Enhancement of Anticancer Effects by Combining 5-Fluorouracil with Refametinib in Human Oral Squamous Cell Carcinoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Cell Treatment
2.3. Cell Viability Assay
2.4. Colony Formation Assay
2.5. Western Blot
2.6. Mitochondrial Membrane Potential Measurement
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
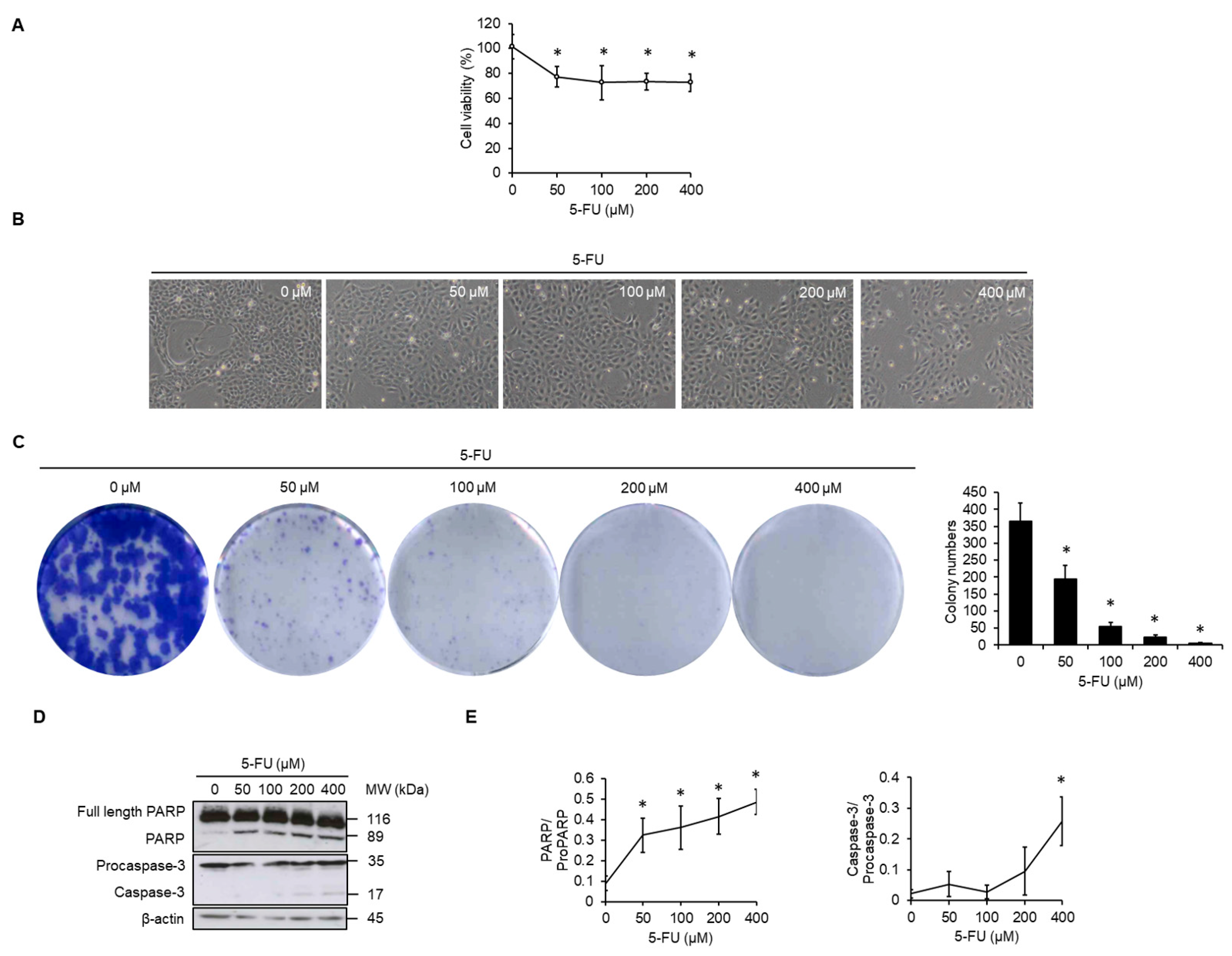
3.1. 5-FU Inhibits Cell Proliferation and Causes Apoptosis in SCC4 Cells
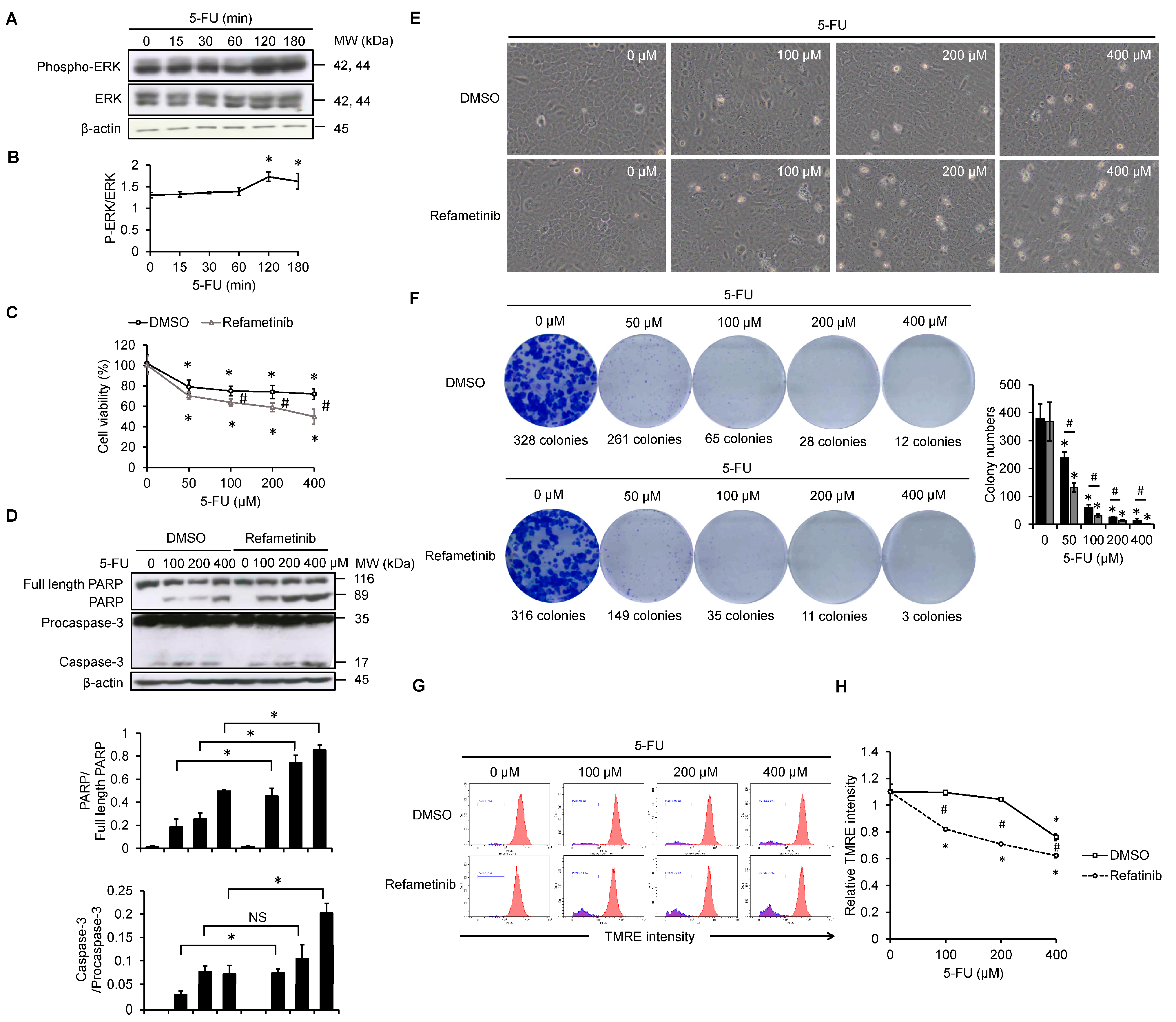
3.2. Refametinib Sensitizes SCC4 Cells to 5-FU Treatment for Suppressing Cell Proliferation and Promoting Apoptosis
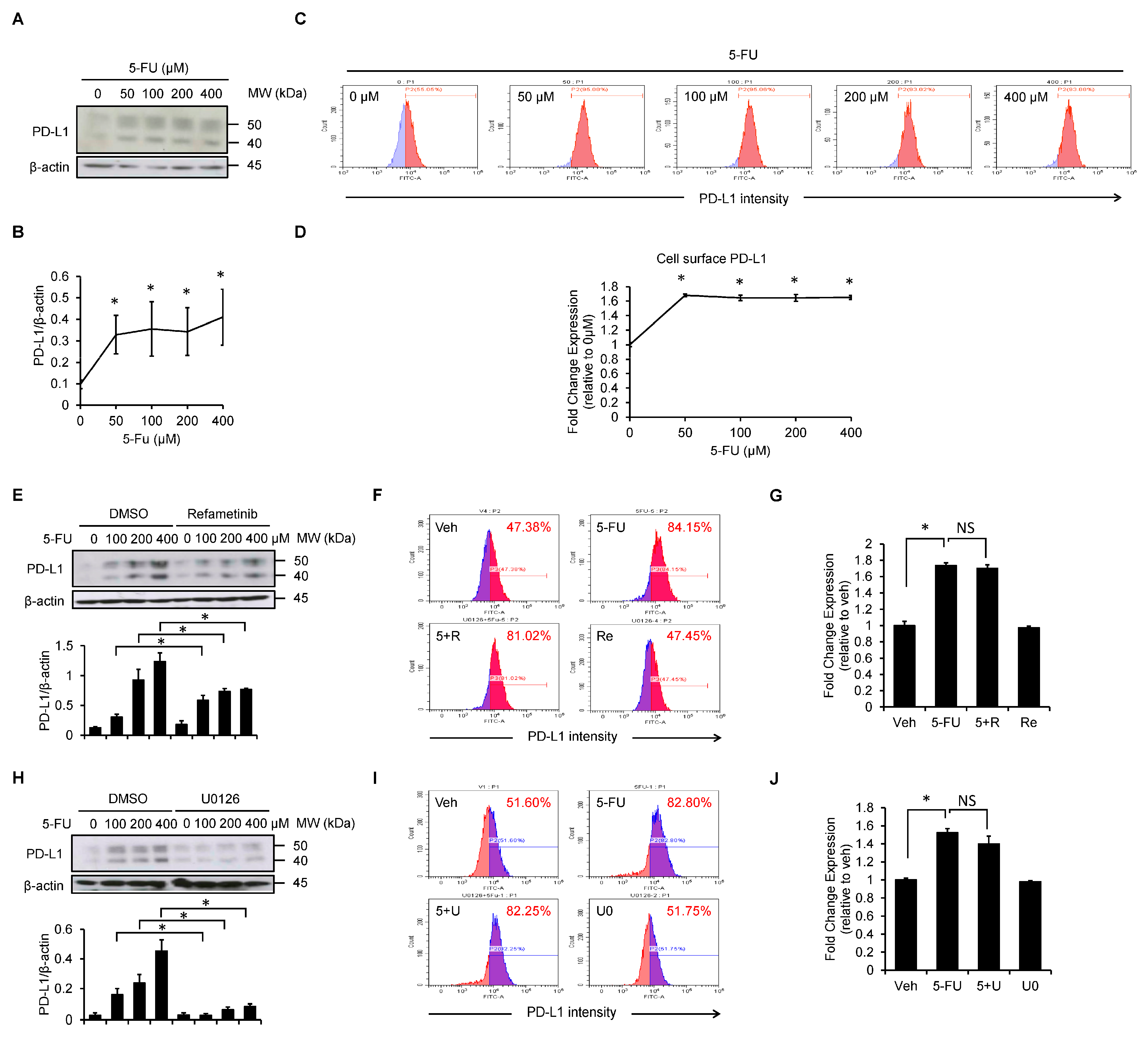
3.3. Refametinib Mitigates 5-FU-Induced PD-L1 Expression in SCC4 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand challenges in oral cancers. Front. Oral. Health 2020, 1, 3. [Google Scholar] [CrossRef]
- Elaiwy, O.; El Ansari, W.; AlKhalil, M.; Ammar, A. Epidemiology and pathology of oral squamous cell carcinoma in a multi-ethnic population: Retrospective study of 154 cases over 7 years in Qatar. Ann. Med. Sur. 2020, 60, 195–200. [Google Scholar] [CrossRef]
- Moreau Bachelard, C.; Coquan, E.; du Rusquec, P.; Paoletti, X.; Le Tourneau, C. Risks and benefits of anticancer drugs in advanced cancer patients: A systematic review and meta-analysis. EClinicalMedicine 2021, 40, 101130. [Google Scholar] [CrossRef]
- Bailly, C.; Thuru, X.; Quesnel, B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer 2020, 2, zcaa002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Tong, D.; Poot, M.; Hu, D.; Oda, D. 5-Fluorouracil-induced apoptosis in cultured oral cancer cells. Oral. Oncol. 2000, 36, 236–241. [Google Scholar] [CrossRef]
- Jacobs, C.; Lyman, G.; Velez-García, E.; Sridhar, K.S.; Knight, W.; Hochster, H.; Goodnough, L.T.; Mortimer, J.E.; Einhorn, L.H.; Schacter, L. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J. Clin. Oncol. 1992, 10, 257–263. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Ueyama, Y. Establishment of 5-fluorouracil-resistant oral squamous cell carcinoma cell lines with epithelial to mesenchymal transition changes. Int. J. Oncol. 2014, 44, 1302–1308. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef] [Green Version]
- Mishima, K.; Yamada, E.; Masui, K.; Shimokawara, T.; Takayama, K.; Sugimura, M.; Ichijima, K. Overexpression of the ERK/MAP kinases in oral squamous cell carcinoma. Mod. Pathol. 1998, 11, 886–891. [Google Scholar] [PubMed]
- Iverson, C.; Larson, G.; Lai, C.; Yeh, L.T.; Dadson, C.; Weingarten, P.; Appleby, T.; Vo, T.; Maderna, A.; Vernier, J.M.; et al. RDEA119/BAY 869766: A potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009, 69, 6839–6847. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Tian, H. Current development status of MEK inhibitors. Molecules 2017, 22, 1551. [Google Scholar] [CrossRef] [Green Version]
- Kiessling, M.K.; Rogler, G. Targeting the RAS pathway by mitogen-activated protein kinase inhibitors. Swiss Med. Wkly. 2015, 145, w14207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steijns, F.; Bracke, N.; Renard, M.; De Backer, J.; Sips, P.; Debunne, N.; Wynendaele, E.; Verbeke, F.; De Spiegeleer, B.; Campens, L. MEK1/2 inhibition in murine heart and aorta after oral administration of refametinib supplemented drinking water. Front. Pharmacol. 2020, 11, 1336. [Google Scholar] [CrossRef]
- Weekes, C.D.; Von Hoff, D.D.; Adjei, A.A.; Leffingwell, D.P.; Eckhardt, S.G.; Gore, L.; Lewis, K.D.; Weiss, G.J.; Ramanathan, R.K.; Dy, G.K.; et al. Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin. Cancer Res. 2013, 19, 1232–1243. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.Y.; Yoon, J.H.; Hsu, C.; Rau, K.M.; Poon, R.T.; Yeo, W.; Park, J.W.; et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 5976–5985. [Google Scholar] [CrossRef] [Green Version]
- Van Laethem, J.L.; Riess, H.; Jassem, J.; Haas, M.; Martens, U.M.; Weekes, C.; Peeters, M.; Ross, P.; Bridgewater, J.; Melichar, B.; et al. Phase I/II study of refametinib (BAY 86-9766) in combination with gemcitabine in advanced pancreatic cancer. Target. Oncol. 2017, 12, 97–109. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Zerdes, I.; Matikas, A.; Bergh, J.; Rassidakis, G.Z.; Foukakis, T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: Biology and clinical correlations. Oncogene 2018, 37, 4639–4661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, K.; Ali, A.; Magalhaes, M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Sci. Rep. 2020, 10, 9705. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; García-Pedrero, J.M. PD-L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Huang, D.; Ramsey, A.J.; Ig-Izevbekhai, K.; Zhang, K.; Lajud, S.A.; O’Malley, B.W.; Li, D. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 122, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Barsoum, I.B.; Truesdell, P.; Cotechini, T.; Macdonald-Goodfellow, S.K.; Petroff, M.; Siemens, D.R.; Koti, M.; Craig, A.W.; Graham, C.H. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016, 7, 10557–10567. [Google Scholar] [CrossRef] [Green Version]
- Lailler, C.; Lamuraglia, M.; Racine, F.; Louandre, C.; Godin, C.; Chauffert, B.; Galmiche, A.; Saidak, Z. DNA damage response- and JAK-dependent regulation of PD-L1 expression in head and neck squamous cell carcinoma (HNSCC) cells exposed to 5-fluorouracil (5-FU). Transl. Oncol. 2021, 14, 101110. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kraak, L.; Goel, G.; Ramanan, K.; Kaltenmeier, C.; Zhang, L.; Normolle, D.P.; Freeman, G.J.; Tang, D.; Nason, K.S.; Davison, J.M.; et al. 5-Fluorouracil upregulates cell surface B7-H1 (PD-L1) expression in gastrointestinal cancers. J. Immunother. Cancer 2016, 4, 65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Su, D.M.; Liang, M.; Fu, J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 2008, 45, 1470–1476. [Google Scholar] [CrossRef]
- Tu, Y.C.; Yeh, W.C.; Yu, H.H.; Lee, Y.C.; Su, B.C. Hedgehog suppresses paclitaxel sensitivity by regulating Akt-mediated phosphorylation of Bax in EGFR wild-type non-small cell lung cancer cells. Front. Pharmacol. 2022, 13, 815308. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Davern, M.; O’ Brien, R.M.; McGrath, J.; Donlon, N.E.; Melo, A.M.; Buckley, C.E.; Sheppard, A.D.; Reynolds, J.V.; Lynam-Lennon, N.; Maher, S.G.; et al. PD-1 blockade enhances chemotherapy toxicity in oesophageal adenocarcinoma. Sci. Rep. 2022, 12, 3259. [Google Scholar] [CrossRef]
- Patel, S.A.; Minn, A.J. Combination cancer therapy with immune checkpoint blockade: Mechanisms and strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakagami, H. Apoptosis-inducing activity and tumor-specificity of antitumor agents against oral squamous cell carcinoma. Jpn. Dent. Sci. Rev. 2010, 46, 173–187. [Google Scholar] [CrossRef] [Green Version]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Simões, A.E.; Gomes, S.E.; Castro, R.E.; Carvalho, T.; Rodrigues, C.M.; Borralho, P.M. MEK5/ERK5 signaling inhibition increases colon cancer cell sensitivity to 5-fluorouracil through a p53-dependent mechanism. Oncotarget 2016, 7, 34322–34340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Chen, Y.; Yang, L.; Pillai, R.; Shirasawa, S.; Fakih, M. MEK162 enhances antitumor activity of 5-Fluorouracil and Trifluridine in KRAS-mutated human colorectal cancer cell lines. Anticancer Res. 2017, 37, 2831–2838. [Google Scholar] [CrossRef] [Green Version]
- Urick, M.E.; Chung, E.J.; Shield, W.P., 3rd; Gerber, N.; White, A.; Sowers, A.; Thetford, A.; Camphausen, K.; Mitchell, J.; Citrin, D.E. Enhancement of 5-fluorouracil-induced in vitro and in vivo radiosensitization with MEK inhibition. Clin. Cancer Res. 2011, 17, 5038–5047. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, Y.; Liu, X.; Chen, C.; Harrington, S.M.; Cao, S.; Xie, T.; Pham, T.; Mansfield, A.S.; Yan, Y.; et al. Targeting B7-H1 (PD-L1) sensitizes cancer cells to chemotherapy. Heliyon 2018, 4, e01039. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.Y.; Li, J.; Tao, L.; Lam, A.K.; Chan, K.W.; Ko, J.; Yu, V.Z.; Wong, M.; Li, B.; Lung, M.L. Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation. Transl. Oncol. 2018, 11, 1323–1333. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Y.; Che, X.; Hou, K.; Zhang, C.; Li, C.; Wen, T.; Wang, S.; Cheng, Y.; Liu, Y.; et al. 5-FU-induced upregulation of exosomal PD-L1 causes immunosuppression in advanced gastric cancer patients. Front. Oncol. 2020, 10, 492. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Su, B.-C.; Ma, T.-L.; Hong, Y.C.; Chen, Y.-W.; Vo, T.T.T.; Wu, L.-Y.; Peng, T.-Y.; Wang, C.-S.; Lee, I.-T. Enhancement of Anticancer Effects by Combining 5-Fluorouracil with Refametinib in Human Oral Squamous Cell Carcinoma Cell Line. Appl. Sci. 2023, 13, 4340. https://doi.org/10.3390/app13074340
Chen P-C, Su B-C, Ma T-L, Hong YC, Chen Y-W, Vo TTT, Wu L-Y, Peng T-Y, Wang C-S, Lee I-T. Enhancement of Anticancer Effects by Combining 5-Fluorouracil with Refametinib in Human Oral Squamous Cell Carcinoma Cell Line. Applied Sciences. 2023; 13(7):4340. https://doi.org/10.3390/app13074340
Chicago/Turabian StyleChen, Po-Chun, Bor-Chyuan Su, Tien-Li Ma, Ying Chui Hong, Yu-Wen Chen, Thi Thuy Tien Vo, Luo-Yun Wu, Tzu-Yu Peng, Ching-Shuen Wang, and I-Ta Lee. 2023. "Enhancement of Anticancer Effects by Combining 5-Fluorouracil with Refametinib in Human Oral Squamous Cell Carcinoma Cell Line" Applied Sciences 13, no. 7: 4340. https://doi.org/10.3390/app13074340
APA StyleChen, P.-C., Su, B.-C., Ma, T.-L., Hong, Y. C., Chen, Y.-W., Vo, T. T. T., Wu, L.-Y., Peng, T.-Y., Wang, C.-S., & Lee, I.-T. (2023). Enhancement of Anticancer Effects by Combining 5-Fluorouracil with Refametinib in Human Oral Squamous Cell Carcinoma Cell Line. Applied Sciences, 13(7), 4340. https://doi.org/10.3390/app13074340






