Abstract
This study analyzed muscle activity during the stand-up paddle stroke, considering the paddling side and the adjacent and opposing muscles relative to the position of the arms during paddling. Methods: Fourteen male paddleboarders performed three trials covering 195 m in which surface electromyography of the upper trapezius, biceps brachii, triceps brachii, tibialis anterior, and gastrocnemius medialis were recorded (four-cycle strokes on each side). The data were processed according to percentage of maximum voluntary contraction (% MVC). The MVC activation values (µV) for each muscle were then calculated and presented as percentage MVC (% MVC). Results: The recovery phase accounted for 60% of the paddle cycle, while the pull phase represented 39%. During right-side paddling, higher % MVC was found in the opposite-side upper trapezius (24.35%, p < 0.01) during the pulling phase and in the adjacent biceps brachii (8.36%, p < 0.03) during the recovery phase. In left-side paddling, greater % MVC was found in the opposite-side upper trapezius (27.60%, p < 0.01) during the pulling phase and in the opposite-side triceps brachii (42.25%, p < 0.04) during the recovery phase. Furthermore, the pulling phase exhibited higher MVC in the opposite-side upper trapezius compared to the recovery phase, both in the right-side (24.35%, p < 0.03) and left-side (27.60%, p < 0.01) paddling. Conclusions: these findings help establish the muscular activity of both sides of the paddling technique and the differences between the upper and lower limbs.
1. Introduction
Stand-up paddle boarding (SUP) is a sport that combines elements of surfing and rowing, allowing paddlers to practice distance paddling and/or surf waves [1]. It has become a popular and accessible activity with numerous benefits, including improvements in body mass index, aerobic and anaerobic fitness, and multidirectional trunk strength, as well as applications for rehabilitation and fall prevention [2,3,4,5,6]. Despite its growing popularity, research on SUP remains limited, with few studies investigating its physiology [7,8], biomechanics [9,10], epidemiology [11,12], and psychology [6] in both recreational and competitive participants [13,14,15].
The biomechanics of SUP paddling is similar to dragon boat racing with an entry, drive, and exit phase [16]. A comparison of paddle stroke mechanics between experienced and inexperienced SUP participants by Schram et al. [14] revealed that inexperienced participants showed higher overall shoulder action and less hip range of motion than experienced participants. Muscle activation during paddling appears to primarily involve the upper extremities, trunk, hip stabilizers, and knees [9,17]. During SUP in an ergometer and at sea, it was demonstrated that muscle activation during the water-based test started sooner and was maintained longer than that during the ergometer test [17]. Tsai et al. [9] evaluating different postures, found higher biceps brachii activity when paddling on the knees on the board and higher activation of the external oblique and triceps brachii in the standing position.
Analyzing the muscles involved in paddling, the upper trapezius is responsible for producing clavicle elevation and retraction relative to the thorax due to its attachment to the distal clavicle [18]. The biceps brachii muscle was found to be primarily activated during the recovery phase and the late pull phase. Its role in this context involves assisting in shoulder flexion, aiding the upper hand (the hand opposite to the one holding the paddle) in lifting the paddle and preparing for the subsequent pull phase [9]. In the case of the triceps brachii, it was observed to mainly engage from the later stage of the recovery phase to the middle stage of the pull phase [9]. The tibialis anterior muscle was noted for its activation in maintaining board balance [19]. Finally, the gastrocnemius medialis muscle was found to be activated during the pull phase, with significantly higher activation levels associated with the task of maintaining balance. This heightened activation can be attributed to the higher center of gravity in the standing position and the increased sway movements, which demanded additional effort to ensure stability [9].
During SUP, paddlers can alternate between left- and right-hand sides for paddling [2], involving pushing cycles, controlling the fluid movement of the board and the relative movements of the paddle and the water, consequently, the paddler has to continuously change his or her basic attitude to adjust trajectory and balance [20]. Therefore, it is important to consider different settings such as the sea and lakes [21]. Thus, during recreational SUP practice, participants often alternate paddling sides based on physical or natural conditions. To date, the understanding of paddling on both sides remains limited. Regardless of SUP being described as a full-body activity, understanding the differences between sides is particularly necessary in groups that comprise most participants in this sport. This study aimed to analyze muscle activity during the stroke cycle, considering the paddling side (left and right), comparing the opposite and adjacent muscles to the paddle stroke, and examining activation in the upper and lower limbs, to better understand muscle activation patterns during paddling.
2. Materials and Methods
2.1. Sample and Ethical Procedures
Fourteen SUP recreational right-handed male participants (24 ± 7.1 years, 1.73 ± 1.22 m, 58 ± 15.5 kg, wingspan 1.79 ± 0.87 m, and body mass index 24.2 ± 4.9 kg/m2; mean ± SD) volunteered to participate in this study after being instructed on the procedures. Participants were only included if they met age requirements (>18 years), had at least 6 months of SUP experience with regular weekly practice (1 to 2 times per week), and were excluded if they had any health risks or conditions that affected paddling performance.
Prior to testing, the participants were informed about the benefits and risks of the investigation and signed an institutionally approved informed consent document. This study was approved by the University Ethics Committee (CE-UBI-Pj-2022-042) and all the procedures were in accordance with the Declaration of Helsinki regarding human research.
2.2. Procedures
2.2.1. Measurements
Muscle activity was assessed on both sides of the body by surface electromyography (EMG) on a wireless EMG system with built-in accelerometers (Miniwave, Cometa, Milano, Italy; EMGandMotionsTools software 8.7.6.0) and probes equipped with a 7-g memory and a sampling rate of 2000 Hz at 16 bits. For each subject, the skin under the electrodes was shaved, rubbed with sandpaper, and cleaned with alcohol so that the interelectrode resistance did not exceed 5 KOhm [22]. Transparent bandages with labels (Hydrofilm®, 10 cm × 12.5 cm, Rock hill, SC, USA) were used to protect the electrodes to isolate them from water [23].
Additionally, participants wore custom-made long-sleeved surf suits (Decathlon, Olaian 3/2 mm, Villeneuve-d’Ascq, France) to protect the electrodes and sensors during the trials.
The EMG sensors were placed according to the SENIAM recommendations (Kendall ™, ECG electrodes, 57 width mm × 34 length mm, gel area 201 mm2, sensor area 80 mm2, Dublin, Ohio, USA) and the inter-electrode distance between each pair was 20 mm [24]. The muscles under analysis in this study were the upper trapezius, biceps brachii, triceps brachii, tibialis anterior, and gastrocnemius medialis according to the importance of these muscles in paddling [9].
2.2.2. Maximal Voluntary Contraction
The maximum voluntary contraction (MVC) test is one of the most commonly used methods of the EMG signal normalization [25]. Prior to the paddle assessment, to determine the MVC, each subject performed three maximal voluntary isometric contractions on dry land for each muscle analyzed, held for 5 s with a minimum rest interval of 30 s between repetitions. A minimum 1-min rest period preceded each new test position. The MVC procedures were conducted with the application of manual resistance by the examiner, according to positioning guidelines based on the guidelines of both Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles (SENIAM) and Noraxon (Scottsdale, AZ, USA.) company [24,26]. To ensure isometric conditions, the examiner made every effort to adjust the counterforce appropriately. For the upper trapezius muscle assessment, participants were positioned in a standing posture, and the examiner applied a downward force to their shoulders [27]. Regarding the MVC for the biceps brachii and triceps brachii, these assessments were carried out with the elbow flexed at approximately 90 degrees, as described in previous studies [28,29]. The examiner provided upper arm stabilization to enhance and standardize activation conditions. For the biceps brachii, the forearm was in a supinated position, while a neutral forearm position was maintained for the triceps brachii. For the tibialis anterior, the foot was held in dorsiflexion, and the toes were not extended, maintaining a neutral position of the foot. For the gastrocnemius medialis, the foot was placed in plantar flexion with an emphasis on elevating the heel more than pushing the forefoot downward. To achieve maximum pressure in this position, pressure was applied against both the forefoot and the calcaneus, ensuring a pointed, plantar-flexed position of the foot. The maximum value of the resulting EMG envelope was determined, and this was averaged across the trials for each test [30].
2.2.3. Stand-Up Paddle Stroke Assessment
All conditions were performed with the same SUP board (Itiwit 10′32″5″), paddle (Itwit 170–220 cm) and in the same location, and before starting, the height of the paddle was individually adjusted (range 1.7–2.2 m) [21]. The protocol was performed on an inland lake without current interference where the participants had to paddle in a straight line (Figure 1). Before initiating data collection with the subjects, the temperature and wind were analyzed to assess whether it was possible to perform the trials without interference from external conditions. All trials were carried out in the direction of the wind, being recorded daily, obtaining an average of 3.4 m.s−1 (gentle breeze) using the Beaufort Wind Scale [31].
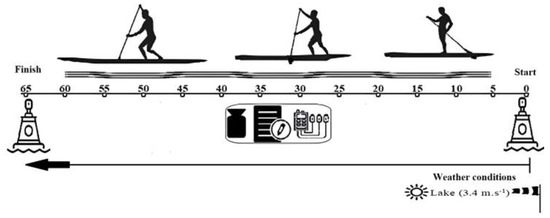
Figure 1.
Schematic overview of the SUP field protocol.
Before initiating the official trials, the participants performed a 5-min warm-up where they self-selected the frequency and rate of the paddling. Each subject performed 3 trials of stand-up paddling on the water at an individual pace. Then, to maintain the forward movement when paddling, the participants were instructed to alternate paddle sides after three strokes [9] during three trials of 65 m, the total distance covered was 195 m in a straight line limited by two floats indicating the beginning and end of the course.
The EMG measurement was synchronized with a digital video camera (Panasonic, DC-FZ 1000II, Osaka, Japan) fixed on a tripod (Falcon eyes, FT-120, Hoogeveen, The Netherlands) positioned at a distance of 20 m, perpendicular to the course, to record the entire procedure. To synchronize the data, the subjects had to stand on the board in a T position for 5 s and then tap on their arm biceps brachii three times before starting the trials. The same gesture of tapping the biceps brachii three times occurred when each subject finished the course.
2.2.4. Data Analysis
Initially, each video was cut according to the trials (Windows, media player) to later synchronize the data with the EMG software (EMG and Motion Tools, V8, Cometa, Bareggio Mi, Italy). The video analysis was connected with the event. In this study, synchronization was processed by identifying visible peaks in the accelerometer signal. In this way, it was possible to find the final and initial time of a propulsive phase in the EMG data through the video sequence, with an accuracy of 33.3 ms on a video frame.
The “pulling” phase is the process in which the blade is completely immersed in water and is swung backward to generate forward power [32]. In the “exit” and “recovery” phases, the blade is pulled out of the water and returned to the starting position before starting the next catch phase [9]. Event times were checked by a second observer to identify errors. After that, four left and right cycle strokes were analyzed. The first six cycle strokes of both sides were excluded from the analysis, as well as the first stroke of each cycle, to eliminate the paddle transferring from side to side, which influences the cycle parameters. After, phase cycle parameters (s) and stroke time parameters (%) were calculated.
Signal processing was started by applying filters to the MVC file. EMG sensors received raw EMG data and the first frequencies were removed with the following filters: (i) a low pass filter with a cutoff frequency of 400 Hz and Butterworth filter with an order of 4; (ii) a high Pass filter with a cutoff frequency of 20 Hz and Butterworth filter with an order of 4. The maximum MVC activation values (µV) for each muscle were then calculated and presented as percentage MVC (% MVC). The last procedure was to apply the same filters to the signal taken from each trial, as well as to apply the MVC to the trial file. Finally, the mean cycles were exported to an Excel file.
2.3. Statistical Analysis
Descriptive statistics were calculated including mean, standard deviation, and coefficient of variation for each phase and side. The normality plot tests of Shapiro–Wilk were applied and, therefore, the Student’s t-test was used to compare the two sides, the right and left sides, relative to the paddling movement. The statistical significance was set to p ≤ 0.05. The Cohen’s D effect size was calculated as an indicator of the magnitude of the effect, with D considered a small effect if <0.2; a medium effect if <0.5; and a large effect if >0.8 [33]. Statistical analysis was carried out using SPSS statistical software (IBM Corp., Armonk, NY, USA, version 20.0).
3. Results
Regarding the time in stroke cycle, the participants spend approximately 60% in the recovery phase and 39% in the pull phase. There were no significant differences in the stroke cycle parameters between left and right paddle stroke cycles (Table 1).

Table 1.
Stroke cycle parameters regarding time (seconds) and percentage (%) of stroke phases (recovery and pull phases) expressed as mean (mean ± SD). p-values and effect sizes are also shown.
We observed higher muscle activation during the recovery phase for the upper limbs on the left paddling side in the biceps brachii adjacent (18.6% MVC), upper trapezius adjacent (18.3% MVC) and opposite (33.1% MVC). During the pull phase, higher activation was observed in the triceps brachii adjacent (38% MVC) and opposite (51% MVC) (Figure 2). Regarding the lower limbs, higher muscle activation was observed in the tibialis anterior for the adjacent and opposite sides of the body, but higher activation in the pull phase compared with the recovery phase (Figure 2).
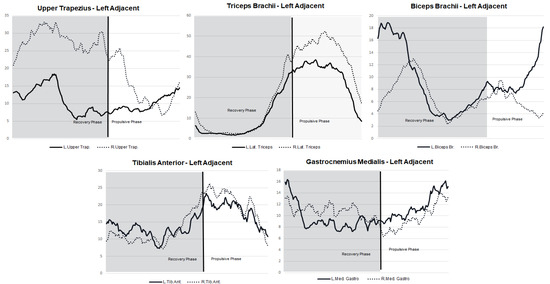
Figure 2.
Muscle activity (% MVC) patterns for the left paddling side stroke concerning the stroke cycle and regarding the muscles of the opposite and adjacent sides of the body.
For the upper limbs on the right paddling side, higher muscle activation was observed during the recovery phase in the muscles: upper trapezius adjacent (27.4% MVC) and opposite (20.4% MVC). During the pull phase, higher activation was observed in the triceps brachii adjacent (39.1% MVC) and opposite (39.49% MVC). The lower limbs had the same behavior for muscle activity as the left paddling side (Figure 3).
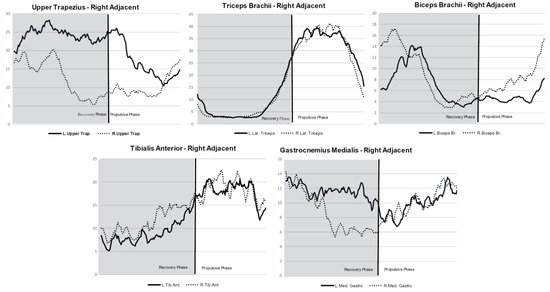
Figure 3.
Muscle activity (%MVC) patterns for the right paddling side stroke concerning the stroke cycle and regarding the muscles of the opposite and adjacent sides of the body.
On the right paddling side, in the pull phase, there were significant differences between the opposite side and the adjacent side in the upper trapezius and, in the recovery phase, differences were also found in the upper trapezius and biceps brachii (Table 2). Regarding the left paddling side, there were significant differences in the pull phase between the opposite side and the adjacent side in the upper trapezius and biceps brachii, and in the triceps brachii during the recovery phase (Table 3).

Table 2.
Mean ± SD of the %MVC for the comparison between the pull and recovery phases of a full stroke cycle, on the right paddling side, on the opposite and adjacent sides of the body during all five studied muscles. p-values and effect sizes are also shown.

Table 3.
Mean ± SD of the %MVC comparison between the pull and recovery phases of a full stroke cycle, on the left paddling side, on the opposite and adjacent sides of the body during all five studied muscles. p-values and effect sizes are also shown.
Differences were found in all muscles of the upper limb and in the tibialis anterior on the opposite side. On the adjacent side, differences were observed only in the triceps brachii (Table 4). On the left paddling side, significant differences were found in the upper trapezius and the biceps brachii on the opposite side, and in the triceps brachii on the adjacent arm (Table 5).

Table 4.
Mean ± SD of muscle activation (%MVC) for the comparison between the pull and recovery phases on the right paddling side. p-values and effect sizes are also shown.

Table 5.
Mean ± SD of muscle activation (%MVC) for the comparison between the pull and recovery phases on the left paddling side. p-values and effect sizes are also shown.
4. Discussion
The results of this study revealed that the participants spent more time in the recovery phase than in the pull phase. Furthermore, the muscles with the highest level of muscle activation were the upper trapezius and triceps brachii muscles of the upper limb, as well as the tibialis anterior muscle of the lower limb. Lastly, these findings showed that the muscles on the opposite side of the stroke had more activity than the adjacent muscles throughout the paddling cycle.
The subjects in this study spent a higher amount of time in the recovery phase (60%) compared to the pull phase (39%). This contrasts with the findings of a previous study conducted by Ruess et al. [17], which reported a distribution of 52% for the power phase exit, and 12.75% for the recovery phase. The differences in these results can be attributed to variations in the experience levels of the study participants, as well as the fact that Ruess et al. [17] used an ergometer. This laboratory setup did not consider external conditions such as water or wind that might introduce additional perturbations and instability to the paddler [14].
Upon comparing these results regarding muscle activation with those of a study conducted by Tsai et. al. [9], it was observed that the results concerning the upper trapezius muscle are similar, especially when the subjects spent more time in the pull phase. This similarity was also observed in the present study in the opposite arm during left and right paddling strokes. For the biceps brachii muscle, the results were similar when the subjects spent more time in the recovery phase, and in this study, the muscle showed higher activation in the adjacent arm in the left and right paddling strokes. Similarly, the results for the gastrocnemius medialis muscles were similar when the subjects spent more time in the recovery phase and showed higher activation in the opposite and adjacent arms in the left and right paddling strokes.
However, the present study showed a disagreement regarding the triceps brachii muscle, which showed higher activation during the recovery phase than the tibialis anterior muscle, whereas in the study of Tsai et. al. [9], the triceps brachii muscle showed higher activation during the pull phase. Trevithick et al. [34] conducted an EMG study using a kayak ergometer and reported that during the pull phase of kayak paddling, a consistent pattern of activity was observed in the supraspinatus, upper trapezius, and latissimus dorsi muscles. Specifically, the supraspinatus muscle showed an increase in activity from 20% to nearly 80% of the average maximum activity during the paddling cycle.
During the exit phase, a consistent pattern of activity was demonstrated in the latissimus dorsi, rhomboid major, and serratus anterior muscles, with this short phase of the paddling cycle oscillating between 15% and 30% of the average maximum activity. Lastly, during the recovery phase, a consistent pattern of activity was demonstrated in the supraspinatus and upper trapezius muscles. The muscles showed an initial rapid linear decrease in activity between 40% and 50% of the average maximum activity, followed by a short-duration, small increase in activity during the mid-recovery phase. The tibialis anterior muscle is highly activated when the ankle joint is perturbed and deviates from the normal trajectory toward plantar flexion, whereas the gastrocnemius is active when the ankle shows increased dorsiflexion [35].
In the current study, it was observed that the triceps brachii muscle in the pull phase showed higher activation in the opposite arm, regardless of whether it was during the right or left paddling stroke. Nevertheless, Tsai et al. [9] observed that the triceps brachii muscle acted mainly from the later stage of the recovery phase to the middle stage of the pull phase. The difference in results can be attributed to the fact that Tsai et al. [9] compared EMG activation in different postures, namely in the standing position, where they found higher triceps brachii activation.
Previous research analyzing the front crawl stroke in swimming using EMG has suggested that the scapular rotators are active throughout the paddling stroke, with the greatest activity occurring during the entry and exit phases and the lowest during the propulsive phase [36,37]. In the present study, it was found that the upper trapezius muscle had higher activity during the recovery phase and its behavior varied depending on the paddling side. Specifically, when paddling on the left side, the upper trapezius exhibited higher activity in the opposite arm, while when paddling on the right side, the upper trapezius showed higher activity in the adjacent arm; this could be due to the fact that all of the participants were right-handed.
The tibialis anterior, during the pull phase, had higher muscle activation than the gastrocnemius medialis in the lower limbs, which contrasts with Tsai et al. [9] findings that the gastrocnemius medialis was more activated in the standing position and during the pull phase, with the tibialis anterior reacting to the instability of the SUP board. When analyzing the upper and lower limbs together, it appears that the lower limbs act as stabilizers, helping to balance the board during the pull phase, while the upper limbs act as propulsors. This suggests that the lower limbs may be more activated during the pull phase due to their role in stabilizing the movement of the upper limbs and the board.
Significant differences were observed in the muscle activation of all upper limb muscles during the pull and recovery phases of the paddling stroke, as well as in the tibialis anterior muscle of the lower limb on the opposite side (right paddling side), indicating that the muscles on the opposite side play an important role during the paddling cycle, as mentioned by Dyson et al. [24]. This could be attributed to the compensatory mechanism of the opposite side to control the oscillations and wobbling of the SUP board, as previously reported by Ruess et al. [5].
Several limitations need to be acknowledged in this study. Firstly, the number of stroke cycles analyzed was relatively small, which may limit the generalizability of the findings. Future studies with a larger number of stroke cycles may provide a more comprehensive understanding of the impact of fatigue on paddling mechanics. Secondly, this study did not analyze the role of core muscles in the paddling stroke cycle, which can be an important factor in the development of fatigue. Incorporating core muscle analysis into future studies may provide a more holistic view of the effects of fatigue on paddling mechanics. Finally, the sample population consisted of recreational paddleboarders, which may not reflect the experiences of more experienced paddleboarders. It would be beneficial to recreate this study with a more diverse sample, including both experienced and inexperienced paddleboarders to better understand the impact of fatigue on paddling mechanics at different levels of expertise.
A better understanding of the role of upper and lower body muscles during the paddling stroke can be valuable for paddleboarders looking to improve their conditioning and technique.
The findings of this study suggest that training programs should be designed considering that stimulation of opposite and adjacent muscles depends on the side of the paddling stroke and stroke phases. Taking into account that the development of force applied in the propulsive and recovery phases can improve muscle recruitment, coaches and athletes should consider the importance of switching movements between the two sides of the stroke and in this way, promote balance in muscle recruitment. Therefore, incorporating these recommendations into a comprehensive training regimen can potentially improve overall paddling performance.
5. Conclusions
The findings of the current study indicate that the muscles on the opposite side of the paddle exhibited higher activity compared to the muscles associated with the paddle side. In SUP, athletes can choose to alternate the paddling side for technical or tactical reasons. Instructors can observe the performance of SUP practitioners and recommend switching movements to promote muscle balance and reduce muscle fatigue on the paddling side. These observations and recommendations can be easily incorporated into SUP training programs to improve overall paddling efficiency and reduce the risk of injury.
Author Contributions
Conceptualization, J.F. and A.C.; methodology, J.F.; software, J.Š.; validation, J.F., H.P.N. and A.C.; formal analysis, D.T.; investigation, J.F.; resources, J.F.; data curation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, J.F., H.P.N., A.C. and H.L.; visualization, J.F.; supervision, L.L.; project administration, D.A.M., H.L. and H.P.N.; funding acquisition, D.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT), I.P., project number UIDB/04045/2020 and Grant/Award Number UIDP/04748/2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Beira Interior (º CE-UBI-Pj-2022-042).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Walker, C.; Nichols, A.; Forman, T. A survey of injuries and medical conditions affecting stand-up paddle surfboarding participants. Clin. J. Sports Med. 2010, 20, 144. [Google Scholar]
- Schram, B.; Hing, W.; Climstein, M. Profiling the Sport of Stand-up Paddle Boarding. J. Sports Sci. 2015, 34, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Schram, B.L.; Hing, W.A.; Climstein, M.; Furness, J.W. A Performance Analysis of a Stand-up Paddle Board Marathon Race. J. Strength Cond. Res. 2017, 31, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Balerdi, E.; León-Guereño, P. Analysis of Stand-up Paddle Boarding: A Systematic Review (Análisis Del Stand up Paddle. Una Revisión Sistemática). Retos 2021, 44, 193–201. [Google Scholar] [CrossRef]
- Ruess, C.; Kristen, K.H.; Eckelt, M.; Mally, F.; Litzenberger, S.; Sabo, A. Stand up Paddle Surfing-an Aerobic Workout and Balance Training. Procedia Eng. 2013, 60, 62–66. [Google Scholar] [CrossRef]
- Schram, B.; Hing, W.; Climstein, M. The Physiological, Musculoskeletal and Psychological Effects of Stand up Paddle Boarding. BMC Sports Sci. Med. Rehabil. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Schram, B.; Hing, W.; Climstein, M. Laboratory- and Field-Based Assessment of Maximal Aerobic Power of Elite Stand-up Paddle-Board Athletes. Int. J. Sports Physiol. Perform. 2016, 11, 28–32. [Google Scholar] [CrossRef]
- Neiva, H.P.; Faíl, L.B.; Marinho, D.A. A 30-Min Test Applied to Stand-up Paddleboarding: A Pilot Study. J. Hum. Sport Exerc. 2020, 15, S1387–S1393. [Google Scholar] [CrossRef]
- Tsai, F.-H.; Wu, W.-L.; Chen, Y.-J.; Liang, J.-M.; Hou, Y.-Y. Electromyography Analysis of Muscle Activation during Stand-up Paddle Boarding: A Comparison of Paddling in Kneeling and Standing Positions. Appl. Sci. 2020, 10, 2356. [Google Scholar] [CrossRef]
- Hibbert, J.E.; Kaufman, C.; Schmidt, D.J. Shoulder, Trunk, and Hip Sagittal Plane Kinematics during Stand-up Paddle Boarding. Sports 2023, 11, 152. [Google Scholar] [CrossRef]
- Furness, J.; Olorunnife, O.; Schram, B.; Climstein, M.; Hing, W. Epidemiology of Injuries in Stand-up Paddle Boarding. Orthop. J. Sports Med. 2017, 5, 232596711771075. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Babarro, A.; Calleja-González, J.; Viribay, A.; Fernández-Lázaro, D.; León-Guereño, P.; Mielgo-Ayuso, J. Relationship between Training Factors and Injuries in Stand-up Paddleboarding Athletes. Int. J. Environ. Res. Public Health 2021, 18, 880. [Google Scholar] [CrossRef] [PubMed]
- Schram, B. The Long-Term Effects of Stand-up Paddle Boarding: A Case Study. Int. J. Sports Exerc. Med. 2017, 3, 065. [Google Scholar] [CrossRef][Green Version]
- Schram, B.; Furness, J.; Kemp-Smith, K.; Sharp, J.; Cristini, M.; Harvie, D.; Keady, E.; Ghobrial, M.; Tussler, J.; Hing, W.; et al. A Biomechanical Analysis of the Stand-up Paddle Board Stroke: A Comparative Study. PeerJ 2019, 7, e8006. [Google Scholar] [CrossRef]
- Balikian, P.; Marinho, A.H.; Gomes de Araujo, G.; Prado, E.S.; Mendes, E.V.; Ryan Geraldes, A.A. Anaerobic Threshold in Stand-up Paddle. J. Strength Cond. Res. 2022, 36, 1896–1900. [Google Scholar] [CrossRef]
- Ho, S.R.; Smith, R.; O’Meara, D. Biomechanical Analysis of Dragon Boat Paddling: A Comparison of Elite and Sub-Elite Paddlers. J. Sports Sci. 2009, 27, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ruess, C.; Kristen, K.H.; Eckelt, M.; Mally, F.; Litzenberger, S.; Sabo, A. Activity of Trunk and Leg Muscles during Stand up Paddle Surfing. Procedia Eng. 2013, 60, 57–61. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Fey, A.J.; Dorn, C.S.; Busch, B.P.; Laux, L.A.; Hassett, D.R.; Ludewig, P.M. Potential torque capabilities of the trapezius. J. Orthop. Sports Phys. Ther. 2007, 37, A44–A45. [Google Scholar]
- Limonta, E.; Squadrone, R.; Rodano, R.; Marzegan, A.; Veicsteinas, A.; Merati, G.; Sacchi, M. Tridimensional Kinematic Analysis on a Kayaking Simulator: Key Factors to Successful Performance. Sport Sci. Health 2010, 6, 27–34. [Google Scholar] [CrossRef]
- Willmott, A.G.B.; Sayers, B.; Brickley, G. The Physiological and Perceptual Responses of Stand-up Paddle Board Exercise in a Laboratory- and Field-Setting. Eur. J. Sport Sci. 2019, 20, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Afsharipour, B.; Soedirdjo, S.; Merletti, R. Two-Dimensional Surface EMG: The Effects of Electrode Size, Interelectrode Distance and Image Truncation. Biomed. Signal Process. Control 2019, 49, 298–307. [Google Scholar] [CrossRef]
- Conceição, A.; Silva, A.J.; Barbosa, T.; Karsai, I.; Louro, H. Neuromuscular Fatigue during 200 M Breaststroke. J. Sports Sci. Med. 2014, 13, 200–210. [Google Scholar] [PubMed]
- Dyson, R.; Buchanan, M.; Farrington, T.A.; Hurrion, P. Electromyographic Activity during Windsurfing on Water. J. Sports Sci. 1996, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Castelein, B.; Cagnie, B.; Parlevliet, T.; Danneels, L.; Cools, A. Optimal Normalization Tests for Muscle Activation of the Levator Scapulae, Pectoralis Minor, and Rhomboid Major: An Electromyography Study Using Maximum Voluntary Isometric Contractions. Arch. Phys. Med. Rehabil. 2015, 96, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaisi, S.; Aghazadeh, F. Electromyography Analysis: Comparison of Maximum Voluntary Contraction Methods for Anterior Deltoid and Trapezius Muscles. Procedia Manuf. 2015, 3, 4578–4583. [Google Scholar] [CrossRef]
- Konrad, P. The ABC of EMG: A Practical Introduction to Kinesiological Electromyography; Version of 1.4 March 2006; Noraxon Inc.: Scottsdale, AZ, USA, 2006; pp. 1–61. Available online: https://www.noraxon.com/wp-content/uploads/2014/12/ABC-EMG-ISBN.pdf (accessed on 12 September 2023).
- Liu, P.; Liu, L.; Martel, F.; Rancourt, D.; Clancy, E.A. Influence of Joint Angle on EMG–Torque Model during Constant-Posture, Quasi-Constant-Torque Contractions. J. Electromyogr. Kinesiol. 2013, 23, 1020–1028. [Google Scholar] [CrossRef]
- Roman-Liu, D.; Bartuzi, P. Influence of Type of MVC Test on Electromyography Measures of Biceps Brachii and Triceps Brachii. Int. J. Occup. Saf. Ergon. 2017, 24, 200–206. [Google Scholar] [CrossRef]
- Boettcher, C.E.; Ginn, K.A.; Cathers, I. Standard Maximum Isometric Voluntary Contraction Tests for Normalizing Shoulder Muscle EMG. J. Orthop. Res. 2008, 26, 1591–1597. [Google Scholar] [CrossRef]
- National Weather Service. The Beaufort Wind Force Scale. Available online: https://www.weather.gov/mfl/beaufort (accessed on 1 May 2022).
- Michael, J.S.; Smith, R.; Rooney, K.B. Determinants of Kayak Paddling Performance. Sports Biomech. 2009, 8, 167–179. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for T-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Trevithick, B.A.; Ginn, K.A.; Halaki, M.; Balnave, R. Shoulder Muscle Recruitment Patterns during a Kayak Stroke Performed on a Paddling Ergometer. J. Electromyogr. Kinesiol. 2007, 17, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.F.; Woollacott, M.H.; Chong, R.K.Y. Control of Reactive Balance Adjustments in Perturbed Human Walking: Roles of Proximal and Distal Postural Muscle Activity. Exp. Brain Res. 1998, 119, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Pink, M.; Perry, J.; Browne, A.; Scovazzo, M.L.; Kerrigan, J. The Normal Shoulder during Freestyle Swimming. Am. J. Sports Med. 1991, 19, 569–576. [Google Scholar] [CrossRef]
- Martens, J.; Daly, D.; Deschamps, K.; Fernandes, R.J.P.; Staes, F. Intra-Individual Variability of Surface Electromyography in Front Crawl Swimming. PLoS ONE 2015, 10, e0144998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).