An Operator Training Simulator to Enable Responses to Chemical Accidents through Mutual Cooperation between the Participants
Abstract
1. Introduction
| Author | Accident Scenario | Applied Technology | Target | Cooperation | Year | Ref. |
|---|---|---|---|---|---|---|
| Brambilla and Manca | Pool evaporating, boiling, and/or ignition, leakage, etc. | Process simulator | Boardman | No | 2011 | [21] |
| Manca et al. | Pool fire | VR, Process simulator | Field operator, Boardman | No | 2013 | [17] |
| Nakai et al. | Fire and/or explosion | VR, Process simulator | Field operator, Boardman | Yes | 2014 | [22] |
| Sharma et al. | Unknown cause | Process simulator | Boardman | No | 2015 | [23] |
| Colombo and Golzio | Leakage, jet fire | VR, Process simulator | Field operator, Boardman | Yes | 2016 | [24] |
| Nakai and Suzuki | Equipment malfunction | AR | Field operator | No | 2016 | [16] |
| Ahmad et al. | Equipment malfunction, fire, etc. | Process simulator | Boardman | No | 2016 | [25] |
| Gerlach et al. | Overflow, clogging of the filtration system | Process simulator | Boardman | No | 2016 | [26] |
| Ouyang et al. | Fire | VR | Field operator | No | 2018 | [27] |
| Puskas et al. | Equipment malfunction | Process simulator | Boardman | No | 2017 | [28] |
| Lee et al. | Overpressure | Process simulator | Field operator | No | 2017 | [29] |
| Pirola et al. | Equipment malfunction | VR, Process simulator | Field operator, Boardman | Yes | 2020 | [30] |
| Yang et al. | Load fluctuation | Process simulator | Boardman | No | 2021 | [19] |
2. Materials and Methods
2.1. Selection of the Training Processes
2.2. Selection of the Chemical Accident Content for Training
2.2.1. Chemical Accident Case Selection
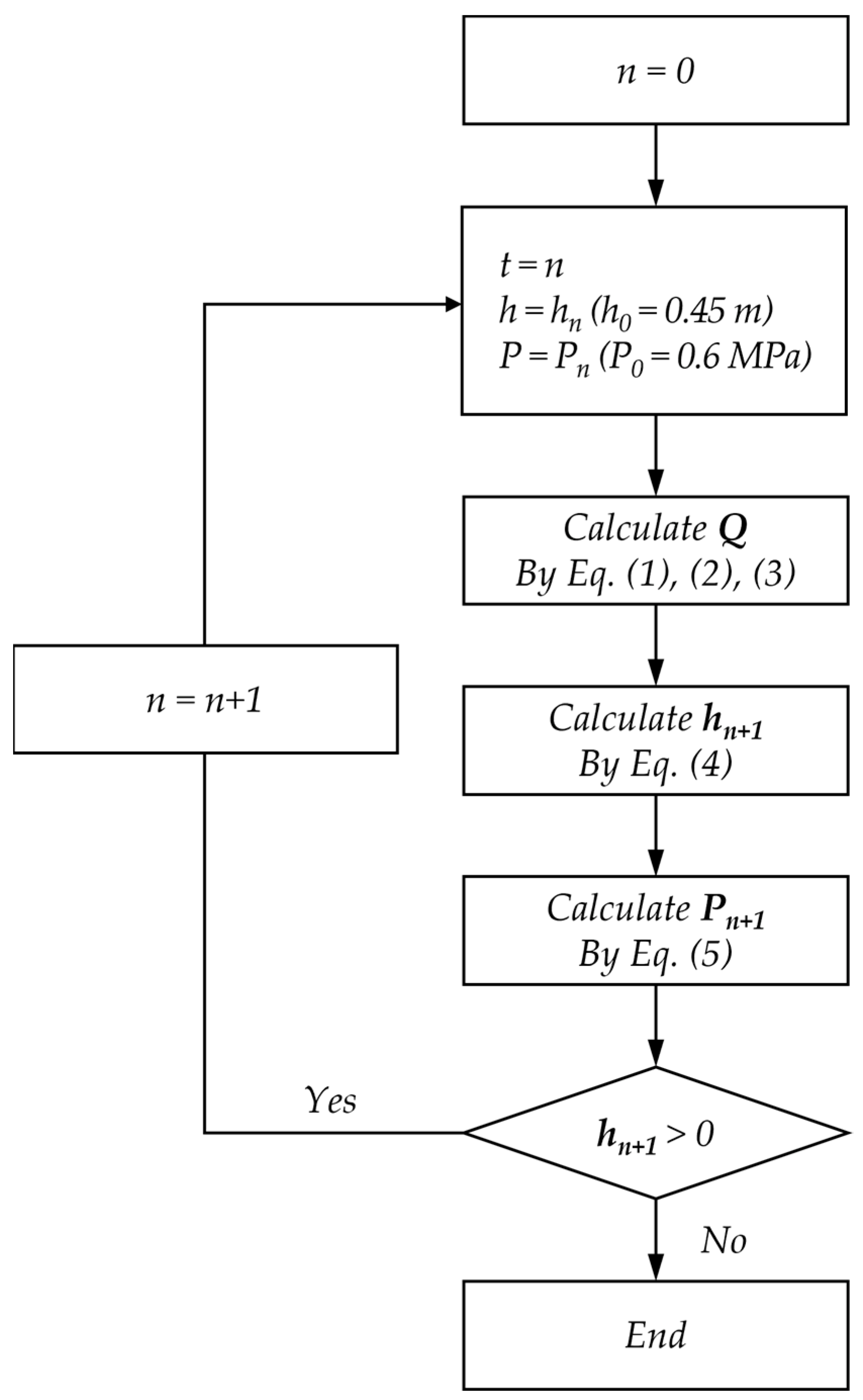
2.2.2. Derivation of Changes to the Facility Status due to a Chemical Accident
2.2.3. Development of the Content for the Chemical Accident Response Cases
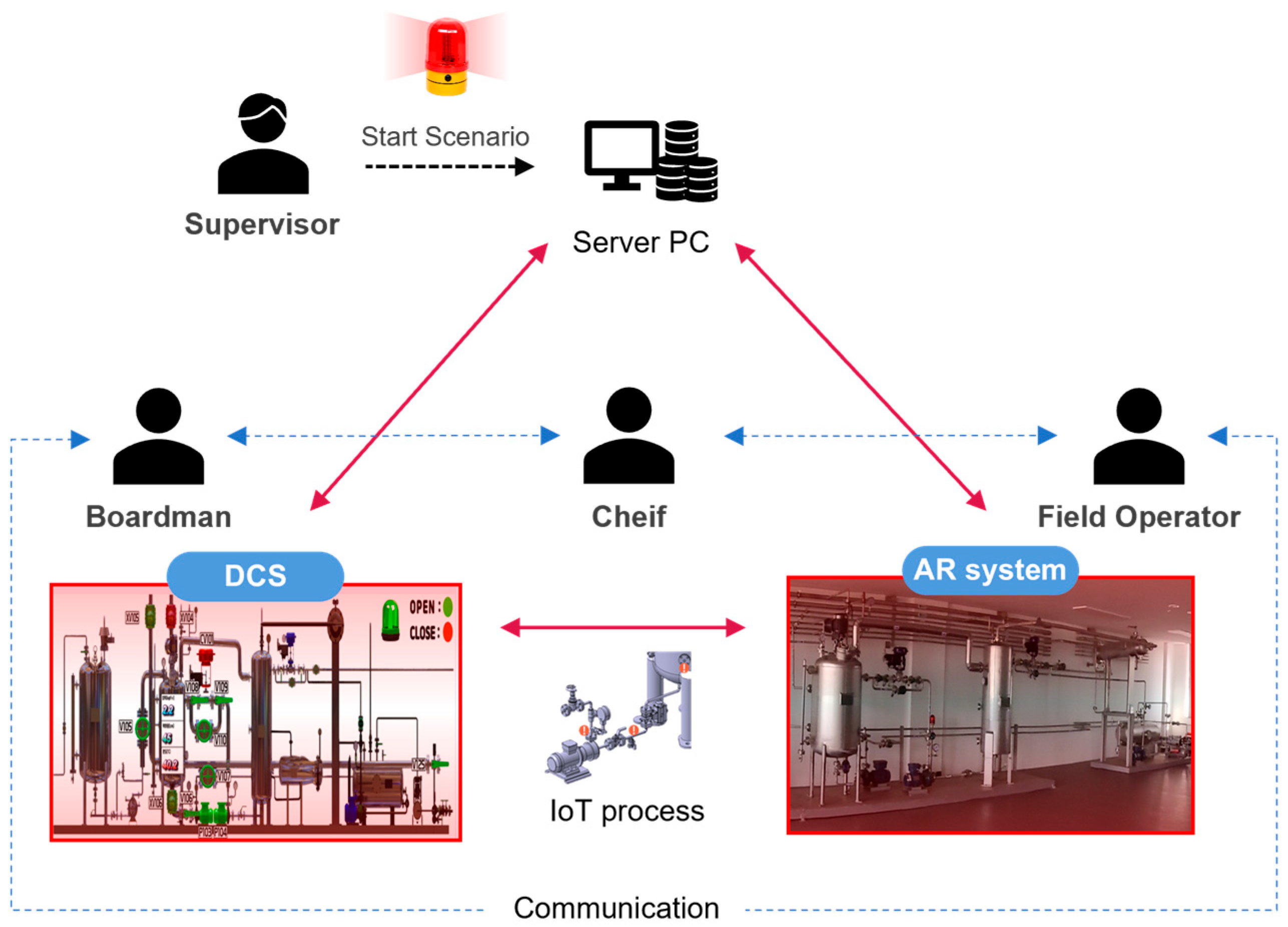
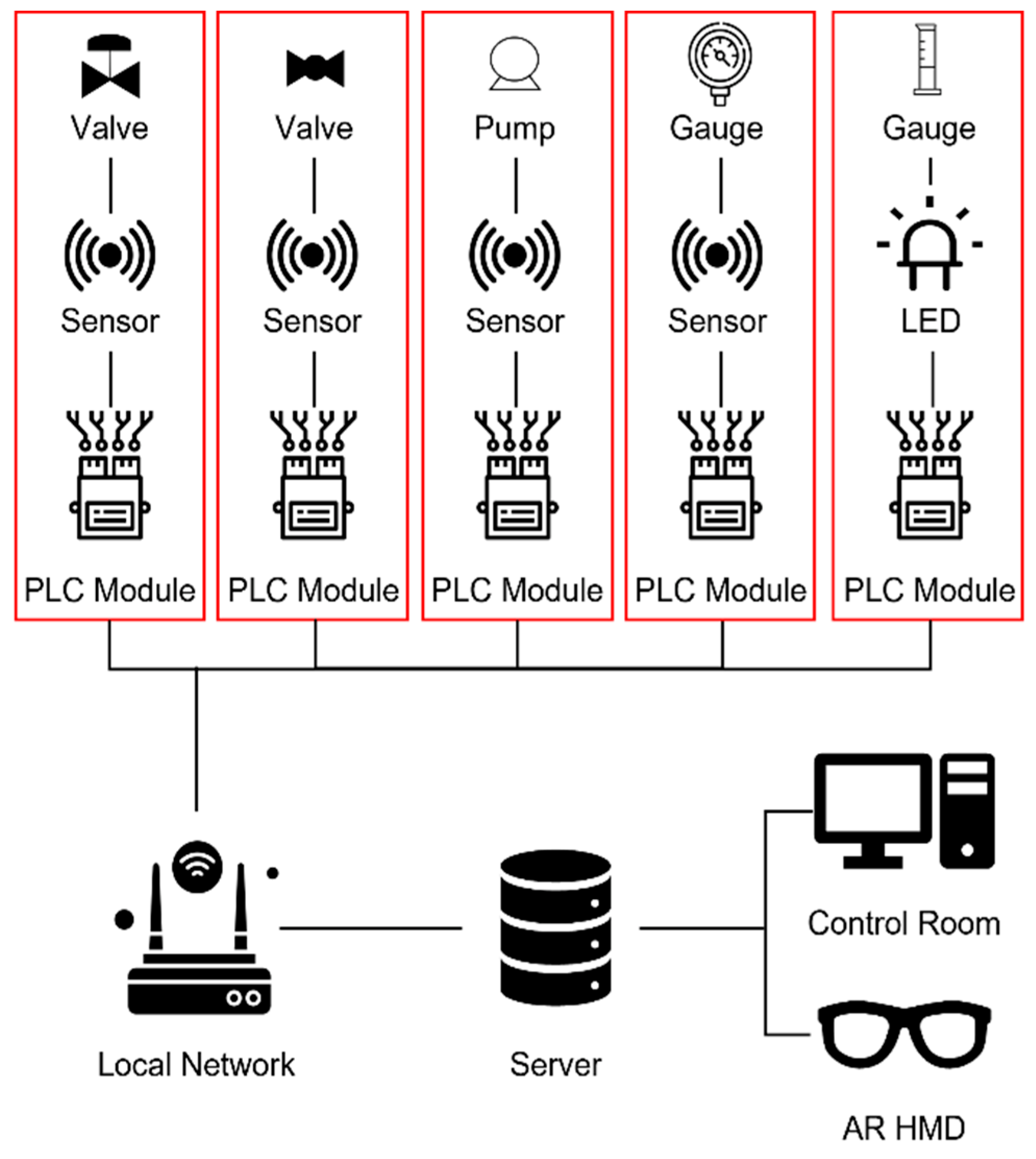
2.3. OTS Infrastructure Construction
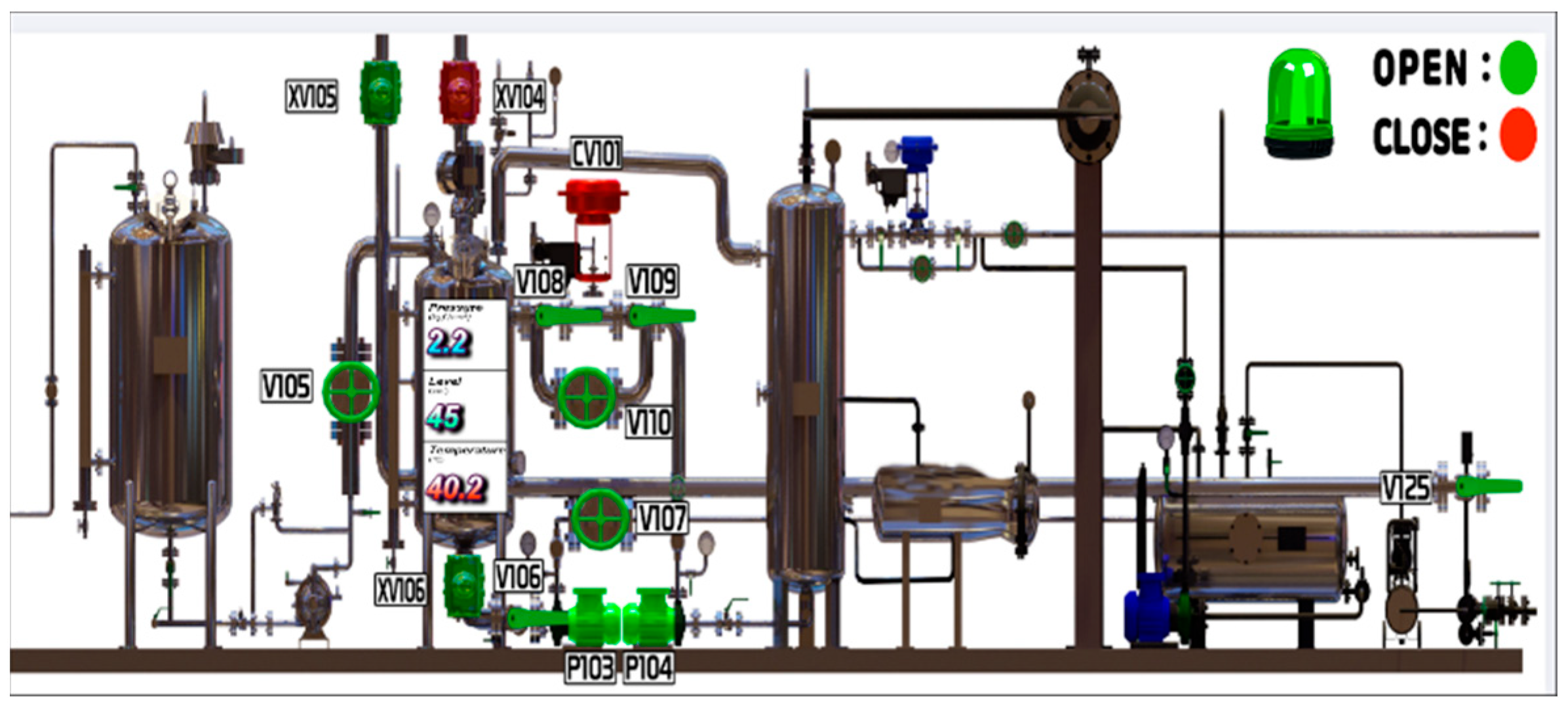
2.3.1. DCS Configuration
2.3.2. Synchronization with the AR System
2.4. Construction of the Pilot Plant and Infrastructure
3. Results
3.1. Development of Changes in the Status of the Facility as Training Contents
3.2. Development of Accident Response Scenarios
3.3. Pilot Operation and Results
3.4. Comparison between the OTS and Traditional Training Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Huang, J.; Lu, Y.; Ma, H.; Li, W.; Chen, J. A New Text-Mining–Bayesian Network Approach for Identifying Chemical Safety Risk Factors. Mathematics 2022, 10, 4815. [Google Scholar] [CrossRef]
- Johnson, C. Why human error modeling has failed to help systems development. Interact Comput. 1999, 11, 517–524. [Google Scholar] [CrossRef]
- Zare, A.; Hoboubi, N.; Farahbakhsh, S.; Jahangiri, M. Applying analytic hierarchy process and failure likelihood index method (AHP-FLIM) to assess human reliability in critical and sensitive jobs of a petrochemical industry. Hellyon 2022, 8, e09509. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Zhang, C.; Chen, X.; Long, E. Statistical analyasis of major industrial accidents in China from 2000 to 2020. Eng. Fail. Anal. 2022, 141, 106632. [Google Scholar] [CrossRef]
- Hemmatian, B.; Abdolhamidzadeh, B.; Darbra, R.; Casal, J. The significance of domino effect in chemical accidents. J. Loss Prevent. Proc. 2014, 29, 30–38. [Google Scholar] [CrossRef]
- Jahangiri, M.; Hoboubi, N.; Rostamabadi, A.; Keshavarzi, S.; Hosseini, A. Human Error Analysis in a Permit to Work System: A Case Study in a Chemical Plant. Saf. Health Work 2016, 7, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Woo, J.; Kang, C. Analysis of severe industrial accidents caused by hazardous chemicals in South Korea from January 2008 to June 2018. Saf. Sci. 2020, 124, 104580. [Google Scholar] [CrossRef]
- De Beer, J.; Depew, C. The role of process engineering in the digital transformation. Comput. Chem. Eng. 2021, 154, 107423. [Google Scholar] [CrossRef]
- Wilk, M.; Rommel, S.; Liauw, M.; Schinke, B.; Zanthoff, H. Education 4.0: Challenges for Education and Advanced Training. Chem. Ing. Tech. 2020, 92, 983–992. [Google Scholar] [CrossRef]
- Kumar, V.; Carberry, D.; Beenfeldt, C.; Andersson, M.; Mansouri, S.; Gallucci, F. Virtual reality in chemical and biochemical engineering education and training. Educ. Chem. Eng. 2021, 36, 143–153. [Google Scholar] [CrossRef]
- Abdelaziz, M. Challenges and Issues in Building Virtual Reality-Based e-Learning System. Int. J. e-Educ. e-Bus. e-Manag. e-Learn. 2014, 4, 320–328. [Google Scholar] [CrossRef]
- Patle, D.; Ahmad, Z.; Rangaiah, G. Operator training simulators in the chemical industry: Review, issues, and future directions. Rev. Chem. Eng. 2014, 30, 199–216. [Google Scholar] [CrossRef]
- Garcia Fracaro, S.; Glassey, J.; Bernaerts, K.; Wilk, M. Immersive technologies for the training of operators in the process industry: A Systematic Literature Review. Comput. Chem. Eng. 2022, 160, 107691. [Google Scholar] [CrossRef]
- Patle, D.S.; Manca, D. Operator Training simulators in virtual reality environment for process operators: A review. Virtual Real. 2019, 23, 293–311. [Google Scholar] [CrossRef]
- Siminovich, C.; Joao, S. Dynamic operator training simulators for sulphuric acid, phosphoric acid, and DAP production units. Procedia Eng. 2014, 83, 215–224. [Google Scholar] [CrossRef]
- Nakai, A.; Suzuki, K. Instructional information system using AR technology for chemical plants. Chem. Eng. Trans. 2016, 53, 199–204. [Google Scholar] [CrossRef]
- Manca, D.; Brambilla, S.; Colombo, S. Bridging between Virtual Reality and accident simulation for training of process-industry operators. Adv. Eng. Softw. 2013, 55, 1–9. [Google Scholar] [CrossRef]
- Szke, I.; Louka, M.; Bryntesen, T.; Edvardsen, S.; Bratteli, J. Comprehensive support for nuclear decommissioning based on 3D simulation and advanced user interface technologies. J. Nucl. Sci. Technol. 2015, 52, 371–387. [Google Scholar] [CrossRef]
- Yang, G.; Shao, Z.; Xu, Z.; Zhang, D.; Lou, H.; Wang, K. Development of a novel type operator training simulator framework for air separation process. In Proceedings of the 2021 China Automation Congress (CAC), Beijing, China, 22–24 October 2021; Volume 2021, pp. 4014–4019. [Google Scholar] [CrossRef]
- Lee, J.; Cameron, I.; Hassall, M. Improving process safety: What roles for digitalization and industry 4.0? Process Saf. Environ. 2019, 132, 325–339. [Google Scholar] [CrossRef]
- Brambilla, S.; Manca, D. Recommended features of an industrial accident simulator for the training of operators. J. Loss Prevent. Proc. 2011, 24, 344–355. [Google Scholar] [CrossRef]
- Nakai, A.; Kaihata, Y.; Suzuki, K. The Experience-Based Safety Training System Using Vr Technology for Chemical Plant. Int. J. Adv. Comput. Sci. Appl. 2014, 5, 63–67. [Google Scholar] [CrossRef]
- Sharma, C.; Bhavsar, P.; Srinivasan, B.; Srinivasan, R. Eye gaze movement studies of control room operators: A novel approach to improve process safety. Comput. Chem. Eng. 2016, 85, 43–57. [Google Scholar] [CrossRef]
- Colombo, S.; Golzio, L. The Plant Simulator as viable means to prevent and manage risk through competencies management: Experiment results. Saf. Sci. 2016, 84, 46–56. [Google Scholar] [CrossRef]
- Ahmad, Z.; Patle, D.; Rangaiah, G. Operator training simulator for biodiesel synthesis from waste cooking oil. Process Saf. Environ. 2016, 99, 55–68. [Google Scholar] [CrossRef]
- Gerlach, I.; Tholin, S.; Hass, V.; Mandenius, C. Operator training simulator for an industrial bioethanol plant. Processes 2016, 4, 34. [Google Scholar] [CrossRef]
- Ouyang, S.; Wang, G.; Yao, J.; Zhu, G.; Liu, Z.; Feng, C. A Unity3D-based interactive three-dimensional virtual practice platform for chemical engineering. Comput. Appl. Eng. Educ. 2018, 26, 91–100. [Google Scholar] [CrossRef]
- Puskás, J.; Egedy, A.; Németh, S. Development of operator training simulator for isopropyl alcohol producing plant. Educ. Chem. Eng. 2018, 22, 35–43. [Google Scholar] [CrossRef]
- Lee, Y.; Ko, C.; Lee, H.; Jeon, K.; Shin, S.; Han, C. Interactive plant simulation modeling for developing an operator training system in a natural gas pressure-regulating station. Pet. Sci. 2017, 14, 529–538. [Google Scholar] [CrossRef]
- Pirola, C.; Peretti, C.; Galli, F. Immersive virtual crude distillation unit learning experience: The EYE4EDU project. Comput. Chem. Eng. 2020, 140, 106973. [Google Scholar] [CrossRef]
- Balaton, M.; Nagy, L.; Szeifert, F. Operator training simulator process model implementation of a batch processing unit in a packaged simulation software. Comput. Chem. Eng. 2013, 48, 335–344. [Google Scholar] [CrossRef]
- De Tommaso, J.; Rossi, F.; Moradi, N.; Pirola, C.; Patience, G.; Galli, F. Experimental methods in chemical engineering: Process simulation. Can. J. Chem. Eng. 2020, 98, 2301–2320. [Google Scholar] [CrossRef]
- Al-Malah Kamal, I.M. Aspen Plus: Chemical Engineering Applications, 1st ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; Volume 160, pp. 106–120. [Google Scholar]
- Kidam, K.; Hurme, M. Analysis of equipment failures as contributors to chemical process accidents. Process. Saf. Environ. 2013, 91, 61–78. [Google Scholar] [CrossRef]
- Center for Chemical Process Safety (CCPS). Guidelines for Consequence Analysis of Chemical Releases, 1st ed.; American Institute of Chemical Engineers: New York, NY, USA, 1999; pp. 15–85. [Google Scholar]
- An, S.; Lim, K.; Go, H.; Jung, G.; Ma, B. A study on development of multi-user training contents for response to chemical accidents based on virtual reality. J. Digit. Contents Soc. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Marcano, L.; Haugen, F.; Sannerud, R.; Komulainen, T. Review of simulator training practices for industrial operators: How can individual simulator training be enabled? Saf. Sci. 2019, 115, 414–424. [Google Scholar] [CrossRef]
- Garcia Fracaro, S.; Hu, Y.; Gallagher, T.; Loenen, S.; Solmaz, S.; Cermak-Sassenrath, D.; Gerven, T. Immersive Tools for Teaching and Training in a Science and Technology Environment—First CHARMING Policy Brief. 2021, pp. 1–7. Available online: https://charming-etn.eu/wp-content/uploads/2021/04/D5.4_CHARMING_Policy_Brief.pdf (accessed on 17 January 2023).
- Letrud, K.; Hernes, S. The diffusion of the learning pyramid myths in academia: An exploratory study. J. Curric. Stud. 2016, 48, 291–302. [Google Scholar] [CrossRef]
- Education System of National Institute of Chemical Safety of Korean Ministry of Environment. Available online: https://edunics.me.go.kr/academy/contents/view.do?contentsNo=14&menuCd=WWW001009001 (accessed on 1 January 2023).





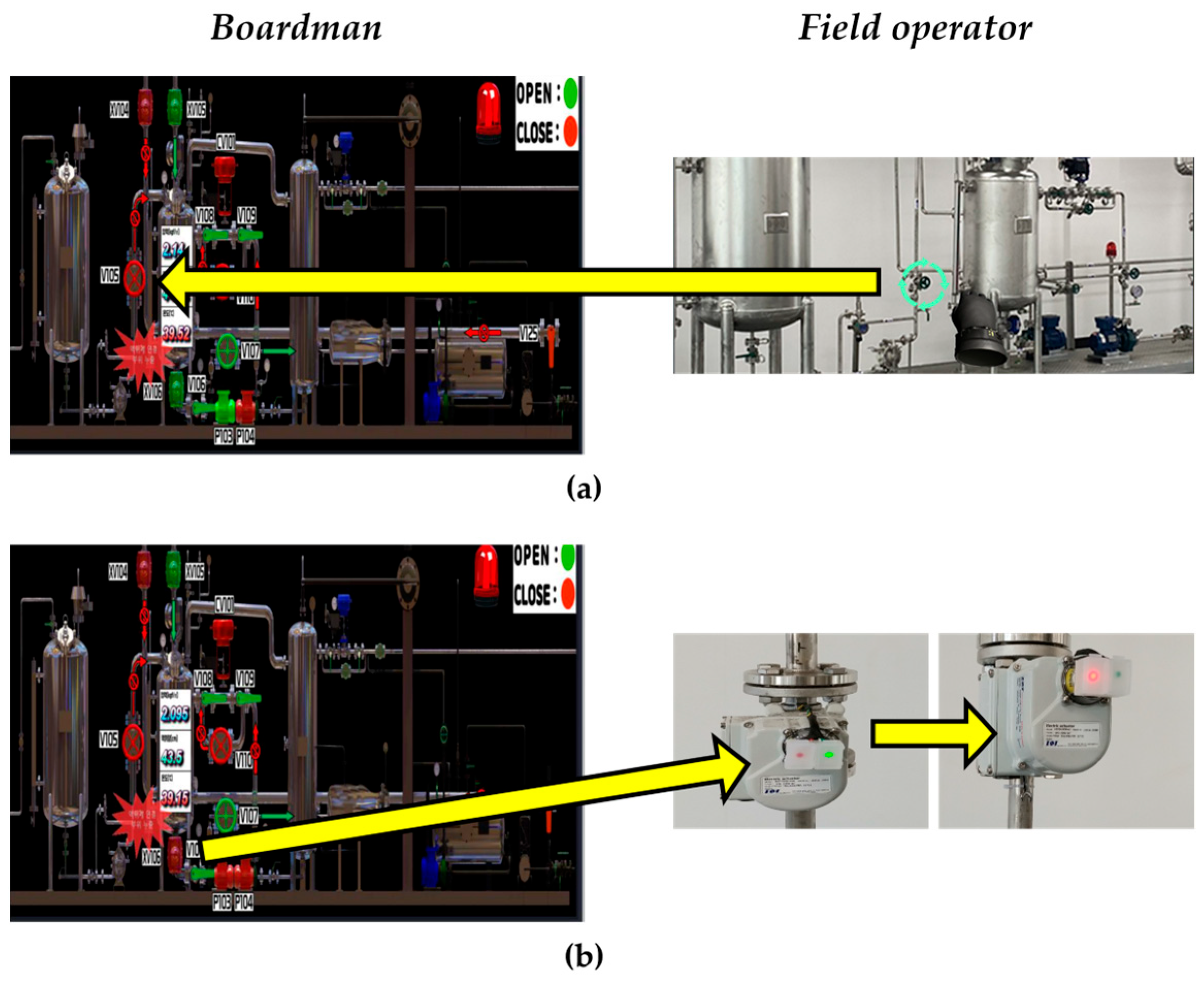
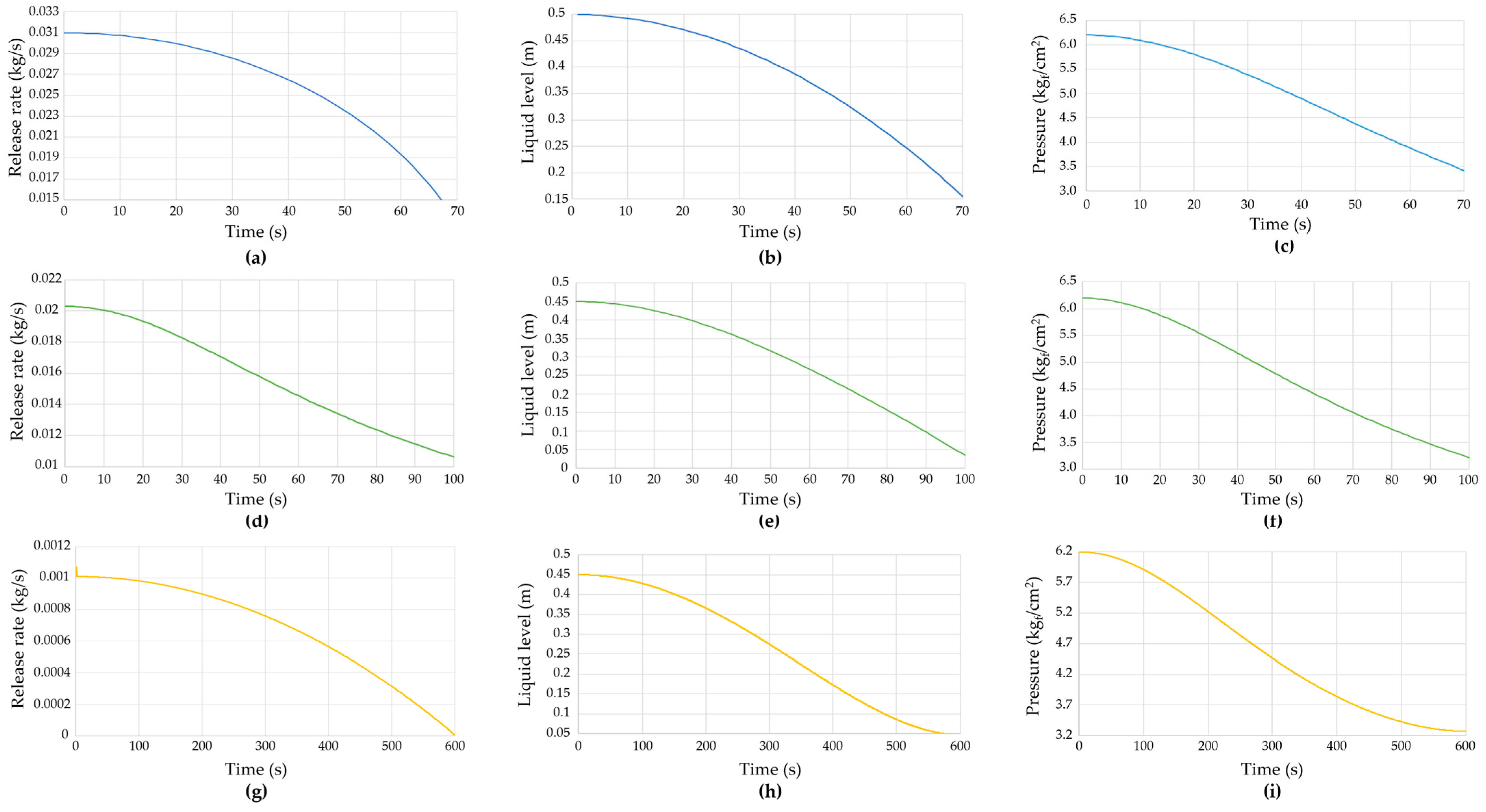
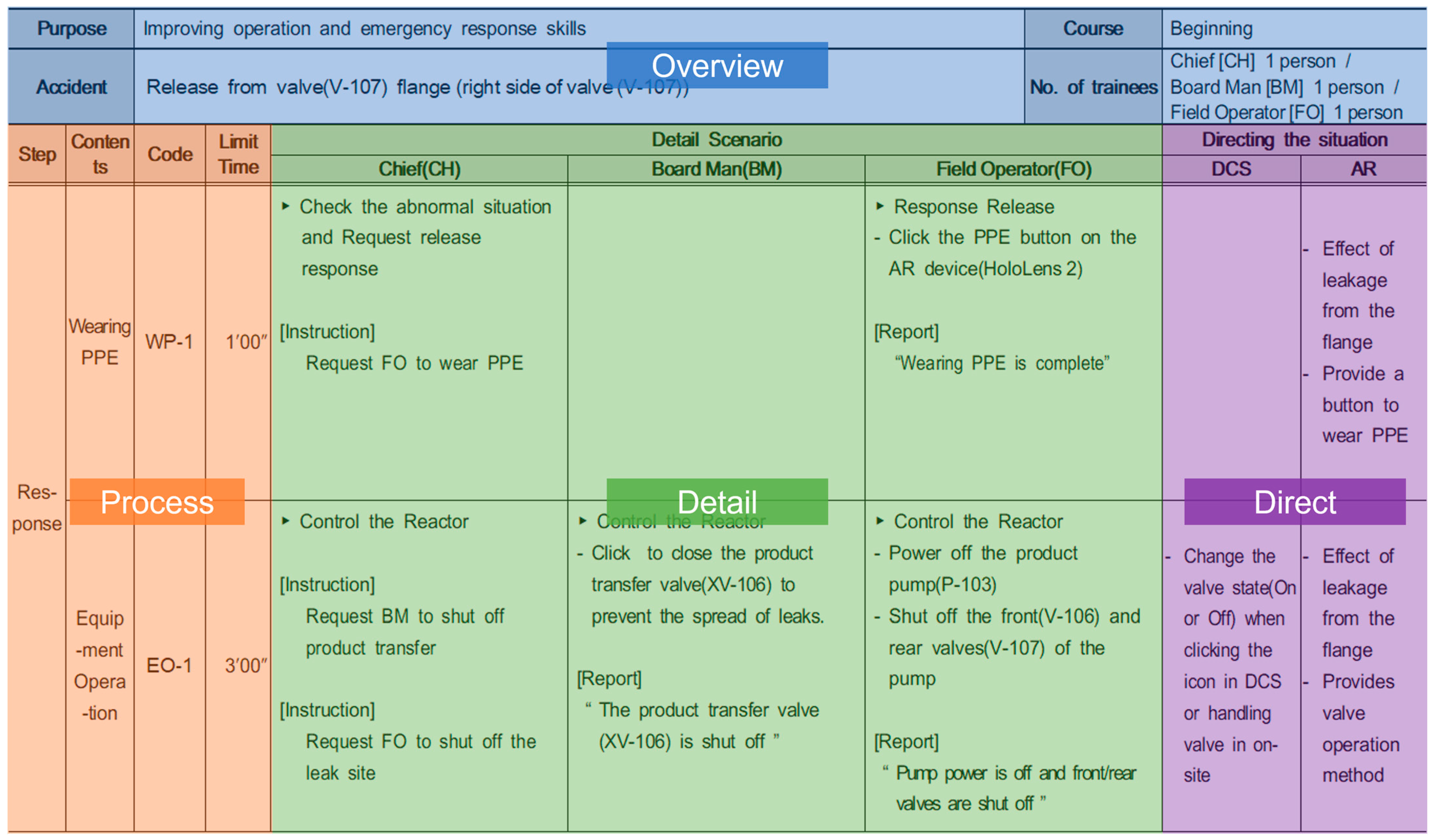

| Equipment Item | Abbreviation | Capacity (m3) | Operating Pressure (MPa) | Operating Temperature (°C) | Phase |
|---|---|---|---|---|---|
| Storage tank | TK-101 | 0.5 | AMB | AMB | Liquid |
| Reactor | R-101 | 0.3 | 0.6 | 70 | Liquid/gas |
| Column | T-101 | 0.1 | 0.3 (1 stage) | - | Liquid/gas |
| Reflux Drum | D-101 | 0.18 | 0.3 | 25 | Liquid/gas |
| Condenser | C-101 | 0.005 | 0.5 | - | Liquid/gas |
| Vessel | V-101 A/B | 0.09/0.55 | 0.8 | AMB | Gas |
| Division | Chemical Formula | CAS No. | Boiling Point (°C) | Vapor Pressure (mmHg) |
|---|---|---|---|---|
| Hexamethyldisilane | Si2C6H18 | 1450-14-2 | 113 | 20.8 |
| Hydrogen chloride | HCl | 7647-01-0 | −85.05 | 35.42 |
| Trimethylsilane | C3H10Si | 993-07-7 | 6.7 | 594 |
| Trimethylchlorosilane | C3H9SiCl | 75-77-4 | 57 | 200 |
| Case No. | Scenario | Situation | Response | Leakage Model |
|---|---|---|---|---|
| 1 | Piping failure 1 | Leakage from a flange gap due to gasket aging. | Close the valve and shut off the pump. | Liquid leakage from the pipe |
| 2 | Piping failure 2 | Leakage due to corrosion of the hydrochloric acid supply pipe. | Close the valve connected to the reactor. | Gas leakage from the pipe |
| 3 | Level gauge leakage | Leakage at the bottom of the reactor due to poor welding. | Block the inflow and outflow by closing the valve connected to the reactor. | Liquid leakage from the vessel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ma, B. An Operator Training Simulator to Enable Responses to Chemical Accidents through Mutual Cooperation between the Participants. Appl. Sci. 2023, 13, 1382. https://doi.org/10.3390/app13031382
Lee J, Ma B. An Operator Training Simulator to Enable Responses to Chemical Accidents through Mutual Cooperation between the Participants. Applied Sciences. 2023; 13(3):1382. https://doi.org/10.3390/app13031382
Chicago/Turabian StyleLee, Junseo, and Byungchol Ma. 2023. "An Operator Training Simulator to Enable Responses to Chemical Accidents through Mutual Cooperation between the Participants" Applied Sciences 13, no. 3: 1382. https://doi.org/10.3390/app13031382
APA StyleLee, J., & Ma, B. (2023). An Operator Training Simulator to Enable Responses to Chemical Accidents through Mutual Cooperation between the Participants. Applied Sciences, 13(3), 1382. https://doi.org/10.3390/app13031382








