Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Characterization
2.3. Measurement of Acid Value
2.4. Direct Preparation of Aqueous Polymer Solutions
2.5. Preparation of Polymer Pre-Solutions in Organic Solvents
2.6. Solubilization of Polymer in Alkaline Water from the Organic-Solvent Solution
2.7. Measurements of Size Distribution
2.8. Measurement of Critical Micelle Concentration (CMC) of Block Copolymer
2.9. Measurement of Viscosity
3. Result and Discussion
3.1. Synthesis and Characterization of Block Co-Polymers
3.2. Dissolution of Polymers in Water
3.3. Dissolution Method with a Water-Soluble Organic Solvent
3.4. Property of Polymer Micelles
3.5. Characterization of Transferring from Pre-Solution
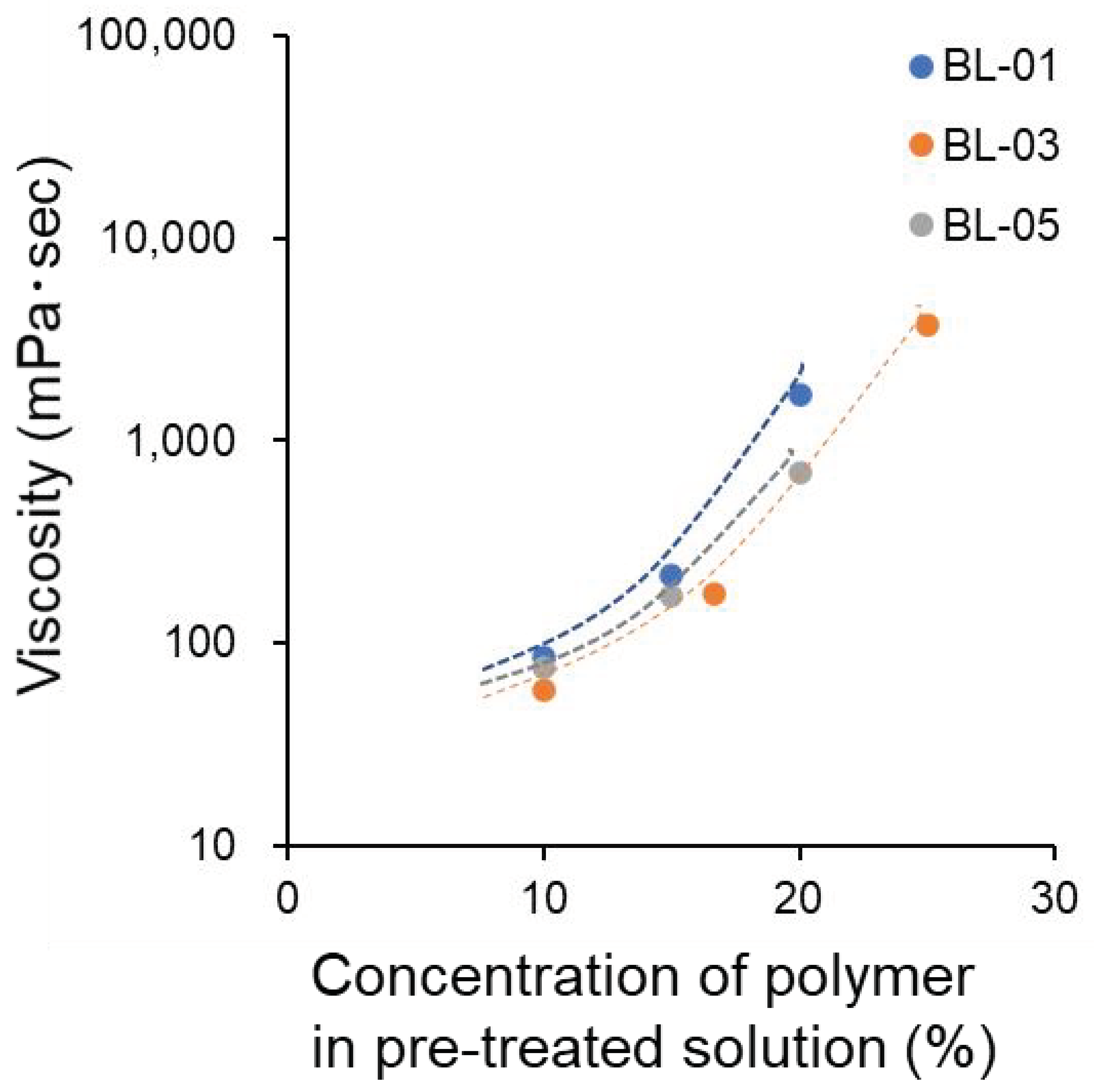
3.6. Preparation of Concentrated Aqueous Solutions from Pre-Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duivenvoorde, F.L.; Laven, J.; van der Linde, R. Diblock copolymer dispersants in polyester powder coatings. Prog. Org. Coat. 2002, 45, 127–137. [Google Scholar] [CrossRef]
- Duivenvoorde, F.L.; van Nostrum, C.F.; Laven, J.; van der Linde, R. Improving pigment dispersing in powder coatings with block copolymer dispersants. J. Coat. Technol. 2000, 72, 145–152. [Google Scholar] [CrossRef]
- Louguet, S.; Kumar, A.C.; Sigaud, G.; Duguet, E.; Lecommandoux, S.; Schatz, C. A physico-chemical investigation of poly (ethylene oxide)-block-poly (L-lysine) copolymer adsorption onto silica nanoparticles. J. Colloid Interface Sci. 2011, 359, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Badila, M.; Brochon, C.; Hébraud, A.; Hadziioannou, G. Encapsulation of TiO2 in poly (4-vinyl pyridine)-based cationic microparticles for electrophoretic inks. Polymer 2008, 49, 4529–4533. [Google Scholar] [CrossRef]
- Ohtake, T.; Ito, H.; Toyoda, N. Amphiphilic block copolymer surfactant-containing quaternized pyridinium salt segments for color dispersion. Polym. J. 2022, 54, 1203–1211. [Google Scholar] [CrossRef]
- Ohtake, T.; Ito, H.; Toyoda, N. Amphiphilic Polymers for Color Dispersion: Toward Stable and Low Viscosity Inkjet Ink. Langmuir 2022, 38, 7618–7627. [Google Scholar] [CrossRef]
- Yoon, C.; Choi, J.H. Synthesizing polymeric dispersants to apply to carbon black pigmented mill bases for use in ink-jet inks. Color. Technol. 2019, 00, 1–15. [Google Scholar]
- Chan, D.H.H.; Deane, O.J.; Kynaston, E.L.; Lindsay, C.; Taylor, P.; Armes, S.P. Sterically Stabilized Diblock Copolymer Nanoparticles Enable Convenient Preparation of Suspension Concentrates Comprising Various Agrochemical Actives. Langmuir 2022, 38, 2885–2894. [Google Scholar] [CrossRef]
- Yatake, M. Inkset for inkjet Recorded Matter. Japanese Patent Application No. 2008-231130, 2008. [Google Scholar]
- van den Haak, H.J.W.; Krutzer, L.L.M. Design of pigment dispersants for high-solids paint systems. Prog. Org. Coat. 2001, 43, 56–63. [Google Scholar] [CrossRef]
- Takahashi, S. Effect of Properties of High-Molecular Weight Polymer on the Characteristics of Polymer-Dispersed Pigment in Water-Based Ink. J. Jpn. Soc. Colour Mater. 2012, 85, 271–278. [Google Scholar]
- Yasuda, K.; Ito, O. Aqueous Ink Composition, Ink Set and Image Formation Method. Japanese Patent Application No. 2016-069583, 2016. [Google Scholar]
- Koike, T.; Kikuta, N.; Mizuno, Y. Aqueous Composite Resin and Its Manufacturing Method. Japanese Patent Application, No. 2018-080311, 2018. [Google Scholar]
- Nagaki, A.; Ashikari, Y.; Takumi, M.; Tamaki, T. Flash Chemistry Makes Impossible Organolithium Chemistry Possible. Chem. Lett. 2021, 50, 485–492. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 Underlies a Sensing Mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar] [CrossRef]
- Nagaki, A.; Miyazaki, A.; Yoshida, J. Synthesis of Polystyrenes−Poly(alkyl methacrylates) Block Copolymers via Anionic Polymerization Using an Integrated Flow Microreactor System. Macromolecules 2010, 43, 8424. [Google Scholar] [CrossRef]
- Nagaki, A. Manufacturing Method of Resin with Mn/Mw Less Than or Equal to 1.25. Japanese Patent Application No. 2009-67999, 2009. [Google Scholar]
- Yoshida, J.; Nagaki, A.; Kamon, Y. Manufacturing Method for Block Copolymer. Japanese Patent Application No. 2010-180353, 2010. [Google Scholar]
- Uemura, Y; Tanaka, H.; Oe, N. Manufacturing Method for Block Copolymer and Block Copolymer Made by the Method. Japanese Patent Application No. 2015-504445, 2015.
- Fukui, H.; Kimura, T.; Uemura, Y.; Tanaka, H. Method for Producing Kneaded Product for Aqueous Pigment Dispersion, and Method for Producing Aqueous Pigment Dispersion and Ink Composition Using the Kneaded Product. Japanese Patent Application No. 2015-147888, 2015. [Google Scholar]
- Matsuoka, H.; Ohnishi, T.; Gohsh, A. pH-Responsive non-surface-active/surface-active transition of weakly ionic amphiphilic diblock copolymers. Colloid. Polym. Sci. 2014, 292, 797–806. [Google Scholar] [CrossRef]
- Youn, L.D.; Hyun, K.J. Preparation of small-sized carboxylated latexes by emulsion polymerization using alkali-soluble random copolymer. J. Appl. Polym. Sci. 1998, 69, 543–550. [Google Scholar]
- Mai, Y.; Eisenberg, A. Self-Assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Lund, R.; Willner, L.; Richter, D.; Lindner, P.; Narayanan, T. Kinetic Pathway of the Cylinder-to-Sphere Transition in Block Copolymer Micelles Observed in Situ by Time-Resolved Neutron and Synchrotron Scattering. ACS Macro Lett. 2013, 2, 1082. [Google Scholar] [CrossRef] [PubMed]
- Tamori, K.; Kihara, K.; Esumi, K.; Meguro, K. Micellar properties and growth of cationic fluorocarbon surfactant; diethanolheptadecafluoro-2-undecanolmethylammonium chloride. Colloid Polym. Sci. 1992, 270, 927–934. [Google Scholar] [CrossRef]
- Satomi, T.; Ueno, K.; Fujita, Y.; Kobayashi, H.; Tateishi, T.; Otsuka, H. Characterization of Polypyridine-graft-PEG Copolymer at Interface. J. Jpn. Soc. Colour Mater. 2006, 79, 475–482. [Google Scholar] [CrossRef]
- Otsuka, H.; Sanbai, T.; Matsukuma, D.; Ikenakga, Y. Self-Assembly of poly(ethylene glycol)-block-polypyridine copolymer into micelles and at silica surface: Effect of molecular architecture on silica dispersion. Colloid. Polym. Sci. 2014, 292, 291–300. [Google Scholar] [CrossRef]
- Nakajima, A. Solvent Effect on the Vibrational Structures of the Fluorescence and Absorption Spectra of Pyrene. Bull. Chem. Soc. Jpn. 1971, 44, 3272–3277. [Google Scholar] [CrossRef]
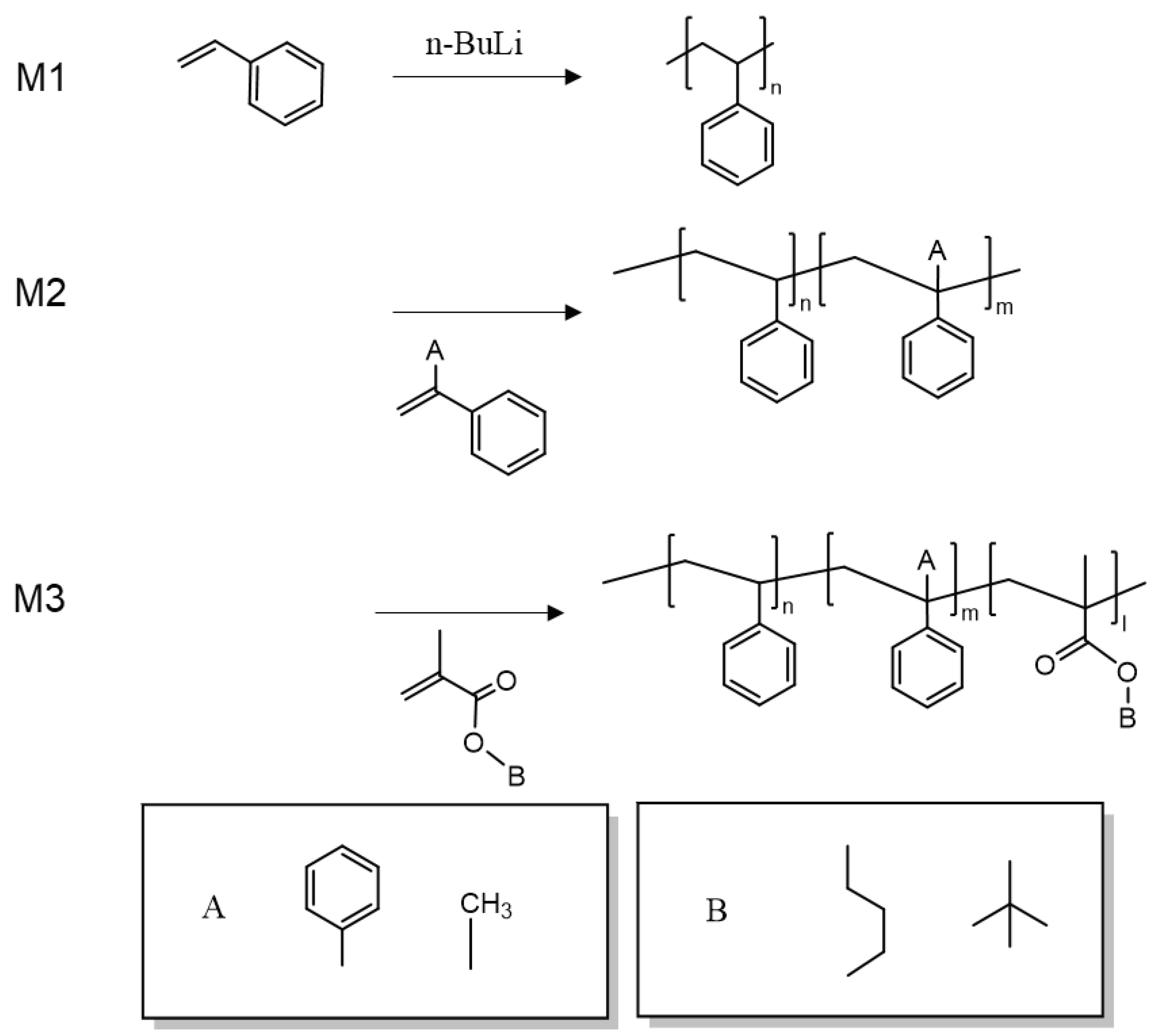
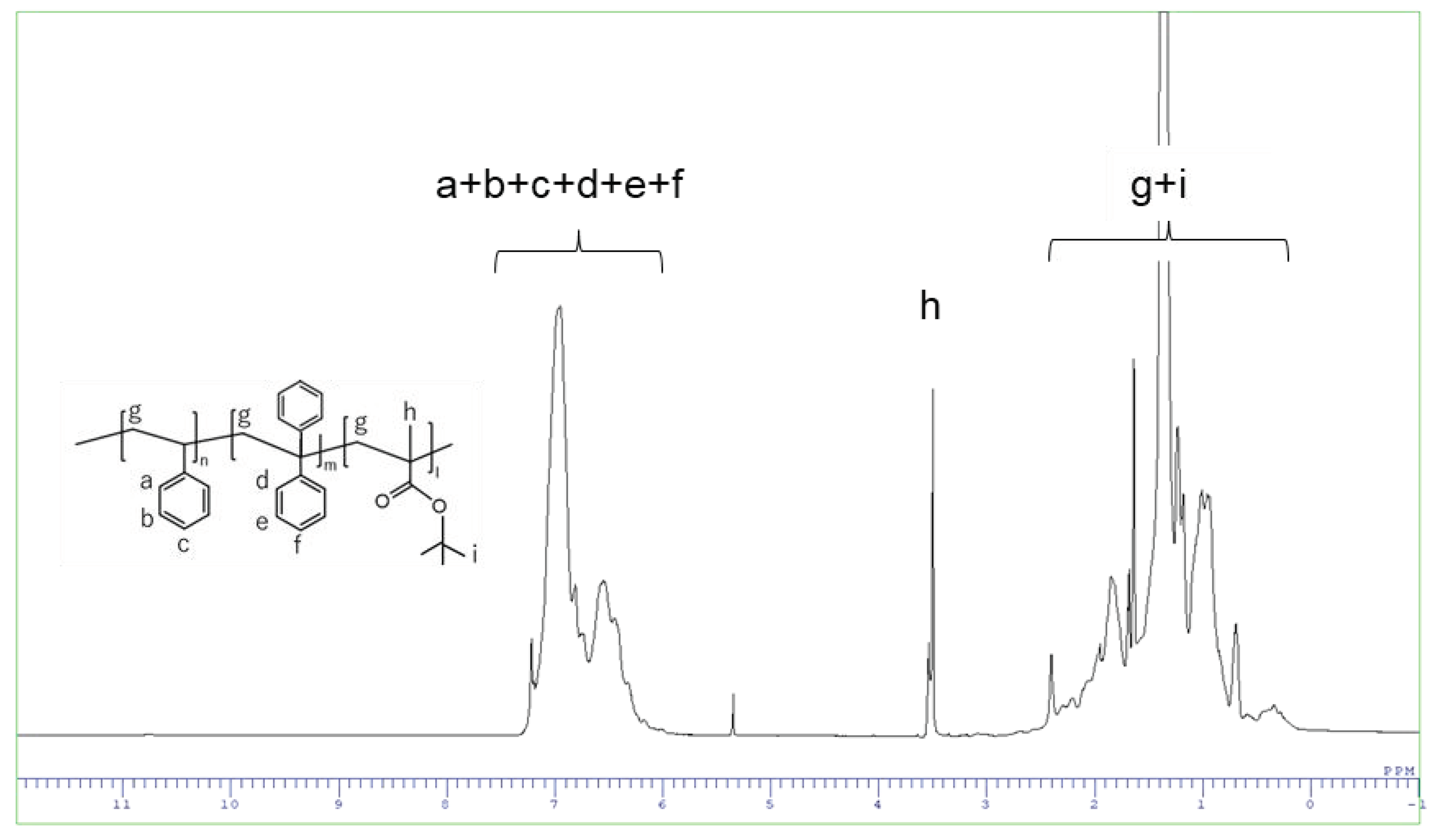

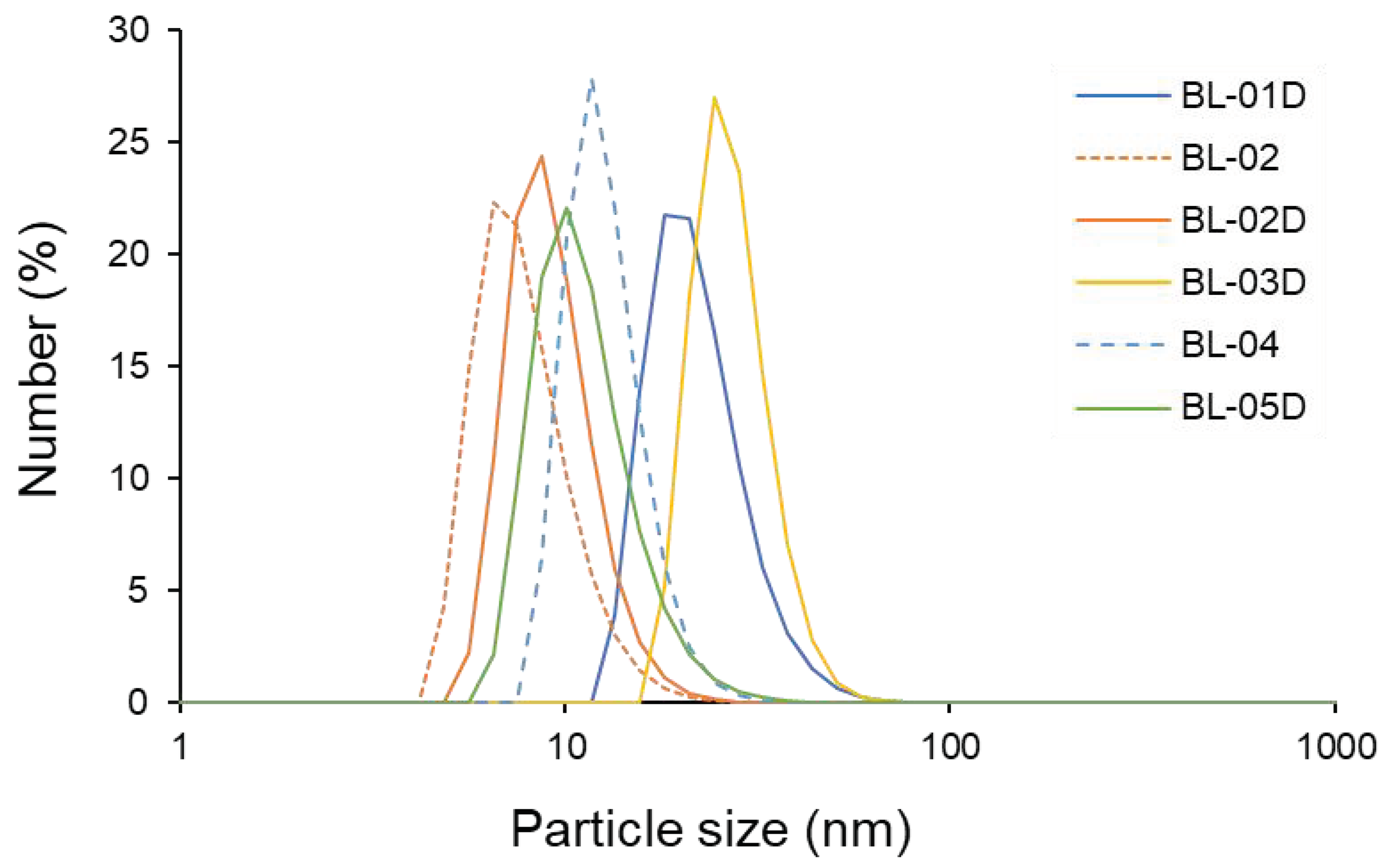
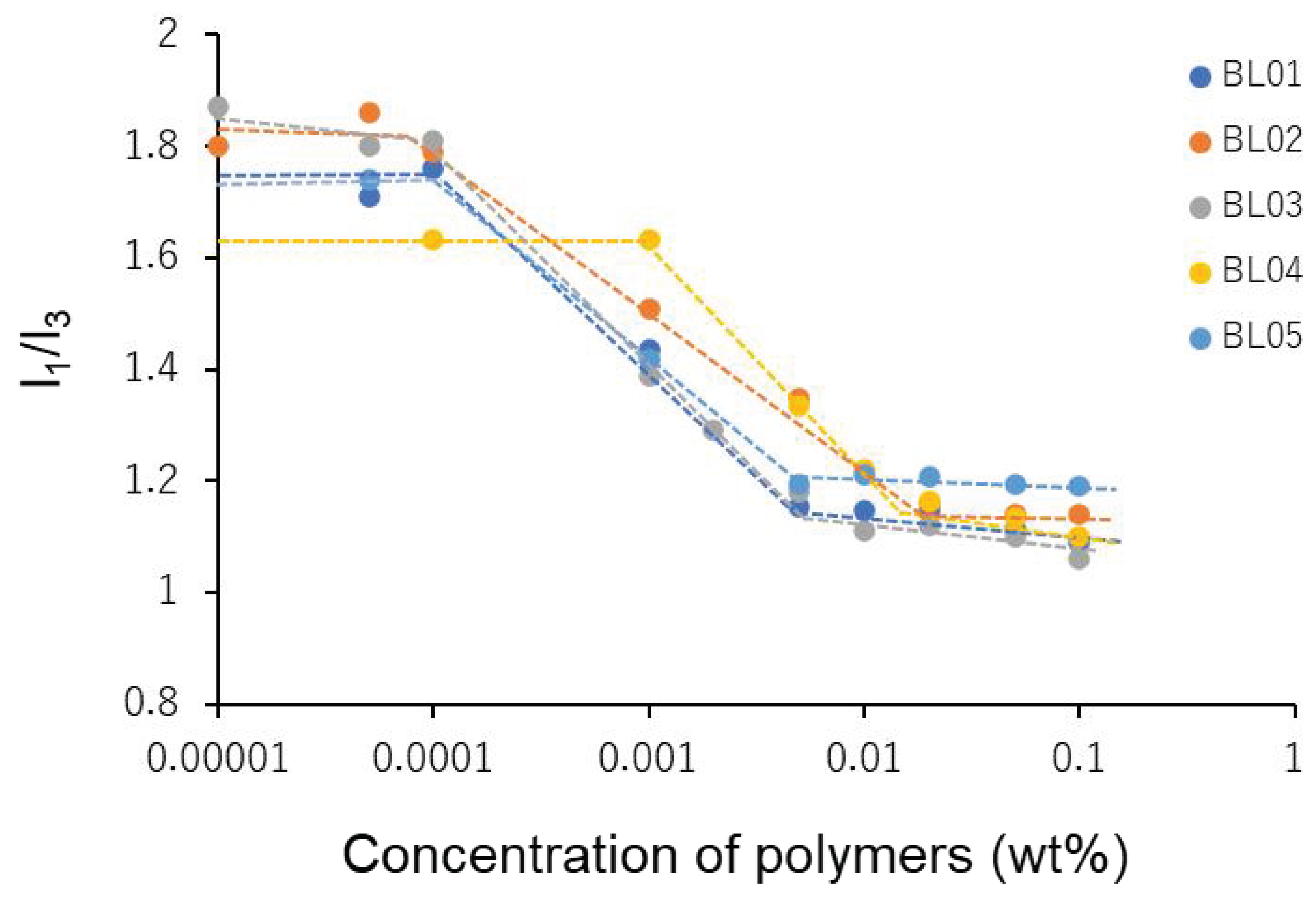


| Sample | Monomers | Mol Ratio | Mn g/mol−1 | Mw g/mol−1 | Mw/Mn | Acid Value |
|---|---|---|---|---|---|---|
| BL-01 | St, DPE, MAA, | 13/1/8 | 2679 | 3107 | 1.16 | 129 |
| BL-02 | St, DPE, n-BMA, MAA | 3.9/1/9.1/8 | 2445 | 4077 | 1.67 | 146 |
| BL-03 | St, α-MeSt, MAA | 6.8/2/5 | 1509 | 1737 | 1.15 | 143 |
| BL-04 | St, α-MeSt, MAA | 8/2/13 | 2685 | 3165 | 1.18 | 271 |
| BL-05 | St, α-MeSt, MAA | 24/2/14 | 3950 | 4752 | 1.20 | 152 |
| Sample | Monomers | Solubility in the KOH Solution * |
|---|---|---|
| BL-01 | St, DPE, MAA, | Insoluble ** |
| BL-02 | St, DPE, n-BMA, MAA | Soluble |
| BL-03 | St, α-MeSt, MAA | Insoluble ** |
| BL-04 | St, α-MeSt, MAA | Soluble |
| BL-05 | St, α-MeSt, MAA | Insoluble ** |
| Sample | Monomer | Solubility * in the Water-Soluble Solvent | |
|---|---|---|---|
| MEK | DEG | ||
| BL-01 | St, DPE, MAA, | Soluble | Soluble(110 °C) |
| BL-02 | St, DPE, n-BMA, MAA | Soluble | Soluble(50 °C) |
| BL-03 | St, α-MeSt, MAA | Soluble | Soluble(50 °C) |
| BL-04 | St, α-MeSt, MAA | Insoluble | Insoluble |
| BL-05 | St, α-MeSt, MAA | Soluble | Soluble(90 °C) |
| Sample | Monomer | Solubility * in | MEK Solution | DEG Solution | |
|---|---|---|---|---|---|
| MEK | DEG | →KOHaq ** | →KOHaq ** | ||
| BL-01 | St, DPE, MAA, | Soluble | Soluble (110 °C) | Insoluble | Soluble |
| BL-02 | St, DPE, n-BMA, MAA | Soluble | Soluble (50 °C) | Insoluble | Soluble |
| BL-03 | St, α-MeSt, MAA | Soluble | Soluble (50 °C) | Insoluble | Soluble |
| BL-04 | St, α-MeSt, MAA | Insoluble | Insoluble | - | |
| BL-05 | St, α-MeSt, MAA | Soluble | Soluble (90 °C) | Insoluble | Soluble |
| Sample | From Polymer Powder | From DEG Solution | ||
|---|---|---|---|---|
| Number Means | Đ | Number Means | Đ | |
| BL-01 | - | - | 21.0 | 0.2 |
| BL-02 | 7.7 | 0.37 | 9.5 | 0.23 |
| BL-03 | - | - | 26.9 | 0.37 |
| BL-04 | 12.6 | 0.32 | - | - |
| BL-05 | - | - | 12.8 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asada, M.; Tanaka, H.; Suwa, Y.; Irifune, S.; Osawa, S.; Otsuka, H. Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation. Appl. Sci. 2023, 13, 1834. https://doi.org/10.3390/app13031834
Asada M, Tanaka H, Suwa Y, Irifune S, Osawa S, Otsuka H. Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation. Applied Sciences. 2023; 13(3):1834. https://doi.org/10.3390/app13031834
Chicago/Turabian StyleAsada, Masahiko, Hisakazu Tanaka, Yukie Suwa, Sachiko Irifune, Shigehito Osawa, and Hidenori Otsuka. 2023. "Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation" Applied Sciences 13, no. 3: 1834. https://doi.org/10.3390/app13031834
APA StyleAsada, M., Tanaka, H., Suwa, Y., Irifune, S., Osawa, S., & Otsuka, H. (2023). Tuning Pre-Solution of an Amphiphilic Polymeric Dispersant with Low Acid-Value toward Colored-Ink Preparation. Applied Sciences, 13(3), 1834. https://doi.org/10.3390/app13031834







