Abstract
In this research, we have developed the approach to controlled synthesis of acrylonitrile-acrylamide copolymers with narrow molecular weight distribution and various monomer sequence distributions. By using dibenzyl trithiocarbonate and batch/semibatch polymerization, we have first synthesized random, gradient, and block-gradient copolymers containing 3.4–10.2 mol. % of acrylamide and revealed the influence of the monomer sequence on the cyclization behavior of poly(acrylonitrile-co-acrylamide) by combination of differential scanning calorimetry and Fourier transform infrared spectroscopy. This allowed us to find differences in cyclization behavior of the copolymers in argon and air atmosphere. Intramolecular cyclization was the main process proceeding in argon atmosphere. The radical mechanism of cyclization was suppressed already at the molar part of acrylamide units in copolymer exceeding ~3 mol. % for random copolymer and ~6 mol. % for block-gradient copolymer. The activation energy of ionic cyclization was equal to 89 ± 3 kJ·mol−1 and was not influenced by both copolymer composition and chain microstructure in contrast to the rate of cyclization. The latter was increased with the rise of acrylamide content, the content of hetero-triads and in the range block-gradient < gradient < random structure. In air atmosphere, the oxidation reactions dominated over cyclization. The oxidation reactions were found to be less sensitive to copolymer composition and chain microstructure.
1. Introduction
Copolymers of acrylonitrile (AN) are widely used for producing precursors of carbon fibers [1,2,3,4,5,6].
The final properties of the carbon fibers depend on the variety of factors including molecular characteristics of the polymer, the parameters of polymer molding, thermo-oxidative stabilization, and carbonization [7]. The molecular characteristics of the polymer include copolymer composition, monomer sequence distribution, molecular weight (MW), and molecular weight distribution (MWD) of the polymer [8]. Copolymer composition, MW, and MWD can be varied in a wide range and are governed by polymerization conditions [8]. The monomer sequence distribution is set up by the monomer reactivity ratio and monomer feed ratio [9]. However, if the monomers have different reactivity in the copolymerization, i.e., they are consumed at different rates, then the monomer feed changes continuously throughout reaction resulting in the formation of macromolecules of various composition and monomer sequence distribution [8]. To overcome this problem, the monomer composition in the reaction mixture should be kept constant. It can be achieved by supplemental addition of less reactive monomer with a given rate providing formation of macromolecules with similar composition during all the copolymerization. However, the synthesis of the copolymers with different monomer sequence distribution, but similar average composition in conditions of free radical copolymerization, seems unlikely. At the same time, it becomes possible in conditions of reversible deactivation radical polymerization (RDRP) [10,11,12]. RDRP differs from conventional free radical polymerization due to appearance of new reversible termination and/or transfer reactions of the propagating radicals with special compounds, which suppress the irreversible termination reaction of the propagating radicals and result in the continuous conversion of deactivated macromolecules into propagating radicals. Thus, RDRP has similar features as a living anionic polymerization, i.e., the number-average molecular weight (Mn) increases linearly with progress in monomer conversion, molecular weight distribution becomes narrow, and the random or gradient copolymers with high compositional homogeneity can form from the monomers with different reactivity [10]. The formation of gradient copolymers with different monomer sequence distribution in RDRP is possible even if the difference between the reactivity ratios of the comonomers is not significant, when monomers are dosed with different rates. So, semibatch reactors can be used to produce gradient copolymers by using different comonomer feed policies. The model was used to predict the following characteristics of copolymers of styrene-methyl methacrylate and acrylonitrile-methyl methacrylate in atom transfer radical polymerization (i.e., one of the RDRP techniques) using semibatch reactors: average MW, dispersity, copolymer composition, and comonomer sequence length at any polymerization time [13]. This simulation has demonstrated the possibility of varying the monomer unit distribution from random to gradient under conditions mentioned above. The combination of RDRP and the use of semibatch reactor was implemented for reversible addition–fragmentation chain transfer (RAFT) copolymerization of AN and N-isopropyl acrylamide (NIPA) for the synthesis of the copolymer with uniform distribution of NIPA units along the chain rather than its gradient distribution [14]. The batch copolymerization of AN and NIPA resulted in the formation of the gradient copolymer with the compositional drift of NIPA along the backbone. In this case, the cyclization reaction in gradient copolymer proceeds in the narrow temperature range with intensive heat release, which are undesirable for production of carbon fibers. Amide group in NIPA unit can probably accelerate the cyclization reaction, as the temperature of cyclization onset was lower than for pure PAN. The semibatch copolymerization leads to the formation of the copolymer with uniform distribution of NIPA along the backbone. The cyclization reaction proceeds in the wide temperature range and with low intensity of heat release, that is preferable for carbon fiber production. The authors relate this effect to the ability of amide moiety to accelerate the cyclization reaction. However, no detailed discussion of this phenomenon was given.
The similar approach was implemented in [15,16] for the copolymers of AN and acrylic acid (AA) produced by RAFT copolymerization. Acrylic acid is able to accelerate the cyclization of nitrile groups similarly to amide-containing AN copolymer [17,18,19]. The batch, continuous, and semi-continuous copolymerizations led to the formation of macromolecules with various drift of AA units along the backbone and to the drastic changes in the properties of the copolymers, i.e., their solubility, spinnability, and cyclization behavior [15,16,20,21].
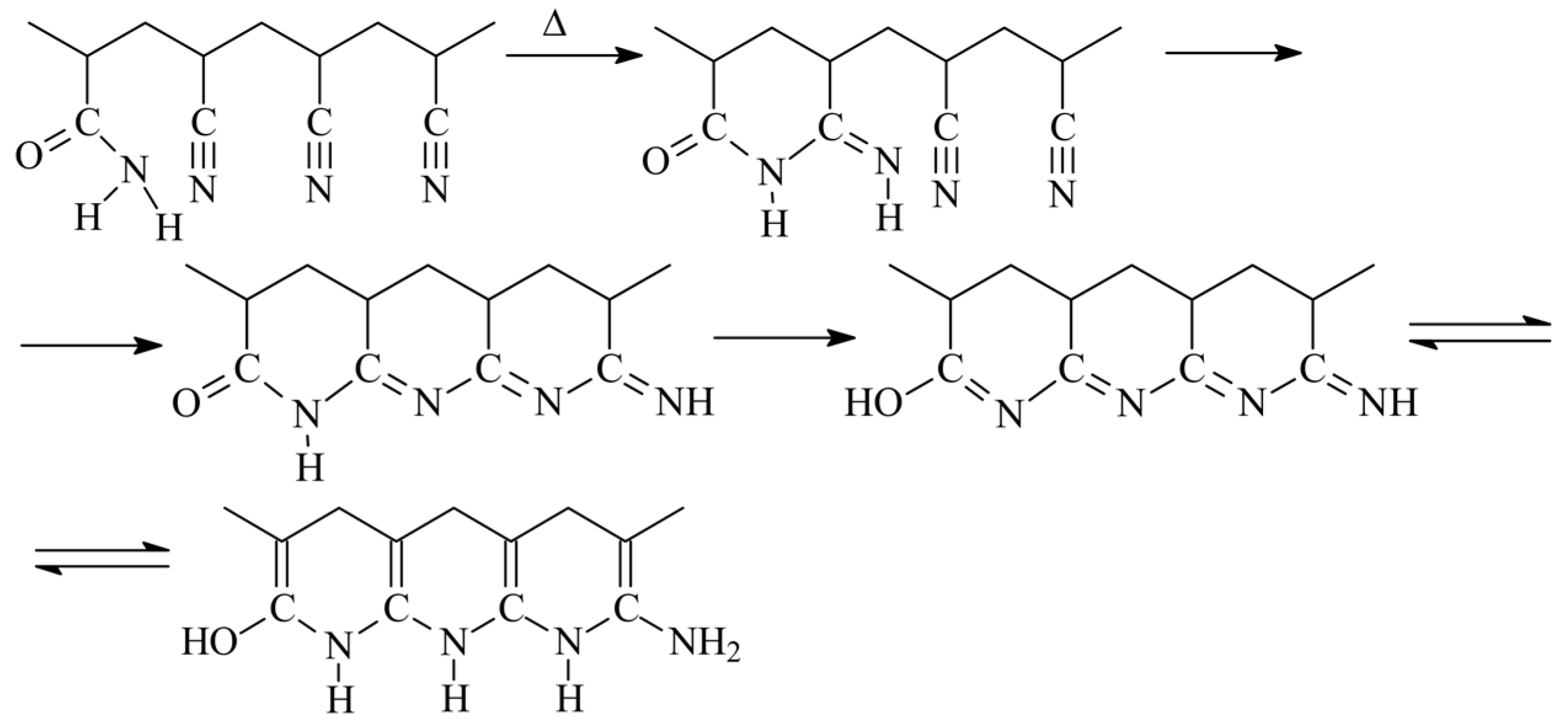
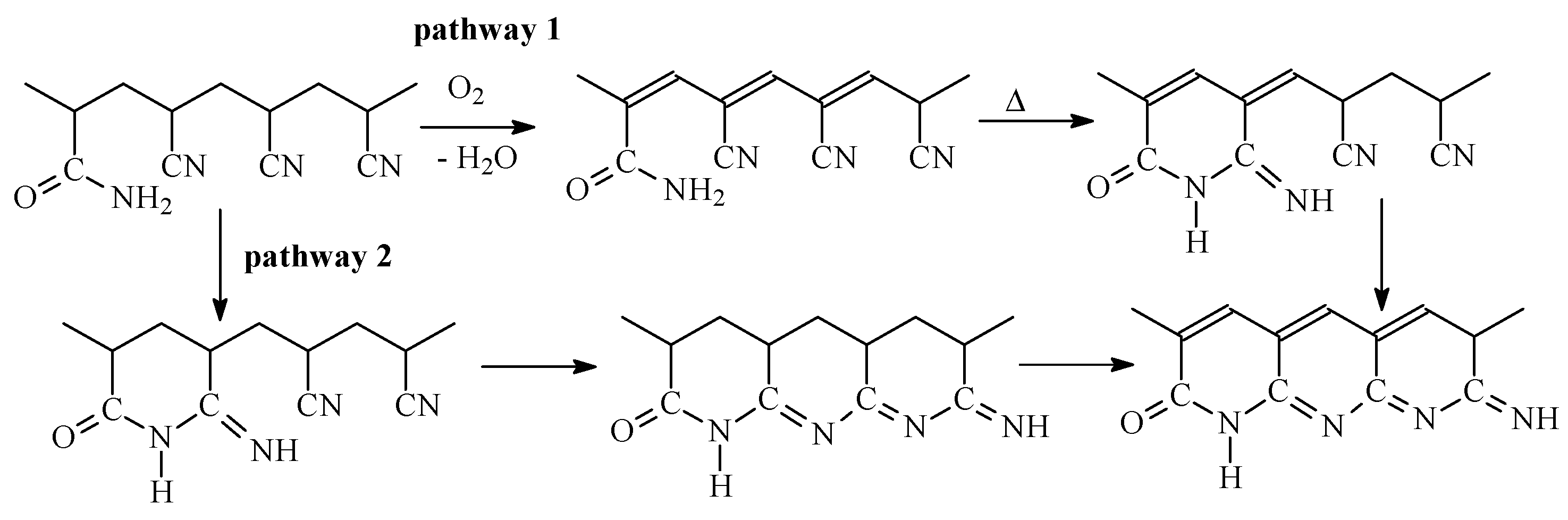
Acrylamide (AAM) is another monomer that can accelerate cyclization reaction. Its ability to accelerate cyclization of nitrile groups was described first in [22] on the example of the copolymers synthesized through copolymerization in water. The copolymers of AN and AAM can be obtained via a radical mechanism only resulting to the formation of atactic copolymer [8]. To eliminate the composition heterogeneity of the copolymers, the synthesis was stopped at low monomer conversions. The nature of impact of AAM on the cyclization was proposed in [23] basing on the study of FTIR spectra of the similar AN and AAM copolymers subjected to thermal treatment under reduced pressure and in air. Cyclization can be observed even at 130 °C after the induction period of 4 h, while at 160 °C the induction period is about 1 h. After the induction period is completed, the intensities of the absorption bands corresponding to CN, CO, and NH groups fall drastically, proving participation of AAM in cyclization. The proposed mechanism of cyclization initiated by AAM is rather complicated and involves several steps (Scheme 1) [22].
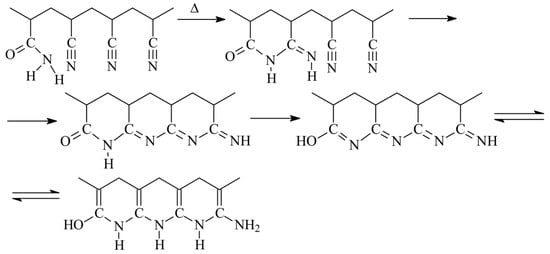
Scheme 1.
The development of ladder structure in the copolymers of AN and AAM.
The influence of the copolymer composition on the cyclization reaction is rather interesting. Below 3 mol. % of AAM in the copolymer, cyclization proceeds slowly at both at 160 and 200 °C, while at AAM content above 5 mol. % the cyclization rate is higher and it does not depend on the copolymer composition indicating that the fraction of the available nitrile and amide groups that have reacted at the given time seems a constant value [24]. Besides, the extent of the coloration of the copolymer films after the same time of treatment is inversely proportional to the concentration of AAM in the copolymer, suggesting that the extent of conjugation decreases with the rise of AAM content in the copolymer. These observations have been achieved for the copolymers obtained by radical copolymerization in water with monomer reactivity ratios equal to rAN = 1.5 and rAAM = 0.3. In this case, the sequence length of AN units is 40 and 9 and AAM units 1 for the monomer feed with 2.5 and 10 mol. % of AAM. However, the simple calculations of the number of AN units that can participate in cyclization reaction based only on the monomer sequence distribution give overrated results. However, if the stereochemistry (iso-/sindiotactic placements) is taken into account, then the experimental and theoretic values become close. In the latter model, the amide group initiates the cyclization reaction if the neighboring nitrile group has isotactic placement, and the conjugation spreads over the backbone in the case of isotactic configuration of AN units (intramolecular cyclization) and transfers to another chain in the case of sindiotactic configuration of AN units (intermolecular cyclization).
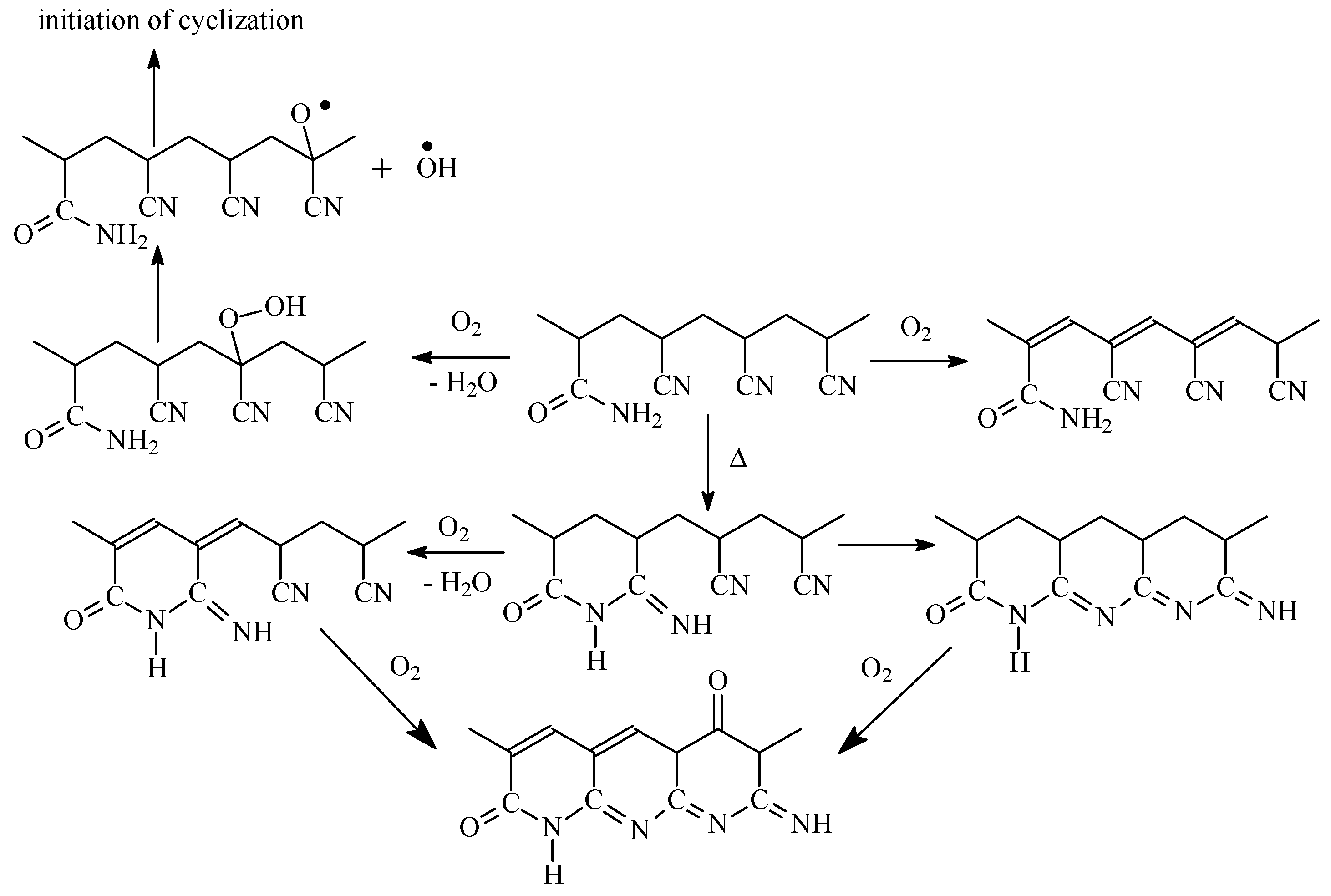
In air atmosphere, new reactions take place along with cyclization in similar temperature range. This conclusion is confirmed by FTIR analysis of the copolymers subjected to thermo-oxidative stabilization, which revealed the slow formation of pyridine structure and slow consumption of NH2 groups [25]. The authors proposed that two processes compete with each other: intramolecular and intermolecular cyclization followed by oxidative reactions, according to Scheme 2. It was supposed that thermal treatment in air leads to the acceleration of dehydration reaction rather than cyclization. Besides, during the short induction period, amide groups react with neighboring nitrile groups forming cyclic structures. After reaching the critical concentration of these structures, the conjugation starts, first through dehydration reaction, followed by cyclization reaction.
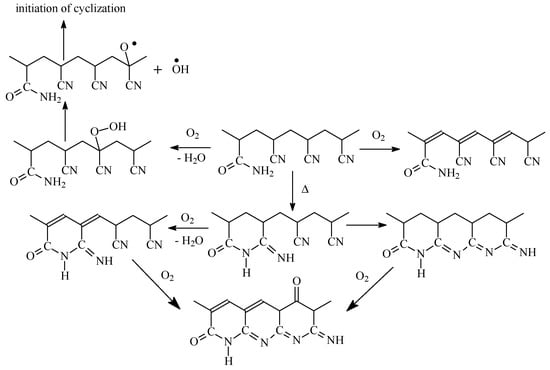
Scheme 2.
The proposed mechanism of thermo-oxidative stabilization.
Cyclization behavior of various AN and AAM copolymers is described [22,23,24,25,26,27,28]. However, they were synthesized in different conditions (solvent, temperature, etc.). Meanwhile, the change of the radical copolymerization method (copolymerization in solution, precipitative copolymerization, etc.) affects the monomer reactivity and changes the monomer sequence distribution. For example, in DMF or DMF–water mixtures (where water is the non-solvent for the AN and AAM copolymer), the monomer reactivity ratios are rAN = 0.86 and rAAM = 0.81 [29]. The polarity of non-solvent gives rise to a large variety of the monomer apparent reactivity ratios for AN and AAM [30]. Copolymerization in DMSO results in rAN = 0.65 and rAAM = 1.68 [31]. As a result, the comparison of the kinetics of cyclization of nitrile groups for the copolymers that differ simultaneously in their composition, monomer sequence distribution, and compositional heterogeneity becomes impossible. So, the question about the optimal molecular structure of AN and AAM copolymer that is suitable for production of carbon fibers remains unsolved.
Previously, we have shown that RAFT copolymerization in dimethylsulfoxide (DMSO) solution may be applied to the AN and AAM and results in the formation of copolymers with Mn = 10–30 kg·mol−1 and dispersity Đ = 1.5–1.6 [32]. We have also demonstrated that rheological and cyclization behavior of RAFT-based copolymers and copolymers containing 2 and 10 mol. % of AAM prepared by conventional free radical copolymerization is different. However, the detailed study of the cyclization behavior of the copolymers was beyond our interest at that time. Recently, we have also studied the influence of alkyl acrylate nature on the rheological and cyclization behavior of ternary copolymers of AN, AAM, and alkyl acrylate of given composition synthesized by RAFT copolymerization [33,34]. We have shown that the cyclization rate and formation of ladder structure increases in the following range of acrylate monomer: methyl acrylate < butyl acrylate < 2-ethylhexyl acrylate < lauryl acrylate.
In the present work, we continued working with the RAFT-based copolymers of AN and AAM, aiming to study in detail cyclization behavior of RAFT-based copolymers. The combination of RAFT mechanism and the controlled addition of AAM in the reaction should provide the copolymer with a tunable composition profile and allows the study of the influence of the molecular structure of the copolymers on their cyclization behavior.
2. Materials and Methods
Acrylonitrile (99 %), supplied by Acros, was distilled before use. Acrylamide (97%), from Fluka (Buchs, Switzerland), was recrystallized from chloroform. Azobisisobutyronitrile (AIBN) used as a radical initiator was recrystallized from ethanol and stored in a dark place at −3 °C. Dibenzyl trithiocarbonate (C6H5CH2–S–C(=S)–S–CH2C6H5, BTC) was synthesized and characterized according to the known procedure [35]. Dimethylsulfoxide (DMSO, 99%) and N,N-dimethylformamide (DMF, HPLC), supplied by Fluka, was distilled under reduced pressure before use.
Polymerization was performed in a 100 mL three-neck flask equipped with an overhead device with an anchor-type stirrer. A solution containing the calculated amounts of AIBN and BTC dissolved in AN and DMSO was loaded into the flask. In the batch process, AAM was added also before the beginning of the polymerization. In the semi-batch process, AAM was loaded in DMSO solution (1/1 wt) with a BYZ-810 syringe pump (Table 1). The detailed formulations are given in the Table S1, ESI. The flask was purged with argon (99.99%) for 15 min, closed, and immersed in a bath heated to 80 °C. At a specified time, samples were taken for analysis. The total monomer conversion was determined gravimetrically by precipitating the polymer in the excess of deionized water.

Table 1.
Formulations of the RAFT copolymerization of AN and AAM.
Samples for analysis by attenuated total reflection Fourier transform infrared (ATR FTIR) spectroscopy and differential scanning calorimetry (DSC) were prepared by dissolving the copolymer (4 wt%) in DMSO and cast onto glass substrate. The samples were then placed in the vacuum oven at 80 °C overnight. The resulted films were washed with water for 24 h to remove the residual DMSO, dried to the constant weight, and separated from the surface. The films with average thickness of 20–50 μm were cut into pieces 20 × 20 mm and analyzed.
The average molecular weights and dispersity (ÐM = Mw/Mn) were determined by the size exclusion chromatography (SEC). The SEC measurements were performed in DMF containing 0.1 wt% of LiBr at 50 °C with a flow rate of 1.0 mL/min using a chromatograph GPC-120 PolymerLabs (Santa Clara, CA, USA) equipped with refractive index and with two columns PLgel 5 µm MIXED B for MW range 5 × 102–1 × 107. The SEC system was calibrated using narrow dispersed linear poly(methyl methacrylate) standards with MW ranging from 800 to 2 × 106 g mol−1. A second-order polynomial was used to fit the log10M versus retention time dependence. The molecular weights were recalculated for poly(acrylonitrile) using known coefficients of the Mark–Houwink equation K1M1α1 = K2M2α2, where K = 39.4 × 10−4, α = 0.75 for PAN, and K = 17.7 × 10−4, α = 0.62 for PMMA [36].
FTIR spectroscopy in ATR mode (diamond crystal) was recorded using Spectrum Two FT-IR Spectrometer (PerkinElmer) in the range of 4000–400 cm−1. For quantitative analysis of the composition of the films of the synthesized copolymers, the calibration curve was used (Figure S1). The calibration curve was obtained from the mixtures of the monomers of the known molar ratio. The ratio of the intensities, A of the characteristics bands assigned to νCO = 1690 cm−1 (AAM) and νCN = 2229 cm−1 (AN) was used [37]. The dependence of ACO/ACN on the molar ratio [AAM]/[AN] was linear. The copolymers of the known composition were used to confirm the validity of the calibration curves.
1H NMR and 13C NMR spectra of copolymers were recorded using pulse Fourier spectrometer Bruker DPX-500 operating at 500 MHz in DMSO-d6. Tetramethyl silane was used as an internal standard.
Differential scanning calorimetry was performed on a Netzsch DSC 204 (Netzsch, Germany) in the atmosphere of dry gas (air, argon) at flow rate of 100 mL·min−1 in the range of 30–500 °C and heating rate from 5 to 30 K·min−1 in air and 10 K·min−1 in argon. The activation energy of the reactions was determined by Kissinger method [38]:
where R—universal gas constant, Tp—peak temperature (K), and ϕ—heating rate (K·min−1).
FTIR spectra in ATR mode (diamond crystal) of the films subjected to thermal treating in argon at 200 and 225 °C and in air at 200 °C were recorded using Spectrum Two FT-IR Spectrometer (PerkinElmer, Waltham, MA, USA) in the range of 4000–400 cm−1. The proportion of the unreacted nitrile groups ϕCN [39] and stabilization index Es were determined according to the equations [40]:
where —intensity of the band assigned to –C≡N group, —intensity of the band assigned to −C=N− group, and —the ratio of the extinction equal to 0.29.
3. Results and Discussion
3.1. CopolymerSynthesis
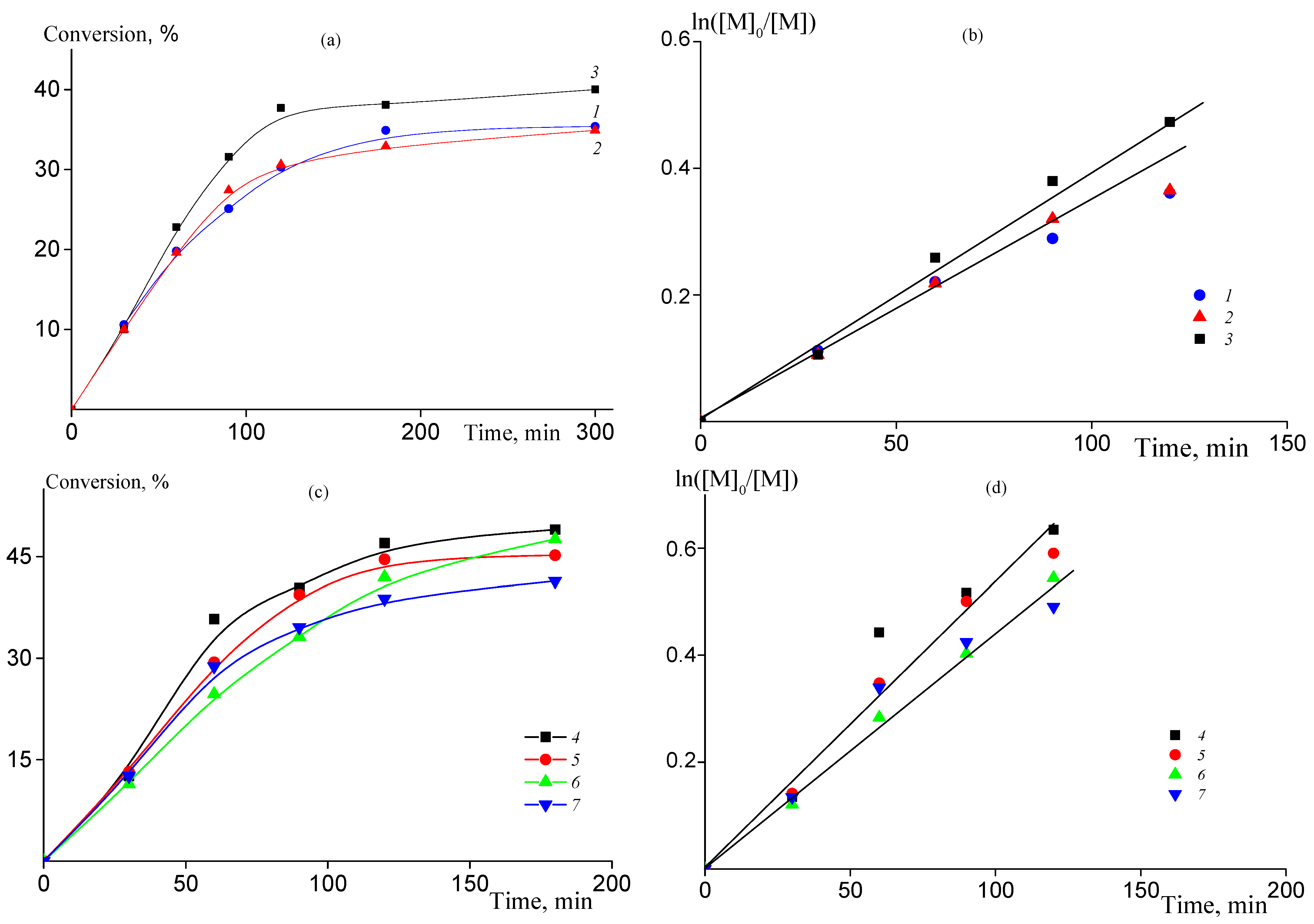
The copolymerization of AN with low amounts of AAM should proceed with similar rate as the homopolymerization of AN. Indeed, in the batch process the content of AAM in the monomer feed in the range of 2–7 mol. % has negligible effect on the copolymerization kinetics (Figure 1a). Simultaneously, the kinetic plots are described by first-order kinetics (Figure 1b), which is typical for RAFT polymerization [41,42,43,44]. In continuous and semi-continuous process, the total amount of AAM introduced into reaction is higher (10–15 mol. %, Table 1). However, due to the slow addition of AAM to the monomer mixture it has no visible effect on the copolymerization rate (Figure 1c). Similarly to the batch reaction, the copolymerization kinetics is first-order with respect to monomer concentration in the wide range of the polymerization time (Figure 1d).
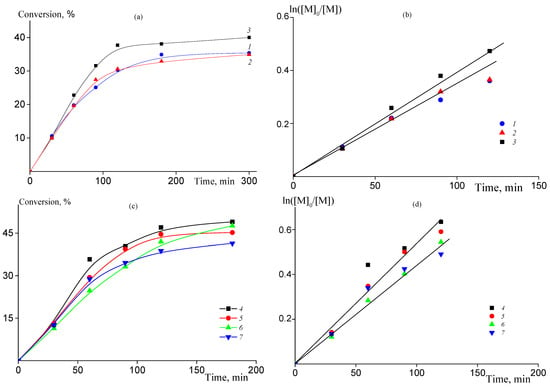
Figure 1.
(a,c) Time-conversion dependences for AN-AAM copolymerization in 60 wt% DMSO solution at 80 °C, [AIBN] = [BTC] = 1 mmol/L (a) and [AIBN] = [BTC] = 2 mmol/L (c); (b,d) corresponding semi-logarithmic dependences of the monomer concentration on the polymerization time. Numbering of the curves corresponds to the numbering of the samples from Table 1.
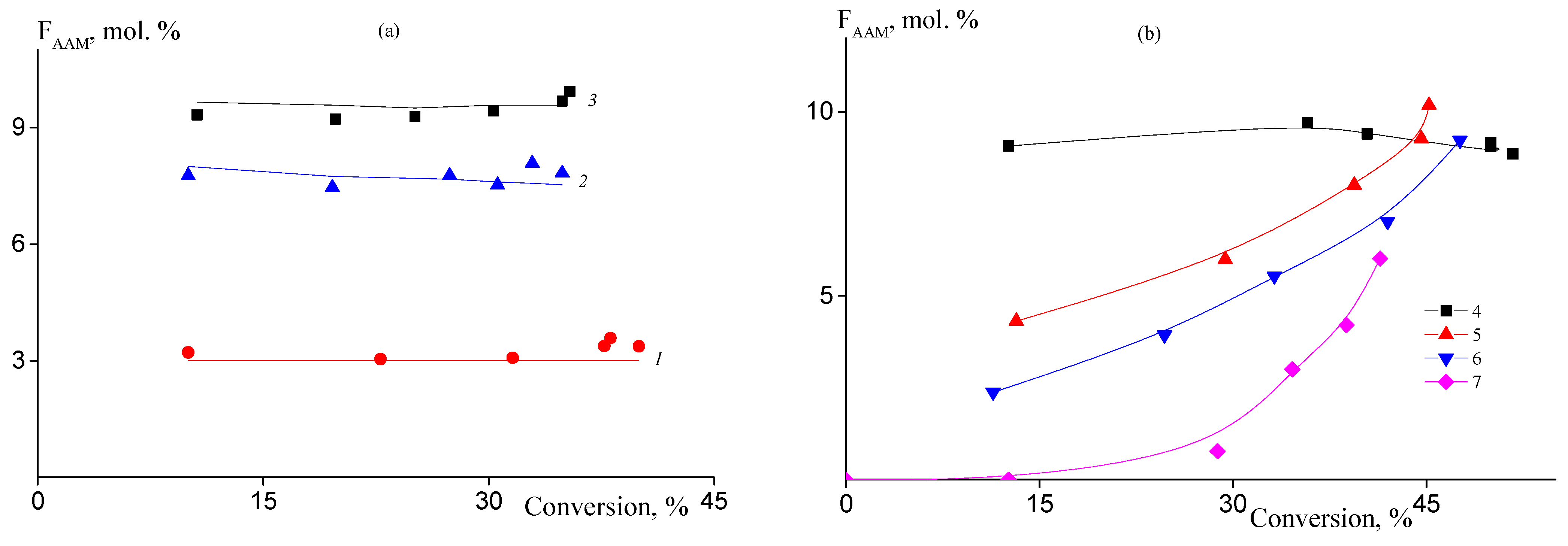
The changes of the copolymer composition throughout copolymerization were studied by ATR FTIR spectroscopy. Figure 2 presents the dependences of the molar part of AAM units in the copolymers formed in batch, continuous, and semi-continuous processes on the monomer conversion. AAM is a more active monomer than AN in solution polymerization in DMSO; the relative monomer activities are rAN = 0.65 and rAAM = 1.98 [31]. In batch copolymerization, the molar part of AAM units in the copolymer remains constant throughout reaction and the experimental values of copolymer composition (points) are in a good accordance with theoretical values (lines) calculated on the basis of rAN and rAAM values (Figure 2a). Continuous and semi-continuous processes give an opportunity to control the introduction of AAM in the copolymer chain (Figure 2b, curves 2–4). The lower the rate of AAM addition to the reaction mixture, the lower the AAM content in the copolymer. The average AAM molar fraction increases with the progress of the monomer conversion. In conditions of the conventional free radical copolymerization, the change of the average copolymer composition is accompanied by the growth of the composition dispersity. It can be illustrated by the following example. Let us suppose that the experiment 7 is conducted in the absence of BTC. In this case, before starting the addition of AAM, the polymer formed should be pure PAN due to the presence of AN in the polymerization mixture only. After AAM is added to the polymerization system, a copolymer should form. As a result, the copolymer formed at the end of the reaction will consist of the macromolecules of different composition. In RAFT copolymerization, the compositional heterogeneity of macromolecules is replaced by the composition heterogeneity along the macromolecule, but all macromolecules have similar composition. So, the copolymer is compositionally homogeneous. Thus, in experiment 7 performed in the presence of BTC, the polymer formed during a period below 0.5 h is also pure PAN, while later it is transferred to block-gradient copolymer due to chain-extension. Thus, combination of batch/continuous/semi-continuous process with RAFT mechanism allows synthesis of the copolymers with various monomer sequence distributions.
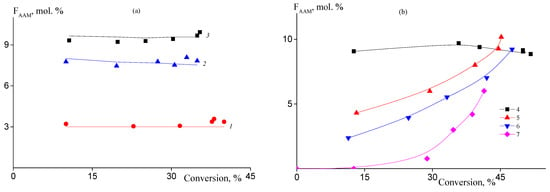
Figure 2.
Dependences of the molar part of AAM in the copolymer on the monomer conversion in the copolymerization of AN and AAM at 80 °C. (a) [AIBN] = [BTC] = 1 mmol/L; (b) [AIBN] = [BTC] = 2 mmol/L. Numbering of the curves corresponds to the numbering of the samples from Table 1.
Being active in cyclization reaction, the AAM unit distribution along the chain should affect the thermal behavior of AN/AAM copolymers. In batch process, triad composition at low monomer conversion and the instantaneous number-average sequence length of AN, <NAN>n, and AAM, <NAAM>n can be evaluated on the assumption of the validity of terminal model kinetics from the Equations (4) and (5) [9,45]:
The accordance between experimental and theoretical values of copolymer composition confirms the validity of the terminal-model kinetics. The calculated values of triad composition and number-average sequence length of AN and AAM units are summarized in Table 2. Among AN centered triads, triads AN-AN-AN prevail, while among AAM-centered triads, triads AN-AAM-AN prevail in the triad composition of macromolecules. Homo-triads AAM-AAM-AAM and hetero-triads AAM-AN-AAM are unfavorable. The number-average sequence length of AN decreases with the rise of the AAM content in monomer feed, while the sequence length of AAM is about 1. Thus, the batch process suggests that AAM units are randomly distributed along macromolecules at fAAM ≤ 7.

Table 2.
Triad composition and number-average sequence length of AN and AAM.
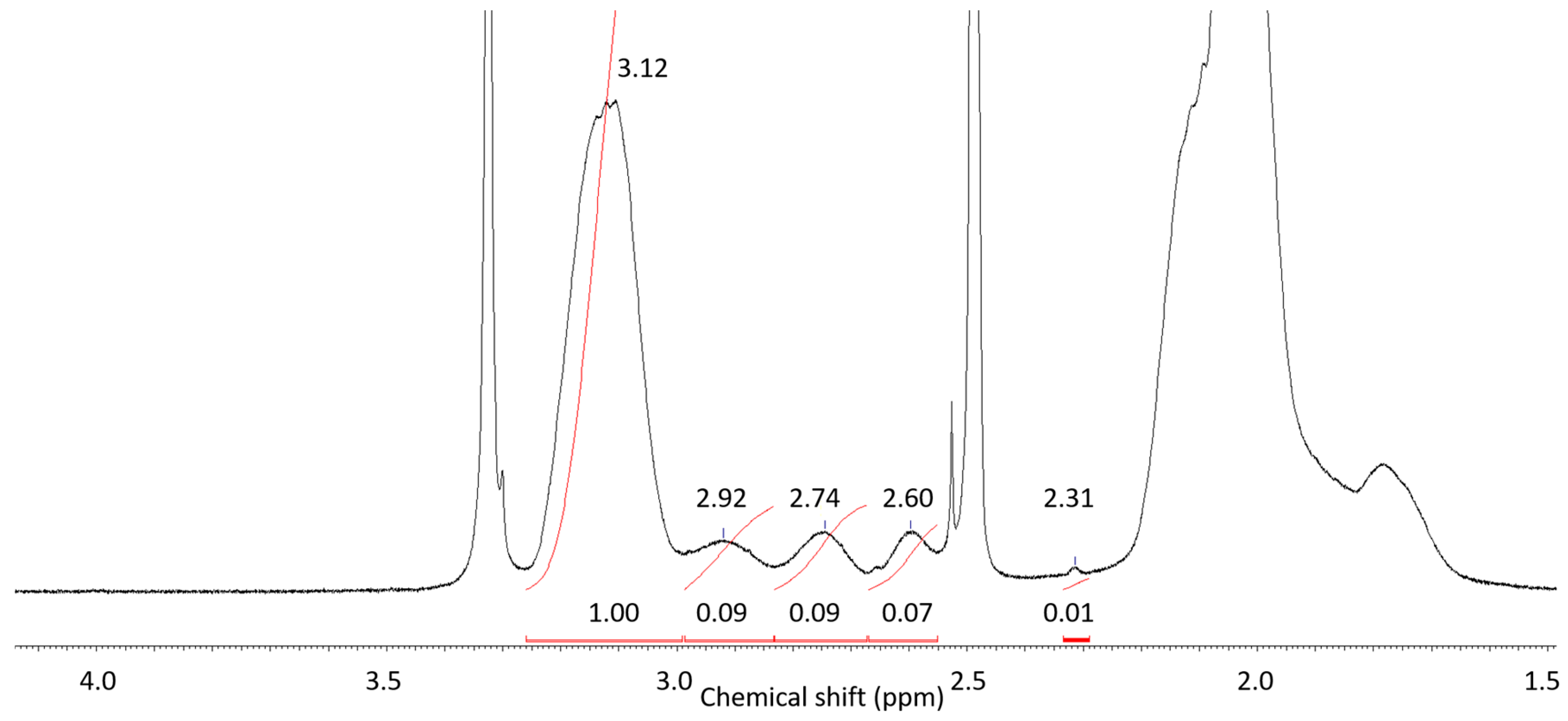
Different microstructures of the copolymers have been confirmed by NMR analysis of the copolymers 4–7 (Table 1) obtained at limited conversions. Figure 3 presents the typical 1H NMR spectrum of copolymer 4 in DMSO-d6 solution at room temperature. According to [46], the signals in the range of ~2–3 ppm correspond to compositional triad sequences: AN-AN-AN (3.12 ppm), AN-AN-AAM (2.92 ppm), AN-AAM-AN and AAM-AN-AAM (2.75 ppm), AN-AAM-AAM (2.60 ppm), and AAM-AAM-AAM (2.31 ppm). The spectra of copolymers 5–7 are given in ESI (Figure S2a–c). 13C NMR spectroscopy is used more frequently to determine polymer chain microstructure. However, the viscosity of copolymer solutions at room temperature was high enough and integration time was 20 min resulting in low intensity of the signals comparable with measurement noise due to limited capacity of the instrument (Figure S2d, ESI). Thus, for analysis of triad composition the results of 1H NMR spectroscopy was used.
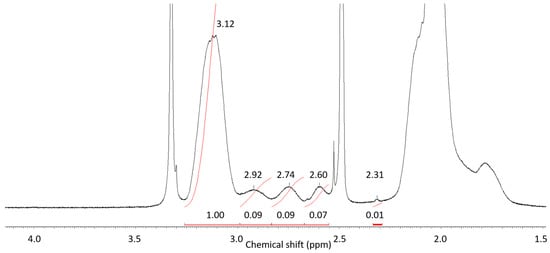
Figure 3.
1H NMR spectrum of copolymer 7 in DMSO–d6 solution at room temperature.
Table 3 presents the data of triad composition of the copolymers 4–7. In all the cases, the molar part of homo-triads AAM-AAM-AAM is negligible. For copolymers 4 and 5, the molar part of hetero-triads is similar. For copolymers 6 and 7, the molar part of homo-triad AN-AN-AN is higher than for copolymers 4 and 5. Thus, it may be concluded that cyclization behavior of the copolymers 4 and 5 may differ from the copolymers 6 and 7.

Table 3.
Triad composition of AN and AAM copolymers 4–7.
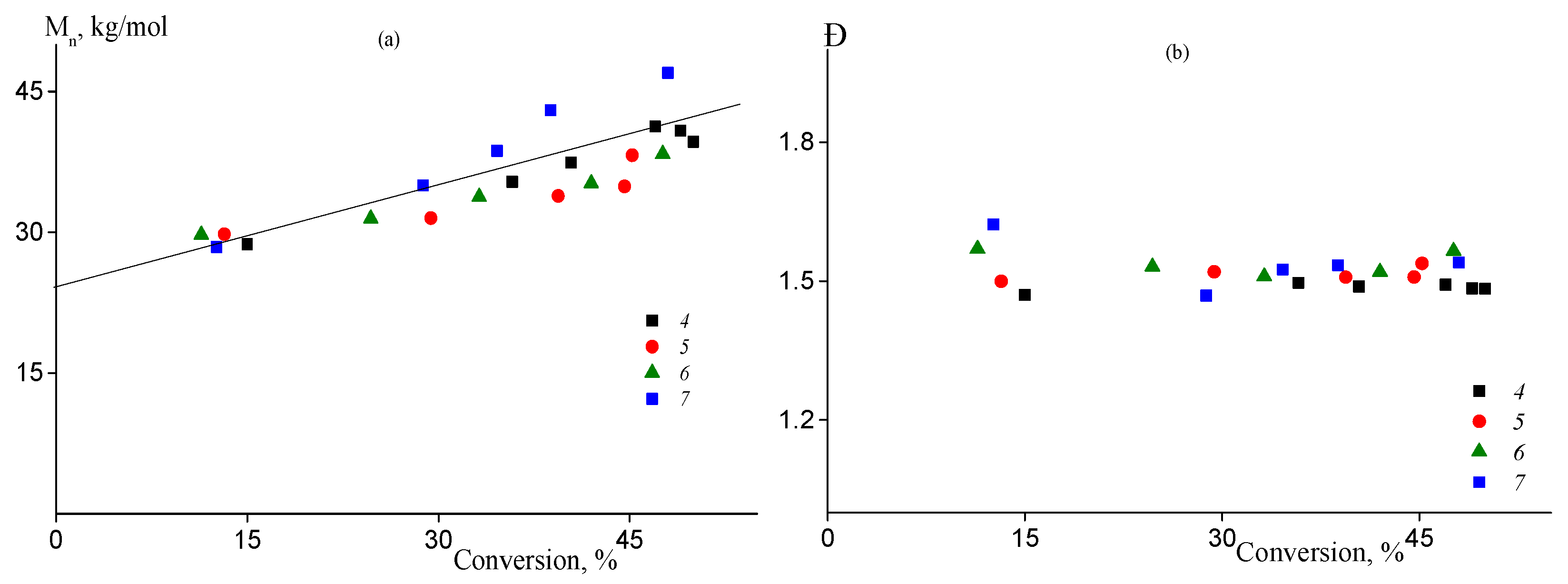
Previously we have shown that batch copolymerization of AN and AAM (fAAM ≤ 10) proceeded through RAFT mechanism in DMSO solution in the presence of BTC. Thus, it is expected that semi-batch processes have similar features. The SEC traces of the copolymers formed are given in ESI (Figure S3). Figure 4a demonstrates that the number-average molecular weight Mn of the copolymers increases linearly with the rise of the monomer conversion confirming RAFT mechanism of the process. Dispersion Đ = Mw/Mn remains constant throughout copolymerization and remains lower (1.4–1.5) compared to the free radical process (Figure 4b).
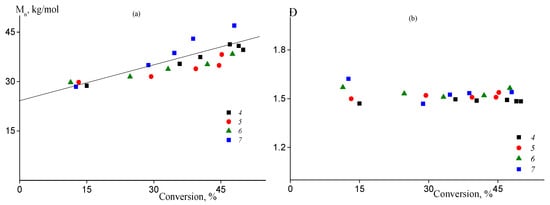
Figure 4.
The dependences of the number-average molecular weight Mn (a) and dispersity Đ (b) on the monomer conversion of the copolymers formed in the RAFT copolymerization of AN and AAM at 80 °C and [AIBN] = [BTC] = 2 mmol/L. Numbering of the legends corresponds to the numbering of the samples from Table 1.
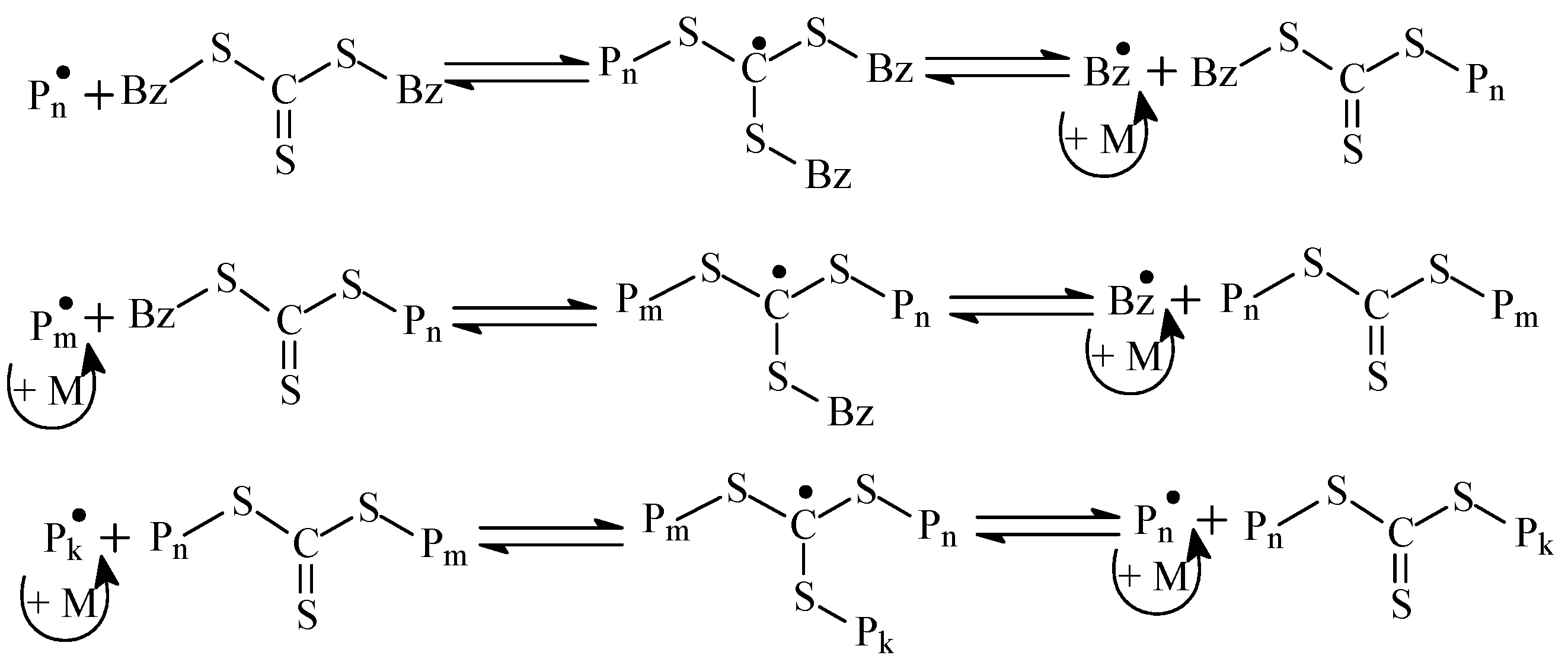
Thus, RAFT copolymerization of AN and AAM allow the synthesis of the range of copolymers with random (experiments 1–4), gradient (experiments 5, 6), and block-gradient (experiment 7) structure. Due to the relatively low content of AAM units in the copolymer, the difference in microstructures of copolymers 4 (random) and 5 (gradient) is negligible. It is more evident for copolymers 4 and 6 (gradient), 4 and 7 (block-gradient), and 6 and 7. In contrast to monofunctional RAFT agents, BTC is bifunctional and has two leaving benzyl groups [47]. As a result, the macromolecule grows from the center to the tails, according to the following reversible chain transfer reactions (Scheme 3).
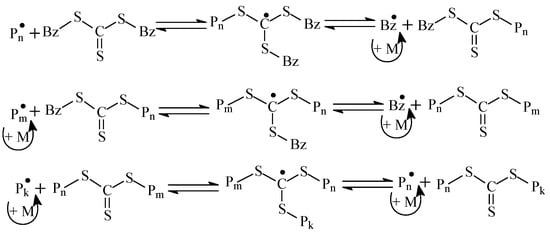
Scheme 3.
The general scenario of the RAFT mechanism in the presence of BTC (Bz = benzyl).
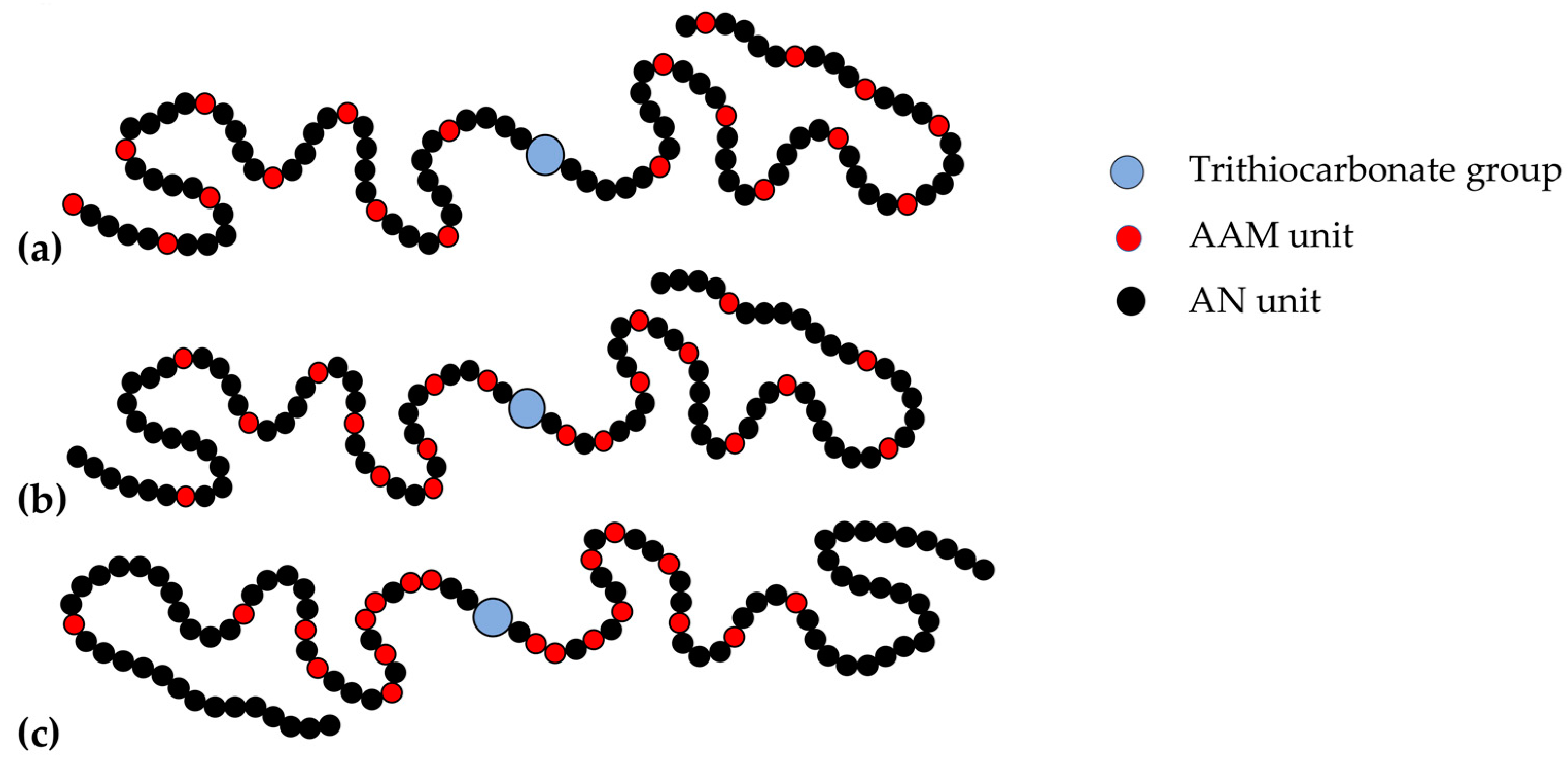
Thus, the structure of random, gradient, and block-gradient copolymers may be depicted as follows (Figure 5).
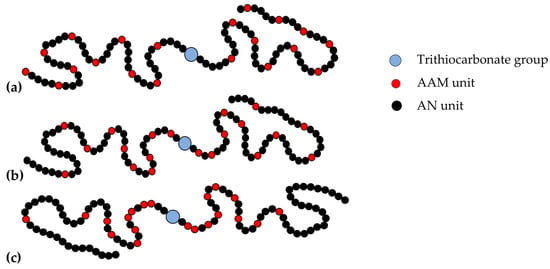
Figure 5.
Schematic presentation of the random (a), gradient (b), and block-gradient (c) copolymers of AN and AAM obtained in the presence of BTC.
Below we shall consider the effect of the different monomer sequence distribution on the thermal behavior of the corresponding copolymers.
3.2. Thermal Treatment of the Copolymers in the Inert Atmosphere: Cyclization Reaction
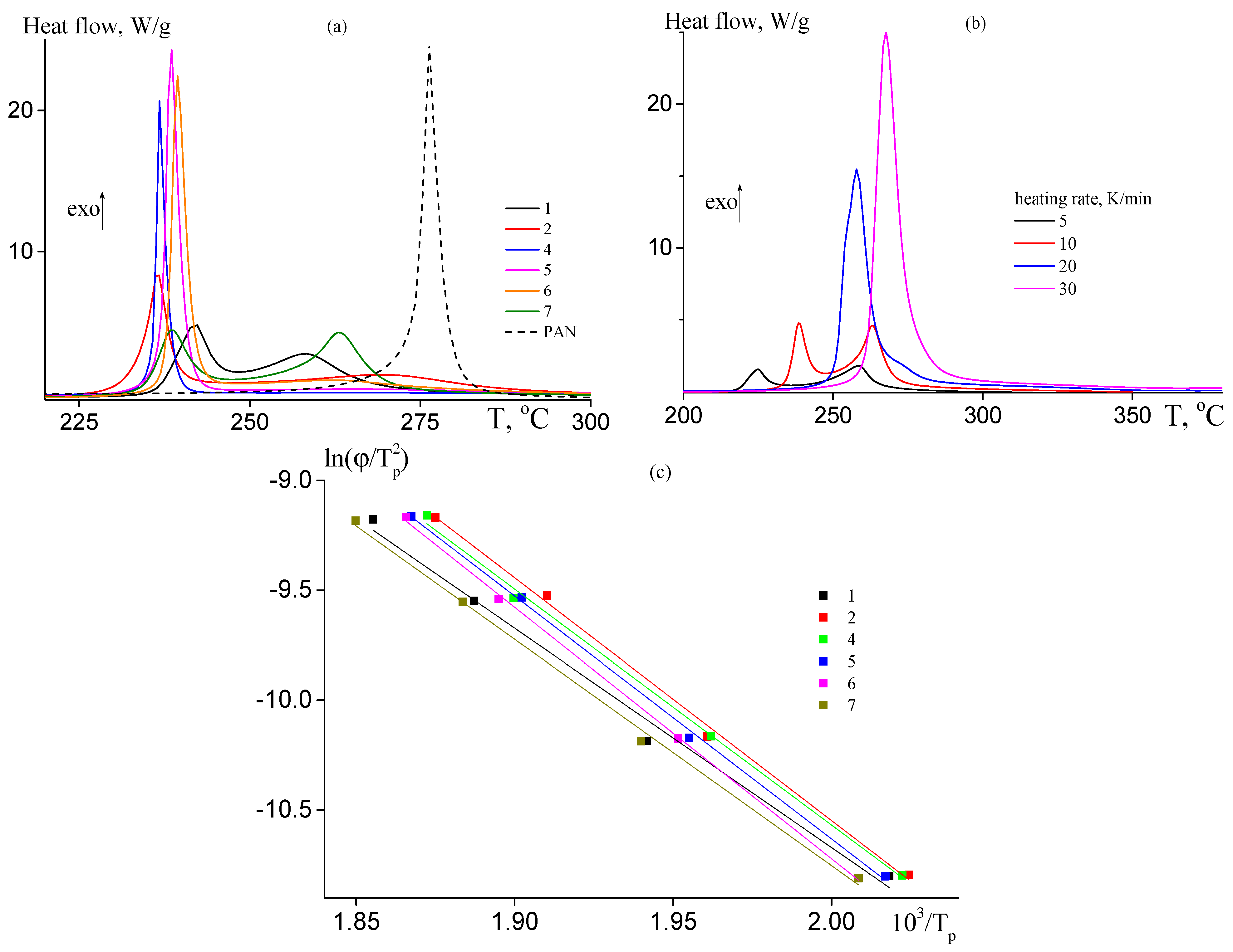
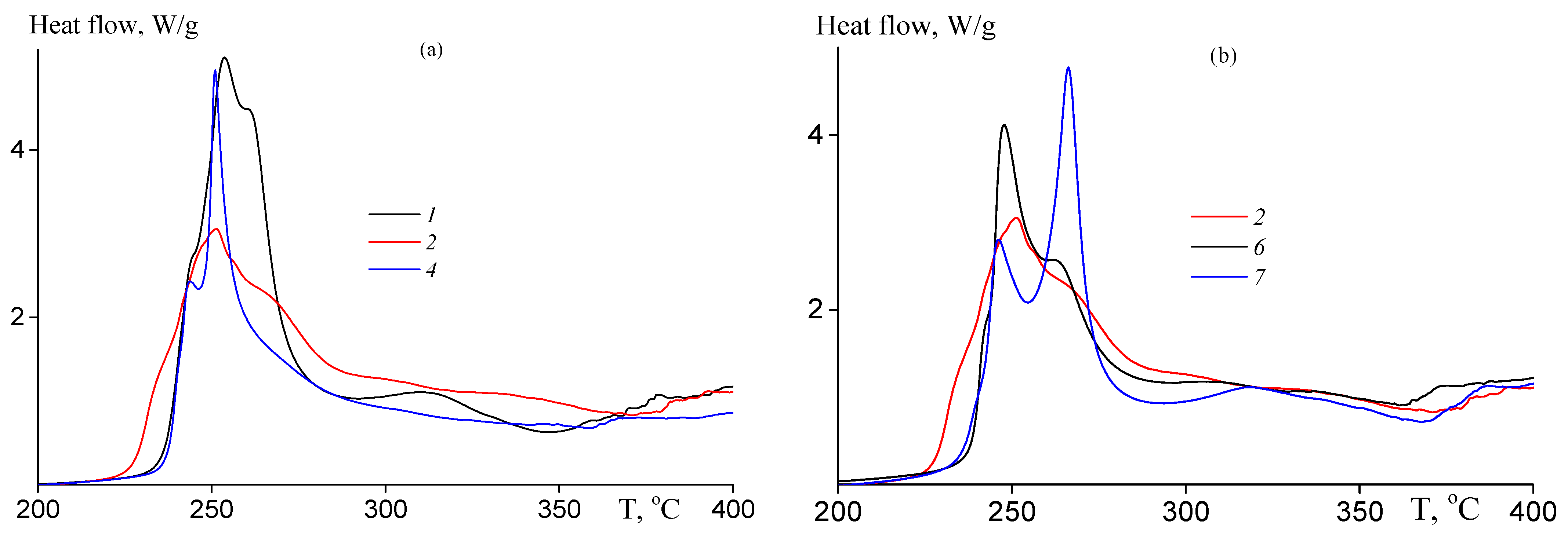
The copolymer films were studied by DSC in the dynamic conditions under argon atmosphere. Figure 6a presents DSC thermograms of the copolymers at the heating rate of 10 K·min−1; the DSC curve of PAN is given for a comparison. Introduction of AAM affects the mechanism and the rate of the cyclization reaction. For random copolymers, at AAM content in the copolymer below ~9 mol. % both radical and ionic mechanisms of cyclization are observed (curves 2 and 3). The low-temperature peak on the DSC thermogram is responsible for ionic mechanism of cyclization, while high-temperature peak is responsible for radical mechanism of cyclization. The ionic mechanism becomes predominant with further increase in the AAM molar part in the copolymer (curve 4). The increase in the AAM content in the copolymer leads to the rise of the heat flow corresponding to the ionic cyclization and to suppress the heat flow corresponding to the radical mechanism (Table 4). The chain unit distribution also affects the ratio of the radical and ionic mechanisms (curves 5–7). For block-gradient copolymer (curve 7), the central block of gradient copolymer is attached to the terminal blocks of PAN, resulting in the separate manifest of ionic and radical mechanisms. In gradient copolymers (samples 5 and 6), the AAM content increases from the center of the macromolecule to its tails. In this case, the radical cyclization is suppressed practically. The DSC data are summarized in Table 4.
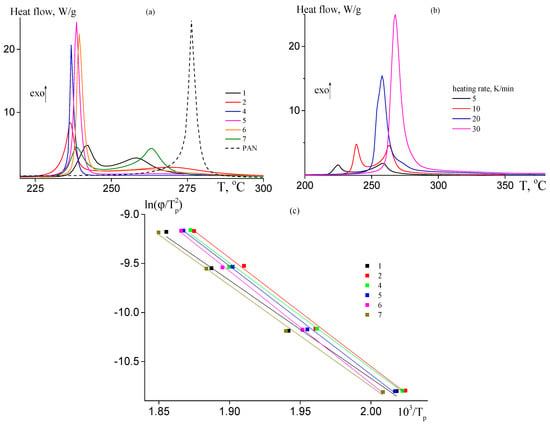
Figure 6.
(a) DCS thermograms of the copolymers 1, 2, 4–7 (Table 1), and PAN registered in argon at heating rate 10 K·min−1; (b) DCS thermograms of samples 7 registered in argon at different heating rates (5–30 K·min−1); and (c) dependence of ln(ϕ/Tp2) on 1/Tp for the copolymers of AN and AAM 1, 2, 4–7.

Table 4.
DSC data for the copolymers of AN and AAM registered in argon atmosphere.
It is interesting to evaluate the activation energy as a function of the monomer unit sequence. Figure 6b presents the DCS thermograms of sample 7 registered at different heating rates. The increase in the heating rate leads to the shift of the thermogram to the region of high temperature and to increase their areas. It is notable that the shape of the thermogram changes. At low heating rate (5 K·min−1), two peaks are observed. The low-temperature peak shifts faster with the rise of the heating rate in contrast to high-temperature peak. As a result, only one low-temperature peak is observed at high heating rate (20 K·min−1), which render it impossible to determine the activation energy of the radical cyclization and allow the estimation of ionic cyclization only. The corresponding thermograms for other copolymers are presented in ESI (Figure S4). To calculate the activation energy, the dependence of ln(ϕ/Tp2) on Tp−1 was plotted (Figure 6c). According to Equation (1), the activation energy of ionic cyclization was determined from the slope of the linear dependence. As is seen from Table 4, the activation energy of radical cyclization (determined for pure PAN) is about 20 kJ·mol−1 higher than that of ionic cyclization initiated by the amide group. The calculated value of ionic cyclization (89 ± 3 kJ·mol−1) is close to what was determined before for AN-AAM—alkyl acrylate systems [33]. In contrast to previous reports [48], the activation energy of ionic cyclization is not influenced by AAM content in the copolymer; also, it is not influenced by the monomer sequence distribution. It may be supposed that for the investigated systems the development of the conjugation starts after achieving a certain number of the cycles formed through the reaction of amide and nitrile groups (Scheme 1).
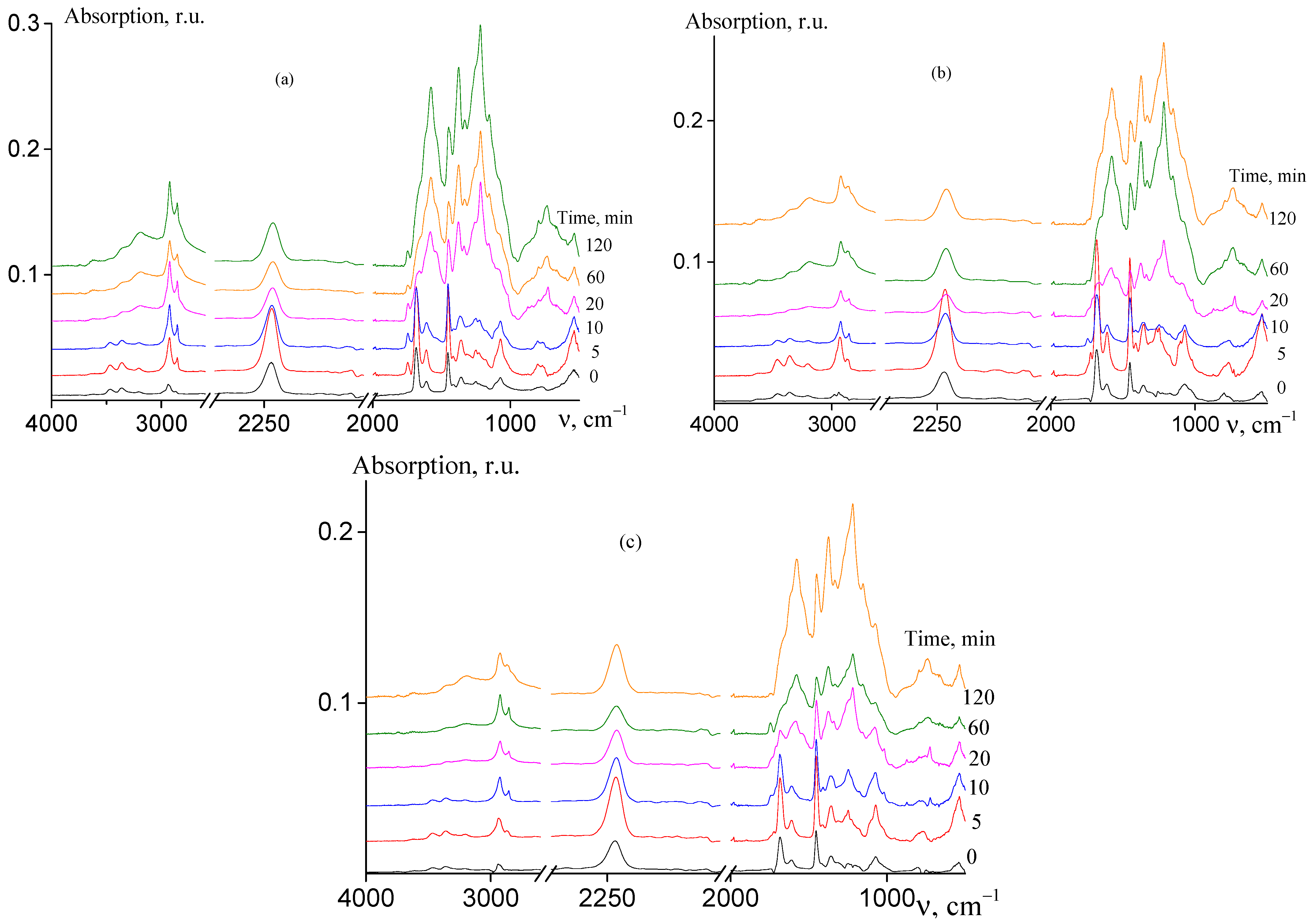
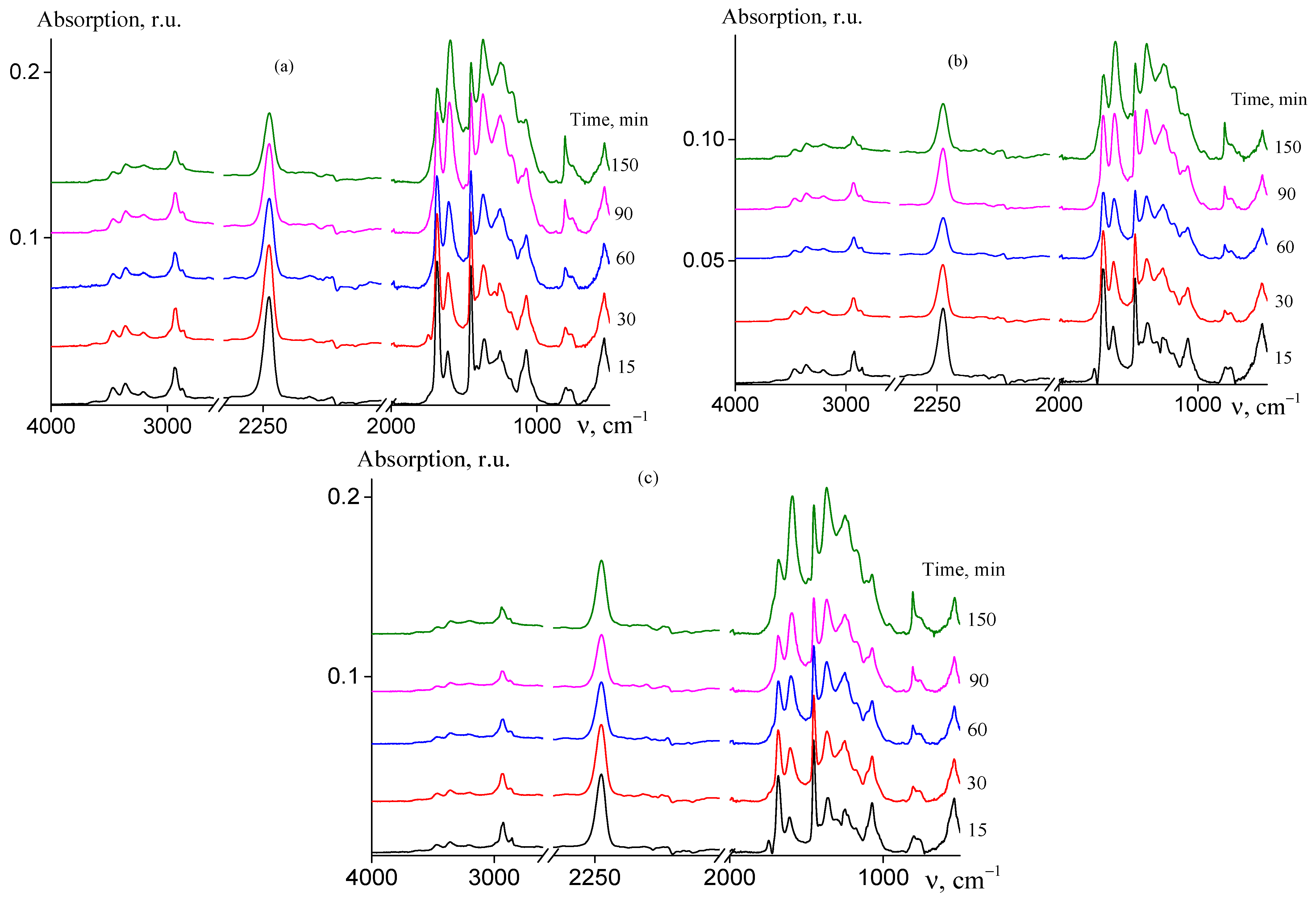
The analysis of the chemical changes that occur during cyclization reaction may lead to a deeper understanding of the formation of the ladder structure in AN and AAM copolymers depending on their microstructure. Figure 7 shows the FTIR spectra of random (a), gradient (b), and block-gradient (c) copolymers registered after isothermal heating of the samples at 200 °C at various times.
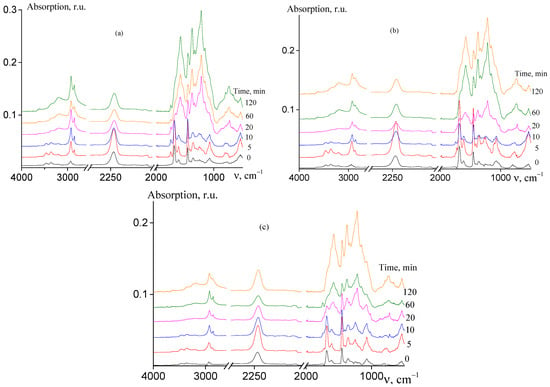
Figure 7.
FTIR spectra of AN and AAM copolymers subjected to thermal treatment in Ar atmosphere at 200 °C: (a) random copolymer 2 (FAAM = 7.8 mol. %); (b) gradient copolymer 6 (FAAM = 9.2 mol. %); and (c) block-gradient copolymer 7 (FAAM = 6.0 mol. %). Time of thermal treatment is indicated in the figures.
In the spectra of non-treated copolymers (curves 1), the characteristic absorbance bands of AN and AAM units are observed. The following bands are observed: stretching vibrations of CN groups at 2244 cm−1, stretching vibrations of CH groups at 2990–2920 cm−1, bending vibrations of CH2 groups are at 1453 and 1360 cm−1, mixed vibrations at 1257 and 1073 cm−1, stretching vibrations of C=O at 1685 and 1613 cm−1, and N–H stretching vibrations at 3462 and 3357 cm−1 [23].
Upon heating at 200 °C, the chemical structure of the copolymers changes and the new bands appear in the spectra and/or the intensity of the existing bands changes (curves 2–6). The reduction of nitrile stretching absorbance at 2244 cm−1 and carbonyl stretching absorbance at 1685 cm−1 is observed as compared with intensity of vibrations of CH and CH2 groups (2990–2920, 1453 cm−1). The similar trend is observed for N–H stretching vibrations at 3462 and 3357 cm−1. A new low intense band appears at 1745 cm−1 and its intensity decreases upon further heating. The bands at 3190, 1581, 1540, 1370, 1247, 1220, 1150, 795, 740, and 660 cm−1 appear and their intensity increases with the rise of duration of heating. These bands, except bands at 1745 and 1220 cm−1, belong to a pyridone-type structure formed in pure PAN. The absence of the absorbance at 1700 cm−1 indicates the existence of amide–iminol tautomerism. It is important to note that the band at 2244 cm−1 resembles its shape and no traces of the band 2200 cm−1 are observed; the latter corresponds to the nitrile group conjugated to the olefinic group. The absence of the bands at 810 and 960 cm−1 referred to CH bending vibrations in olefins along with the absence of the band at 2200 cm−1 confirms that no dehydration takes place. Similar trends are observed for other studied copolymers and for all the samples upon the heating at 250 °C (Figures S5 and S6, ESI). All these changes are typical for cyclization reaction of AN and AAM copolymers [22,23]. Thus, the copolymer composition and copolymer microstructure have no impact on the chemical changes of the copolymers during cyclization. However, the rate of formation of ladder structure depends on the factors mentioned above (Figure 8).
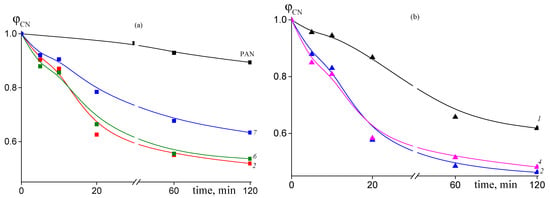
Figure 8.
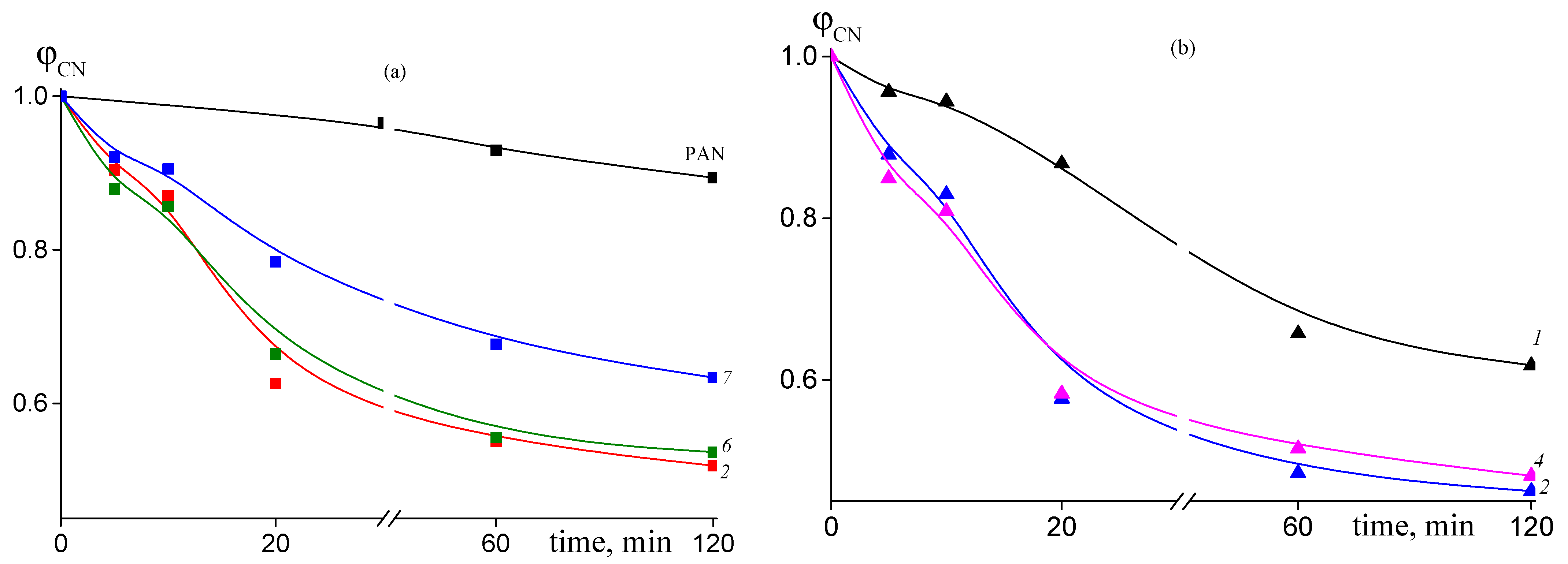
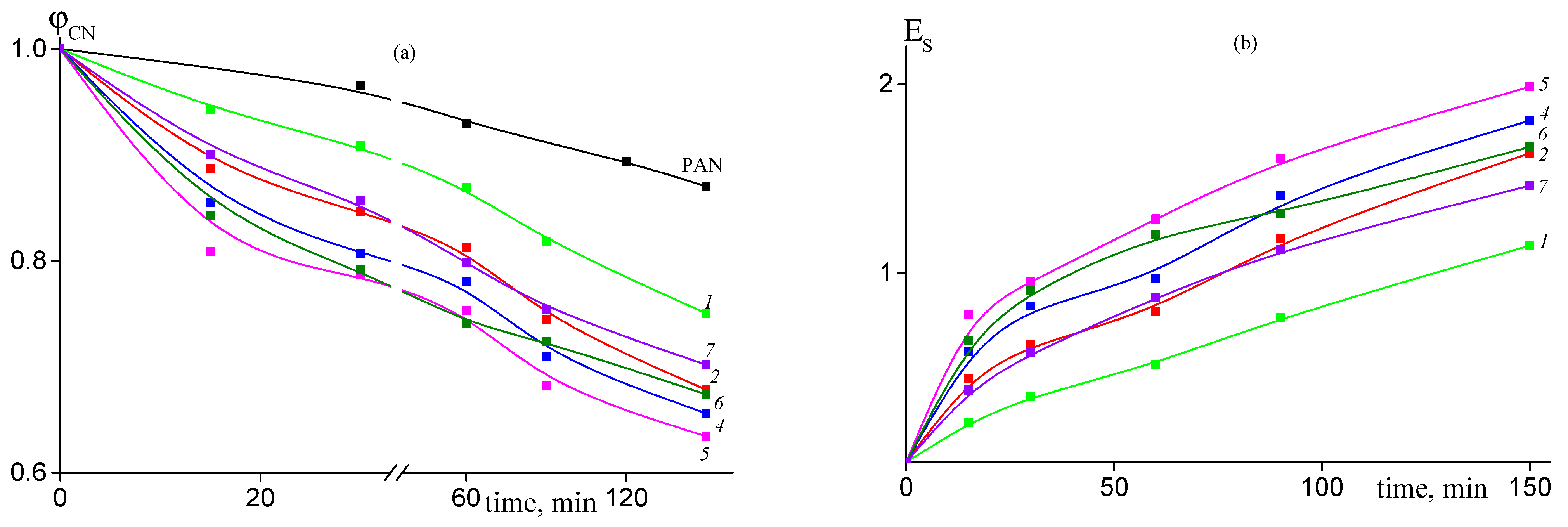
Dependence of the proportion of the unreacted nitrile groups ϕCN on the time of heat treatment at 200 °C for: (a) PAN, random copolymer 2 (FAAM = 7.8 mol. %) (2), gradient copolymer 6 (FAAM = 9.2 mol. %) (6), and block-gradient copolymer 7 (FAAM = 6.0 mol. %) (7); (b) random copolymer 1 (FAAM = 3.4 mol. %) (1), random copolymer 2 (FAAM = 8.8 mol. %) (2), and gradient copolymer 4 (FAAM = 10.2 mol. %) (4).
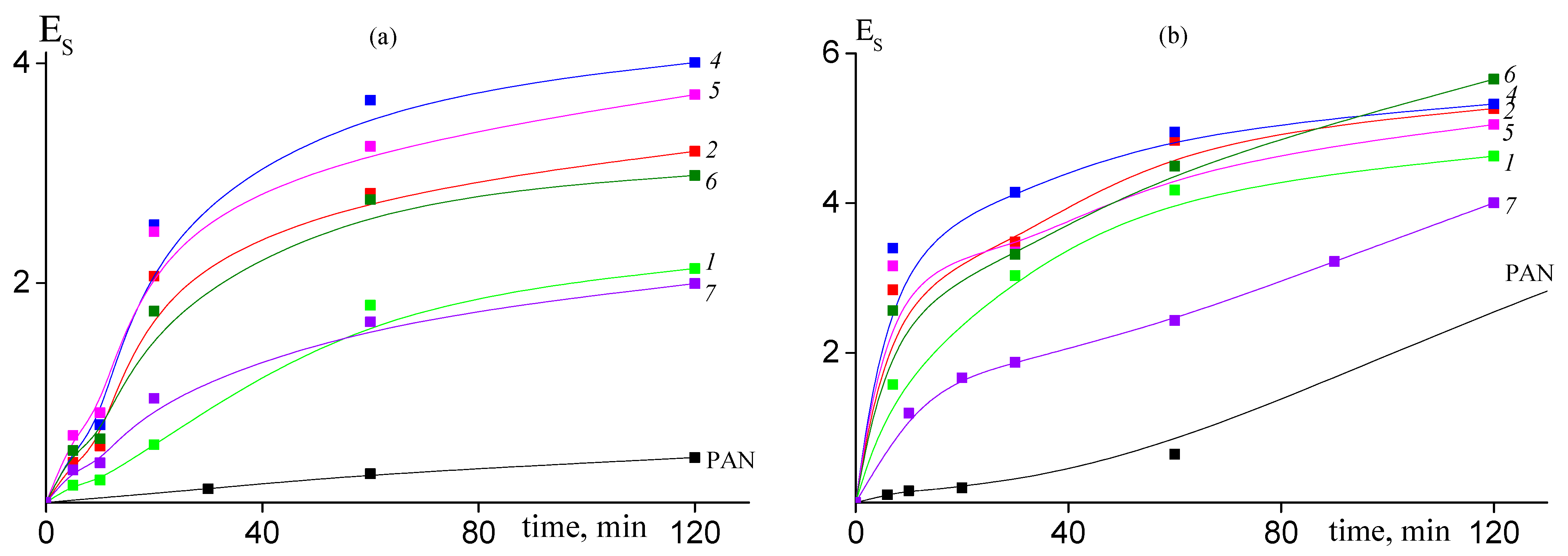
The addition of AAM in the PAN structure results in noticeable acceleration of cyclization reaction. The conversion of nitrile groups increases with the rise of content of AAM in the copolymer (Figure 8). However, if we compare copolymers of various microstructures, we can see that conversion of nitrile groups decreases in the following order of copolymers: random > gradient > block-gradient. Another way to the quantitative analysis of the kinetics of the cyclization reaction is the analysis of the dependence of the stabilization index Es on the time of heat treatment, which gives information about development of the ladder structure. Figure 9 presents corresponding dependences for copolymers obtained at 200 (a) and 250 °C (b). The higher the Es value, the more developed the ladder structure. At 200 °C both the copolymer composition and microstructure affect the Es (Figure 9a). The dependences have S-shape. The rise of AAM content in the copolymer leads to the growth of Es value. A similar trend is observed: the Es value decreases from block-gradient to gradient to random copolymer. Thus, the increase in the molar part of homo-triad AN-AN-AN and decrease in the molar parts of hetero-triads (Table 2) result in the retardation of the development of the ladder structure at 200 °C. The increase in the temperature up to 250 °C resembles the difference between PAN and copolymers (Figure 9b). However, the impact of the copolymer composition and microstructure becomes less pronounced due to the higher cyclization rate. The influence of the copolymer composition on the cyclization rate is clear and is due to the increase in the initiation rate (Scheme 1). The influence of chain microstructure is less evident. From our point of view, the optimal ratio of homo- and hetero-triads provides the rapid development of the ladder cyclization via ionic mechanism. The decrease in the molar part of AN-AAM-AN and AAM-AN-AAM triads has a stronger effect on the retardation of cyclization. The rise of the temperature leads to the increase in the initiation rate and shortens its duration. Thus, it becomes difficult to observe the influence of the copolymer composition and microstructure on the cyclization rate.
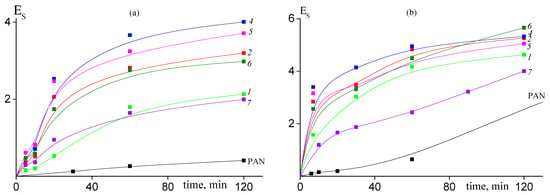
Figure 9.
Dependence of the stabilization index Es on the time of heat treatment at 200 °C (a) and 225 °C (b) for PAN and AN/AAM copolymers. Numeration of the curves corresponds to the numeration of the samples in Table 1.
3.3. Thermal Treatment of the Copolymers in Air
The similar experiments in dynamic and isothermal conditions have been conducted in air atmosphere. Figure 10 presents DSC thermograms of random copolymers with various compositions (a) and copolymers with various microstructures (b).
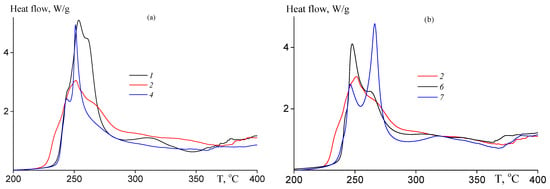
Figure 10.
DCS thermograms registered in air at heating rate 10 K·min−1 of (a) the random copolymers 1, 2, 4; (b) random 2, gradient 6 and block-gradient copolymer 7 (Table 1).
The exo-effect can be observed at lower temperatures as compared with experiment in argon atmosphere and its intensity at the temperature of the maximum of heat release is several times less (see Figure 6a,b); the multiple peaks corresponding to various chemical processes are observed. Both chemical composition and chain microstructure affect the shape of the thermogram, i.e., the contribution of the chemical reactions proceeding upon thermal treatment in air atmosphere (Table 5). The increase in the number of the chemical reactions causes the rise of the value of the enthalpy of the process.

Table 5.
DSC data for the copolymers of AN and AAM registered in air.
Upon heating at 200 °C in air atmosphere, the chemical structure of the copolymers changes. These changes can be followed by FTIR spectroscopy (Figure 11). No visible qualitative differences are observed during transformation of FTIR spectra of random (a), gradient (b), and block-gradient (c) copolymers. However, the differences are observed by comparison of Figure 7 and Figure 11, conforming to changes in the chemical structure of the copolymers after heating during the same period in argon (Figure 7) and in air (Figure 11). In argon, the pyridone type structure becomes dominant after 30 min. In air, the number of new bands is less, and the most noticeable changes are due to the band at 1590 cm−1 that stands out above the band of initial amide group at 1680 cm−1 only after 90 min. Simultaneously, the bands at 816 and 956 cm−1 (CH bending vibrations in olefins) become visible upon treatment in air and are absent in argon. The reduction of intensity of the nitrile group at 2244 cm−1 and NH in amide group at 3480 cm−1 occurs slowly.
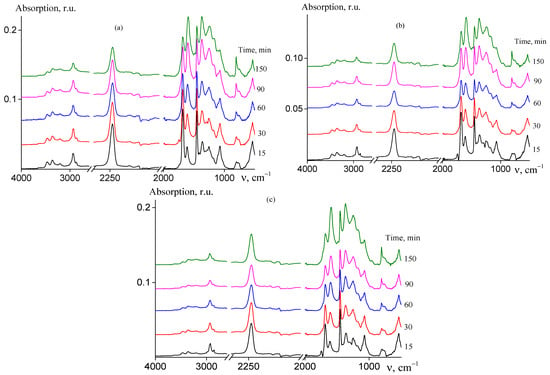
Figure 11.
FTIR spectra of AN and AAM copolymers subjected to thermal treatment in air at 200 °C for 15 (1), 30 (2), 60 (3), 90 (4), and 150 min (6): (a) random copolymer 2 (FAAM = 7.8 mol. %); (b) gradient copolymer 6 (FAAM = 9.2 mol. %); and (c) block-gradient copolymer 7 (FAAM = 6.0 mol. %).
The lower conversion of nitrile groups is confirmed by Figure 12a. The rate of intramolecular cyclization reaction in air atmosphere is smaller as compared to experiment in argon atmosphere (Figure 8). Thus, cyclization is not a dominant reaction upon heating the copolymers at 200 °C in air. Moreover, it is less sensitive to the amount of amide groups in the copolymer, except the sample 1, containing least value of AAM units (3.4 mol. %), and to the copolymer microstructure. As a consequence, the stabilization index reflecting the development of the ladder structure is relatively low.
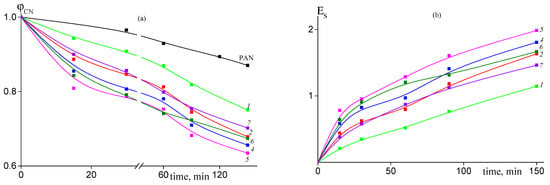
Figure 12.
Dependence of the proportion of the unreacted nitrile groups ϕCN (a) and stabilization index Es (b) on the time of heat treatment at 200 °C for PAN and AN/AAM copolymers. Numeration of the curves corresponds to the numeration of the samples in Table 1.
Summarizing FTIR data, it may be proposed that in oxidative conditions (upon heating in air), the formation of the conjugated C=C bonds occurs faster than that of the C=N bonds for AN/AAM copolymers (Scheme 4). As a result, the ladder structure develops slowly, at least at 200 °C. The important role of oxidation reaction is in accordance with [25].
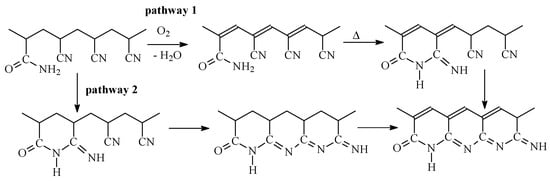
Scheme 4.
The proposed mechanism of thermo-oxidative stabilization for the copolymers of AN and AAM.
4. Conclusions
In the present study we have synthesized first a number of copolymers of AN and AAM with desired MW, narrow MWD, and various monomer sequences (random, gradient, and block-gradient). These RAFT-based copolymers were used to clarify the influence of chain microstructure on the cyclization behavior of the copolymers in inert and air atmosphere.
We have demonstrated that cyclization reaction dominated in argon atmosphere. The ionic mechanism of cyclization prevailed over radical one if the molar part of AAM units in copolymer exceeded ~3 mol. % for random copolymer and ~6 mol. % for block-gradient copolymer. It was demonstrated first that the activation energy of ionic cyclization was not influenced by both copolymer composition and chain microstructure. Besides, we have found that the ratio of homo- and hetero-triads affects the rate of the cyclization reaction. In particular, the decrease in the molar part of AN-AAM-AN and AAM-AN-AAM triads is followed by the decrease in the cyclization rate.
On the contrary, the oxidation reactions dominated in air atmosphere. In this case, the formation of conjugated C=C bonds developed along with conjugated C=N bonds resulting in the lower values of the conversion of nitrile groups. It was shown first that the oxidative reactions were found to be less sensitive for copolymer composition and chain microstructure.
Thus, it may be proposed that the low content of AAM units (~3 mol. %) randomly distributed along the chain is enough to perform successful thermo-oxidative stabilization of AN copolymer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13063734/s1, Figure S1: Calibration curve for determination copolymer composition; Figure S2: SEC traces of the copolymers formed in the experiments 4–7 (Table 1) at various conversions; Figure S3: 1H NMR spectra of copolymers 5 (a), 6 (b), 7 (c), and 12C NMR spectrum of copolymer 7 (d); Figure S4: DCS thermograms of samples 1, 2, 4–6 registered in argon at different heating rates; Figure S5: FTIR spectra of AN and AAM copolymers subjected to thermal treatment in Ar atmosphere at 200 °C for 0 (1), 5 (2), 10 (3) 20 (4), 60 (5), and 120 min (6); Figure S6: FTIR spectra of AN and AAM copolymers subjected to thermal treatment in Ar atmosphere at 225 °C for 0 (1), 7 (2), 30 (3) 60 (4), and 120 min (5); Table S1: Formulations of the RAFT copolymerization of AN and AAM.
Author Contributions
Conceptualization, E.V.C.; methodology, R.V.T. and A.Y.G.; investigation, R.V.T., M.S.B. and A.V.P.; writing—original draft, E.V.C., writing—editing, N.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, D.-C.J.M.; Buchmeiser, M.R. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Kaur, J.; Millington, K.; Smith, S. Producing high-quality precursor polymer and fibers to achieve theoretical strength in carbon fibers: A review. J. Appl. Polym. Sci. 2016, 133, 43963. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Recent progress in fabrication, structure and properties of carbon fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Y.; Lu, C. Effect of acrylonitrile sequence distribution on th thermal stabilization reactions and carbon yields of poly(acrylonitrile-co-methyl acrylate). Polym. Degrad. Stab. 2018, 147, 89–96. [Google Scholar] [CrossRef]
- Morgan, P. (Ed.) Carbon Fibers and Their Composites; Taylor and Francis: New York, NY, USA, 2005; pp. 185–259. [Google Scholar]
- Odian, G. Principles of Polymerization, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004; pp. 198–506. [Google Scholar]
- Tirell, D.A. Copolymer Composition. In Comprehensive Polymer Science and Supplements; Allen, G., Bevington, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3, pp. 195–206. [Google Scholar]
- Chernikova, E.V.; Mineeva, K.O. Reversible Deactivation Radical Copolymerization: Synthesis of Copolymers with Controlled Unit Sequence. Polym. Sci. Ser. B 2022, 64, 1–25. [Google Scholar] [CrossRef]
- Borisova, O.; Billon, L.; Zaremski, M.; Grassl, B.; Bakaeva, Z.; Lapp, A.; Stepanek, P.; Borisov, O. Synthesis and pH- and salinity-controlled self-assembly of novel amphiphilic block-gradient copolymers of styrene and acrylic acid. Soft Matter. 2012, 8, 7649–7659. [Google Scholar] [CrossRef]
- Kozhunova, E.Y.; Plutalova, A.V.; Chernikova, E.V. RAFT Copolymerization of Vinyl Acetate and Acrylic Acid in the Selective Solvent. Polymers 2022, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, M.; Khan, M.J.; Abbasi, S.H.; Soares, J.B.P. Gradient Copolymers by ATRP in Semibatch Reactors: Dynamic Monte Carlo Simulation. Macromol. React. Eng. 2009, 3, 148–159. [Google Scholar] [CrossRef]
- Moskowitz, J.D.; Wiggins, J.S. Semibatch RAFT copolymerization of acrylonitrile and Nisopropylacrylamide: Effect of comonomer distribution on cyclization and thermal stability. Polymer 2016, 84, 311–318. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Shaova, A.A.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Grebenkina, N.A.; Chernikova, E.V. Copolymers of Acrylonitrile and Acrylic Acid: Effect of Composition and Distribution of Chain Units on the Thermal Behavior of Copolymers. Polym. Sci. Ser. B 2020, 62, 102–115. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Berkovich, A.K.; Chernikova, E.V. Influence of Synthesis Method on the Properties of Carbon Fiber Precursors Based on Acrylonitrile and Acrylic Acid Copolymers. Polym. Sci. Ser. B 2020, 62, 447–457. [Google Scholar] [CrossRef]
- Bahrami, S.H.; Bajaj, P.; Sen, K. Thermal Behavior of Acrylonitrile Carboxylic Acid Copolymers. J. Appl. Polymer Sci. 2003, 88, 685–698. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.P.R.; Sivadasan, P.; Katherine, B.K.; Ninan, K.N. Cyclization reaction in poly(acrylonitrile/itaconic acid) copolymer: An isothermal differential scanning calorimetry kinetic study. J. Appl. Polymer Sci. 2003, 88, 915–920. [Google Scholar] [CrossRef]
- Sivy, G.T.; Coleman, M.M. Fourier transform IR studies of the degradation of polyacrylonitrile copolymers—II: Acrylonitrile/methacrylic acid copolymers. Carbon 1981, 19, 127–131. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Chernikova, E.V.; Kulichikhin, V.G.; Varfolomeeva, L.A.; Kuzin, M.S.; Toms, R.V.; Prokopov, N.I. The effect of synthetic procedure of acrylonitrile–acrylic acid copolymers on the rheological properties of solutions and features of fiber spinning. Materials 2020, 13, 3454. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Varfolomeeva, L.A.; Kuzin, M.S.; Vashchenko, A.F.; Chernikova, E.V.; Toms, R.V.; Kulichikhin, V.G. Effect of the comonomer addition sequence in the synthesis of an acrylonitrile terpolymer on the solution reology and fiber properties. Mendeleev Commun. 2022, 32, 652–654. [Google Scholar] [CrossRef]
- Coleman, M.M.; Sivy, G.T. Fourier transform ir studies of the degradation of polyacrylonitrile copolymers—I: Introduction and comparative rates of the degradation of three copolymers below 200 °C and under reduced pressure. Carbon 1981, 19, 123–126. [Google Scholar] [CrossRef]
- Sivy, G.T.; Coleman, M.M. Fourier transform IR studies of the degradation of polyacrylonitrile copolymers—IV: Acrylonitrile/acrylamide copolymers. Carbon 1981, 19, 137–139. [Google Scholar] [CrossRef]
- Coleman, M.M.; Sivy, G.T.; Painter, P.C.; Snyder, R.W.; Gordon, B., III. Studies of the degradation of acrylonitrile/acrylamide copolymers as a function of composition and temperature. Carbon 1983, 21, 255–267. [Google Scholar] [CrossRef]
- Sivy, G.T.; Gordon, B., III; Coleman, M.M. Carbon, Studies of the degradation of copolymers of acrylonitrile and acrylamide in air at 200 oC. Speculations of the role of the preoxidation step in carbon fiber formation. Carbon 1983, 21, 573–578. [Google Scholar] [CrossRef]
- Sun, J.; Wang, K.; Wang, J.; Qin, C.; Dai, L. Pre-oxidation nanofibers from acrylonitrile-acrylamide copolymers synthesized by solvent-water polymerization. Adv. Mater. Res. 2011, 175–176, 164–169. [Google Scholar] [CrossRef]
- Cheng, R.; Zhou, Y.; Wang, J.; Cheng, Y.; Ryu, S.; Jin, R. High Char-Yield in AN-AM Copolymer by Acidic Hydrolysis of Homopolyacrylonitrile. Carbon Lett. 2013, 14, 34–39. [Google Scholar] [CrossRef]
- Han, N.; Zhang, X.-X.; Wang, X.-C. Various Comonomers in Acrylonitrile Based Copolymers: Effects on Thermal Behavior. Iran. Polym. J. 2010, 19, 243–253. [Google Scholar]
- Takahashi, A.; Tanaka, H.; Kagawa, I. The Analysis and Solubility of Acrylonitrile-Acrylamide Copolymers. Kogyo Kagaku Zasshi 1967, 70, 988–992. [Google Scholar] [CrossRef]
- Chapiro, A.; Perec-Spitzer, L. Influence des solvants sur la copolymerisation de l’acrylamide avec l’acrylonitrile. Eur. Polym. J. 1975, 11, 59–69. [Google Scholar] [CrossRef]
- Hou, C.; Ying, L.; Wang, C. Determination of monomer apparent reactivity ratios for acrylonitrile-acrylamide copolymerization system. J. Mater. Sci. 2005, 40, 609–612. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Kishilov, S.M.; Plutalova, A.V.; Kostina, Y.V.; Bondarenko, G.N.; Baskakov, A.A.; Il’in, S.O.; Nikolaev, A.Y. Specific Features of the Copolymerization of Acrylonitrile and Acrylamide in the Presence of Low-Molecular-Mass and Polymeric Trithiocarbonates and Properties of the Obtained Copolymers. Polym. Sci. Ser. B 2014, 56, 553–565. [Google Scholar] [CrossRef]
- Maksimov, N.M.; Toms, R.V.; Balashov, M.S.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Kuzin, M.S.; Skvortsov, I.Y.; Kulichikhin, V.G.; Chernikova, E.V. Novel Potential Precursor of Carbon Fiber Based on Copolymers of Acrylonitrile, Acrylamide, and Alkyl Acrylates. Polym. Sci. Ser. B 2014, 64, 670–687. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Maksimov, N.M.; Kuzin, M.S.; Varfolomeeva, L.A.; Toms, R.V.; Chernikova, E.V.; Kulichikhin, V.G. Ifluence of acryl acrylate nature on rheological properties polyacrylonitrile terpolymers solutions, spinnability and mechanical characteristics of fibers. Materials 2023, 16, 107. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Terpugova, P.S.; Garina, E.S.; Golubev, V.B. Controlled radical polymerization of styrene and n -butyl acrylate mediated by trithiocarbonates. Polym. Sci. Ser. A 2007, 49, 108–119. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) Polymer Handbook; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Holland-moritz, K.; Siesler, H.W. Infrared Spectroscopy of Polymers. Appl. Spectrosc. Rev. 1976, 11, 1–55. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Collins, G.L.; Thomas, N.W.; Williams, G.E. Kinetic relationships between heat generation and nitrile consumption in the reaction of poly(acrylonitrile) in air at 265 °C. Carbon 1988, 26, 671–679. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E. (Eds.) RAFT Polymerization: Methods, Synthesis, Applications; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Barner-Kowollik, C. (Ed.) Handbook of RAFT Polymerization; Wiley: Weinheim, Germany, 2008. [Google Scholar]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT process–A Third Update. Aust. J. Chem. 2012, 65, 985–1076. [Google Scholar] [CrossRef]
- Moad, G. Mechanism and Kinetics of Dithiobenzoate-Mediated RAFT Polymerization–Status of the Dilemma. Macromol. Chem. Phys. 2014, 215, 9–26. [Google Scholar] [CrossRef]
- Van Doremaele, G.H.J.; German, A.L.; de Vries, N.K.; van der Velden, G.P.M. 1H and 13C NMR investigation of the intramolecular structure of solution and emulsion styrene-methyl acrylate copolymers. Macromolecules 1990, 23, 4206–4215. [Google Scholar] [CrossRef]
- Mukherjee, M.; Chatterjee, S.K.; Brar, A.S. One- and two-dimensional nuclear magnetic resonance studies on the compositional sequence and the microstructure of acrylamide/acrylonitrile copolymers. J. Appl. Polym. Sci. 1999, 73, 55–67. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Sivtsov, E.V. Reversible Addition-Fragmentation Chain-Transfer Polymerization: Fundamentals and Use in Practice. Polym. Sci. Ser. B 2017, 59, 117–146. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Lu, C.; Ling, L. Thermo-chemical reactions and structural evolution of acrylamide-modified polyacrylonitrile. Chin. J. Polym. Sci. 2010, 28, 367–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).