, relatively modest levels of antagonistic activity in the fermented broth were reported. By keeping the ideal value of semolina flour, Figure 2C shows the antagonistic action of MgSO4 and peanut root extract. The graph shows that the intermediate range of MgSO4 and peanut root extract levels supports the maximum value of fermented broth antagonistic activity, while lower and higher levels support relatively modest levels of inhibitory action.
3.3. Study of the Metabolism of the B. velezensis BP-1 Strain in Submerged Fermentation
During the fermentation process, the total sugar, amino nitrogen content, pH, and dry mycelium weight were measured. In order to characterize the relationships between the independent variables, linear and exponential models were used. The total sugar concentration was high at the beginning of the fermentation process, but it gradually dropped due to increased usage. A linear regression model (R2 = 0.9609) depicts the relationship between total sugar and time. The exponential decay model yielded the best-fit regression, as shown in Figure 3A. The amino nitrogen level relationship with time (R2 = 0.7748) was found to have an up-and-down tendency, as described by linear regression. The best-fit regression by the exponential decay model is indicated by lines and equations (Figure 3B). The weight of dried mycelium increased as fermentation progressed. It reached a maximum after 84 h and declined after 120 h. The interaction between dry mycelium weight and time is described by a linear regression model with an R2 value of 0.9104. The best-fit regression line is provided by an exponential decay model (Figure 3C). Furthermore, the pH of the fermentation process did not remain consistent, fluctuating between 5.5 and 6.6. Linear regression is used to describe the pH–time relationship. In this situation, the R2 was 0.878. The best-fit regression line is provided by an exponential decay model (Figure 3D).
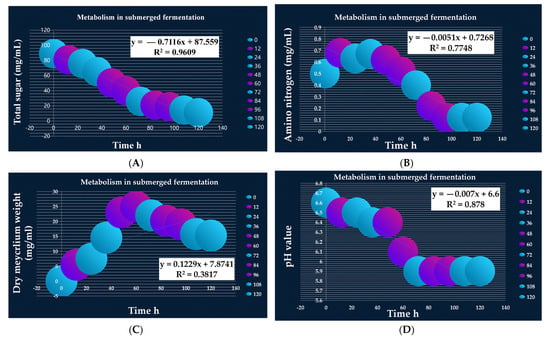
Figure 3.
Metabolism study of submerged fermentation batch of B. velezensis BP-1. Total sugar consumption (A), amino nitrogen production (B), dry mycelium weight (C), and pH values (D). The correlation between the parameters regarding metabolism with culturing time. In order to analyze the data, all treatments were repeated three times.
3.4. In Vitro Assay of the B. velezensis BP-1 Strain against P. arachidicola
In comparison to the antagonistic potential of the basic medium fermentation broth, which was 70.66 percent, the antagonistic potential of the optimized medium fermentation broth against P. arachidicola was 88.35 percent. Interestingly, the antagonistic potential of both the optimized and basic medium was minimal when compared to the commercial control fungicide. As shown in Figure 4A, 91.27 percent of the antagonistic potential of the control group suppresses fungus development.
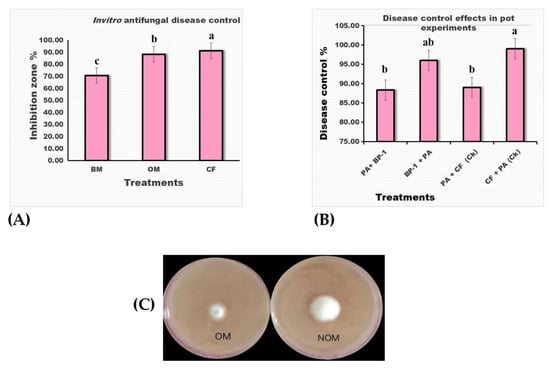
Figure 4.
In vitro antagonistic activity of B. velezensis BP-1; (A) commercial fungicide (CF) was used as the control value. Optimized fermentation medium (OM) broth and basic medium (BM) broth; (B) disease control effects in pot experiment: B. velezensis BP-1 (BP-1), commercial fungicide (CF), P. arachidicola (PA). All of the values are comparisons of mean ± SE. According to Tukey’s HSD, a p > 0.05 means that the values share the same letter in a column and are statistically insignificant. a, b, and c superscripts indicate pairwise comparisons, whether they are statistically different. In order to analyze the data, all treatments were repeated three times. (C) Validation of the optimal formulation for the inhibition rate of P. arachidicola growth by the fermented broth of B. velezensis BP-1 OM (optimized medium) and NOM (nonoptimized medium).
3.5. Disease Control Efficiency of the B. velezensis BP-1 Strain in Pot Experiments
Disease control efficiency in the pot experiments revealed that in the trial B. velezensis BP-1 (BP-1) + P. arachidicola (PA) inoculates had significant 96 percent disease control, whereas the trial (PA) inoculates + BP-1 treatment had less efficient 88.33 percent disease control. Similarly, in the control trials, the CF + PA inoculates showed strong disease control effects that were statistically significant, whereas the PA inoculates and CF both had low efficacy in controlling leaf web blotch (Figure 4B). Figure 4C is a summary of the in-vitro antagonistic results, which show that optimizing the fermentation medium increased the BP-1 strain’s ability to fight off P. arachidicola.
4. Discussion
We screened and optimized the submerged fermentation medium nutrients to synthesize secondary metabolites from the B. velezensis BP-1 strain. These secondary metabolites were evaluated against P. arachidicola to control web blotch disease in peanut plants. It is stated that B. velezensis has a strong ability to control fungal phytopathogens [38,39,40,41]. As previously described by Jensen et al. and Collinge et al. [42,43], fungus pathogens acquire resistance to a number of antagonists. In order to accomplish this task, new strains of antagonistic bacteria and new formulations are urgently needed. In the current study, a novel nutrient media for the fermentation process was introduced.
According to our results, the B. velezensis BP-1 strain grown in semolina and peanut root extract-containing medium had more significant antifungal effects than the other fermentation nutrients. The results proved that semolina flour and peanut root extract were the most significant optimal carbon and nitrogen resources in the fermentation medium, respectively. Purama et al., 2008, and Tays et al., 2018 [44,45], discovered that the ratio of the substrate medium, which is made up of diverse carbon, nitrogen, and inorganic micro-element sources, has a substantial influence on microbial production and bioactivity. Semolina flour had a significant influence on the antifungal activity of B. velezensis BP-1 when used as a carbon source in a submerged fermentation medium. Semolina flour in a fermentation broth may boost the antifungal activities and biomass production of the B. velezensis BP-1 strain. A recent study indicates that semolina has more protein and gluten than other grains [46]. The findings indicated that the composition of peanut root extract has the potential to replace tryptone extract as a nitrogen source in the fermentation of the BP-1 strain, hence sustaining and enhancing the strain’s biological control activity. Richard et al. [47] said that the root metabolites of certain plant species could be changed to support a certain microbial strain with the desired abilities.
Similarly, MgSO4, an inorganic salt that has been optimized, may effectively replace NaCl as the base medium. It has been shown that Mg2+ plays a significant role in the manufacturing of proteins by activating a variety of enzymes and taking part in the synthesis of amino acids, the transcription and translation of genes, the generation of proteins that serve as the structural components of ribosomes, and other processes [48,49]. The outcomes demonstrated that the experimental layout considerably influences the synthesis of secondary metabolites that conceal B. velezensis BP-1’s antagonistic action against P. arachidechola. The studies confirmed the effectiveness of the response surface methodology’s central composite design. In order to identify the ideal parameters for improvement, the response surface approach is an appropriate mathematical and statistical methodology. Designing an experimental design could be helpful for demonstrating the interactions between different parameters.
Applications of response surface methodology to enhance fermentation parameters have recently become very common [50,51]. The results were analyzed to figure out the optimal medium content level and its interaction with the other three significant parameters, which were later optimized using the central composite design via RSM. Because of its efficacy and longevity, the central composites design is the most appropriate design for fermentation [31,52]. The fermentation batch’s metabolism indicated that as the number of bacteria rose, the total sugar levels declined but any decreasing sugar levels climbed, showing that B. velezensis BP-1 was consuming both total sugar and amino nitrogen to promote its own development. Eventually, the rate of development of the B. velezensis BP-1 strain was reduced owing to a concentration of secondary metabolites and other metabolites in the fermentation batch as well as a reduction in pH. Total sugar consumption increased the amount of fermentation component synthesis, improved pH, and had a negative influence on the dry mycelium. Streptomyces has been researched lately for a similar mechanism [37].
The in vitro antagonistic activity of B. velezensis BP-1 revealed that the optimized fermentation broth had potent antifungal effects against P. arachidecola, which might aid in the control of peanut web blotch disease. For high secondary metabolite antifungal activity, semolina flour, peanut root extract, and ammonium sulfate were the optimized components. According to a number of studies [53,54], the optimization of fermentation parameters increases the antifungal efficacy of the Bacillus species to counter fungal pathogens, such as B. pumilus HR10 and B. amyloliquefaciens BLB369. The effectiveness of disease control in pot experiments revealed that treatment with the BP-1 spray had a significant impact on peanut web blotch control, even if inoculation occurred later. Nevertheless, early inoculation using P. arachidicola inoculate followed by BP-1 spray treatment had a lower impact. This suggests that B. velezensis BP-1 might control the disease more effectively and build plant immunity via pretreatment. This might be effective for addressing the early stages of a peanut disease epidemic and other related symptoms. According to Caulier et al. and Tsalgatidou et al. [55,56], endophytic bacteria, particularly the Bacillus species, have the ability to inhibit plant diseases as well as stimulate plant growth. By finding and investigating novel endophytic Bacillus species, it may be possible to produce microbial biocontrol agents that inhibit fungal infections before and after harvest [57]. Based on our findings, we conclude that the novel fermentation nutritional medium components semolina flour and peanut root extract may boost B. velezensis BP-1’s ability to synthesize novel antifungal metabolites. Until the present, no Bacillus species have been recorded to inhibit peanut web blotch in China; this is the first investigation in this field.
5. Conclusions
This work highlighted the screening and optimization of the fermentation medium to produce efficient metabolites for the biological control of P. arachidicola. Six fermentation recipes were screened, and LB was used as a comparison. On the basis of its antifungal activity, LB was selected as the basic fermentation medium. The LB medium was used to screen for the best carbon, nitrogen, and inorganic salt components. For the different carbon sources, semolina flour was introduced as the novel component, while for the nitrogen sources, peanut root extract was used. On the basis of the antifungal activity of fermentation, semolina flour was screened as the optimum sole carbon source, while peanut root extract was selected as the sole nitrogen source in the submerged fermentation process. Similarly, MgSO4 was screened as an inorganic salt. The central composite design of the response surface methodology determined the optimum composition: 15 g/L of semolina flour as the carbon source, 13.68 g/L of peanut root extract as the nitrogen source, and 0.50 g/L of magnesium sulfate as the inorganic salt, with 90% inhibition of P. arachidicola achieved. The in vitro bioassays of the inhibitory action revealed that the growth of P. arachidicola was significantly inhibited by the fermentation broth of B. velezensis BP-1 using the improved medium composition. In the pot experiments, the disease management effects showed that B. velezensis BP-1 had substantial efficiency in controlling the web blotch disease in peanut plants. Our findings emphasize that the B. velezensis BP-1 strain could be a potent biocontrol agent, and novel nutrient medium compositions can boost the antagonistic efficiency of the strain.
Author Contributions
Conceptualization, T.A. and C.L.; methodology, T.A. and C.L.; software, C.Z.; validation, C.L. and C.Z.; formal analysis, T.A., S.Y., J.X. and X.L.; investigation, T.A. and C.Z.; resources, C.L.; data curation, C.Z. and X.P.; writing—original draft preparation, T.A. and M.U.; writing—review and editing, T.A., Y.L., C.L. and M.U.; visualization, T.A. and C.Z.; supervision, C.Z. and C.L.; project administration, C.Z. and C.L.; funding acquisition, C.Z. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the President’s Foundation Project of the Liaoning Academy of Agricultural Sciences (2020QN2407), National Key Research and Development Program of China (2017YFD0201100), as well as the Liaoning Province Youth Top Talent Project (XLYC1907181), Shenyang Young and Middle-aged Scientific and Technological Innovation Talents Support Plan (RC200195), the basic scientific research business expenses of the Liaoning Academy of Agricultural Sciences (2022XTCX0502003), and the basic research expenses of the Liaoning Academy of Agricultural Sciences (2021GQ1903).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
A very special thanks to Chaoqun Zang for providing the Bacillus strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Lyu, J.; Chen, D. Performance assessment of peanut production in China. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2022, 72, 176–188. [Google Scholar] [CrossRef]
- Yu, S.; Yu, G.; Ren, L.; Sun, H.; Cui, X.; You, S.; Wang, H.; Shi, P.; Yu, H.; Liaoning Sandy Land Amelioration and Utilization Research Institute. The Effect of single-seed precision sowing on peanut yield under different planting density. Liaoning Agric. Sci. 2018, 6, 19–22. [Google Scholar]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, D.; Zhou, R.; Yang, F.; Su, W. Analysis of occurrence and epidemic dynamics of peanut web blotch disease in Liaoning Province. Chin. J. Oil Crop Sci. 2013, 35, 80–83. [Google Scholar]
- Li, S.; Xue, X.; Gao, M.; Wang, N.; Cui, X.; Sang, S.; Fan, W.; Wang, Z. Genome Resource for Peanut Web Blotch Causal Agent Peyronellaea arachidicola Strain YY187. Plant Dis. 2021, 105, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Lichtveld, M.; Mazet, J.A.; Togami, E.; Miller, S.A. Plant health and its effects on food safety and security in a One Health framework: Four case studies. One Health Outlook 2021, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Al-Raish, S.M.; Saeed, E.E.; Sham, A.; Alblooshi, K.; El-Tarabily, K.A.; AbuQamar, S.F. Molecular characterization and disease control of stem canker on royal poinciana (Delonix regia) caused by Neoscytalidium dimidiatum in the United Arab Emirates. Int. J. Mol. Sci. 2020, 21, 1033. [Google Scholar] [CrossRef]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of fungicides and factors affecting their fate and removal efficacy: A review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Behlau, F. An overview of citrus canker in Brazil. Trop. Plant Pathol. 2021, 46, 1–12. [Google Scholar] [CrossRef]
- Zapata-Sarmiento, D.H.; Palacios-Pala, E.F.; Rodríguez-Hernández, A.A.; Melchor, D.L.M.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Trichoderma asperellum, a potential biological control agent of Stemphylium vesicarium, on onion (Allium cepa L.). Biol. Control 2020, 140, 104105. [Google Scholar] [CrossRef]
- Amin, A.; Akbar, M.; Khalil, T.; Akram, W.; Ahmad, A. Antifungal activity of Alternanthera philoxeroides organic solvent extract against plant pathogenic fungi. Pak. J. Bot. 2022, 54, 337–344. [Google Scholar]
- Baptista, J.P.; Teixeira, G.M.; de Jesus, M.L.A.; Bertê, R.; Higashi, A.; Mosela, M.; da Silva, D.V.; de Oliveira, J.P.; Sanches, D.S.; Brancher, J.D.; et al. Antifungal activity and genomic characterization of the biocontrol agent Bacillus velezensis CMRP. Sci. Rep. 2022, 12, 17401. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Bailey, K.; Boyetchko, S.; Längle, T. Social and economic drivers shaping the future of biological control: A Canadian perspective on the factors affecting the development and use of microbial biopesticides. Biol. Control 2010, 52, 221–229. [Google Scholar] [CrossRef]
- Zou, Q.; Ren, Z.; Gao, S.; Zhou, H.; Zhao, J.; Liu, E. Isolation and Identification of Bacillus subtilis YN145 against Magnaporthe oryzae and Its Antimicrobial Activities. Chin. J. Biol. Control 2017, 33, 421. [Google Scholar]
- Zhang, C.; Zhao, Y.; Xie, J.; Lin, Y.; Pei, X.; Liu, X. Screening and Identification of Bacillus velezensis Strain BP-1 and the Field Control Efficiency against Peanut Web Blotch. Chin. J. Biol. Control 2021, 37, 259–265. [Google Scholar]
- Schisler, D.; Slininger, P.; Behle, R.; Jackson, M. Formulation of Bacillus spp. for biological control of plant diseases. Phytopathology 2004, 94, 1267–1271. [Google Scholar] [CrossRef]
- Gomes, R.J.; Ida, E.I.; Spinosa, W.A. Nutritional supplementation with amino acids on bacterial cellulose production by Komagataeibacter intermedius: Effect analysis and application of response surface methodology. Appl. Biochem. Biotechnol. 2022; online ahead of print. [Google Scholar]
- Vlajkov, V.; Anđelić, S.; Pajčin, I.; Grahovac, M.; Budakov, D.; Jokić, A.; Grahovac, J. Medium for the production of Bacillus-based biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms 2022, 10, 1165. [Google Scholar] [CrossRef]
- Dioha, I.; Ikeme, C.; Nafi’u, T.; Soba, N. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef]
- Link, H.; Weuster-Botz, D. Medium Formulation and Development. In Comprehensive Biotechnology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 119–134. [Google Scholar] [CrossRef]
- Shafi, J.; Mingshan, J.; Zhiqiu, Q.; Xiuwei, L.; Zumin, G.; Xinghai, L.; Yang, Z.; Peiwen, Q.; Hongzhe, T.; Wunan, C.; et al. Optimization of Bacillus aerius strain JS-786 cell dry mass and its antifungal activity against Botrytis cinerea using response surface methodology. Arch. Biol. Sci. 2017, 69, 469–480. [Google Scholar] [CrossRef]
- Marin, D.H.; Romero, R.A.; Guzman, M.; Sutton, T.B. Black Sigatoka: An increasing threat to banana cultivation. Plant Dis. 2003, 87, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, C.; Chairez, I.; Fernández-Linares, L. A novel culture medium designed for the simultaneous enhancement of biomass and lipid production by Chlorella vulgaris UTEX 26. Bioresour. Technol. 2016, 212, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zou, P.; Miao, L.; Qi, J.; Song, L.; Zhu, L.; Xu, X. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Environ. Sci. Pollut. Res. 2014, 21, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Roslee, A.; Khalil, K.A.; Napis, S.; Convey, P.; Gomez-Fuentes, C.; Ahmad, S. Response surface methodology optimization and kinetics of diesel degradation by a cold-adapted Antarctic bacterium, Arthrobacter sp. strain AQ5-05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Granato, D.; de Araújo Calado, V.M. The use and importance of design of experiments (DOE) in process modelling in food science and technology. In Mathematical and Statistical Methods in Food Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014; Volume 1, pp. 1–18. [Google Scholar]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Sa, R. The Antimicrobial Activity Substances and Disease Prevention Mechanism of the Endophytic Antagonistic Bacterium N6–34 from Poplar; Shandong Agricultural University: Taian, China, 2018. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers Pt Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- BMG LABTECH GmbH. Using the SPECTROstar Nano for Testing Free Amino Nitrogen Content in Alcoholic Beverages. News-Medical. Available online: https://www.bmglabtech.com (accessed on 1 February 2023).
- Wen, Z.; Liu, Z.; Hou, Y.; Liu, C.; Gao, F.; Zheng, Y.; Chen, F. Ethanol induced astaxanthin accumulation and transcriptional expression of carotenogenic genes in Haematococcus pluvialis. Enzym. Microb. Technol. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Gao, X.; He, Q.; Jiang, Y.; Huang, L. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efficacies against Fulvia fulva and Botryosphaeria dothidea. J. Phytopathol. 2016, 164, 155–165. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Wu, Y.; Irfan, M. Application of response surface methodology for optimization of medium components for the production of secondary metabolites by Streptomyces diastatochromogenes KX85AMB. Express 2017, 7, 96. [Google Scholar]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.-T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Matar, S.; Abo-Zaid, G. Production of Bacillus velezensis Strain GB1 as a Biocontrol Agent and Its Impact on Bemisia tabaci by Inducing Systemic Resistance in a Squash Plant. Horticulturae 2022, 8, 511. [Google Scholar] [CrossRef]
- Mosela, M.; Andrade, G.; Massucato, L.R.; Almeida, S.R.d.A.; Nogueira, A.F.; Filho, R.B.D.L.; Zeffa, D.M.; Mian, S.; Higashi, A.Y.; Shimizu, G.D.; et al. Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef] [PubMed]
- Garkovenko, A.V.; Vasilyev, I.Y.; Ilnitskaya, E.V.; Radchenko, V.V.; Asaturova, A.M.; Kozitsyn, A.E.; Tomashevich, N.S.; Milovanov, A.V.; Grigoreva, T.V.; Shternshis, M.V. Draft genome sequence of Bacillus velezensis BZR 336g, a plant growth-promoting antifungal biocontrol agent isolated from winter wheat. Microbiol. Resour. Announc. 2020, 9, e00450-20. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 429–471. [Google Scholar]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Purama, R.K.; Goyal, A. Screening and optimization of nutritional factors for higher dextransucrase production by Leuconostocmesenteroides NRRL B-640 using statistical approach. Bioresour. Technol. 2008, 99, 7108–7114. [Google Scholar] [CrossRef] [PubMed]
- Tays, C.; Guarnieri, M.T.; Sauvageau, D.; Stein, L.Y. Combined effects of carbon and nitrogen source to optimize growth of proteobacterial methanotrophs. Front. Microbiol. 2018, 9, 2239. [Google Scholar] [CrossRef]
- Grant, C.; Cubadda, F.; Carcea, M.; Pogna, N.E.; Gazza, L. Vitamins, Minerals, and Nutritional Value of Durum Wheat. In Durum Wheat; Elsevier: Amsterdam, The Netherlands, 2012; pp. 125–137. [Google Scholar]
- Jacoby, R.P.; Martyn, A.; Kopriva, S. Exometabolomic profiling of bacterial strains as cultivated using Arabidopsis root extract as the sole carbon source. Mol. Plant-Microbe Interact. 2018, 31, 803–813. [Google Scholar] [CrossRef]
- Yoon, H.; Warshel, A. Simulating the fidelity and the three Mg mechanism of pol η and clarifying the validity of transition state theory in enzyme catalysis. Proteins Struct. Funct. Bioinform. 2017, 85, 1446–1453. [Google Scholar] [CrossRef]
- Tian, Z.; Hou, L.; Hu, M.; Gao, Y.; Li, D.; Fan, B.; Wang, F.; Li, S. Optimization of Sporulation Conditions for Bacillus subtilis BSNK-5. Processes 2022, 10, 1133. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Jia, R.; Hu, L.; Wang, Y. Response Surface Methodology (RSM) Mediated Optimization of Medium Components for Mycelial Growth and Metabolites Production of Streptomyces Alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef]
- Nouby, M.; Mathivanan, D.; Srinivasan, K. A combined approach of complex eigenvalue analysis and design of experiments (DOE) to study disc brake squeal. Int. J. Eng. Sci. Technol. 2009, 1, 254–271. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Li, P. The relations among reducing sugar, pH, dry weight of mycelium and production during the liquid fermentation of Marasmius androsaceus. Edible Fungi China 2002, 21, 37–38. [Google Scholar]
- Dai, Y.; Wang, Y.-H.; Li, M.; Zhu, M.-L.; Wen, T.-Y.; Wu, X.-Q. Medium optimization to analyze the protein composition of Bacillus pumilus HR10 antagonizing Sphaeropsis sapinea. AMB Express 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 Culture Medium by Response Surface Methodology for Low Cost Production of Antifungal Activity. Microorganisms 2022, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Tsalgatidou, P.C.; Thomloudi, E.E.; Baira, E.; Papadimitriou, K.; Skagia, A.; Venieraki, A.; Katinakis, P. Integrated genomic and metabolomic analysis illuminates key secreted metabolites produced by the novel endophyte Bacillus halotolerans Cal. l. 30 involved in diverse biological control activities. Microorganisms 2022, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient biotic strategy to control postharvest diseases of fruits and vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).