Fungi-Templated Silver Nanoparticle Composite: Synthesis, Characterization, and Its Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks
2.2. Preparation of Leaf Extract
2.3. Growth and Harvest of Fungal Mycelia
2.4. Synthesis of AgNPs
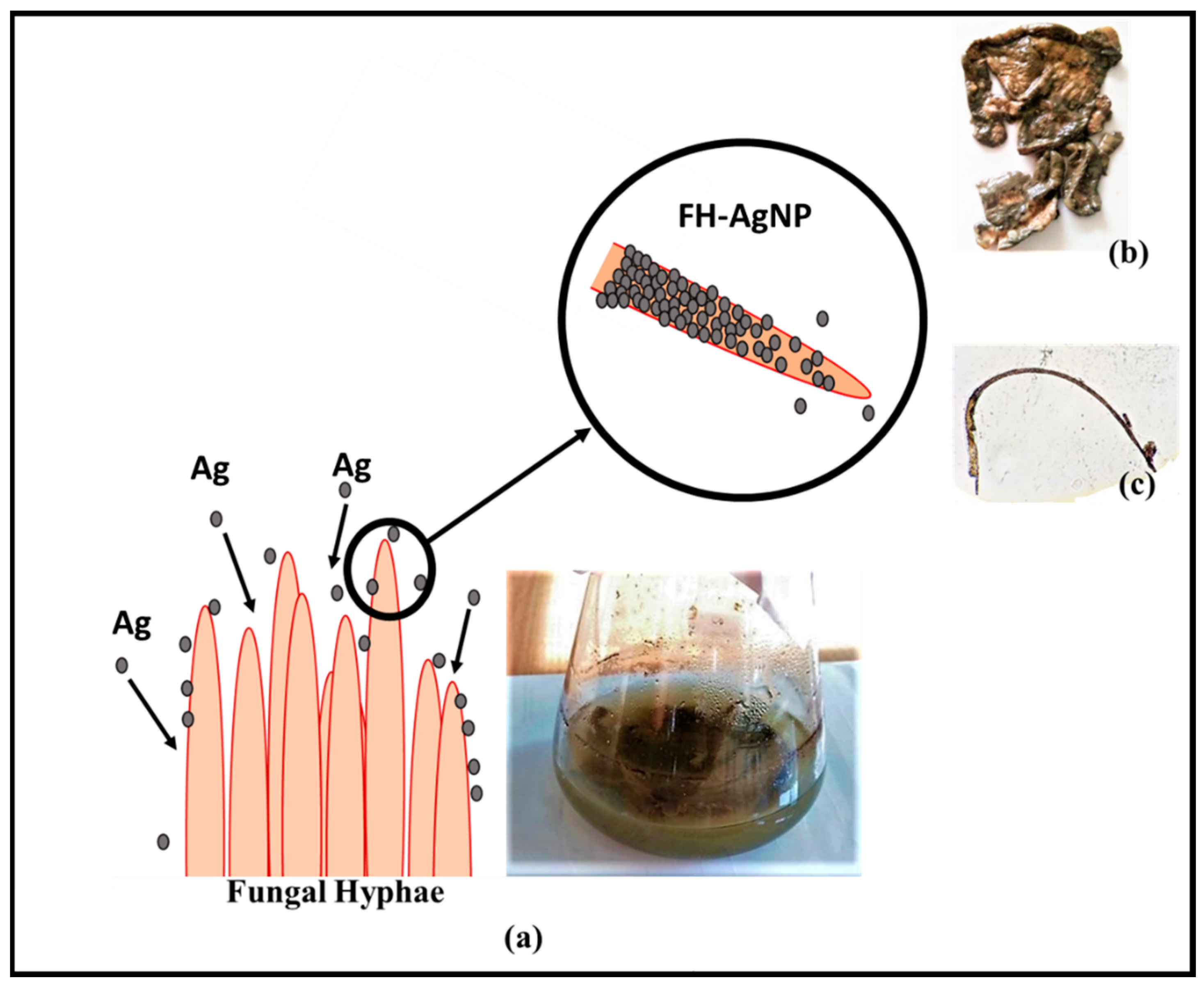
2.5. Preparation of Fungal Hyphae–Silver Nanoparticle (FH-AgNP) Composite
2.6. Degradation Studies
2.7. Antibacterial Studies
3. Results and Discussion
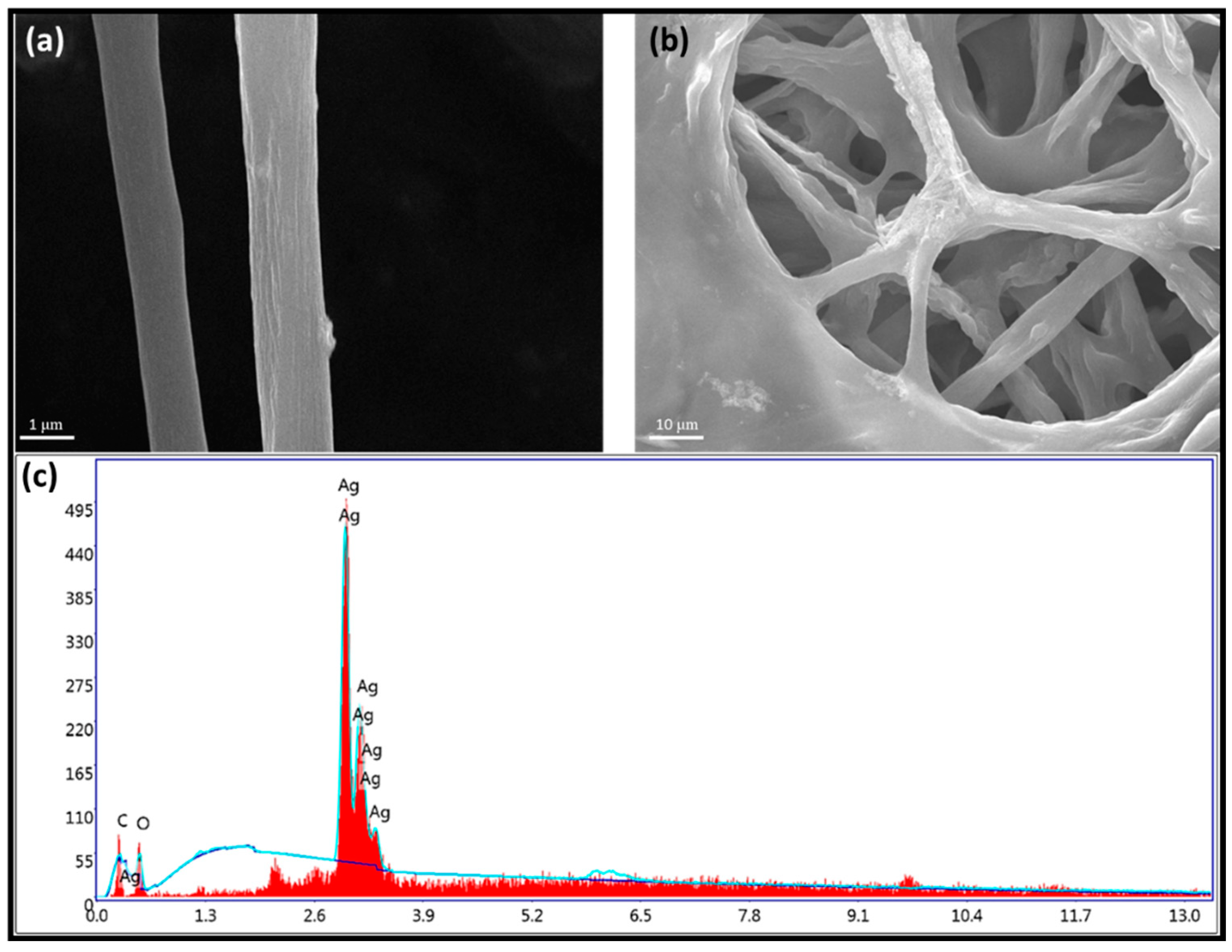
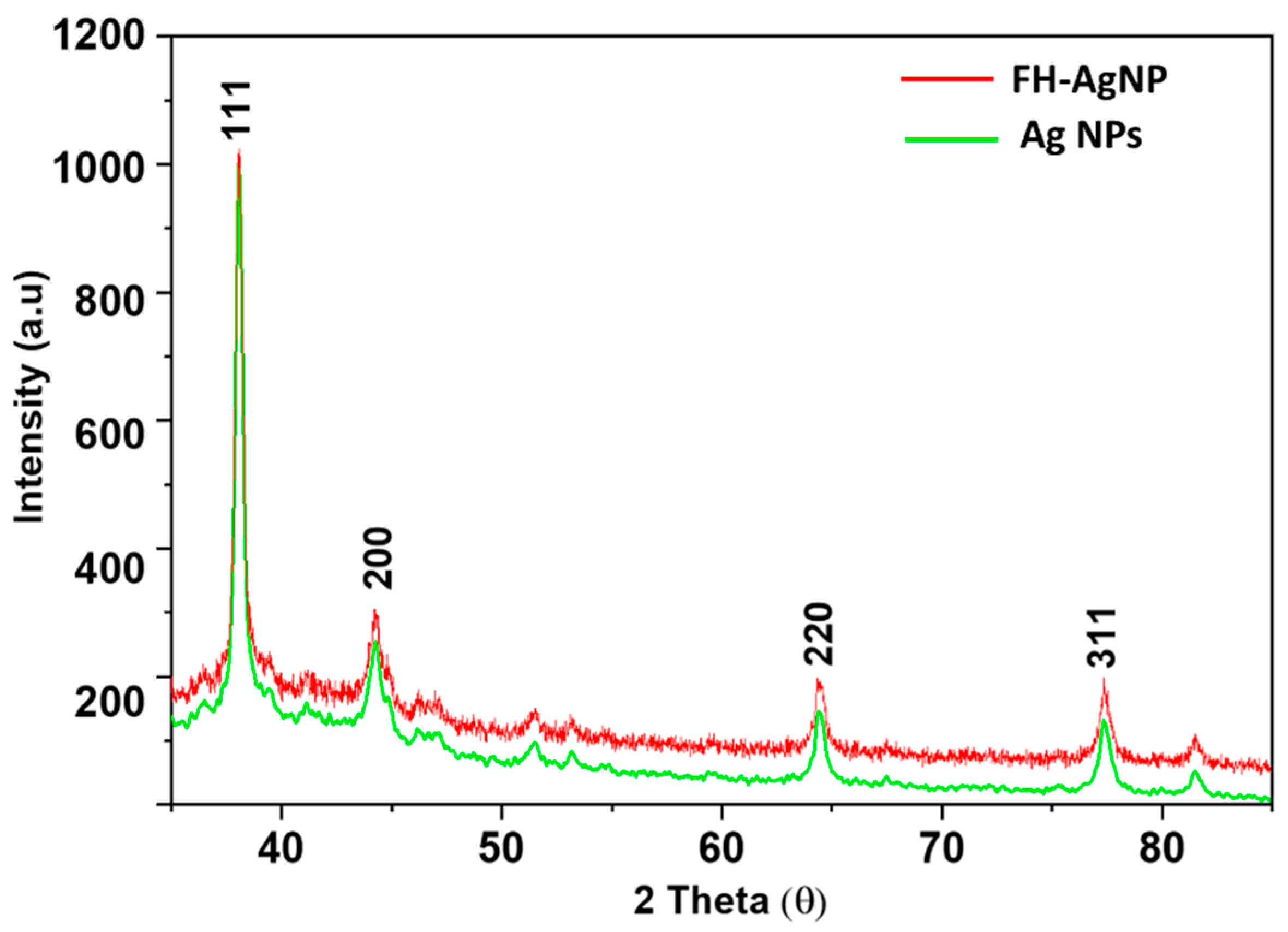
3.1. Morphological Characterization
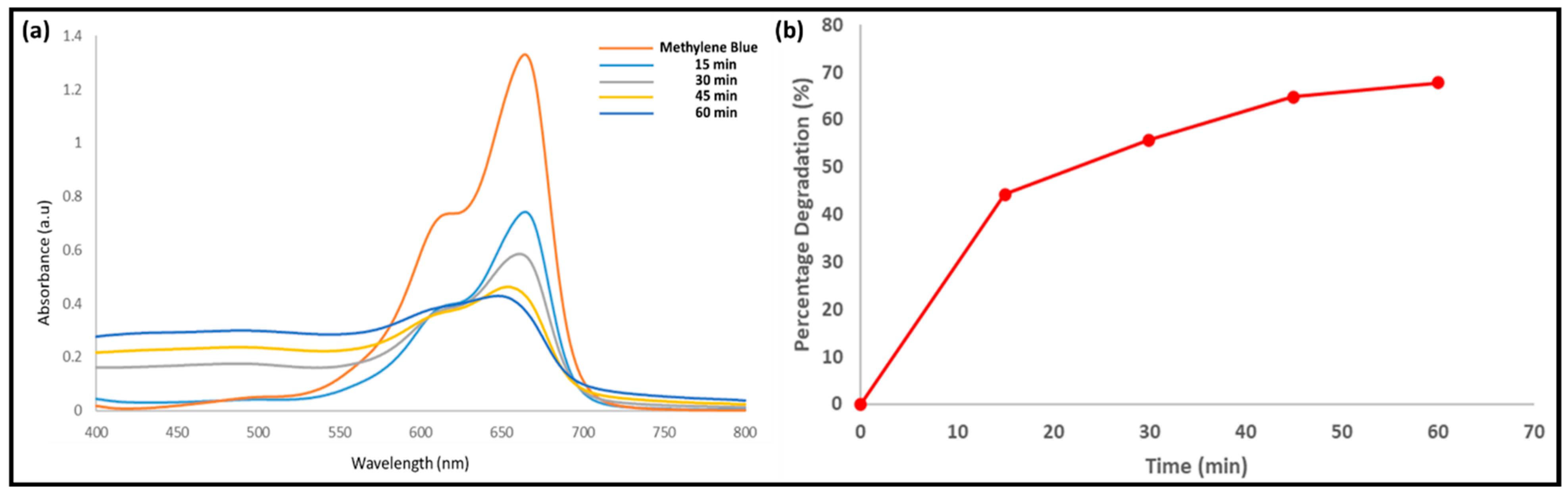
3.2. Photocatalytic Degradation of Methylene Blue
3.3. Antibacterial Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozden, S.; MacWan, I.G.; Owuor, P.S.; Kosolwattana, S.; Autreto, P.A.S.; Silwal, S.; Vajtai, R.; Tiwary, C.S.; Mohite, A.D.; Patra, P.K.; et al. Bacteria as Bio-Template for 3D Carbon Nanotube Architectures. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-Templating: An Emerging Synthetic Technique for Catalysts. A Review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Zheng, X.T.; Xu, H.V.; Tan, Y.N. Bioinspired Design and Engineering of Functional Nanostructured Materials for Biomedical Applications. ACS Symp. Ser. 2017, 1253, 123–152. [Google Scholar] [CrossRef]
- Selvakumar, R.; Seethalakshmi, N.; Thavamani, P.; Naidu, R.; Megharaj, M. Recent Advances in the Synthesis of Inorganic Nano/Microstructures Using Microbial Biotemplates and Their Applications. RSC Adv. 2014, 4, 52156–52169. [Google Scholar] [CrossRef]
- Kubo, A.M.; Gorup, L.F.; Toffano, L.; Amaral, L.S.; Rodrigues-Filho, E.; Mohan, H.; Aroca, R.; Camargo, E.R. Nanostructured Assemblies of Gold and Silver Nanoparticles for Plasmon Enhanced Spectroscopy Using Living Biotemplates. Colloids Interfaces 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Kubo, A.M.; Gorup, L.F.; Amaral, L.S.; Filho, E.R.; Camargo, E.R. Kinetic Control of Microtubule Morphology Obtained by Assembling Gold Nanoparticles on Living Fungal Biotemplates. Bioconjug. Chem. 2016, 27, 2337–2345. [Google Scholar] [CrossRef]
- Kubo, A.M.; Gorup, L.F.; Amaral, L.S.; Rodrigues-Filho, E.; de Camargo, E.R. Heterogeneous Microtubules of Self-Assembled Silver and Gold Nanoparticles Using Alive Biotemplates. Mater. Res. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Rehman, A.; Majeed, M.I.; Ihsan, A.; Hussain, S.Z.; Saif-Ur-Rehman; Ghauri, M.A.; Khalid, Z.M.; Hussain, I. Living Fungal Hyphae-Templated Porous Gold Microwires Using Nanoparticles as Building Blocks. J. Nanopart. Res. 2011, 13, 6747–6754. [Google Scholar] [CrossRef]
- Zhu, W.K.; Cong, H.P.; Guan, Q.F.; Yao, W.T.; Liang, H.W.; Wang, W.; Yu, S.H. Coupling Microbial Growth with Nanoparticles: A Universal Strategy to Produce Functional Fungal Hyphae Macrospheres. ACS Appl. Mater. Interfaces 2016, 8, 12693–12701. [Google Scholar] [CrossRef]
- Sabah, A.; Kumar, P.; Mohammed, W.S.; Dutta, J. Visible-Light-Induced Directed Gold Microwires by Self-Organization of Nanoparticles on Aspergillus Niger. Part. Part. Syst. Charact. 2013, 30, 473–480. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Khattak, W.A.; Shahzad, A.; Ul-Islam, M.; Yang, G. Biotemplate-Mediated Green Synthesis and Applications of Nanomaterials. Curr. Pharm. Des. 2020, 26, 5819–5836. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; Zhang, Q.; Wang, J.H.; Wu, X.L.; Wang, S.Y.; Peng, Y.Z. Contributions of Functional Groups and Extracellular Polymeric Substances on the Biosorption of Dyes by Aerobic Granules. Bioresour. Technol. 2011, 102, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Abdi, O.; Kazemi, M. A Review Study of Biosorption of Heavy Metals and Comparison between Different Biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal Biosynthesis of Gold Nanoparticles: Mechanism and Scale Up. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef]
- Li, Z.; Chung, S.-W.; Nam, J.-M.; Ginger, D.S.; Mirkin, C.A. Living Templates for the Hierarchical Assembly of Gold Nanoparticles. Angew. Chem. 2003, 115, 2408–2411. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Pan, G.; Qian, P.; Ni, Z. Photocatalytic Degradation of Methylene Blue with a Nanocomposite System: Synthesis, Photocatalysis and Degradation Pathways. Phys. Chem. Chem. Phys. 2015, 17, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Samari, F.; Salehipoor, H.; Eftekhar, E.; Yousefinejad, S. Low-Temperature Biosynthesis of Silver Nanoparticles Using Mango Leaf Extract: Catalytic Effect, Antioxidant Properties, Anticancer Activity and Application for Colorimetric Sensing. New J. Chem. 2018, 42, 15905–15916. [Google Scholar] [CrossRef]
- Res, J.M.B.; Sarsar, V.; Selwal, K.K.; Selwal, M.K. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Mangifera Indica and Evaluation of Their Antimicrobial Activity. J. Microbiol. Biotech. Res. 2013, 3, 27–32. [Google Scholar]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.H. Thermal Degradation and Fire Properties of Fungal Mycelium and Mycelium—Biomass Composite Materials. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Pal, U.; Castillo López, D.N.; Carcaño-Montiel, M.G.; López-Reyes, L.; Díaz-Nuñez, P.; Peña-Rodríguez, O. Nanoparticle-Assembled Gold Microtubes Built on Fungi Templates for SERS-Based Molecular Sensing. ACS Appl. Nano Mater. 2019, 2, 2533–2541. [Google Scholar] [CrossRef]
- Feofilova, E.P. The Fungal Cell Wall: Modern Concepts of Its Composition and Biological Function. Microbiology 2010, 79, 711–720. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, Y.; Guo, C.; Zhang, C. Preparation and Characterization of Silver-Doped Graphene-Reinforced Silver Matrix Bulk Composite as a Novel Electrical Contact Material. Appl. Phys. A Mater. Sci. Process. 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Taufiq-Yap, Y.H.; Ng, C.H.; Vo, D.V.N.; Ibrahim, M.L.; Hasan, M.M.; Khan, M.A.R.; Nur, A.S.M.; Awual, M.R. Step towards the Sustainable Toxic Dyes Removal and Recycling from Aqueous Solution- A Comprehensive Review. Resour. Conserv. Recycl. 2021, 175, 105849. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A.S. Plasmon-Induced Photocatalytic Degradation of Methylene Blue Dye Using Biosynthesized Silver Nanoparticles as Photocatalyst. Environ. Technol. 2018, 41, 1520–1534. [Google Scholar] [CrossRef]
- Jose, A.; Sunaja Devi, K.R.; Pinheiro, D.; Lakshmi Narayana, S. Electrochemical Synthesis, Photodegradation and Antibacterial Properties of PEG Capped Zinc Oxide Nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 187, 25–34. [Google Scholar] [CrossRef] [PubMed]

| Concentration (μg) | Zone of Inhibition—E. coli (mm) | Zone of Inhibition—S. aureus (mm) | |
|---|---|---|---|
| FH-AgNPs | 50 | 5 | 5.5 |
| 100 | 5.8 | 5.7 | |
| 200 | 6 | 6.5 | |
| 400 | 6 | 6.5 | |
| Ampicillin (Standard) | 50 | 6.6 | 6.2 |
| 100 | 7 | 7.5 | |
| 200 | 7.4 | 7.5 | |
| 400 | 8 | 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joy, F.; Devasia, J.; Nizam, A.; Lakshmaiah, V.V.; Krishna, S.B.N. Fungi-Templated Silver Nanoparticle Composite: Synthesis, Characterization, and Its Applications. Appl. Sci. 2023, 13, 2158. https://doi.org/10.3390/app13042158
Joy F, Devasia J, Nizam A, Lakshmaiah VV, Krishna SBN. Fungi-Templated Silver Nanoparticle Composite: Synthesis, Characterization, and Its Applications. Applied Sciences. 2023; 13(4):2158. https://doi.org/10.3390/app13042158
Chicago/Turabian StyleJoy, Francis, Jyothis Devasia, Aatika Nizam, Vasantha Veerappa Lakshmaiah, and Suresh Babu Naidu Krishna. 2023. "Fungi-Templated Silver Nanoparticle Composite: Synthesis, Characterization, and Its Applications" Applied Sciences 13, no. 4: 2158. https://doi.org/10.3390/app13042158
APA StyleJoy, F., Devasia, J., Nizam, A., Lakshmaiah, V. V., & Krishna, S. B. N. (2023). Fungi-Templated Silver Nanoparticle Composite: Synthesis, Characterization, and Its Applications. Applied Sciences, 13(4), 2158. https://doi.org/10.3390/app13042158







