Image-Guided Surgical and Pharmacotherapeutic Routines as Part of Diligent Medical Treatment
Abstract
:1. Introduction
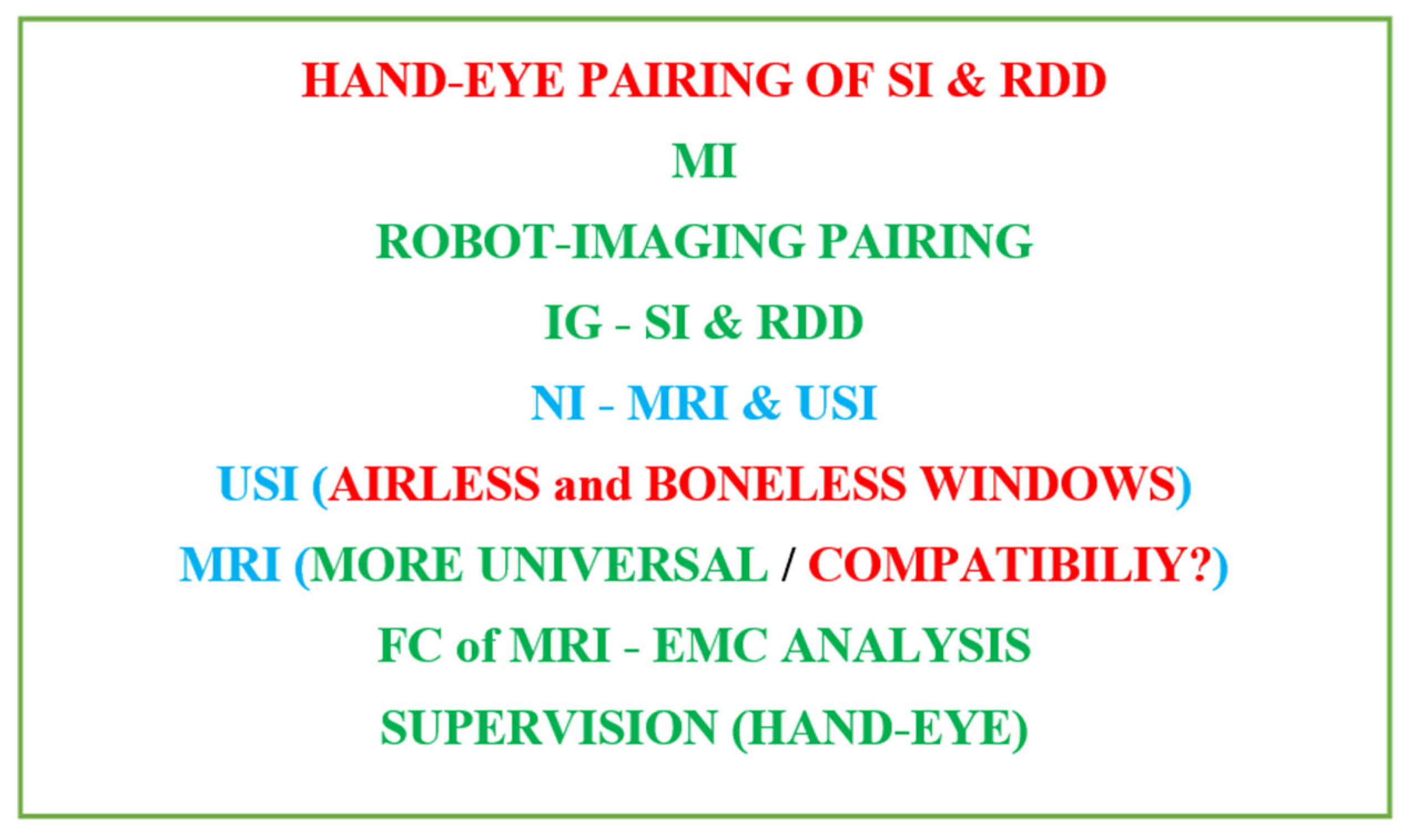
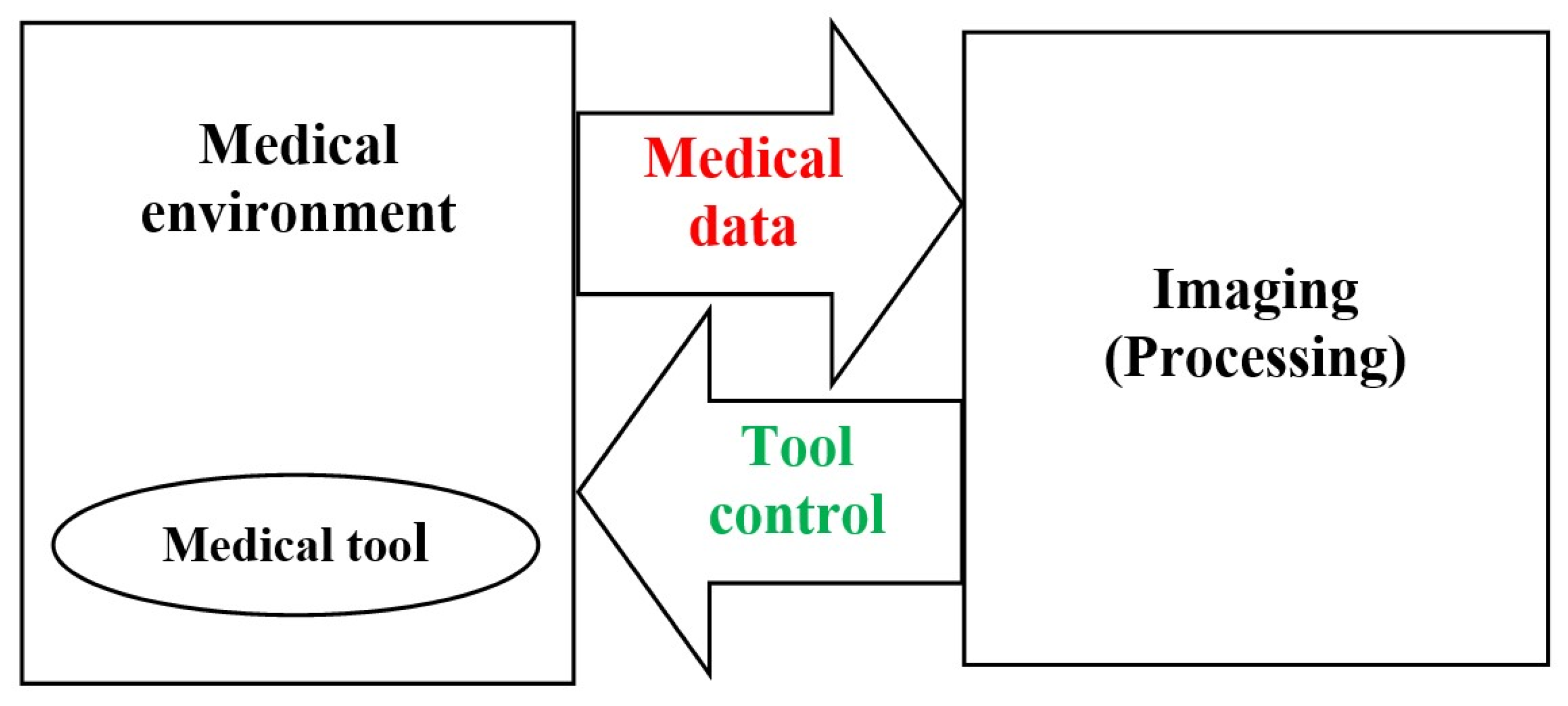
2. Image Guided Medical Procedures
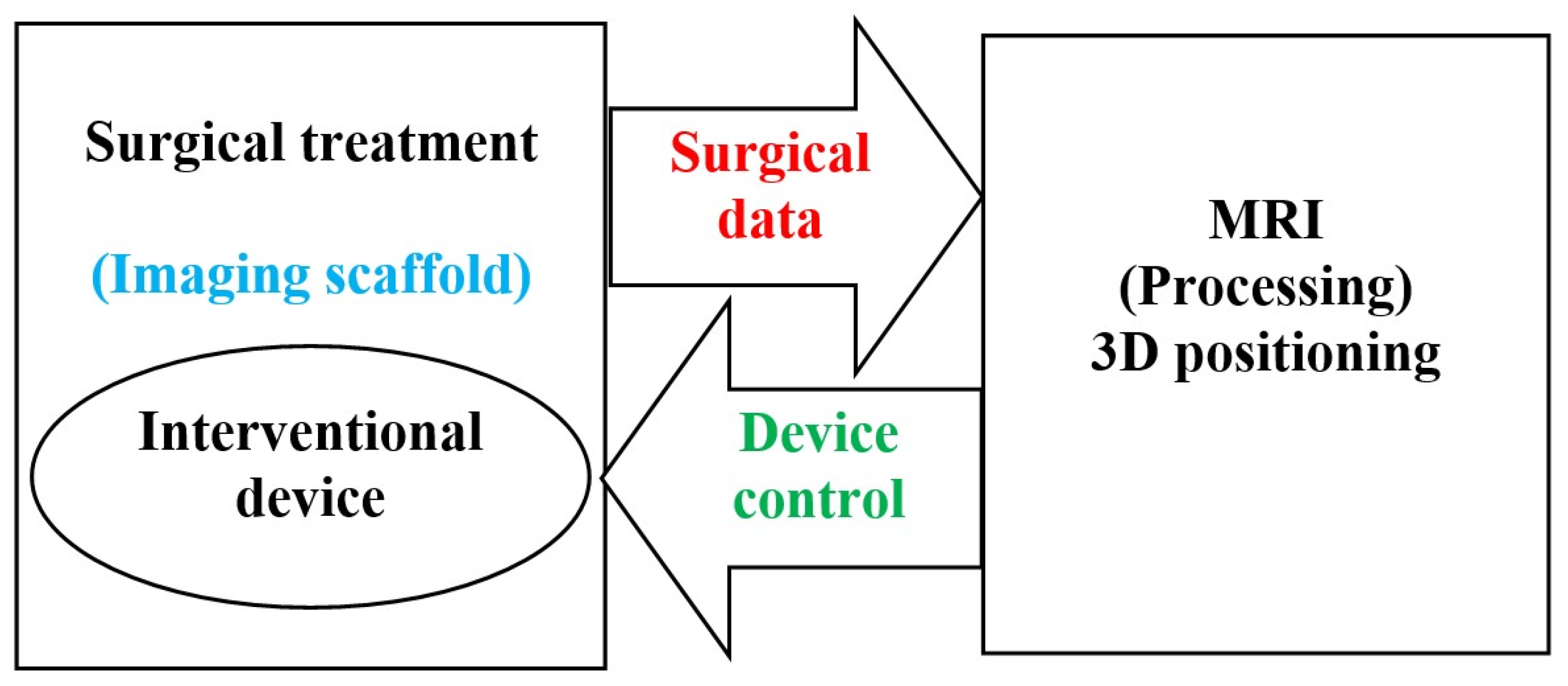
2.1. Image Guided Surgical Interventions
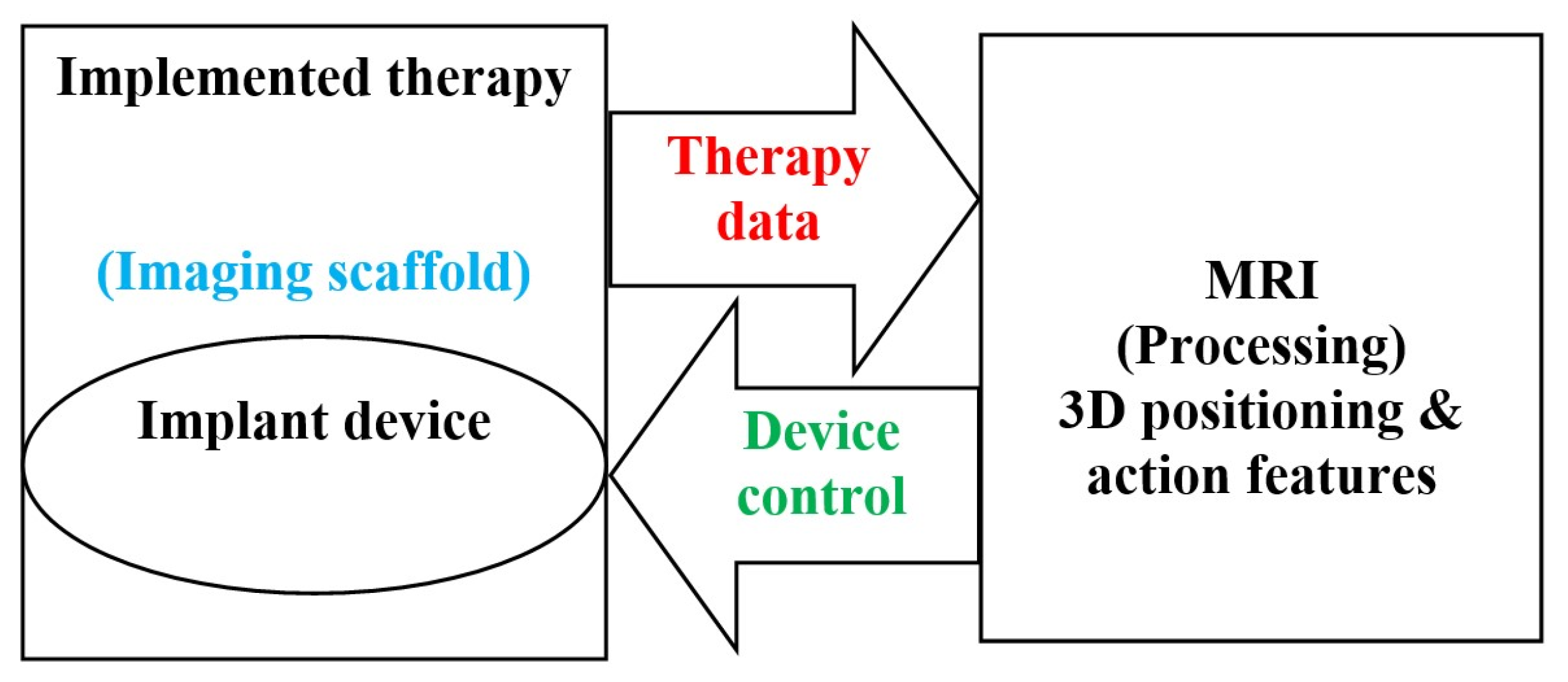
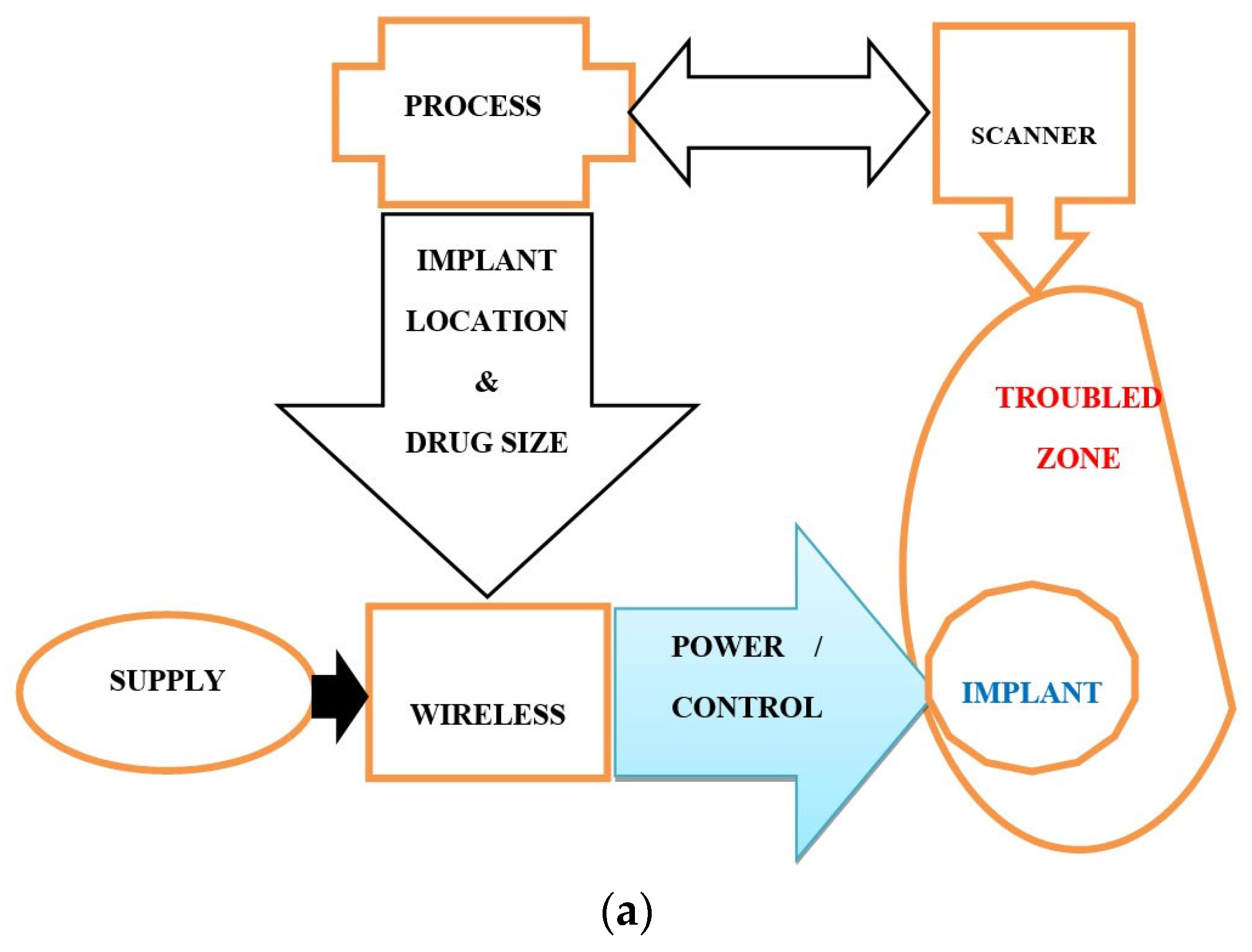
2.2. Implantable Drug Delivery Schemes
2.3. The Necessary Specifications of Surgeries and Drug Deliveries
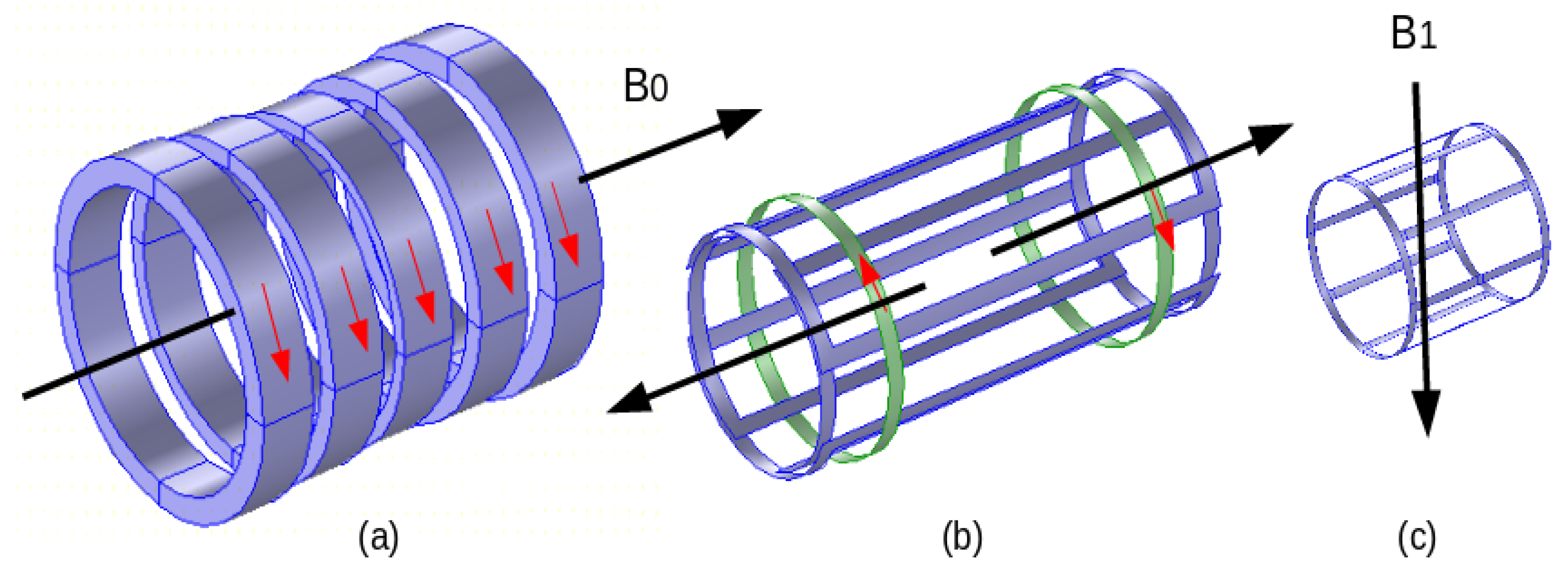
3. MRI Field Components
Safety in MRI
4. MRI-Controlled SI and RDD—Performance and Compliance
4.1. Compatibility Compliance Check
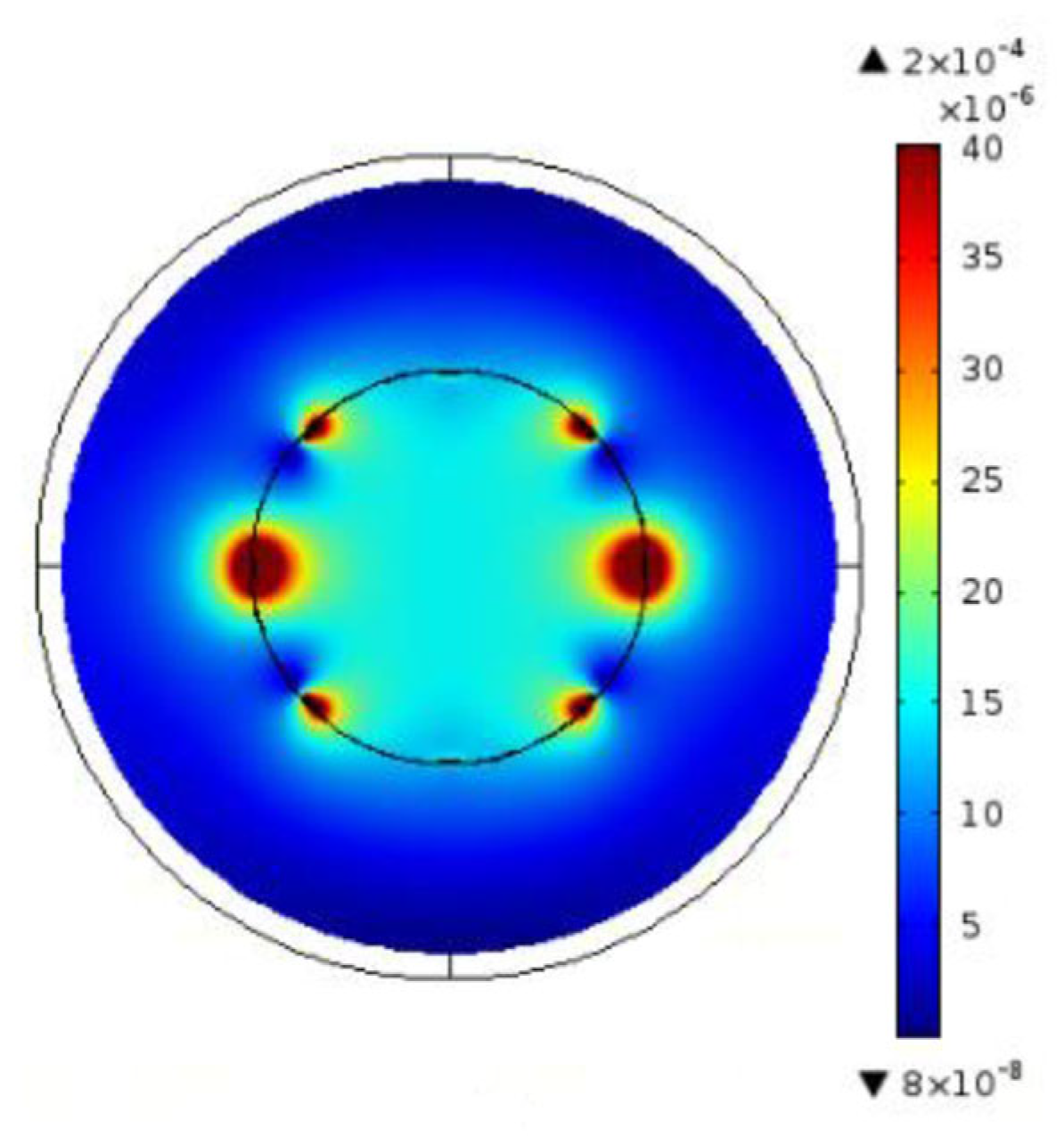
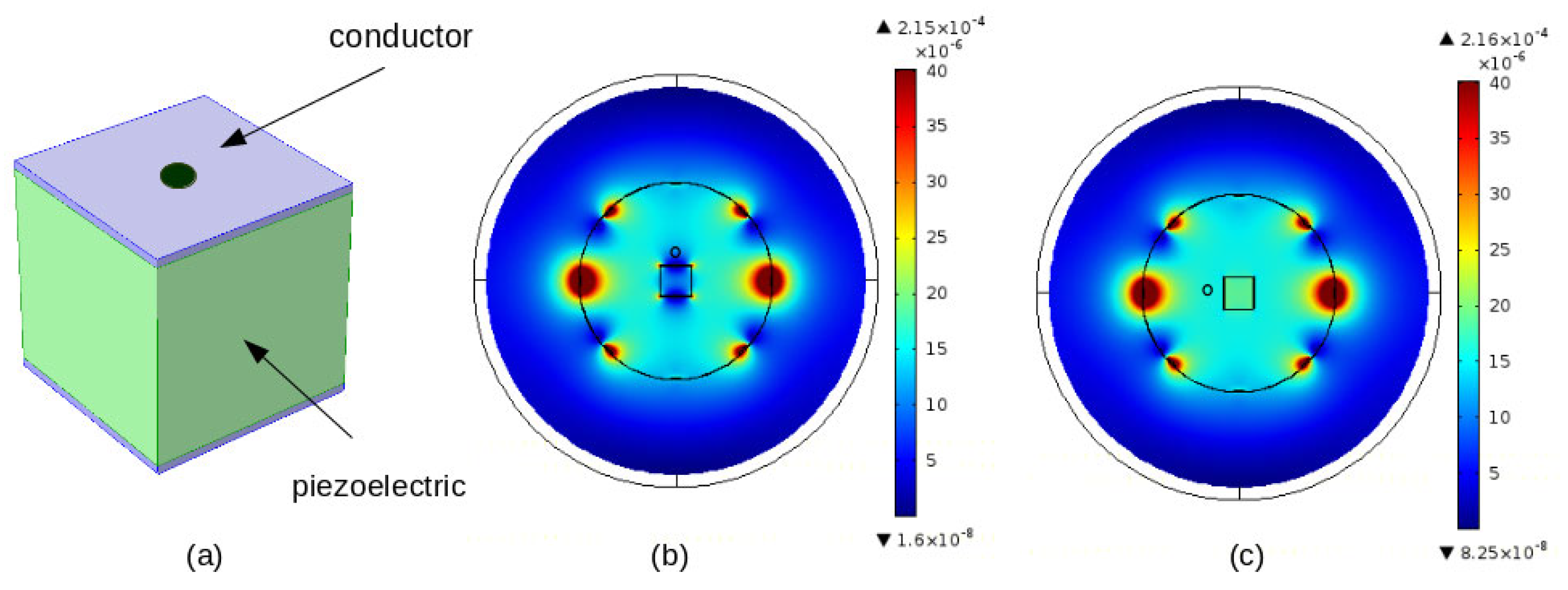
4.2. Electromagnetic Field Perturbations
4.3. EMC Conformity Control
4.4. Application Example
5. Discussion
6. Conclusions
- Image-assisted robotics, non-ionized, minimally invasive, and locally restrictive means;
- Physical phantoms based on the actual biological properties of the body;
- Digital twins under human control;
- Artificial intelligence tools and robotics assisted by augmented reality.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiss, S.; Wäscher, K.; Caglar Özen, A.; Lottner, T.; Heidt, T.; von Zur Mühlen, C.; Bock, M. Quantifying myocardial perfusion during MR-guided interventions without exogenous contrast agents: Intra-arterial spin labeling. Z. Med. Phys. 2023. in print. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lou, C.; Zhou, Y.; He, Z.; Jin, X.; Feng, Y.; Anzhu Gao, A.; Yang, G.Z. MRI-guided robot intervention—Current state-of-the-art and new challenges. Med-X 2023, 1, 4. [Google Scholar] [CrossRef]
- Faoro, G.; Maglio, S.; Pane, S.; Iacovacci, V.; Menciassi, A. An Artificial Intelligence-Aided Robotic Platform for Ultrasound-Guided Transcarotid Revascularization. IEEE Robot. Autom. Lett. 2023, 8, 2349–2356. [Google Scholar] [CrossRef]
- Morris, E.D.; O’Connell, D.P.; Gao, Y.; Cao, M. MR safety considerations for MRI-guided radiotherapy. In Advances in Magnetic Resonance Technology and Applications; Academic Press: Cambridge, MA, USA, 2022; Volume 8, pp. 81–100. [Google Scholar] [CrossRef]
- Su, H.; Kwok, K.W.; Cleary, K.; Iordachita, I.; Cavusoglu, M.C.; Desai, J.P.; Fischer, G.S. State of the art and future opportunities in MRI-guided robot-assisted surgery and interventions. Proc. IEEE 2022, 110, 968–992. [Google Scholar] [CrossRef] [PubMed]
- Padhan, J.; Tsekos, N.; Al-Ansari, A.; Abinahed, J.; Deng, Z.; Navkar, N.V. Dynamic Guidance Virtual Fixtures for Guiding Robotic Interventions: Intraoperative MRI-guided Transapical Cardiac Intervention Paradigm. In Proceedings of the 2022 IEEE 22nd International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 7–9 November 2022; pp. 265–270. [Google Scholar] [CrossRef]
- Singh, S.; Torrealdea, F.; Bandula, S. MR Imaging—Guided Intervention: Evaluation of MR Conditional Biopsy and Ablation Needle Tip Artifacts at 3T Using a Balanced Fast Field Echo Sequence. J. Vasc. Interv. Radiol. 2021, 32, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kuah, T.; Vellayappan, B.A.; Makmur, A.; Nair, S.; Song, J.; Tan, J.H.; Kumar, N.; Quek, S.T. State-of-the-Art Imaging Techniques in Metastatic Spinal Cord Compression. Cancers 2022, 14, 3289. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.S.; Coblentz, A.C.; Deshpande, V.S.; Peeters, J.M.; Itriago-Leon, P.M.; Chavhan, G.B. State-of-the-art magnetic resonance imaging sequences for pediatric body imaging. Pediatr. Radiol. 2023, 53, 1285–1299. [Google Scholar] [CrossRef]
- Attenberger, U.I.; Biber, S.; Wichtmann, B.D. Technological Advances of Magnetic Resonance Imaging in Today’s Health Care Environment. Investig. Radiol. 2020, 55, 531–542. [Google Scholar] [CrossRef]
- Sennimalai, K.; Selvaraj, M.; Kharbanda, O.P.; Kandasamy, D.; Mohaideen, K. MRI-based cephalometrics: A scoping review of current insights and future perspectives. Dentomaxillofac. Radiol. 2023, 52, 20230024. [Google Scholar] [CrossRef]
- Chianca, V.; Vincenzo, B.; Cuocolo, R.; Zappia, M.; Guarino, S.; Di Pietto, F.; Del Grande, F. MRI Quantitative Evaluation of Muscle Fatty Infiltration. Magnetochemistry 2023, 9, 111. [Google Scholar] [CrossRef]
- Wang, X.; Guo, S.; Li, Z.; Luo, Q.; Dai, Y.; Zhang, H.; Ye, Y.; Gong, Q.; Luo, K. Amphiphilic branched polymer-nitroxides conjugate as a nanoscale agent for potential magnetic resonance imaging of multiple objects in vivo. J. Nanobiotechnol. 2021, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Takeuchi, T.; Fuju, A.; Takahashi, M.; Hashimoto, M.; Okawa, R.; Hayashi, N. MRI safety for leave-on powdered hair thickeners under 1.5-T and 3.0-T MRI: Measurement of deflection force, MRI artifact, and evaluation of preexamination screening. Phys. Eng. Sci. Med. 2023, 46, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, G.; Istanbullu, O. Analysing the effects of metallic biomaterial design and imaging sequences on MRI interpretation challenges due to image artefacts. Phys. Eng. Sci. Med. 2022, 45, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Germann, C.; Nanz, D.; Sutter, R. Magnetic Resonance Imaging Around Metal at 1.5 Tesla: Techniques from Basic to Advanced and Clinical Impact. Investig. Radiol. 2021, 56, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Germann, C.; Falkowski, A.L.; von Deuster, C.; Nanz, D.; Sutter, R. Basic and Advanced Metal-Artifact Reduction Techniques at Ultra-High Field 7-T Magnetic Resonance Imaging-Phantom Study Investigating Feasibility and Efficacy. Investig. Radiol. 2022, 57, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Inaoka, T.; Kitamura, N.; Sugeta, M.; Nakatsuka, T.; Ishikawa, R.; Kasuya, S.; Sugiura, Y.; Nakajima, A.; Nakagawa, K.; Terada, H. Diagnostic Value of Advanced Metal Artifact Reduction Magnetic Resonance Imaging for Periprosthetic Joint Infection. J. Comput. Assist. Tomogr. 2022, 46, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Haskell, M.W.; Nielsen, J.F.; Noll, D.C. Off-resonance artifact correction for MRI: A review. NMR Biomed. 2023, 36, e4867. [Google Scholar] [CrossRef]
- Spronk, T.; Kraff, O.; Kreutner, J.; Schaefers, G.; Quick, H.H. Development and evaluation of a numerical simulation approach to predict metal artifacts from passive implants in MRI. Magma 2022, 35, 485–497. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, V.Y.; Yang, Y.; Hu, P.; Sheng, K.; Lee, P.P.; Kishan, A.U.; Raldow, A.C.; O’Connell, D.P.; Woods, K.E.; et al. Practical Safety Considerations for Integration of Magnetic Resonance Imaging in Radiation Therapy. Pract. Radiat. Oncol. 2020, 10, 443–453. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, V.Y.; Yang, Y.; Hu, P.; Sheng, K.; Lee, P.P.; Kishan, A.U.; Raldow, A.C.; O’Connell, D.P.; Woods, K.E.; et al. Rapid safety assessment and mitigation of radiofrequency induced implant heating using small root mean square sensors and the sensor matrix Qs. Magn. Reason. Med. 2022, 87, 509–527. [Google Scholar] [CrossRef]
- Fierens, G.; Standaert, N.; Peeters, R.; Glorieux, C.; Verhaert, N. Safety of active auditory implants in magnetic resonance imaging. J. Otol. 2021, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Ulloa, P.; Jansen, O.; Hoevener, J.B. Metallic Implants in MRI—Hazards and Imaging Artifacts. Met. Implant. MRT Gefahren Bildartefakte. Rofo. 2021, 193, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, Y.; Du, H. Design and control of a novel magnetic resonance imaging-compatible breast intervention robot. Int. J. Adv. Robot. Syst. 2020, 17, 1729881420927853. [Google Scholar] [CrossRef]
- Carter, L.N.; Addison, O.; Naji, N.; Seres, P.; Wilman, A.H.; Shepherd, D.E.T.; Grover, L.; Cox, S. Reducing MRI susceptibility artefacts in implants using additively manufactured porous Ti-6Al-4V structures. Acta Biomater. 2020, 107, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, I.; Brinkmann, I.M.; Lin, D.J.; Bruno, M.; Johnson, P.M.; Knoll, F.; Keerthivasan, M.B.; Chandarana, H.; Fritz, J. New-Generation Low-Field Magnetic Resonance Imaging of Hip Arthroplasty Implants Using Slice Encoding for Metal Artifact Correction: First In Vitro Experience at 0.55 T and Comparison with 1.5 T. Investig. Radiol. 2022, 57, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Ibrahim, E.S.H.; Dudek, N.; Lu, J.C.; Kalia, V.; Runge, M.; Srinivasan, A.; Stojanovska, J.; Agarwal, P.P. Improving MR image quality in patients with metallic implants. Radiographics 2021, 41, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Manso Jimeno, M.; Vaughan, J.T.; Geethanath, S. Superconducting magnet designs and MRI accessibility: A review. NMR Biomed. 2023, 36, e4921. [Google Scholar] [CrossRef]
- Amin, N.; Pai, I.; Touska, P.; Connor, S.E.J. Utilization of SEMAC-VAT MRI for Improved Visualization of Posterior Fossa Structures in Patients with Cochlear Implants. Otol. Neurotol. 2021, 42, 451–458. [Google Scholar] [CrossRef]
- Kumar, N.; Alathur Ramakrishnan, S.; Lopez, K.G.; Wang, N.; Madhu, S.; Vellayappan, B.A.; Hallinan, J.T.P.D.; Fuh, J.Y.H.; Kumar, A.S. Design and 3D printing of novel titanium spine rods with lower flexural modulus and stiffness profile with optimised imaging compatibility. Eur. Spine J. 2023, 32, 1953–1965. [Google Scholar] [CrossRef]
- Rajiah, P.S.; François, C.J.; Leiner, T. Cardiac MRI: State of the Art. Radiology 2023, 307, e223008. [Google Scholar] [CrossRef]
- Basit, A.; Inam, O.; Omer, H. Accelerating GRAPPA reconstruction using SoC design for real-time cardiac MRI. Comput. Biol. Med. 2023, 160, 107008. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, P.; Erden, O.K.; Ferhanoglu, O.; Tümer, M.; Yalcinkaya, A.D. MRI Compatible Fiber Optic Multi Sensor Platform for Real Time Vital Monitoring. J. Light. Technol. 2021, 39, 4138–4144. [Google Scholar] [CrossRef]
- Wang, F.; Qi, H.; De Goyeneche, A.; Heckel, R.; Lustig, M.; Shimron, E. K-band: Self-supervised MRI Reconstruction via Stochastic Gradient Descent over K-space Subsets. arXiv 2023, arXiv:2308.02958. [Google Scholar] [CrossRef]
- Lee, K.-H.; Fu, K.C.D.; Guo, Z.; Dong, Z.; Leong, M.C.W.; Cheung, C.L.; Lee, A.P.W.; Luk, W.; Kwok, K.W. MR Safe Robotic Manipulator for MRI-Guided Intracardiac Catheterization. IEEE ASME Trans. Mechatron. 2018, 23, 586–595. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ko, S.Y. A Decade of MRI Compatible Robots: Systematic Review. Trans. Robot. 2023, 39, 862–884. [Google Scholar] [CrossRef]
- Farooq, M.U.; Ko, S.Y.; Seung, S.; Kim, C.; Cha, K.; Oh, S.S.; You, H. An MRI-compatible endonasal surgical robotic system: Kinematic analysis and performance evaluation. Mechatronics 2023, 94, 103029. [Google Scholar] [CrossRef]
- Manjila, S.; Rosa, B.; Price, K.; Manjila, R.; Mencattelli, M.; Dupont, P.E. Robotic Instruments Inside the MRI Bore: Key Concepts and Evolving Paradigms in Imaging-enhanced Cranial Neurosurgery. World Neurosurg. 2023, 176, 127–139. [Google Scholar] [CrossRef]
- Zhao, Z.; Carvalho, P.A.; Tang, H.; Pooladvand, K.; Gandomi, K.Y.; Nycz, C.J.; Furlong, C.; Fischer, G.S. Preliminary Characterization of a Plastic Piezoelectric Motor Stator Using High-Speed Digital Holographic Interferometry. In Advancement of Optical Methods & Digital Image Correlation in Experimental Mechanics. Conference Proceedings of the Society for Experimental Mechanics Series; Lin, M.T., Furlong, C., Hwang, C.H., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Tang, H.; Razavi, P.; Pooladvand, K.; Castro, W.C.; Gandomi, K.Y.; Zhao, Z.; Nycz, C.J.; Furlong, C.; Fischer, G.S. Study of MRI Compatible Piezoelectric Motors by Finite Element Modeling and High-Speed Digital Holography. In Advancement of Optical Methods & Digital Image Correlation in Experimental Mechanics. Conference Proceedings of the Society for Experimental Mechanics Series; Lin, M.T., Furlong, C., Hwang, C.H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Williams, R.P.; Karzova, M.M.; Yuldashev, P.V.; Kaloev, A.Z.; Nartov, F.A.; Khokhlova, V.A.; Cunitz, B.W.; Morrison, K.P.; Khokhlova, T.D. Dual-Mode 1-D Linear Ultrasound Array for Image-Guided Drug Delivery Enhancement without Ultrasound Contrast Agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 693–707. [Google Scholar] [CrossRef]
- Williams, R.P.; Karzova, M.M.; Yuldashev, P.V.; Kaloev, A.Z.; Nartov, F.A.; Khokhlova, V.A.; Cunitz, B.W.; Morrison, K.P.; Khokhlova, T.D. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040. [Google Scholar] [CrossRef]
- Nazary Abrbekoh, F.; Salimi, L.; Saghati, S.; Amini, H.; Fathi Karkan, S.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar] [CrossRef]
- Arno, M.C.; Simpson, J.D.; Blackman, L.D.; Brannigan, R.P.; Thurecht, K.J.; Dove, A.P. Enhanced drug delivery to cancer cells through a pH-sensitive polycarbonate platform. Biomater. Sci. 2023, 11, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, C.A.; Zink, J.I. Spatial, temporal, and dose control of drug delivery using noninvasive magnetic stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, K. Nanoparticles-based strategies to improve the delivery of therapeutic small interfering RNA in precision oncology. Pharmaceutics 2022, 14, 1586. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, D.; Zhang, C.; Li, C. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J. Nanobiotechnol. 2021, 19, 384. [Google Scholar] [CrossRef]
- Zhao, J.F.; Zou, F.L.; Zhu, J.F.; Huang, C.; Bu, F.; Zhu, Z.; Yuan, R. Nano-drug delivery system for pancreatic cancer: A visualization and bibliometric analysis. Front. Pharmacol. 2022, 13, 1025618. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, J.; Berangi, M.; Yiannakou, C.; Silva, E.; Francischello, R.; Kuehne, A.; Niendorf, T.; Könneker, S.; Willumeit-Römer, R.; Seitz, J.M. Evaluating metallic artefact of biodegradable magnesium-based implants in magnetic resonance imaging. Bioact. Mater. 2022, 15, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Rashidi, A.; de Cesar Netto, C. Magnetic Resonance Imaging of Total Ankle Arthroplasty: State-of-The-Art Assessment of Implant-Related Pain and Dysfunction. Foot Ankle Clin. 2023, 28, 463–492. [Google Scholar] [CrossRef]
- Berangi, M.; Kuehne, A.; Waiczies, H.; Niendorf, T. MRI of Implantation Sites Using Parallel Transmission of an Optimized Radiofrequency Excitation Vector. Tomography 2023, 9, 603–620. [Google Scholar] [CrossRef]
- Canzi, P.; Magnetto, M.; Simoncelli, A.; Manfrin, M.; Aprile, F.; Lafe, E.; Carlotto, E.; Avato, I.; Scribante, A.; Preda, L.; et al. The role of cochlear implant positioning on MR imaging quality: A preclinical in vivo study with a novel implant magnet system. Eur. Arch. Otorhinolaryngol. 2022, 279, 2889–2898. [Google Scholar] [CrossRef]
- Kimura, M.; Kaku, N.; Kubota, Y.; Tagomori, H.; Tsumura, H. Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Adverse Local Tissue Reactions near Metal Implants after Total Hip Arthroplasty: A Preliminary Report. Clin. Orthop. Surg. 2021, 13, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Fierens, G.; Walraevens, J.; Peeters, R.; Glorieux, C.; Verhaert, N. Metal artefact reduction sequences for a piezoelectric bone conduction implant using a realistic head phantom in MRI. arXiv 2023, arXiv:2306.03767. [Google Scholar] [CrossRef]
- Winchester, A.; Kay-Rivest, E.; Bruno, M.; Hagiwara, M.; Moonis, G.; Jethanamest, D. Image Quality and Artifact Reduction of a Cochlear Implant with Rotatable Magnets. Otol. Neurotol. 2023, 44, e223–e229. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.-M. Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices: Safety and Image Quality. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2023. Available online: https://helda.helsinki.fi/bitstream/handle/10138/352340/Vuorinen_Aino-Maija_dissertation2023.pdf?sequence=1&isAllowed=y (accessed on 1 November 2023).
- Canzi, P.; Carlotto, E.; Simoncelli, A.M.; Lafe, E.; Minervini, D.; Balzi, D.; Delasio, A.; Malpede, S.; Robotti, C.; Manfrin, M.; et al. Recent advances in managing MRI artifacts caused by auditory implants: The effect of Metal Artifact Reduction Sequences. Audiol. Foniatr. 2022, 7, 25–33. [Google Scholar] [CrossRef]
- Traverson, M.; Heiden, M.; Stanciu, L.A.; Nauman, E.A.; Jones-Hall, Y.; Breur, G.J. In Vivo Evaluation of Biodegradability and Biocompatibility of Fe30Mn Alloy. Vet. Comp. Orthop. Traumatol. 2018, 31, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Venezuela, J.; Dargusch, M. Biodegradable shape memory alloys: Progress and prospects. Biomaterials 2021, 279, 121215. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, G.; Wang, P.; Huang, J.; Wen, C. Nutrient alloying elements in biodegradable metals: A review. J. Mater. Chem. B 2021, 9, 9806–9825. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Progress in manufacturing and processing of degradable Fe-based implants: A review. Prog. Biomater. 2022, 11, 163–191. [Google Scholar] [CrossRef]
- Babacan, N.; Kochta, F.; Hoffmann, V.; Gemming, T. Effect of silver additions on the microstructure, mechanical properties and corrosion behavior of biodegradable Fe-30Mn-6Si. Mater. Today Commun. 2021, 28, 102689. [Google Scholar] [CrossRef]
- Tai, C.-C.; Lo, H.-L.; Liaw, C.-K.; Huang, Y.-M.; Huang, Y.-H.; Yang, K.-Y.; Huang, C.-C.; Huang, S.-I.; Shen, H.-H.; Lin, T.-H.; et al. Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model. Int. J. Mol. Sci. 2021, 22, 7368. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hadisi, Z.; Iqbal, N. Characterization and biological properties of nanostructured clinoenstatite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2021, 259, 123969. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Liu, N.; Wang, H.; Liang, C. Laser-modified fe–30 mn surfaces with promoted biodegradability and biocompatibility toward biological applications. J. Mater. Sci. 2021, 56, 13772–13784. [Google Scholar] [CrossRef]
- Saliba, L.; Sammut, K.; Tonna, C.; Pavli, F. FeMn and FeMnag Biodegradable Alloys: An In Vitro and In Vivo Investigation. Available online: https://ssrn.com/abstract=4325636 (accessed on 1 November 2023). [CrossRef]
- Hao, S.; Yang, T.; Zhang, A.; Wang, P.; Jiang, H.; Shen, D.; Guo, L.; Ye, M. Evaluation of Biodegradable Alloy Fe30Mn0.6N in Rabbit Femur and Cartilage through Detecting Osteogenesis and Autophagy. Biomed Res. Int. 2023, 18, 3626776. [Google Scholar] [CrossRef] [PubMed]
- Biffi, C.A.; Fiocchi, J.; Bregoli, C.; Gambaro, S.; Copes, F.; Mantovani, D.; Tuissi, A. Ultrashort Laser Texturing for Tuning Surface Morphology and Degradation Behavior of the Biodegradable Fe–20Mn Alloy for Temporary Implants. Adv. Eng. Mater. 2022, 24, 2101496. [Google Scholar] [CrossRef]
- Putra, N.E.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zhou, J.; Zadpoor, A.A. Extrusion-based 3D printing of ex situ-alloyed highly biodegradable MRI-friendly porous iron-manganese scaffolds. Acta Biomater. 2021, 134, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, Y.; Du, H.; Yu, Y. Experimental Study of Double Cable-Conduit Driving Device for Mri Compatible Biopsy Robots. J. Mech. Med. Biol. 2021, 21, 2140014. [Google Scholar] [CrossRef]
- Li, X.; Young, A.S.; Raman, S.S.; Lu, D.S.; Lee, Y.H.; Tsao, T.C.; Wu, H.H. Automatic needle tracking using Mask R-CNN for MRI-guided percutaneous interventions. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1673–1684. [Google Scholar] [CrossRef]
- Publicover, J.; Gallop, D.; Elliott, C.; Abed, J. A Navigation Strategy for Patient-Specific Needle Deflection in Transperineal MRI-Guided Prostate Interventions. Brachytherapy 2011, 10, S65. [Google Scholar] [CrossRef]
- Scheer, J.K.; Hamelin, T.; Chang, L.; Lemkuil, B.; Carter, B.S.; Chen, C.C. Real-time Magnetic Resonance Imaging-Guided Biopsy Using SmartFrame® Stereotaxis in the Setting of a Conventional Diagnostic Magnetic Resonance Imaging Suite. Oper. Neurosurg. 2017, 13, 329–337. [Google Scholar] [CrossRef]
- Wartenberg, M.; Schornak, J.; Carvalho, P.; Patel, N.; Iordachita, I.; Tempany, C.; Hata, N.; Tokuda, J.; Fischer, G.S. Closed-loop Autonomous Needle Steering during Cooperatively Controlled Needle Insertions for MRI-guided Pelvic Interventions. In The Hamlyn Symposium; Imperial College: London, UK, 2017. [Google Scholar] [CrossRef]
- Wartenberg, M.; Patel, N.; Li, G.; Gregory, S.; Fischer, G.S. Towards synergistic control of hands-on needle insertion with automated needle steering for MRI-guided prostate interventions. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Wu, D.; Li, G.; Patel, N.; Yan, J.; Hu Kim, G.; Monfaredi, R.; Cleary, K.; Iordachita, I. Remotely Actuated Needle Driving Device for MRI-Guided Percutaneous Interventions: Force and Accuracy Evaluation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Minko, T. Drug delivery systems. In Martin’s Physical Pharmacy and Pharmaceutical Sciences; Sinko, P.J., Ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2006; pp. 629–680. [Google Scholar]
- Stewart, S.A.; Dominguez-Robles, J.; Donnelly, R.F.; Larraneta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Sinn, A.W.M.; Bariana, M.; Kumeria, T.; Wang, Y.; Losic, D. Drug-releasing implants: Current progress, challenges and perspectives. J. Mater. Chem. B 2014, 2, 6157–6182. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Pierstorff, E. Reservoir-based polymer drug delivery systems. J. Lab. Autom. 2012, 17, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview. In Nanostructures for the Engineering of Cells, Tissues and Organs; Grumezescu, A.M., Ed.; William Andrew Applied Science Publishers: Oxford, UK, 2018; pp. 473–511. [Google Scholar] [CrossRef]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, N.; Yoon, E.S.; Cho, I.J. MEMS devices for drug delivery. Adv. Drug Deliv. Rev. 2018, 128, 132–147. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the functionalized carbon nanotubes as smart nanocarriers for drug delivery applications. In Fullerens, Graphenes and Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–133. [Google Scholar] [CrossRef]
- Antonino, R.S.C.M.Q.; Ruggiero, M.; Song, Z.; Nascimento, T.H.; Lima, E.M.; Bohr, A.; Knopp, M.M.; Löbmann, K. Impact of drug loading in mesoporous silica-amorphous formulations on the physical stability of drugs with high recrystallization tendency. Int. J. Pharm. X 2019, 1, 100026. [Google Scholar] [CrossRef]
- Yang, P.M.; Mu, H.Z.; Zhang, Y.S.; Wang, W.C.; Liu, C.; Zhang, S.Y. Sequential release of immunomodulatory cytokines binding on nano-hydroxyapatite coated titanium surface for regulating macrophage polarization and bone regeneration. Med. Hypotheses 2020, 144, 110241. [Google Scholar] [CrossRef] [PubMed]
- Häner, J.D.; Räber, L.; Windecker, S. Biodegradable vs. permanent polymer drug-eluting stents: The need for a new nomenclature to classify drug-eluting stent technology. Eur. Heart J. 2019, 40, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Razek, A. Towards an image-guided restricted drug release in friendly implanted therapeutics. Eur. Phys. J. Appl. Phys. 2018, 82, 31401. [Google Scholar] [CrossRef]
- Razek, A. Assessment of Supervised Drug Release in Cordial Embedded Therapeutics. Athens J. Technol. Eng. 2019, 6, 77–91. [Google Scholar] [CrossRef]
- Razek, A. Thermal effects of electromagnetic origin from heating processes to biological disturbances due to field exposure—A review. Therm. Sci. Eng. 2023, 6, 20–33. [Google Scholar] [CrossRef]
- Cirimele, V.; Freschi, F.; Giaccone, L.; Pichon, L.; Repetto, M. Human Exposure Assessment in Dynamic Inductive Power Transfer for Automotive Applications. IEEE Trans. Magn. 2017, 53, 5000304. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields for low frequencies (1 Hz–100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Bourland, J.D.; Nyenhuis, J.A.; Ham, C.L.; Engels, J.M.; Hebrank, F.X.; Frese, G.; Schaefer, D.J. Comparison of the threshold for peripheral nerve stimulation during gradient switching in whole body MR systems. J. Magn. Reason. Imaging 2002, 15, 520–525. [Google Scholar] [CrossRef]
- Mohith, S.; Upadhya, A.R.; Navin, K.P.; Kulkarni, S.M.; Rao, M. Recent trends in piezoelectric actuators for precision motion and their applications: A review. Smart Mater. Struct. 2020, 30, 013002. [Google Scholar] [CrossRef]
- Gao, X.; Yang, J.; Wu, J.; Xin, X.; Li, Z.; Yuan, X.; Shen, X.; Dong, S. Piezoelectric Actuators and Motors: Materials, Designs, and Applications. Adv. Mater. Technol. 2020, 5, 1900716. [Google Scholar] [CrossRef]
- Qiao, G.; Li, H.; Lu, X.; Wen, J.; Cheng, T. Piezoelectric stick-slip actuators with flexure hinge mechanisms: A review. J. Intell. Mater. Syst. Struct. 2022, 33, 1879–1901. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Jin, H.; Ren, K.; Guo, J.; Qiao, L.; Qiu, C.; Chen, W.; He, Y.; Dong, S.; et al. Miniaturized electromechanical devices with multi-vibration modes achieved by orderly stacked structure with piezoelectric strain units. Nat. Commun. 2022, 13, 6567. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.K.; Fan, P.Q.; Yuan, T.; Wang, Y.S. A novel hybrid mode linear ultrasonic motor with double driving feet. Rev. Sci. Instrum. 2022, 93, 025003. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Han, H.; Su, Z.; Sun, H. Design and characteristic analysis of multi-degree-of-freedom ultrasonic motor based on spherical stator. Rev. Sci. Instrum. 2022, 93, 025004. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, S.; Zhang, X.; Xu, P.; Zhang, Z.; Ren, L. Bionic Stepping Motors Driven by Piezoelectric Materials. J. Bionic Eng. 2023, 20, 858–872. [Google Scholar] [CrossRef]
- Hernandez, C.; Bernard, Y.; Razek, A. Design and manufacturing of a piezoelectric traveling-wave pumping device. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1949–1956. [Google Scholar] [CrossRef]
- Zhang, S.J.; Liu, Y.; Deng, J.; Gao, X.; Li, J.; Wang, W.Y.; Xun, M.X.; Ma, X.F.; Chang, Q.B.; Liu, J.K.; et al. Piezo robotic hand for motion manipulation from micro to macro. Nat. Commun. 2023, 14, 500. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Tang, J.; Huang, H.; Zhao, H.; Cheng, Y.; Liu, S.; Li, C.; Xiong, M. A Bionic Stick–Slip Piezo-Driven Positioning Platform Designed by Imitating the Structure and Movement of the Crab. J. Bionic Eng. 2023, 20, 2590–2600. [Google Scholar] [CrossRef]
- Virtanen, J. Enhancing the Compatibility of Surgical Robots with Magnetic Resonance Imaging. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2006. Available online: http://urn.fi/urn:isbn:9514280660 (accessed on 1 November 2023).
- Chinzei, K.; Kikinis, R.; Jolesz, F.A. MR compatibility of mechatronic devices: Design criteria. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI’99, Cambridge, UK, 19–22 September 1999; Volume 1679, pp. 1020–1030. [Google Scholar] [CrossRef]
- Chinzei, K.; Hata, N.; Jolesz, F.A.; Kikinis, R. Surgical assist robot for the active navigation in the intraoperative MRI: Hardware design issues. In Proceedings of the 2000 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2000) (Cat. No.00CH37113), Takamatsu, Japan, 31 October–5 November 2000; Volume 1, pp. 727–732. [Google Scholar] [CrossRef]
- Tsekos, N.V.; Khanicheh, A.; Christoforou, E.; Mavroidis, C. Magnetic resonance-compatible robotic and mechatronics systems for image guided interventions and rehabilitation: A Review Study. Annu. Rev. Biomed. Eng. 2007, 9, 351–387. [Google Scholar] [CrossRef]
- Tada, M.; Sasaki, S.; Ogasawara, T. Development of an optical 2-axis force sensor usable in MRI environments. In Proceedings of the SENSORS, 2002, Orlando, FL, USA, 12–14 June 2002; Volume 2, pp. 984–989. [Google Scholar] [CrossRef]
- Tada, M.; Kanade, T. An MR-Compatible Optical Force Sensor for Human Function Modeling. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2004; Barillot, C., Haynor, D.R., Hellier, P., Eds.; Lecture Notes in Computer Science, 3217; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Sedaghat, F.; Tuncali, K. Enabling Technology for MRI-Guided Intervention. Top Magn. Reason. Imaging 2018, 27, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Jolesz, F.A.; Morrison, P.R.; Koran, S.J.; Kelley, R.J.; Hushek, S.G.; Newman, R.W.; Fried, M.P.; Melzer, A.; Seibel, R.M.; Jalahej, H. Compatible instrumentation for intraoperative MRI: Expanding resources. J. Magn. Reason. Imaging 1998, 8, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G. Pocket Guide to MR Procedures and Metallic Objects: Update 1998; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1998; Available online: https://archive.org/details/pocketguidetomrp0000shel_y5n3 (accessed on 1 November 2023).
- Schenck, J.F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef] [PubMed]
- Razek, A. Assessment of EMF Troubles of Biological and Instrumental Medical Questions and Analysis of Their Compliance with Standards. Standards 2023, 3, 227–239. [Google Scholar] [CrossRef]
- Razek, A. Assessment and Categorization of Biological Effects and Atypical Symptoms Owing to Exposure to RF Fields from Wireless Energy Devices. Appl. Sci. 2023, 13, 1265. [Google Scholar] [CrossRef]
- Khairi, R.; Razek, A.; Bernard, L.; Corcolle, R.; Bernard, Y.; Pichon, L.; Poirier-Quinot, M.; Ginefri, J.C. EMC analysis of MRI environment in view of Optimized performance and cost of image guided interventions. Int. J. Appl. Electromag. Mech. 2016, 51, S67–S74. [Google Scholar] [CrossRef]
- Nunes, A.S.; Dular, P.; Chadebec, O.; Kuo-Peng, P. Subproblems Applied to a 3-D Magnetostatic Facet FEM Formulation. IEEE Trans. Magn. 2018, 54, 7402209. [Google Scholar] [CrossRef]
- Batra, T.; Schaltz, E.; Ahn, S. Effect of ferrite addition above the base ferrite on the coupling factor of wireless power transfer for vehicle applications. J. Appl. Phys. 2015, 117, 17D517. [Google Scholar] [CrossRef]
- Piriou, F.; Razek, A. Numerical simulation of a nonconventional alternator connected to a rectifier. IEEE Trans. Energy Convers. 1990, 5, 512–518. [Google Scholar] [CrossRef]
- Padilha, J.B.; Kuo-Peng, P.; Sadowski, N.; Batistela, N.J. Vector Hysteresis Model Associated to FEM in a Hysteresis Motor 691 Modeling. IEEE Trans. Magn. 2017, 53, 7402004. [Google Scholar] [CrossRef]
- Ren, Z.; Razek, A. A coupled electromagnetic-mechanical model for thin conductive plate deflection analysis. IEEE Trans. Magn. 1990, 26, 1650–1652. [Google Scholar] [CrossRef]
- Hariri, H.; Bernard, Y. Razek, 2-D Traveling Wave Driven Piezoelectric Plate Robot for Planar Motion. IEEE ASME Trans. Mechatron. 2018, 23, 242–251. [Google Scholar] [CrossRef]
- Li, C.; Ren, Z.; Razek, A. An approach to adaptive mesh refinement for three-dimensional eddy-current computations. IEEE Trans. Magn. 1994, 30, 113–117. [Google Scholar] [CrossRef]
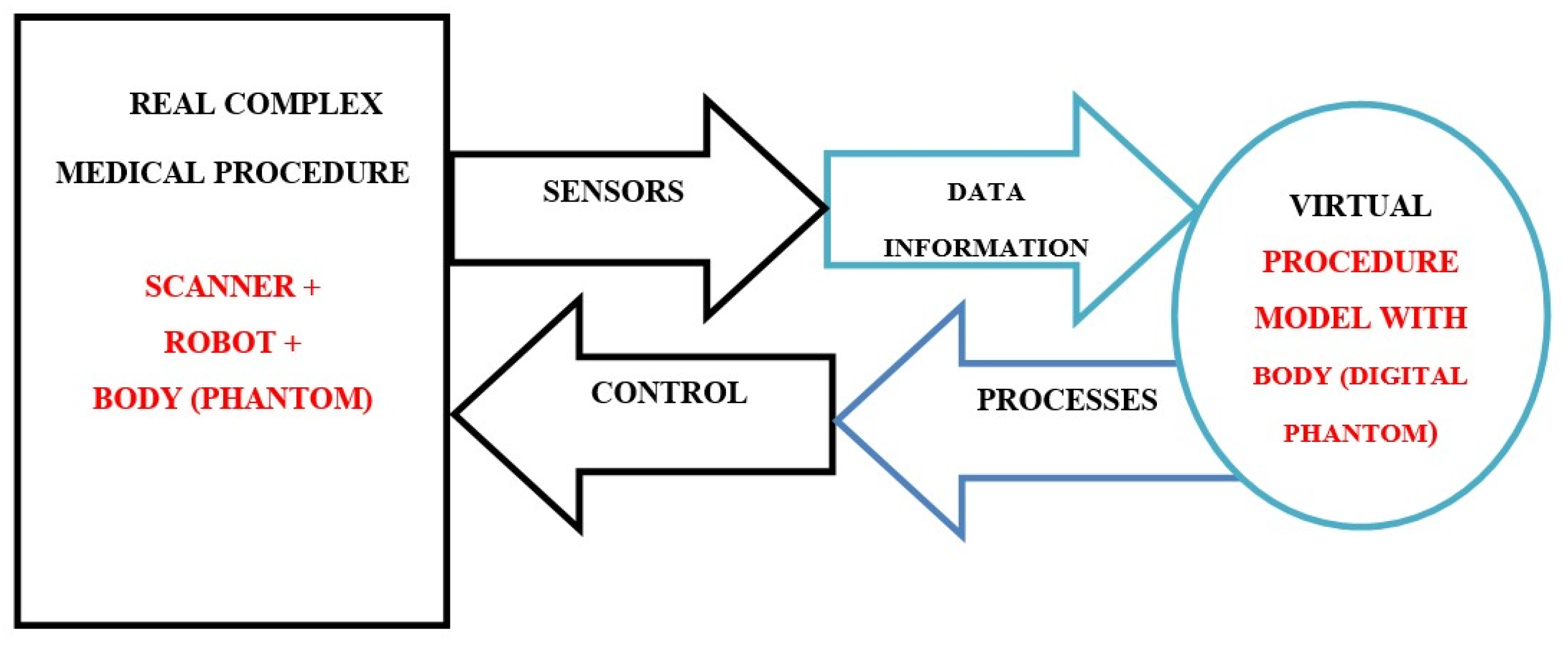
- Grieves, M.; Vickers, J. Digital Twin: Mitigating Unpredictable, Undesirable Emergent Behavior in Complex Systems. In Transdisciplinary Perspectives on Complex Systems; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Tao, F.; Sui, F.; Liu, A.; Qi, Q.; Zhang, M.; Song, B.; Guo, Z.; Lu, S.; Nee, A. Digital twin-driven product design framework. Int. J. Prod. Res. 2019, 57, 3935–3953. [Google Scholar] [CrossRef]
- He, B.; Bai, K. Digital twin-based sustainable intelligent manufacturing: A review. Adv. Manuf. 2020, 9, 1–21. [Google Scholar] [CrossRef]
- Rassõlkin, A.; Rjabtšikov, V.; Kuts, V.; Vaimann, T.; Kallaste, A.; Asad, B.; Partyshev, A. Interface Development for Digital Twin of an Electric Motor Based on Empirical Performance Model. IEEE Access 2022, 10, 15635–15643. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Li, Z. Digital twin in healthcare: Recent updates and challenges. Digit. Health 2023, 9, 20552076221149651. [Google Scholar] [CrossRef]
- Sun, T.; He, X.; Song, X.; Shu, L.; Li, Z. The Digital Twin in Medicine: A Key to the Future of Healthcare? Front. Med. 2022, 9, 907066. [Google Scholar] [CrossRef]
- De Benedictis, A.; Mazzocca, N.; Somma, A.; Strigaroet, C. Digital twins in healthcare: An architectural proposal and its application in a social distancing case study. IEEE J. Biomed. Health Inform. 2022, 27, 5143–5154. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Exploring the revolution in healthcare systems through the applications of digital twin technology. Biomed. Technol. 2023, 4, 28–38. [Google Scholar] [CrossRef]
- Mohamed, N.; Al-Jaroodi, J.; Jawhar, I.; Kesserwan, N. Leveraging Digital Twins for Healthcare Systems Engineering. IEEE Access 2023, 11, 69841–69853. [Google Scholar] [CrossRef]
- Mohamed, N.; Al-Jaroodi, J.; Jawhar, I.; Kesserwan, N. How Healthcare Systems Engineering Can Benefit from Digital Twins? In Proceedings of the 2023 IEEE International Systems Conference (SysCon), Vancouver, BC, Canada, 17–20 April 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Ricci, A.; Croatti, A.; Montagna, S. Pervasive and Connected Digital Twins—A Vision for Digital Health. IEEE Internet Comput. 2022, 26, 26–32. [Google Scholar] [CrossRef]
- Okegbile, S.D.; Cai, J. Edge-assisted human-to-virtual twin connectivity scheme for human digital twin frameworks. In Proceedings of the 2022 IEEE 95th Vehicular Technology Conference: (VTC2022-Spring), Helsinki, Finland, 19–22 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Das, C.; Mumu, A.A.; Ali, M.F.; Sarker, S.; Muyeen, S.; Kumar Das, S.; Das, P.; Hasan, M.M.; Tasneem, Z.; Islam, M.M.; et al. Toward IoRT Collaborative Digital Twin Technology Enabled Future Surgical Sector: Technical Innovations, Opportunities and Challenges. IEEE Access 2022, 10, 129079–129104. [Google Scholar] [CrossRef]
- Moodley, D.; Seebregts, C. Re-imagining health and well-being in low resource African settings using an augmented AI system and a 3D digital twin. arXiv 2023, arXiv:2306.01772. [Google Scholar] [CrossRef]
- Strobel, G.; Möller, F.; van der Valk, H. Healthcare in the Era of Digital Twins: Towards a Domain-Specific Taxonomy. Tech. Rep. Healthcare IT, European Conference on Information Systems. 2022. Available online: https://api.semanticscholar.org/CorpusID:251034187 (accessed on 1 November 2023).
- Song, Y. Human digital twin, the development and impact on design. J. Comput. Inf. Sci. Eng. 2023, 23, 060819. [Google Scholar] [CrossRef]
- Burattini, S.; Montagna, S.; Croatti, A.; Gentili, N.; Ricci, A.; Leonardi, A.; Pandolfini, S.; Tosi, S. An Ecosystem of Digital Twins for Operating Room Management. In Proceedings of the 2023 IEEE 36th International Symposium on Computer-Based Medical Systems (CBMS), L’Aquila, Italy, 22–24 June 2023; pp. 770–775. [Google Scholar] [CrossRef]
- Hagmann, K.; Hellings-Kuß, A.; Klodmann, J.; Richter, R.; Stulp, F.; Leidner, D. A Digital Twin Approach for Contextual Assistance for Surgeons During Surgical Robotics Training. Front. Robot AI 2021, 8, 735566. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The Dielectric Properties of Biological Tissues: II. Measurements in the Frequency Range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Barchanski, A.; Steiner, T.; De Gersem, H.; Clemens, M.; Weiland, T. Local Grid Refinement for low-Frequency Current Computations in 3-D Human Anatomy Models. IEEE Trans. Magn. 2006, 42, 1371–1374. [Google Scholar] [CrossRef]
- Noetscher, G.M. The CAD-Compatible VHP-Male Computational Phantom. In Brain and Human Body Modeling 2020: Computational Human Models Presented at EMBC 2019 and the BRAIN Initiative® 2019 Meeting; Makarov, S.N., Noetscher, G.M., Nummenmaa, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 309–323. [Google Scholar] [CrossRef]
- Makarov, S.N.; Noetscher, G.M.; Yanamadala, J.; Piazza, M.W.; Louie, S.; Prokop, A.; Nazarian, A.; Nummenmaa, A. Virtual Human Models for Electromagnetic Studies and Their Applications. IEEE Rev. Biomed. Eng. 2017, 10, 95–121. [Google Scholar] [CrossRef]
- Shimron, E.; Perlman, O. AI in MRI: Computational Frameworks for a Faster, Optimized, and Automated Imaging Workflow. Bioengineering 2023, 10, 492. [Google Scholar] [CrossRef]
- Seetohul, J.; Shafiee, M.; Sirlantzis, K. Augmented Reality (AR) for Surgical Robotic and Autonomous Systems: State of the Art, Challenges, and Solutions. Sensors 2023, 23, 6202. [Google Scholar] [CrossRef] [PubMed]
- Avrumova, F.; Lebl, D.R. Augmented reality for minimally invasive spinal surgery. Front. Surg. 2023, 9, 1086988. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, J.; Deguet, A.; Taylor, R.H.; Dou, Q. Integrating Artificial Intelligence and Augmented Reality in Robotic Surgery: An Initial dVRK Study Using a Surgical Education Scenario. In Proceedings of the 2022 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 13–15 April 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Fu, J.; Rota, A.; Li, S.; Zhao, J.; Liu, Q.; Iovene, E.; Ferrigno, G.; De Momi, E. Recent Advancements in Augmented Reality for Robotic Applications: A Survey. Actuators 2023, 12, 323. [Google Scholar] [CrossRef]
- Qian, L.; Wu, J.Y.; DiMaio, S.P.; Navab, N.; Kazanzides, P. A Review of Augmented Reality in Robotic-Assisted Surgery. IEEE Trans. Med. Robot. Bionics 2020, 2, 1–16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razek, A. Image-Guided Surgical and Pharmacotherapeutic Routines as Part of Diligent Medical Treatment. Appl. Sci. 2023, 13, 13039. https://doi.org/10.3390/app132413039
Razek A. Image-Guided Surgical and Pharmacotherapeutic Routines as Part of Diligent Medical Treatment. Applied Sciences. 2023; 13(24):13039. https://doi.org/10.3390/app132413039
Chicago/Turabian StyleRazek, Adel. 2023. "Image-Guided Surgical and Pharmacotherapeutic Routines as Part of Diligent Medical Treatment" Applied Sciences 13, no. 24: 13039. https://doi.org/10.3390/app132413039
APA StyleRazek, A. (2023). Image-Guided Surgical and Pharmacotherapeutic Routines as Part of Diligent Medical Treatment. Applied Sciences, 13(24), 13039. https://doi.org/10.3390/app132413039






