Investigating Salinity Effects in Brackish Aquaponics Systems: Evidencing the Co-Cultivation of the Halophyte Crithmum maritimum with the Euryhaline Sparus aurata
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
- -
- fish and plant observations;
- -
- water level and water quality monitoring;
- -
- airflow and dissolved oxygen saturation;
- -
- salinity and temperature monitoring;
- -
- pump function of the systems;
- -
- cleaning the sponges of the mechanical filter from fish waste.
2.2. Fish Histology
2.3. SDS-PAGE and Immunoblot Analysis
2.4. Plant and Fish Growth Performance Indicators
2.5. Statistical Analysis
3. Results
3.1. Rearing Conditions
3.2. Fish and Plant Growth Performance
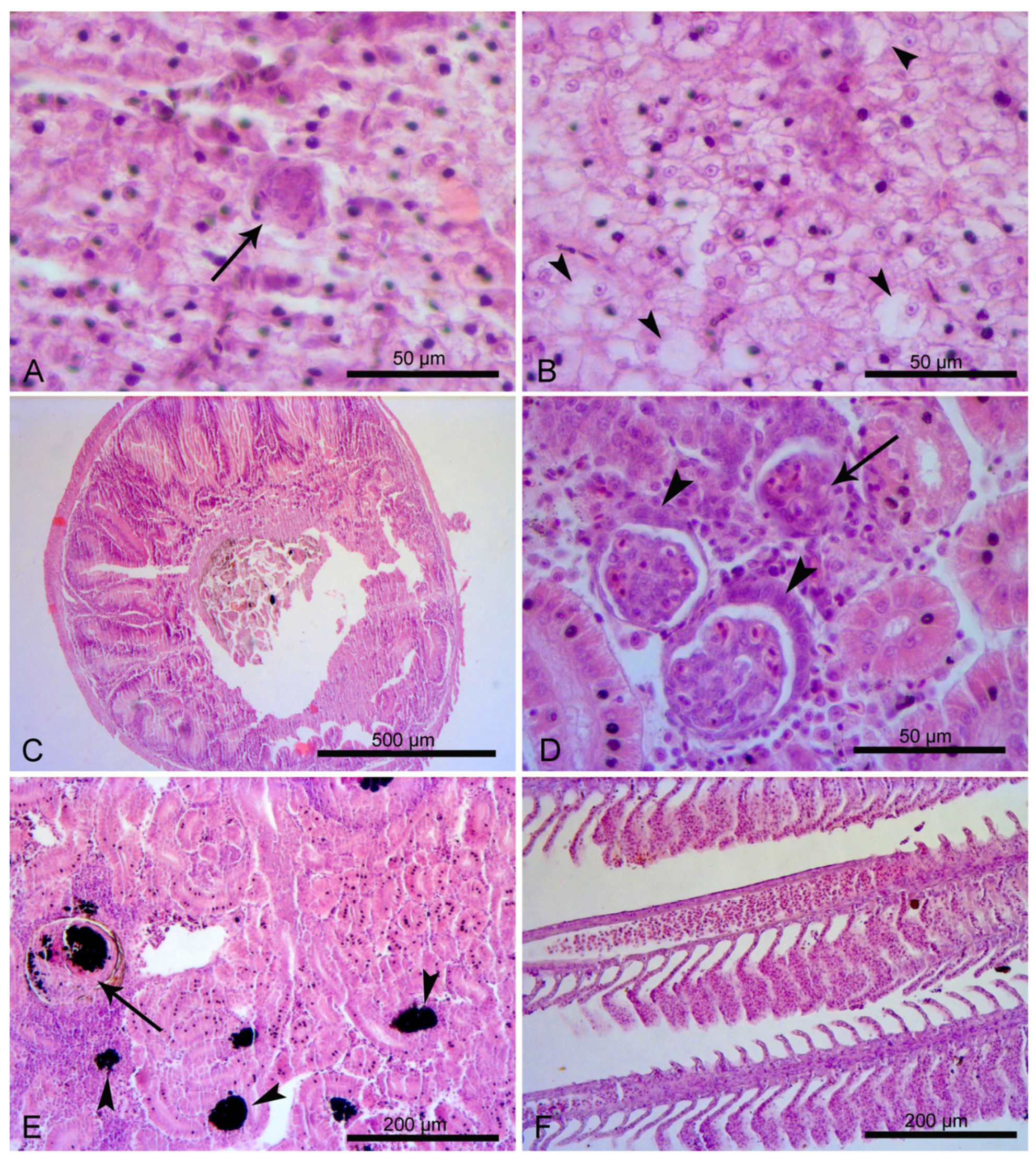
3.3. Fish Histology
3.3.1. Liver
3.3.2. Midgut
3.3.3. Kidney
3.3.4. Gills
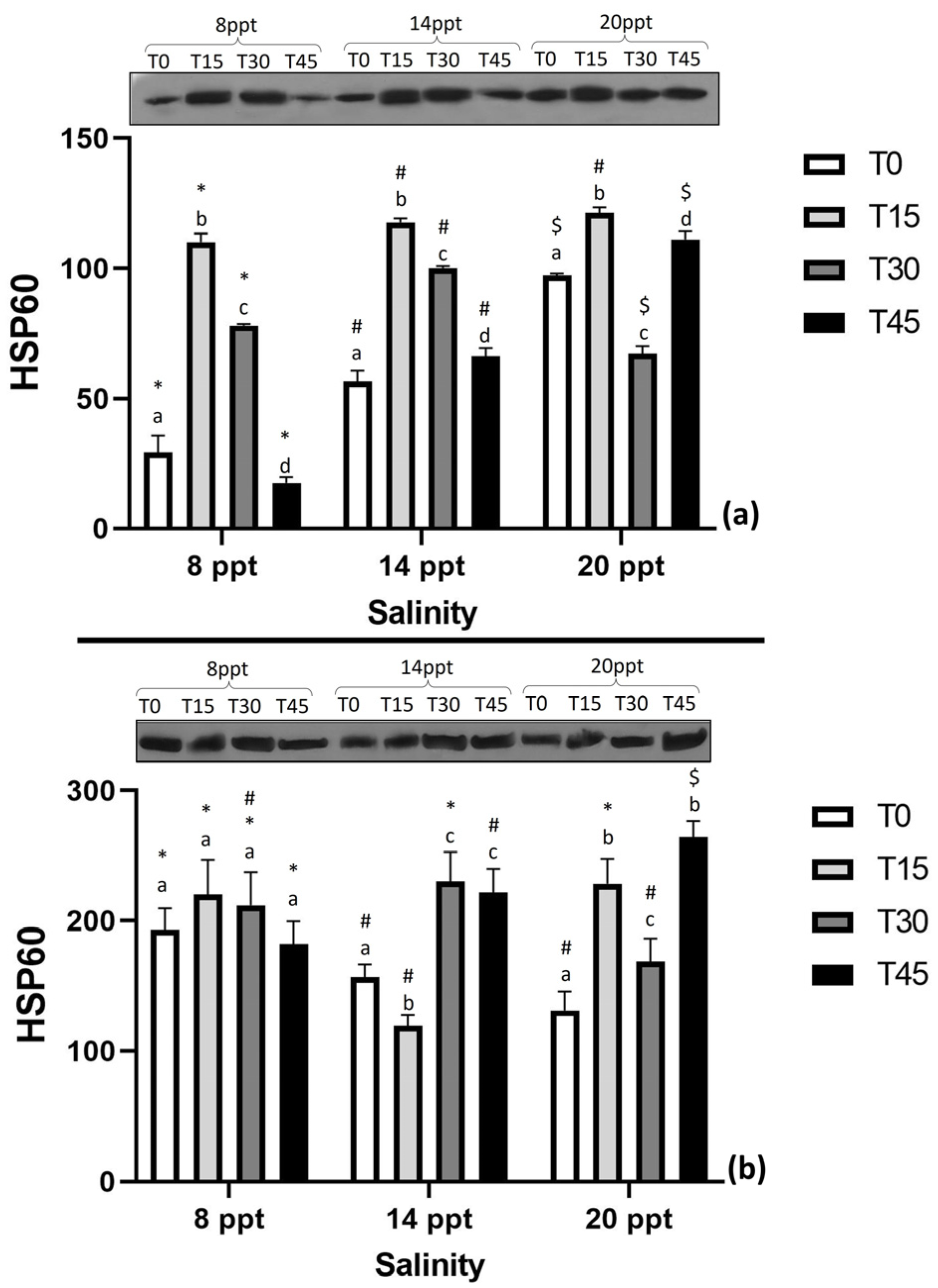
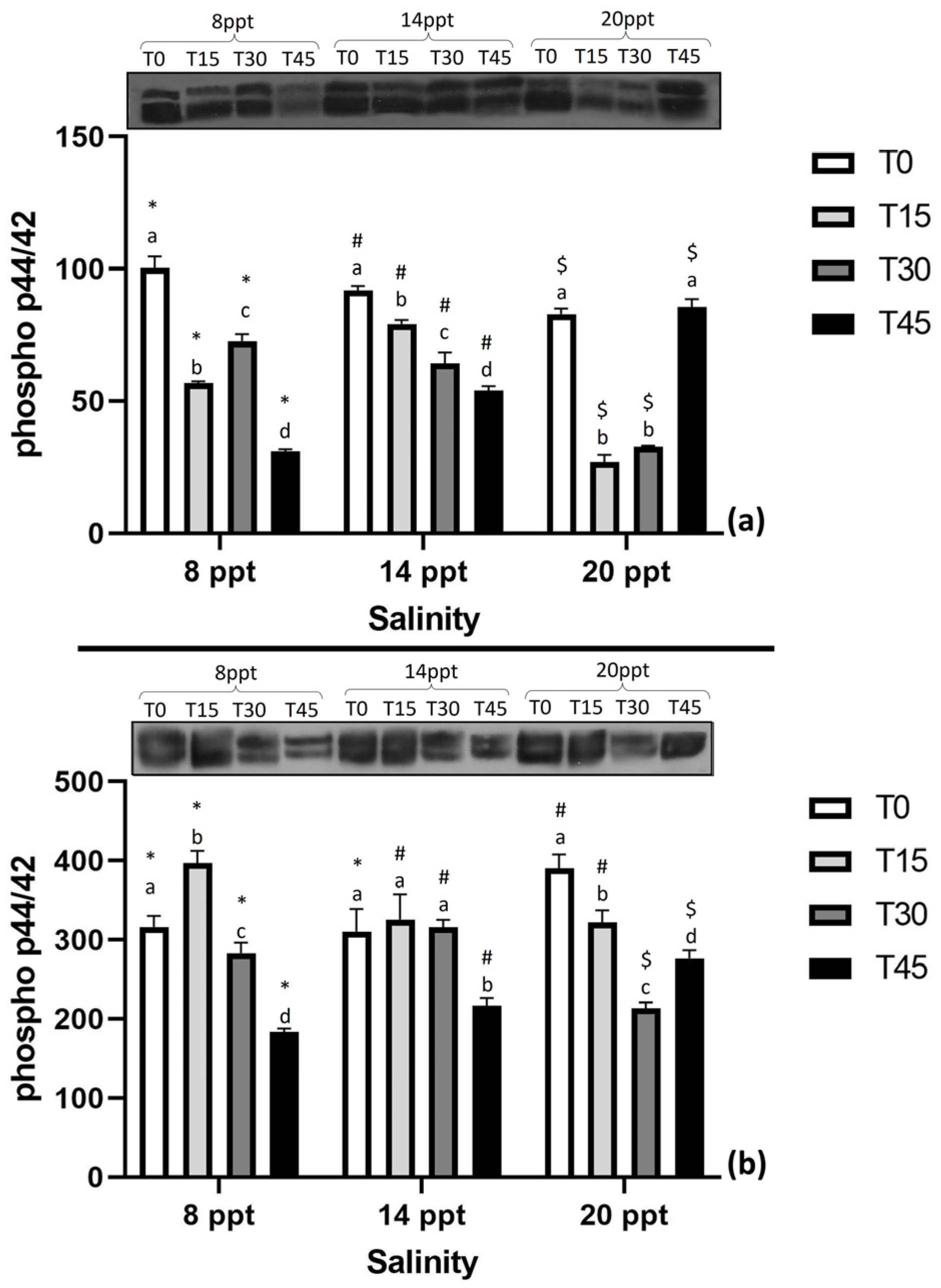
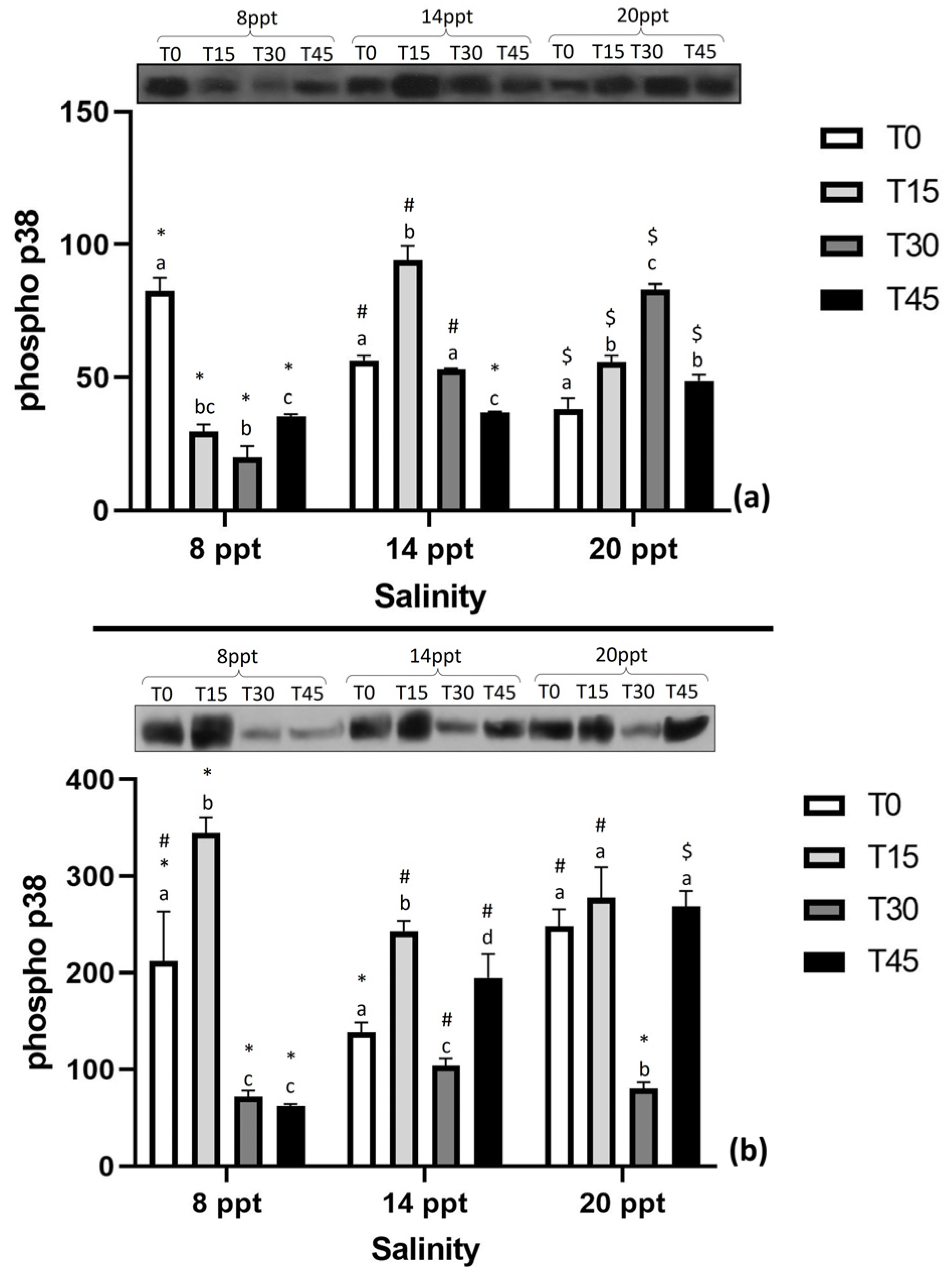
3.4. Expression of Stress-Related Proteins
4. Discussion
4.1. Rearing Condition
4.2. Fish and Plant Growth Performance
4.3. Fish Histology
4.4. Expression of Stress-Related Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, B.P.; Rahmstorf, S.; Engelhart, S.E.; Kemp, A.C. Expert assessment of sea-level rise by AD 2100 and AD 2300. Quat. Sci. Rev. 2014, 84, 1–6. [Google Scholar] [CrossRef]
- van Wijk, E.M.; Rintoul, S.R. Freshening drives contraction of Antarctic bottom water in the Australian Antarctic Basin. Geophys. Res. Lett. 2014, 41, 1657–1664. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Wall, D.H.; Adams, B.J.; Virginia, R.A.; Ball, B.A.; Gooseff, M.N.; McKnight, D.M. The ecology of pulse events: Insights from an extreme climatic event in a polar desert ecosystem. Ecosphere 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.W.; Knaus, U.; Applebaum, S.; Strauch, S.M.; Kotzen, B. Coupled aquaponic systems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.B., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 163–199. [Google Scholar] [CrossRef]
- Goddek, S.; Joyse, A.; Kotzen, B.; Burnell, G. Aquaponics Food Production Systems. Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyse, A., Kotzen, B., Burnell, G., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 3–619. [Google Scholar]
- Vlahos, N.; Levizou, E.; Stathopoulou, P.; Berillis, P.; Antonopoulou, E.; Bekiari, V.; Krigas, N.; Kormas, K.; Mente, E. An experimental brackish aquaponic system using juvenile gilthead sea bream (Sparus aurata) and rock samphire (Crithmum maritimum). Sustainability 2019, 11, 4820. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Asimaki, A.; Berillis, P.; Vlahos, N.; Levizou, E.; Katsoulas, N.; Karapanagiotidis, I.T.; Rumbos, C.I.; Athanassiou, C.G.; Mente, E. Aqua-Ento-Ponics: Effect of insect meal on the development of sea bass, Dicentrarchus labrax, in co-culture with lettuce. Fishes 2022, 7, 397. [Google Scholar] [CrossRef]
- Nelson, R.L. Aquaponic Food Production: Growing Fish and Vegetables for Food and Profit; Nelson, R.L., Pade, J.S., Eds.; Nelson and Pade, Inc.: Montello, WI, USA, 2008; p. 224. [Google Scholar]
- Fronte, B.; Galliano, G.; Bibbiani, C. From freshwater to marine aquaponic: New opportunities for marine fish species production. In Proceedings of the 4th Conference with International Participation Conference VIVUS-on Agriculture, Environmentalism, Horticulture and Floristics, Food Production and Processing and Nutritioh “With Knowledge and Experience to New Entrepreneurial Opportunities”, Slovenia, Poland, 20–21 April 2016. [Google Scholar]
- Stathopoulou, P.; Berillis, P.; Vlahos, N.; Nikouli, E.; Kormas, K.; Levizou, E.; Katsoulas, N.; Mente, E. Freshwater-adapted sea bass Dicentrarchus labrax feeding frequency impact in a lettuce Lactuca sativa aquaponics system. PeerJ 2021, 9, e11522. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO: Rome, Italy, 2014; pp. 16–18, 22–26, 75–81. [Google Scholar]
- Gunning, D. Cultivating Salicornia europaea (marsh samphire). In BIM; Watson, L., Ed.; Irish Sea Fisheries Board Marine Research Station & University College Cork: Dublin, Ireland, 2016; Volume 4, pp. 1–95. [Google Scholar]
- Delaide, B.; Delhaye, G.; Dermience, M.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Plant and fish production performance, nutrient mass balances, energy and water use of the PAFF Box, a small-scale aquaponic system. J. Aquac. Eng. Fish. Res. 2017, 78, 130–139. [Google Scholar] [CrossRef]
- Colt, J.; Schuur, M.A.; Weaver, D.; Semmens, K. Engineering design of aquaponics systems. Rev. Fish. Sci. Aquac. 2021, 30, 33–80. [Google Scholar] [CrossRef]
- Li, G.; Taoa, L.; Lia, X.; Penga, L.; Songa, C.; Daia, L.; Wub, L.; Xieb, L. Design and performance of a novel rice hydroponic biofilter in a pond-scale aquaponic recirculating system. Ecol. Eng. 2018, 125, 1–10. [Google Scholar] [CrossRef]
- Nozzi, V.; Parisi, G.; Di Crescenzo, D.; Giordano, M.; Carnevali, O. Evaluation of Dicentrarchus labrax meats and the vegetable quality of Beta vulgaris var. cicla farmed in freshwater and saltwater aquaponic systems. Water 2016, 8, 423. [Google Scholar] [CrossRef]
- Theodorakaki, E.; Papadakis, G.; Kaloussias, S.; Mente, E.; Vlahos, N. Leaping grey mullet Liza saliens as an alternative and candidate fish species to be used in a brackish aquaponic system co-cultivating with plant Eruca sativa. In Proceedings of the 4th International Congress on Applied Ichthyology, Oceanography & Aquatic Environment, HydroMediT 2021, Virtual Congress, Mytilene, Greece, 4–6 November 2021. [Google Scholar]
- Thomas, R.M.; Verma, A.K.; Krishna, H.; Prakash, S.; Kumar, A.; Peter, R.M. Effect of salinity on growth of Nile tilapia (Oreochromis niloticus) and spinach (Spinacia oleracea) in aquaponic system using inland saline groundwater. Aquac. Res. 2021, 52, 6288–6298. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Tsoumalakou, E.; Levizou, E.; Vanikiotis, T.; Zaoutso, S.S.; Berillis, P. Iron and potassium fertilization improve rocket growth without affecting Tilapia growth and histomorphology characteristics in aquaponics. Appl. Sci. 2021, 11, 5681. [Google Scholar] [CrossRef]
- Suarez-Caceres, G.P.; Quevedo-Ruiz, F.J.; Lobillo-Eguíbar, J.; Fernandez-Cabanas, V.M.; Quevedo-Ruiz, F.J.; Perez-Urrestarazua, L. Polyculture production of vegetables and red hybrid tilapia for self-consumption by means of micro-scale aquaponic systems. Aquac. Eng. 2021, 95, 1–11. [Google Scholar] [CrossRef]
- Holmes, W.N.; Donaldson, E.M. The body compartments and the distribution of electrolytes. Fish Physiol. 1969, 1, 1–89. [Google Scholar] [CrossRef]
- Kültz, D. 2—Osmosensing. Fish Physiol. 2012, 32, 45–68. [Google Scholar] [CrossRef]
- Nishida, E.; Gotoh, Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 1993, 18, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D.; Burg, M. Evolution of osmotic stress signaling via MAP kinase cascades. J. Exp. Biol. 1998, 201, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Tutar, L.; Tutar, Y. Heat shock proteins: An overview. Curr. Pharm. Biotechnol. 2010, 11, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Danaher, J.J. Preliminary evaluation of organic wastes from two aquaponic systems as a source of inorganic nutrients for hydroponics. Acta Hortic. 2007, 742, 201–208. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Kumar Khanal, S. Nitrogen transformations in aquaponic systems: A review. J. Aquac. Eng. Fish. Res. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Morphological and anatomical adaptations of halophytes: A review. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Cham, Switzerland, 2021; pp. 1079–1221. [Google Scholar]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol. Plant. 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Rena, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, P.; Song, H.; Chen, X.; Li, X.; Li, Y. Comparative proteomic analysis of differentially expressed proteins in shoots of Salicornia europaea under different salinity. J. Proteome Res. 2009, 8, 3331–3345. [Google Scholar] [CrossRef] [PubMed]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Flowers, T.J.; Comler, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Laiz-Carrión, Ρ.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Martín del Río, M.P.; Soengas, J.L.; Mancera, J.M. Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: Implications for osmoregulation and energy metabolism. Aquaculture 2005, 250, 849–861. [Google Scholar] [CrossRef]
- Deering, M.J.; Fielder, D.R.; Hewitb, D.R. Growth and fatty acid composition of juvenile leader prawns, Penaeus monodon, fed different lipids. Aquaculture 1997, 151, 131–141. [Google Scholar] [CrossRef]
- Johnson, R.; Wolf, J.; Braunbeck, T. Guidance Document on the Diagnosis of Endocrine-Related Histopathology in Fish Gonads; Organisation for Economic Co-Operation and Development: Paris, France, 2009; p. 96. [Google Scholar]
- Antonopoulou, E.; Chatzigiannidou, I.; Feidantsis, K.; Kounna, C.; Chatzifotis, S. Effect of water temperature on cellular stress responses in meagre (Argyrosomus regius). Fish Physiol. Biochem. 2020, 46, 1075–1091. [Google Scholar] [CrossRef]
- Mente, E.; Solovyev, M.M.; Vlahos, N.; Rotllant, G.; Gisbert, E. Digestive enzymes activity during initial ontogeny and after feeding diets with different protein sources in zebra cichlid Archocentrus nigrofasciatus. J. World Aquac. Soc. 2016, 48, 831–848. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Wan-Nik, W.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: London, UK, 1999; p. 718. [Google Scholar]
- Kotzen, B.; Appelbaum, S. An investigation of aquaponics using brackish water resources in the Negev Desert. J. Appl. Aquac. 2010, 22, 297–320. [Google Scholar] [CrossRef]
- Appelbaum, S.; Kotzen, B. Further investigations of aquaponics using brackish water resources of the Negev desert. Ecocycles 2016, 2, 26–35. [Google Scholar] [CrossRef]
- Tasiou, K. The Production Process of Sea Bass in an Aquaponic System. Master’s Thesis, Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, University of Thessaly, Volos, Greece, 2019. [Google Scholar]
- Spotte, S. Captive Seawater Fishes; John Wiley & Sons: New York, NY, USA, 1992; p. 942. [Google Scholar]
- Endut, A.; Jusoh, A.; Ali, N.; Wan Nik, W.N.; Hassan, A. Effect of flow rate on water quality parameters and plant growth of water spinach (Ipomoea aquatica) in an aquaponic recirculating system. Desalin. Water Treat. 2009, 5, 19–28. [Google Scholar] [CrossRef]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of Tilapia and basil: Comparing a batch and staggered cropping system. Acta Hortic. 2004, 648, 63–69. [Google Scholar] [CrossRef]
- Lennard, W.A.; Leonard, B.V. A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an aquaponic test system. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Lawrence, L.A.; Boardman, D.G.; Patnaik, S.; Marsh, L.; George, J.; Flick, J. Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2010, 303, 28–33. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002; pp. 67–86. [Google Scholar]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef]
- Resh, M.H. (Ed.) 2013 Hobby Hydroponics; Taylor and Francis Group: Boca Raton, FL, USA, 2013; p. 129. [Google Scholar]
- Sadek, S.S.; Fathy Osman, M.; Adel Mansour, M. Growth, survival and feed conversion rates of sea bream (Sparus aurata) cultured in earthen brackish water ponds fed different feed types. Aquac. Int. 2004, 12, 409–421. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Laiz-Carrión, R.; Martín del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Influence of cortisol on osmoregulation and energy metabolism in gilthead sea bream Sparus aurata. J. Exp. Zool. 2003, 298A, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247260. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kardan, J. Morphological and physiological responses of some halophytes to salinity stress. Ann. Univ. Mariae Curie Sklodowska Sect. C Biol. 2016, 70, 31. [Google Scholar] [CrossRef]
- Khan, Μ.A.; Gul, Β.; Weber, J.D. Effect of salinity on the growth and ion content of Salicornia rubra. Soil. Sci. Plant. Anal. 2001, 32, 2965–2977. [Google Scholar] [CrossRef]
- Ben Amor, N.; Ben Hamed, K.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Herman, R.L. Systemic Noninfectious Granulomatoses of Fishes; National Biological Service, Leetown Science Center, National Fish Health Research Laboratory: Kearneysville, WV, USA, 1996; p. 9. [Google Scholar]
- Baudin-Laurencin, F.; Messager, J.L. Granulomatous hypertyrosonaemia. In ICES (International Council for the Exploration of the Sea) Identification Leaflets for Diseases and Parasites of Fish and Shellfish; DK-1261; ICES: Copenhagen, Denmark, 1991; Volume 41, p. 4. ISSN 0109-2510. [Google Scholar]
- Nebel, C.; Romestand, B.; Nègre-Sadargues, G.; Grousset, E.; Aujoulat, F.; Bacal, J.; Charmantier, G. Differential freshwater adaptation in juvenile sea-bass Dicentrarchus labrax involves gills and urinary system. J. Exp. Biol. 2005, 208, 3859–3871. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. A Ecol. 2020, 333, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Deane, E.E.; Kelly, S.P.; Luk, J.C.; Woo, N. Chronic salinity adaptation modulates hepatic heat shock protein and insulin-like growth factor I expression in black sea bream. J. Mar. Biotechnol. 2002, 4, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Jiang, K.; Wang, J.; Shen, X. Effects of salinity on growth, feeding and the mRNA expression of Na+/K+-ATPase and HSP 90 in Liza haematocheila. Ecol. Res. 2015, 3, 51–59. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N. Advances and perspectives on the regulation and expression of piscine heat shock proteins. Rev. Fish Biol. Fish. 2011, 21, 153–185. [Google Scholar] [CrossRef]
- De Wachter, B.; Scholliers, A.; Blust, R. Semiquantitative immunoblot detection of 70 kDa stress proteins in the carp Cyprinus carpio. Bull. Environ. Contam. Toxicol. 1998, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.N.; Winton, J.R.; Dickhoff, W.W. Tissue-specific induction of Hsp90 mRNA and plasma cortisol response in chinook salmon following heat shock, seawater challenge, and handling challenge. J. Mar. Biotechnol. 2000, 2, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; An, K.W. Cloning and expression of Na+/K+-ATPase and osmotic stress transcription factor 1 mRNA in black porgy, Acanthopagrus schlegeli during osmotic stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Deane, E.E.; Woo, N.Y. Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1054–R1063. [Google Scholar] [CrossRef] [PubMed]
- Feidantsis, K.; Poertner, H.O.; Antonopoulou, E.; Michaelidis, B. Synergistic effects of acute warming and low pH on cellular stress responses of the gilthead seabream Sparus aurata. J. Comp. Physiol. 2015, 185, 185–205. [Google Scholar] [CrossRef]
- Tsakoumis, E.; Tsoulia, T.; Feidantsis, K.; Mouchlianitis, F.A.; Berillis, P.; Bobori, D.; Antonopoulou, E. Cellular stress responses of the endemic freshwater fish species Alburnus vistonicus Freyhof & Kottelat, 2007 in a constantly changing environment. Appl. Sci. 2021, 11, 11021. [Google Scholar] [CrossRef]
- Fiol, D.F.; Kültz, D. Osmotic stress sensing and signaling in fishes. FEBS Lett. 2007, 274, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Naguro, I.; Ichijo, H.; Watanabe, K. Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2037–2052. [Google Scholar] [CrossRef]
- Kültz, D.; Avila, K. Mitogen-activated protein kinases are in vivo transducers of osmosensory signals in fish gill cells. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wen, H.; Qi, X.; Zhang, X.; Li, Y. Identification of mapk gene family in Lateolabrax maculatus and their expression profiles in response to hypoxia and salinity challenges. Gene 2019, 684, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Christensen, S.T.; Hoffmann, E.K. Effects of osmotic stress on the activity of MAPKs and PDGFR-β-mediated signal transduction in NIH-3T3 fibroblasts. Am. J. Physiol. Cell Physiol. 2008, 294, C1046–C1055. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Hamad, D.; Gustin, M.C. MAP kinases and the adaptive response to hypertonicity: Functional preservation from yeast to mammals. Am. J. Physiol. Renal Physiol. 2004, 287, F1102–F1110. [Google Scholar] [CrossRef] [PubMed]
| 20 ppt | 14 ppt | 8 ppt | p-Value | |
|---|---|---|---|---|
| TANin | 0.51 ± 0.13 a | 0.50 ± 0.11 a | 0.70 ± 0.24 a | 0.498 |
| TANout | 0.48 ± 0.09 a | 0.46 ± 0.08 a | 0.67 ± 0.22 a | 0.678 |
| ΝO3−in | 114.61 ± 14.73 a | 122.38 ± 11 a | 120 ± 11.12 a | 0.903 |
| ΝO3−out | 112.08 ± 16.05 a | 111.41 ± 17.83 a | 109.76 ± 16.09 a | 0.971 |
| ΝO2− | 0.17 ± 0.04 a | 0.14 ± 0.04 a | 0.18 ± 0.96 a | 0.895 |
| PO4−in | 0.51 ± 0.18 a | 0.52 ± 0.16 a | 0.52 ± 0.18 a | 0.918 |
| PO4−out | 0.42 ± 0.15 a | 0.47 ± 0.18 a | 0.43 ± 0.10 a | 0.955 |
| pHFT | 6.0 ± 0.41 a | 6.1 ± 0.41 a | 6.5 ± 0.38 a | 0.690 |
| pHGB | 6.3 ± 0.4 a | 6.5 ± 0.34 a | 6.6 ± 0.29 a | 0.860 |
| 20 ppt | 14 ppt | 8 ppt | p-Value | |
|---|---|---|---|---|
| Initial mean weight (Win, g) | 3.89 ± 0.05 a | 3.85 ± 0.04 a | 3.97 ± 0.05 a | 0.321 |
| Final mean weight (Wfin, g) | 19.44 ± 0.31 a | 18.52 ± 0.28 a | 19.71 ± 0.36 b | 0.024 |
| Weight gain (WG, g) | 15.6 ± 0.32 a | 14.67 ± 0.28 a | 15.7 ± 0.38 a | 0.173 |
| Specific growth rate (SGR, %/d) | 3.60 ± 0.05 b | 3.09 ± 0.14 a | 3.58 ± 0.06 b | 0.032 |
| Survival (%) | 90± 6.74 a | 97 ±1.33 a | 96 ±2.30 a | 0.436 |
| Body weight increase (BWI %) | 412.8 ± 11.88 a | 387.3 ± 10.24 a | 408.9 ± 13.70 a | 0.268 |
| Initial coefficient factor (Kin) | 1.24 ± 0.01 a | 1.28 ± 0.01 a | 1.26 ± 0.01 a | 0.178 |
| Final coefficient factor (Kfin) | 1.49 ± 0.01 a | 1.48 ± 0.01 a | 1.54 ± 0.01 b | 0.02 |
| Initial mean length (Lin, cm) | 6.77 ± 0.03 a | 6.70 ± 0.03 a | 6.80 ± 0.03 a | 0.123 |
| Final mean length (Lfin, cm) | 10.71 ± 0.18 a | 10.76 ± 0.05 a | 10.82 ± 0.06 a | 0.787 |
| Food conversion ration (FCR) | 0.70 ± 0.01 a | 0.70 ± 0.01 a | 0.74 ± 0.04 a | 0.121 |
| Daily food intake (DFI %/d) | 1.54 ± 0.02 a | 1. 55 ± 0.04 a | 1.56 ± 0.04 a | 0.945 |
| 20 ppt | 14 ppt | 8 ppt | p-Value | |
|---|---|---|---|---|
| Initial number of branches | 8.38 ± 0.44 a | 7.33 ± 0.49 a | 9.11 ± 0.74 a | 0.100 |
| Final number of branches | 14.22 ± 1.93 a | 13.38 ± 1.26 a | 18.94 ± 2.55 a | 0.114 |
| Final height (cm) | 10.22 ± 0.56 a | 14.66 ± 0.78 b | 20.27 ± 1.2 c | 0.027 |
| Rate of height gain (cm/d) | 2.82 ± 0.42 a | 2.62 ± 0.45 a | 8.97 ± 0.92 b | 0.000 |
| Final fresh weight of the aerial part | 8.99 ± 0.88 a | 21.26 ± 3.15 b | 57.03 ± 586 c | 0.000 |
| Liver | Midgut | Kidney | Gills | |
|---|---|---|---|---|
| 20‰ salinity | 1 | 1 | 1 | 1 |
| 14‰ salinity | 1 | 0 | 2 | 2 |
| 8‰ salinity | 1 | 0 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlahos, N.; Berillis, P.; Levizou, E.; Patsea, E.; Panteli, N.; Demertzioglou, M.; Morfesis, K.; Voudouri, G.; Krigas, N.; Kormas, K.; et al. Investigating Salinity Effects in Brackish Aquaponics Systems: Evidencing the Co-Cultivation of the Halophyte Crithmum maritimum with the Euryhaline Sparus aurata. Appl. Sci. 2023, 13, 3385. https://doi.org/10.3390/app13063385
Vlahos N, Berillis P, Levizou E, Patsea E, Panteli N, Demertzioglou M, Morfesis K, Voudouri G, Krigas N, Kormas K, et al. Investigating Salinity Effects in Brackish Aquaponics Systems: Evidencing the Co-Cultivation of the Halophyte Crithmum maritimum with the Euryhaline Sparus aurata. Applied Sciences. 2023; 13(6):3385. https://doi.org/10.3390/app13063385
Chicago/Turabian StyleVlahos, Nikolaos, Panagiotis Berillis, Efi Levizou, Efstathia Patsea, Nikolas Panteli, Maria Demertzioglou, Konstantinos Morfesis, Georgia Voudouri, Nikos Krigas, Kostantinos Kormas, and et al. 2023. "Investigating Salinity Effects in Brackish Aquaponics Systems: Evidencing the Co-Cultivation of the Halophyte Crithmum maritimum with the Euryhaline Sparus aurata" Applied Sciences 13, no. 6: 3385. https://doi.org/10.3390/app13063385
APA StyleVlahos, N., Berillis, P., Levizou, E., Patsea, E., Panteli, N., Demertzioglou, M., Morfesis, K., Voudouri, G., Krigas, N., Kormas, K., Antonopoulou, E., & Mente, E. (2023). Investigating Salinity Effects in Brackish Aquaponics Systems: Evidencing the Co-Cultivation of the Halophyte Crithmum maritimum with the Euryhaline Sparus aurata. Applied Sciences, 13(6), 3385. https://doi.org/10.3390/app13063385










