Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
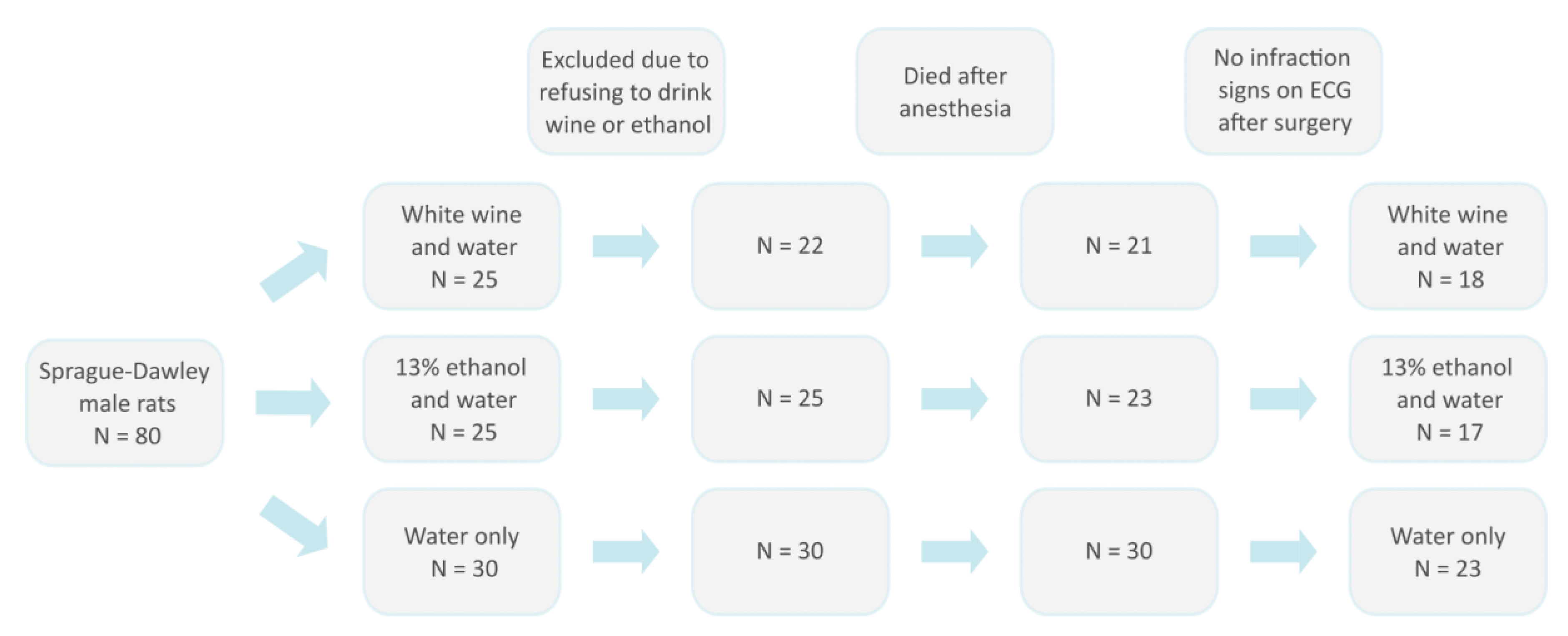
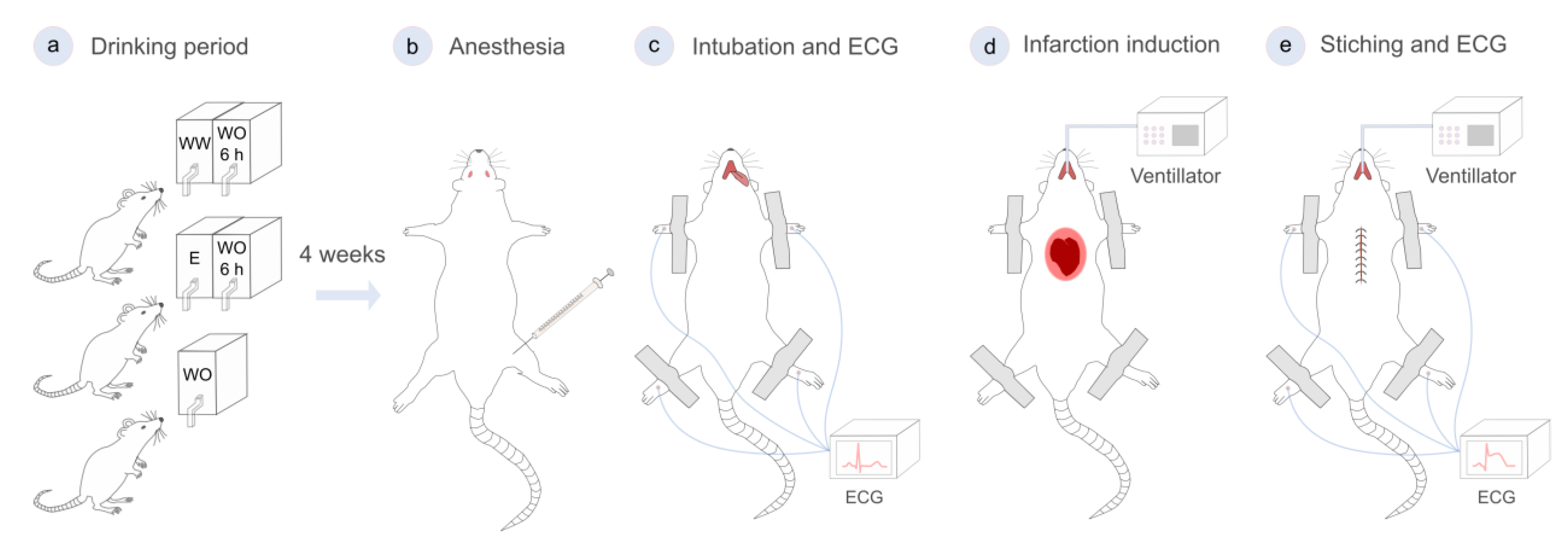
2.2. Animal Model and Experimental Design
2.3. Surgical Procedure
2.4. Data Analysis
3. Results
3.1. Survival Rates after Myocardial Infarction
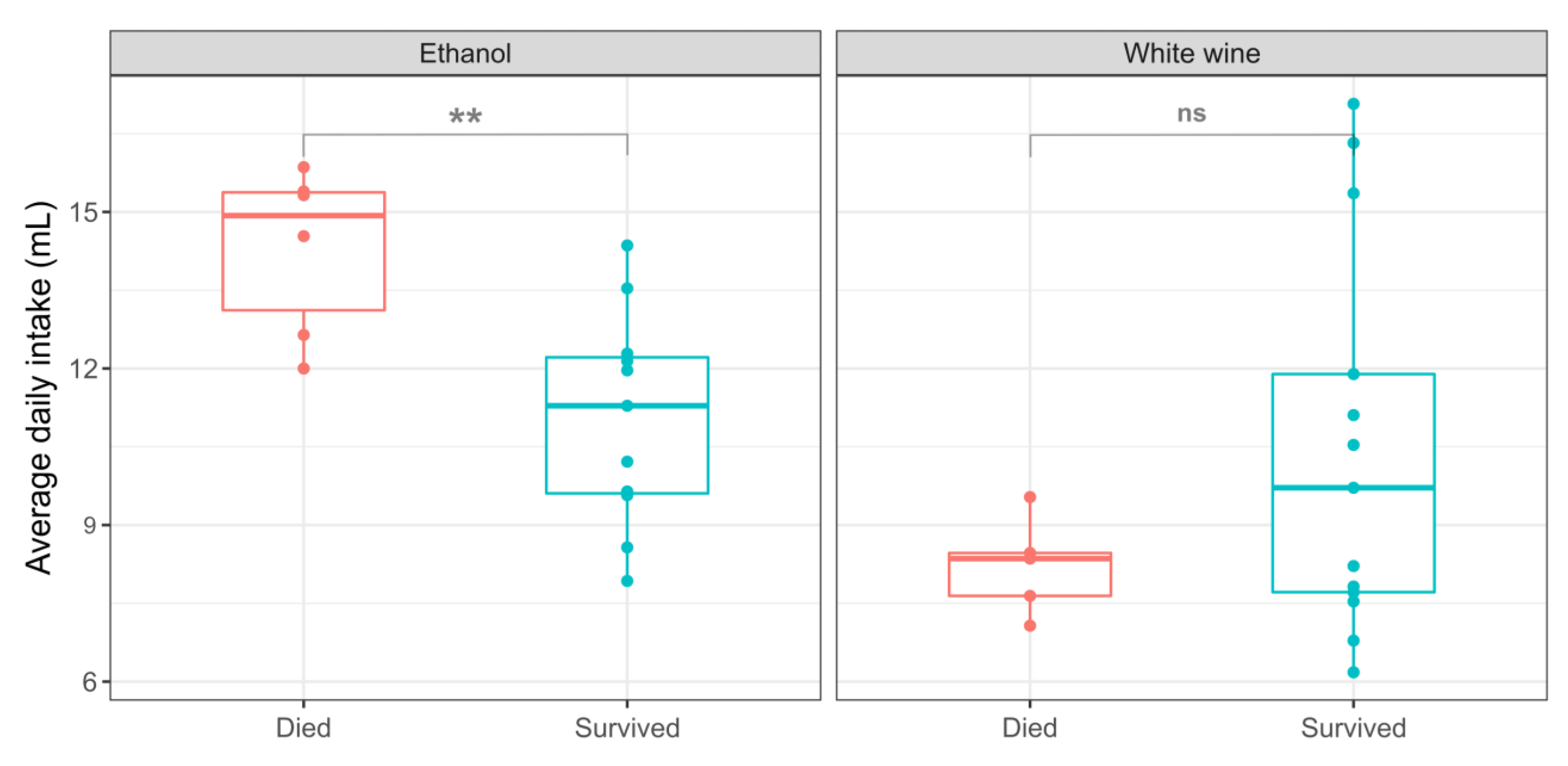
3.2. The Effect of Consumed Volume of Wine and Ethanol on Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases Data. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 14 December 2022).
- Slavin, S.D.; Khera, R.; Zafar, S.Y.; Nasir, K.; Warraich, H.J. Financial Burden, Distress, and Toxicity in Cardiovascular Disease. Am. Heart J. 2021, 238, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Manthey, J.; Rylett, M.; Probst, C.; Wettlaufer, A.; Parry, C.D.H.; Rehm, J. National, Regional, and Global Burdens of Disease from 2000 to 2016 Attributable to Alcohol Use: A Comparative Risk Assessment Study. Lancet Public Health 2020, 5, e51–e61. [Google Scholar] [CrossRef]
- Perl, R. Alcohol and Longevity; Alfred, A., Ed.; Knopf: New York, NY, USA, 1926. [Google Scholar]
- di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; de Gaetano, G. Meta-Analysis of Wine and Beer Consumption in Relation to Vascular Risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef]
- Pai, J.K.; Mukamal, K.J.; Rimm, E.B. Long-Term Alcohol Consumption in Relation to All-Cause and Cardiovascular Mortality among Survivors of Myocardial Infarction: The Health Professionals Follow-up Study. Eur. Heart J. 2012, 33, 1598–1605. [Google Scholar] [CrossRef]
- Janszky, I.; Ljung, R.; Ahnve, S.; Hallqvist, J.; Bennet, A.M.; Mukamal, K.J. Alcohol and Long-Term Prognosis after a First Acute Myocardial Infarction: The SHEEP Study. Eur. Heart J. 2007, 29, 45–53. [Google Scholar] [CrossRef]
- Gronbaek, M.; Deis, A.; Sorensen, T.I.A.; Becker, U.; Schnohr, P.; Jensen, G. Mortality Associated with Moderate Intakes of Wine, Beer, or Spirits. BMJ 1995, 310, 1165–1169. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Maclure, M.; Muller, J.E.; Sherwood, J.B.; Mittleman, M.A. Prior Alcohol Consumption and Mortality Following Acute Myocardial Infarction. JAMA 2001, 285, 1965. [Google Scholar] [CrossRef]
- Miyamae, M.; Diamond, I.; Weiner, M.W.; Camacho, S.A.; Figueredo, V.M. Regular Alcohol Consumption Mimics Cardiac Preconditioning by Protecting against Ischemia–Reperfusion Injury. Proc. Natl. Acad. Sci. USA 1997, 94, 3235–3239. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health–New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between Clinically Recorded Alcohol Consumption and Initial Presentation of 12 Cardiovascular Diseases: Population Based Cohort Study Using Linked Health Records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef] [PubMed]
- Cruijsen, E.; de Ruiter, A.J.; Küpers, L.K.; Busstra, M.C.; Geleijnse, J.M. Alcohol Intake and Long-Term Mortality Risk after Myocardial Infarction in the Alpha Omega Cohort. Am. J. Clin. Nutr. 2022, 115, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, J.S.; Novak, E.; Mukamal, K.J.; Kizer, J.R.; Psaty, B.M.; Stein, P.K.; Brown, D.L. Association of Alcohol Consumption After Development of Heart Failure With Survival Among Older Adults in the Cardiovascular Health Study. JAMA Netw. Open 2018, 1, e186383. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Cui, J.; Tosaki, A.; Cordis, G.; Bertelli, A.A.E.; Bertelli, A.; Maulik, N.; Das, D.K. Cardioprotective Abilities of White Wine. Ann N. Y. Acad. Sci. 2002, 957, 308–316. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Samuel, S.M.; Akita, Y.; Zhan, L.; Bertelli, A.A.E.; Maulik, G.; Maulik, N. White Wine Induced Cardioprotection against Ischemia-Reperfusion Injury Is Mediated by Life Extending Akt/FOXO3a/NFκB Survival Pathway. J. Agric. Food Chem. 2008, 56, 6733–6739. [Google Scholar] [CrossRef]
- Milat, A.M.; Mudnić, I.; Grković, I.; Ključević, N.; Grga, M.; Jerčić, I.; Jurić, D.; Ivanković, D.; Benzon, B.; Boban, M. Effects of White Wine Consumption on Weight in Rats: Do Polyphenols Matter? Oxid Med. Cell Longev. 2017, 2017, 8315803. [Google Scholar] [CrossRef]
- Aljinović, J.; Vukojević, K.; Kosta, V.; Guić, M.M.; Saraga-Babić, M.; Grković, I. Histological Differences in Healing Following Experimental Transmural Infarction in Rats. Histol. Histopathol. 2010, 25, 1507–1517. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Westerterp, K.R. Alcohol and Body Weight. In Health Issues Related to Alcohol Consumption; McDonald, I., Ed.; ILSI Europe: Brussels, Belgium, 1999; pp. 103–123. [Google Scholar]
- Hayes, D.P. Nutritional Hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.P. Adverse Effects of Nutritional Inadequacy and Excess: A Hormetic Model. Am. J. Clin. Nutr. 2008, 88, 578S–581S. [Google Scholar] [CrossRef]
- Chiva-blanch, G.; Badimon, L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Buring, J.E.; Breslow, J.L.; Goldhaber, S.Z.; Rosner, B.; VanDenburgh, M.; Willett, W.; Hennekens, C.H. Moderate Alcohol Intake, Increased Levels of High-Density Lipoprotein and Its Subfractions, and Decreased Risk of Myocardial Infarction. N. Engl. J. Med. 1993, 329, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Vitalini, S.; Burlini, N.; Bernasconi, S.; Iriti, M. Phytosterols in Grapes and Wine, and Effects of Agrochemicals on Their Levels. Food Chem. 2013, 141, 3473–3479. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Cardioprotective Effects of Moderate Red Wine Consumption: Polyphenols vs. Ethanol. J. Appl. Biomed. 2014, 12, 193–202. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. White Wine Polyphenols and Health. In White Wine Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 205–220. [Google Scholar]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Paul, D.; Maulik, N. Akt/FOXO3a/SIRT1-Mediated Cardioprotection by n-Tyrosol against Ischemic Stress in Rat in Vivo Model of Myocardial Infarction: Switching Gears toward Survival and Longevity. J. Agric. Food Chem. 2008, 56, 9692–9698. [Google Scholar] [CrossRef]
- Chernyshova, G.A.; Plotnikov, M.B.; Smol’yakova, V.I.; Golubeva, I.V.; Aliev, O.I.; Tolstikova, T.G.; Krysin, A.P.; Sorokina, I.V. Antiarrhythmic Activity of N-Tyrosol during Acute Myocardial Ischemia and Reperfusion. Bull. Exp. Biol. Med. 2007, 143, 689–691. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-Protective and Antioxidant Properties of Caffeic Acid and Chlorogenic Acid: Mechanistic Role of Angiotensin Converting Enzyme, Cholinesterase and Arginase Activities in Cyclosporine Induced Hypertensive Rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Forman, H.J.; Ursini, F. Para-Hormesis: An Innovative Mechanism for the Health Protection Brought by Antioxidants in Wine. Nutr. Aging 2014, 2, 117–124. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis versus Free Radical Scavenging in Vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic Shifting of Redox Environment by Pro-Oxidative Resveratrol Protects Cells against Stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef]
- Li, J.; Lee, D.H.; Hu, J.; Tabung, F.K.; Li, Y.; Bhupathiraju, S.N.; Rimm, E.B.; Rexrode, K.M.; Manson, J.A.E.; Willett, W.C.; et al. Dietary Inflammatory Potential and Risk of Cardiovascular Disease Among Men and Women in the U.S. J. Am. Coll. Cardiol. 2020, 76, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on Modulation of Gut Microbiota by Wine Polyphenols: From Isolated Cultures to Omic Approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Global White Wine Market. Available online: https://www.factmr.com/report/159/white-wine-market (accessed on 14 December 2022).
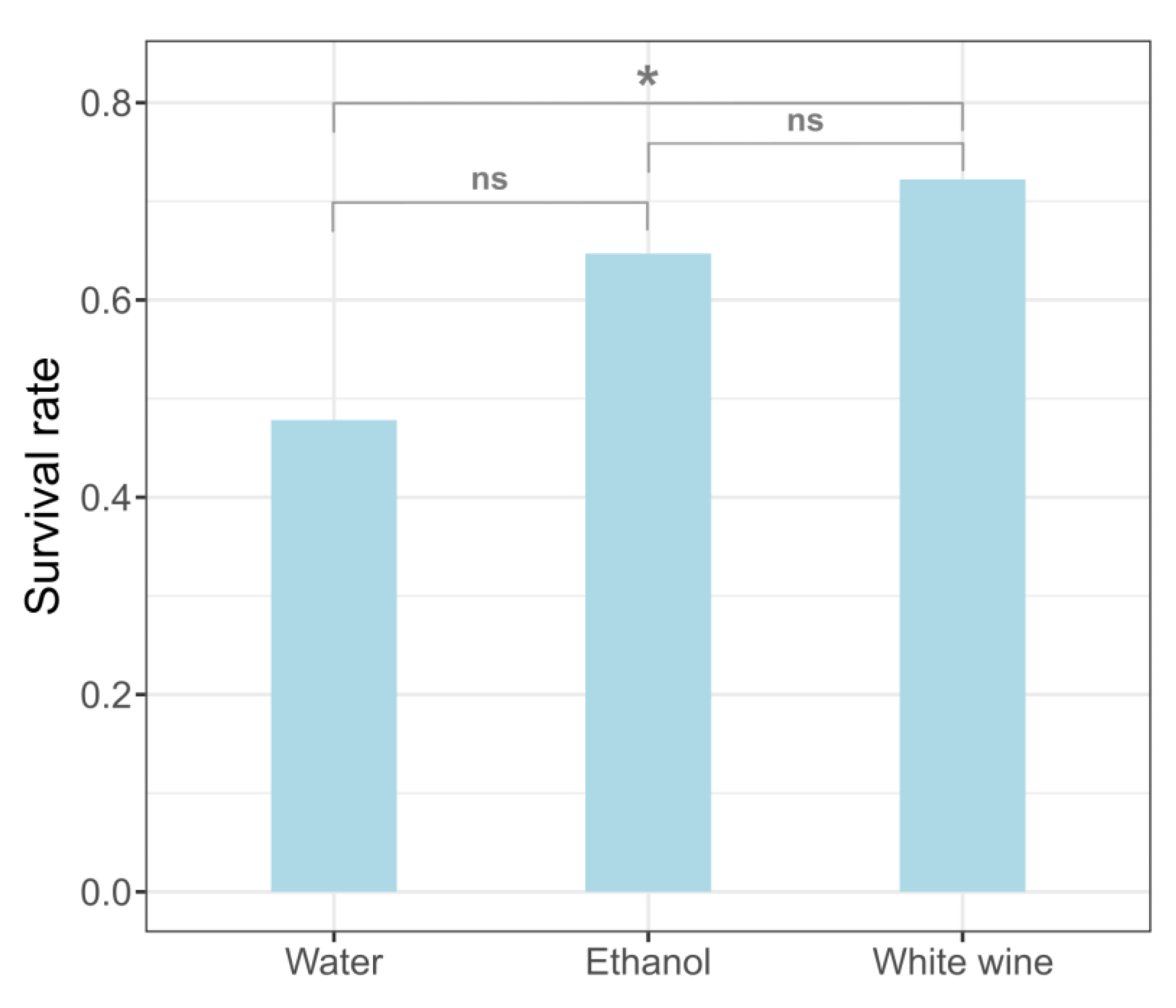
| Treatment | Survived | Died | Total |
|---|---|---|---|
| White wine | 13 (72.2%) | 5 (27.8%) | 18 |
| Ethanol | 11 (64.7%) | 6 (35.3%) | 17 |
| Water | 11 (47.8%) | 12(52.2%) | 23 |
| White Wine | Ethanol | |||||
|---|---|---|---|---|---|---|
| Wine (mL) | Water (mL) | Mass (g) | Ethanol (mL) | Water (mL) | Mass (g) | |
| Survived | 10.5 3.7 | 20.1 1.2 | 329 13 | 11.0 2.0 | 12.1 2.1 | 330 37 |
| Died | 8.21 0.93 | 19.4 8.2 | 351 11 | 14.3 1.6 | 11.9 2.3 | 345 1 |
| Significance | ns | ns | ns | ** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boban, D.; Dželalija, A.M.; Gujinović, D.; Benzon, B.; Ključević, N.; Boban, Z.; Mudnić, I.; Grković, I. Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction. Appl. Sci. 2023, 13, 1450. https://doi.org/10.3390/app13031450
Boban D, Dželalija AM, Gujinović D, Benzon B, Ključević N, Boban Z, Mudnić I, Grković I. Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction. Applied Sciences. 2023; 13(3):1450. https://doi.org/10.3390/app13031450
Chicago/Turabian StyleBoban, Danica, Ana Marija Dželalija, Diana Gujinović, Benjamin Benzon, Nikola Ključević, Zvonimir Boban, Ivana Mudnić, and Ivica Grković. 2023. "Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction" Applied Sciences 13, no. 3: 1450. https://doi.org/10.3390/app13031450
APA StyleBoban, D., Dželalija, A. M., Gujinović, D., Benzon, B., Ključević, N., Boban, Z., Mudnić, I., & Grković, I. (2023). Differential Effects of White Wine and Ethanol Consumption on Survival of Rats after a Myocardial Infarction. Applied Sciences, 13(3), 1450. https://doi.org/10.3390/app13031450






