Abstract
In addressing eutrophication and enhancing water quality, this study builds upon previous research involving the development of an Efficient Phosphorus Removal Composite (EPRC), a material created using modified industrial wastes (steel slag and fly ash) as adsorbent substrates, supplemented with a binder and porosity-forming agent. In this investigation, the EPRC was further enhanced through the addition of zirconium oxychloride octahydrate, resulting in the production of Zr-EPRC particles as reinforced phosphorus removal materials. Comparative experiments were conducted to assess different methods for preparing Zr-EPRC, the static adsorption performance, and dynamic adsorption behavior. The optimal preparation of Zr-EPRC was achieved by separately modifying the base materials, steel slag and fly ash. Loading with mass ratios of zirconium chloride octahydrate to fly ash and steel slag at 0.4 and 0.6, respectively, for a duration of 12 h at a pH of 10 yielded the best results. In static adsorption experiments conducted at temperatures of 15 °C, 25 °C, and 35 °C, Zr-EPRC exhibited saturated phosphorus adsorption capacities of 11.833 mg/g (variance = 0.993), 12.550 mg/g (variance = 0.993), and 13.462 mg/g (variance = 0.996), respectively. Zr-EPRC emerges as a cost-effective and readily available solution with promising stability for general wastewater treatment applications, contributing significantly to the mitigation of eutrophication and the improvement of water quality.
1. Introduction
Eutrophication, a consequence of human activities, involves the excessive input of nutrients like nitrogen and phosphorus into slow-moving water bodies, such as lakes, estuaries, and bays. This nutrient influx triggers the rapid proliferation of algae and zooplankton, depleting dissolved oxygen levels and causing a decline in water quality, ultimately leading to mass fish mortality [1]. Additionally, eutrophication has been linked to increased greenhouse gas emissions and global warming [2,3]. This phenomenon has evolved into a global environmental challenge [4,5,6,7], necessitating a comprehensive understanding of its causes, the exploration of remediation strategies, and the development of innovative treatment technologies for the sustainable management of eutrophic water bodies.
Phosphorus, which is predominantly present in water as orthophosphate, metaphosphate, and organic phosphorus, is widely recognized as the primary driver of eutrophication, with elevated concentrations being particularly problematic [8,9,10,11,12]. The critical threshold for eutrophication in lake water is often defined as a total phosphorus (TP) concentration exceeding 0.02 mg/L, accompanied by a total nitrogen (TN) concentration surpassing 0.2 mg/L. Rapid plankton and algae growths become evident when the TP exceeds 0.1 mg/L [13].
Commonly used methods of phosphorus removal are physical, chemical, and biological methods, adsorption, and so on [14,15,16,17]. Among the various phosphorus removal methods, adsorption stands out due to its low energy consumption, high capacity, and minimal environmental impact [18,19]. This study delves into a comparative assessment of adsorption and phosphorus removal capabilities of a previously developed efficient phosphorus removal material based on fly ash and steel slag, which has been further modified with zirconium oxychloride octahydrate. The investigation explores different modification techniques and their influences on the material’s decolorization and phosphorus removal performance.
Following our group’s previous research findings, the optimal EPRC substrate ratio was determined to be 12:2:1 (fly ash/steel slag/cement), with a particle size of 5 mm demonstrating the most effective phosphorus removal [20,21]. Consequently, this study adheres to the optimal composition and particle size, employing three distinct modification methods to produce Zr-EPRC phosphorus removal materials with enhanced performance. Subsequently, a comprehensive analysis of the phosphorus removal properties, stability, and safety of Zr-EPRC materials is conducted.
The contribution of this study lies in the novel use of zirconium oxychloride octahydrate-modified materials to prepare Zr-EPRC. This innovative approach enhances the adsorption and phosphorus removal efficiency of the material, providing a valuable advancement in the field.
2. Materials and Methods
2.1. Test Materials
The materials essential for crafting Zr-EPEC particles encompass steel slag, fly ash, 525R common silicate cement, and a plant-based blowing agent. These materials are categorized into two groups: adsorption substrates and auxiliary components. Steel slag and fly ash serve as the principal substrates, while the auxiliary materials consist of a binder and a foaming agent. Specifically, 525R ordinary silicate cement is chosen as the binder, while the addition of a plant-based foaming agent enhances the material’s specific surface area. These material selections have been thoughtfully made, drawing from prior research insights and considering key factors like material strength, porosity, and moldability.
For this study, steel slag and fly ash were sourced from a steel plant and a power plant, respectively. Detailed physical properties of these materials are presented in Table 1, while Table 2 provides information on their chemical compositions, as analyzed through X-ray fluorescence (XRF). Additionally, Table 3 offers insights into the particle size distribution of both steel slag and fly ash.

Table 1.
Physical properties of steel slag and fly ash.

Table 2.
Chemical compositions of steel slag and fly ash.

Table 3.
The size distributions of the substrate.
In the realm of material preparation, the binder plays a pivotal role in facilitating bonding and curing, ultimately enhancing the mechanical strength of the material. After careful consideration, 525R ordinary silicate cement was chosen as the most suitable binder. This decision stems from its advantageous attributes, including rapid maturation, robust compressive strength, broad applicability, and cost-effectiveness, all of which are elucidated in Table 4.

Table 4.
Main components of 525R ordinary silicate cement.
Combined with the research results of the previous group, a plant-based foaming agent is still selected as the porogenic agent in the experiment [22]. This agent is characterized as a yellow-brown viscous liquid with a straightforward production process and cost-efficiency. Its primary merit lies in its remarkable compatibility with cement, facilitating reactions with calcium ions to generate insoluble salts. This reaction simultaneously renders the material’s structure porous and loose within the foam while enhancing its stability, all without inducing secondary pollution.
To enhance the phosphorus removal efficiency of the original EPRC material, this experiment introduced a modification utilizing zirconium chloride octahydrate (ZrOCl2-8H2O). ZrOCl2-8H2O is characterized as a white crystalline compound that readily dissolves in polar solvents such as water, ethanol, and ether. Its robust hydrolysis properties facilitate the conversion to zirconium hydroxide when exposed to aqueous environments. As a result, it is widely employed in adsorption and phosphorus removal processes.
2.2. Preparation Process
In accordance with the findings of our group’s prior research, the optimal ratio for EPRC particle preparation is determined as follows: fly ash/steel slag/cement = 12:2:1. Notably, in terms of particle size selection, EPRC particles with a 5 mm diameter have demonstrated the most effective phosphorus removal. As such, the experimental materials in this study adhere to the previously established optimal ratio and are prepared with a particle size of 5 mm. Furthermore, based on our past investigations, the plant-based foaming agent has exhibited the most favorable results, with an optimal dosage of 6 mL per 750 g of material. Consequently, the dosage of the plant-based foaming agent remains consistent in this experiment at 6 mL per 750 g of material.
The initial step involves mechanically grinding the steel slag into a powdered form. This powder is then mixed with an appropriate quantity of fly ash, binder, and the plant-based foaming agent. Following this mixing, a modest amount of tap water is added to facilitate thorough blending. Subsequently, granulation is achieved utilizing a drum granulator. Once granulation is completed, the composite particles are carefully arranged on a level surface and allowed to stand for a duration of 6–8 h. Following this resting period, the particles are regularly moistened by sprinkling with water over several days to maintain a consistent surface humidity level. The granulation process is visually depicted in Figure 1.
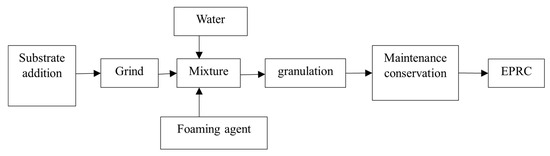
Figure 1.
EPRC preparation process flow.
2.3. Research Method
2.3.1. Comparison of Zr-EPRC Preparation Methods
In this study, the EPRC composites underwent a vital enhancement process through the introduction of zirconium oxychloride octahydrate. This modification aimed to bolster their adsorption and phosphorus removal capabilities. Given the practicality of large-scale production in future applications, it was imperative that the modification method remain straightforward. Consequently, the impregnation method was selected as the most suitable approach. The specific modification methods were classified into three distinct approaches: direct modification of EPRC particles, concurrent modification of substrates followed by granulation, and separate modification of adsorption substrates followed by granulation.
- Direct modification of EPRC to prepare Zr-EPRC
A solution with a concentration of 0.1 mol/L of “ZrOCl2·8H2O” was prepared. Subsequently, the EPRC particles were immersed in this zirconium-containing solution and subjected to continuous agitation at a constant temperature of 25 °C for a duration of 12 h. Following this immersion, the particles were carefully air-dried in a well-ventilated environment. This process resulted in the creation of Zr-EPRC particles, denoted as No.1 Zr-EPRC.
- Preparation of Zr-EPRC via co-modification of adsorption substrates
To create the zirconium-containing solution with a concentration of 0.1 mol/L, “ZrOCl2·8H2O” was prepared. The substrates, namely steel slag and fly ash, intended for EPRC production, were immersed together in the zirconium-containing solution, following the prescribed proportions. This mixture was then placed in a constant temperature oscillator operating at 25 °C for a duration of 12 h. Subsequently, the substrates were rinsed with distilled water until neutral, subjected to extraction and filtration under reduced pressure, and finally, they were dried in an oven at 105 °C. This process yielded substrate powder. The next step involved the creation of Zr-EPRC particles, accomplished by adding cement, a blowing agent, and other necessary materials to the substrate. These modified particles were designated as No. 2 Zr-EPRC.
- Preparation of Zr-EPRC via separate modification of adsorption substrates
A solution with a concentration of 0.1 mol/L of “ZrOCl2·8H2O” was prepared. Subsequently, steel slag and fly ash were separately immersed in this zirconium-containing solution. After undergoing 12 h of continuous agitation at 25 °C, the two modified substrates were prepared. This involved washing, drying, and grinding the substrates. Following these steps, cement, foaming agent, and other requisite materials were added in accordance with the specified proportions. This process resulted in the production of Zr-EPRC particles, denoted as No. 3 Zr-EPRC.
Static adsorption experiments were conducted on the modified adsorbent materials, as well as the original EPRC adsorbent materials prepared through the three distinct methods. The investigation aimed to assess their respective phosphorus removal efficiencies. The experimental procedure was as follows: 1 g of EPRC particles and three types of Zr-EPRC particles were measured and placed into separate conical flasks. Subsequently, 200 mL of a 5 mg/L phosphorus solution was introduced into each flask. The flasks were then subjected to an adsorption test within a constant temperature water bath shaker (at 25 ± 1 °C and 150 rpm). Following a specific duration, the supernatant was aspirated from the solution, and the residual phosphorus concentration in the adsorbed liquid was determined.
- Investigation of Optimal Modification Conditions
To determine the optimal loading conditions, five conical flasks were prepared, each containing 100 mL of deionized water. In each flask, 3 g of fly ash was weighed, and zirconium oxychloride octahydrate was added in accordance with various mass ratios of zirconium oxide octahydrate (m1) to fly ash (m2) at values of 0.2, 0.4, 0.6, 0.8, and 1.0. The mixture was kept in suspension until complete dissolution of “ZrOCl2·8H2O”. The suspension was then adjusted to a pH level of 10 using a 1 mol/L NaOH solution. Subsequently, the conical flasks were placed within a constant temperature oscillator set at 25 °C and oscillated at a speed of 150 rpm for a duration of 10 h. Following the completion of the reaction, solid–liquid separation was achieved via centrifugation. The solid powder obtained was thoroughly rinsed with deionized water until the cleaning solution reached a neutral pH. Finally, the solid powder was subjected to drying in an oven at 105 °C, and the zirconium-modified fly ash was subsequently crushed and ground to yield Zr-FA, which was recorded as such. Static adsorption experiments were conducted on the modified fly ash.
In a similar fashion, zirconium-modified steel slag was prepared by employing zirconium oxychloride octahydrate (m1) and steel slag (m2) with mass ratios of 0.2, 0.4, 0.6, 0.8, and 1.0, respectively. These modified steel slag samples were designated as Zr-SS. Static adsorption experiments were conducted on the modified steel slag powder.
- Determination of Optimal Loading pH
Building upon the optimal loading conditions established in investigation of optimal modification conditions experiment, the pH of the suspension was adjusted to values of 3, 5, 7, 10, and 12 by introducing 1 mol/L HCl and 1 mol/L NaOH solutions as necessary. Subsequently, loading was conducted at a constant temperature for a duration of 12 h. Once the loading process was finalized, the materials were washed until they reached a neutral pH, followed by drying and milling. This process led to the formation of zirconium-loaded steel slag (Zr-SS) and zirconium-loaded fly ash (Zr-FA) at different loading pH levels. Static adsorption experiments were then performed on these adsorbent materials.
- Determination of Optimal Loading Time
After introducing steel slag and fly ash based on the optimal loading conditions determined in investigation of optimal modification conditions experiment, the suspension was adjusted to a pH of 10 and underwent loading for varying durations of 2 h, 4 h, 6 h, 10 h, and 12 h. Following the loading process, the materials were washed until they reached a neutral pH, and subsequently, they were dried and ground. This process resulted in the production of zirconium-loaded steel slag (Zr-SS) and zirconium-loaded fly ash (Zr-FA) samples with different loading modification times. These materials were then subjected to static adsorption experiments.
2.3.2. Adsorption Isotherm Model
Data analysis and model fitting were performed using Origin software (2022). The version number of Origin 2022 is 9.9.0.225.
- Langmuir Adsorption Isotherm Model
The Langmuir isotherm is an empirical model that assumes the thickness of the adsorption layer is one molecule (monolayer adsorption), and the adsorption is homogeneous, with each molecule having adsorption activation energy and constant enthalpy. All adsorption sites have the same affinity for the adsorbate, and there is no migration of adsorbate on the surface plane where no adsorbate is present. Therefore, as adsorption progresses, the number of adsorption sites gradually decreases, and the adsorption rate gradually slows down until reaching adsorption equilibrium [23], as shown in Equation (1):
Converted to a linear form:
In the provided equations, the symbols have the following meanings: Qe denotes the adsorbed amount (mg/g) of adsorbate per unit mass of the adsorbent at adsorption equilibrium, Qm represents the maximum adsorbed amount in the saturated state (mg/g), Ce signifies the concentration of the adsorbate in the solution at adsorption equilibrium (mg/L), and KL is the Langmuir constant (L/mg), which reflects the affinity of the adsorbent for the intercept.
- Freundlich Adsorption Isotherm Model:
The Freundlich model is an empirical model that assumes that the adsorption process is a monolayer adsorption that occurs on an uneven surface and is suitable for lower concentration. The disadvantage is that the maximum adsorption capacity cannot be estimated [24], as shown in Equation (3):
Converted to a linear form:
In the given equations, Kf and n are Freundlich constants. The magnitude of Kf serves as an indicator of the adsorption affinity and the maximum adsorption capacity of the adsorbent to a certain extent. A higher Kf suggests a greater maximum adsorption capacity. The value of n reflects the level of difficulty in adsorption, with a larger n indicating easier adsorption. Typically, the value of n for an effective adsorbent falls within the range of 2 to 10 [25]. By plotting log Qe against log Ce and applying linear fitting, n can be determined from the slope, and Kf can be obtained from the intercept [26].
2.3.3. Adsorption Kinetics Model
Data analysis and model fitting were performed using Origin software (2022) v.9.9.0.225.
Pseudo-First-Order Adsorption Kinetic Model: The pseudo-first-order adsorption kinetic model, introduced by Lagergren, is designed to describe single-layer adsorption predominantly accomplished through boundary diffusion. It effectively captures the rapid reaction phase of the adsorption process, not the entire process. The specific expression is as follows:
In the equation above, Qt represents the adsorption capacity at time t (mg/g). Qe represents the adsorption capacity at equilibrium (mg/g). k1 is the pseudo-first-order rate constant (1/h).
When plotting against t, a linear fit with a correlation coefficient approaching 1 indicates conformity to the pseudo-first-order kinetic model. The slope of the line allows for the calculation of the pseudo-first-order rate constant (k1), while the intercept provides the adsorption capacity at equilibrium.
- Pseudo-Second-Order Adsorption Kinetic Model
The pseudo-second-order adsorption kinetic model, developed by HoYS and McKayG, primarily considers chemical adsorption limitations and provides a comprehensive representation of the entire adsorption process. Its mathematical expression is
Among these equations, k2 represents the pseudo-second-order rate constant (g/mg·h).
Upon plotting against t, the slope of the fitted line yields the value of k2, while the intercept provides the adsorption capacity at equilibrium. These models offer valuable insights into the material’s adsorption kinetics, shedding light on the complex mechanisms governing the process.
2.3.4. Experimental Setup and Methods for Static Adsorption Phosphorus Removal Tests
- Isothermal Adsorption Experiments
Nine portions of 1 g Zr-EPRC particles were meticulously weighed and placed into 250 mL conical flasks. Subsequently, 0.5 mg/L, 5 mg/L, 10 mg/L, 30 mg/L, 60 mg/L, 100 mg/L, 150 mg/L, 200 mg/L, and 300 mg/L of “KH2PO4” solution were separately added to these flasks. These solutions were subjected to an adsorption test within a constant temperature water bath shaker operating at 25 ± 1 °C and 150 rpm. After reaching adsorption equilibrium, the supernatant of each solution was aspirated to determine the remaining phosphorus concentration in the adsorbed liquid.
- Adsorption Kinetics Experiments
Different Initial Concentrations: Four portions, each containing 3 g of Zr-EPRC particles, were weighed and placed into separate 250 mL conical flasks. Next, 250 mL of “KH2PO4” solution with concentrations of 5 mg/L, 10 mg/L, 15 mg/L, and 20 mg/L were added to the respective conical flasks. These flasks were then placed within a constant temperature water bath shaker at 25 ± 1 °C and 150 rpm. At intervals of 1 h, 3 h, 6 h, 9 h, 12 h, 18 h, 24 h, 36 h, and 48 h, the supernatant of each solution was aspirated, and the residual phosphorus concentration in the adsorbed liquid was determined.
Different Initial Temperatures: Three portions, each containing 3 g of Zr-EPRC particles, were weighed and placed into separate 250 mL conical flasks. In these flasks, 250 mL of 20 mg/L “KH2PO4” solution was added. The conical flasks were then positioned within a thermostatic water bath shaker (150 rpm) set at temperatures of 15 °C, 25 °C, and 35 °C. The adsorption time was maintained at 1 h, 3 h, 6 h, 9 h, 12 h, 18 h, 24 h, 36 h, and 48 h, and the supernatant of each solution was aspirated at these intervals to determine the residual phosphorus concentration in the adsorbed liquid.
- One-way Adsorption Experiments
Dosage Experiment: Various quantities (ranging from 0.5 g to 4.0 g) of Zr-EPRC particles were weighed into 250 mL conical flasks. To each flask, 250 mL of 5 mg/L “KH2PO4” solution was added. The adsorption was allowed to proceed for 48 h within a constant temperature water bath shaker (25 ± 1 °C, 150 rpm). The supernatant of the adsorbent was then extracted, and the phosphorus concentration in the adsorbent was determined using ammonium molybdate spectrophotometry. Subsequently, the adsorbed amount of phosphorus and the phosphorus removal rate were calculated.
pH: Five portions of 5 g Zr-EPRC adsorbent material were immersed in a phosphorus solution with an initial concentration of 10 mg/L. The pH levels of these solutions were adjusted to 3, 5, 7, 10, and 12 using 1 mol/L NaOH and 1 mol/L hydrochloric acid solutions. Static adsorption was carried out at a constant temperature of 25 °C. At intervals of 3 h, 6 h, 9 h, 12 h, 24 h, 36 h, and 48 h, the phosphorus concentration in the solution was determined.
Co-existing Anions: Solutions with a phosphorus concentration of 10 mg/L were prepared by introducing various anions such as “NaHCO3”, “NaCl”, “Na2SO4”, and “NaNO3” into the phosphorus-containing solution. This was carried out to achieve ratios of phosphate to anion of 1:1 and 1:5 in the solution. A solution containing only phosphate served as a blank control. In each solution, 1 g of Zr-EPRC particles was added, and static adsorption experiments were conducted to assess changes in the adsorption capacity of Zr-EPRC materials.
2.3.5. Adsorption Study Methodology
In this study, the phosphorus concentration was measured using the ammonium molybdate spectrophotometric method. The adsorption performance of the adsorbent was assessed based on two crucial parameters: adsorption capacity and removal efficiency.
- Experimental Methodology for Phosphorus Analysis
The adsorption capacity (q) of the adsorbent for phosphorus was determined using the following equation:
Calculation of Phosphorus Removal Efficiency by the Adsorbent:
The removal efficiency (R) of the adsorbent for phosphorus was calculated using the following equation:
Among these equations, q represents the adsorption capacity of the adsorbent for phosphorus (mg/g). C0 is the initial phosphorus concentration in the solution before adsorption (mg/L). Ce is the residual phosphorus concentration in the solution after adsorption (mg/L). V is the volume of the solution (L). m is the mass of the adsorbent used (mg). R represents the removal efficiency of the adsorbent for phosphorus (%).
3. Results and Discussion
3.1. Comparison of Zr-EPRC Preparation Methods
3.1.1. Comparison of Modification Methods
As illustrated in Figure 2, the adsorption capacity of all three groups of Zr-EPRC particles for phosphorus surpasses that of the original EPRC particles, demonstrating a significant enhancement in the phosphorus removal efficiency through the introduction of a zirconium oxychloride octahydrate modification. This enhancement can be attributed to the loading process in which zirconium oxychloride octahydrate becomes attached to the adsorbent material in the form of zirconium oxide or zirconium hydroxide, thereby creating a zirconium-based adsorbent. The substantial specific surface area of amorphous-hydrated zirconium oxide, coupled with the rapid exchange between the hydroxyl group in the zirconium tetramer and the phosphate ions in water, results in a considerable increase in both the rate and capacity of phosphate adsorption. This optimization of adsorption leads to improved phosphorus removal performance compared to the original EPRC particles.
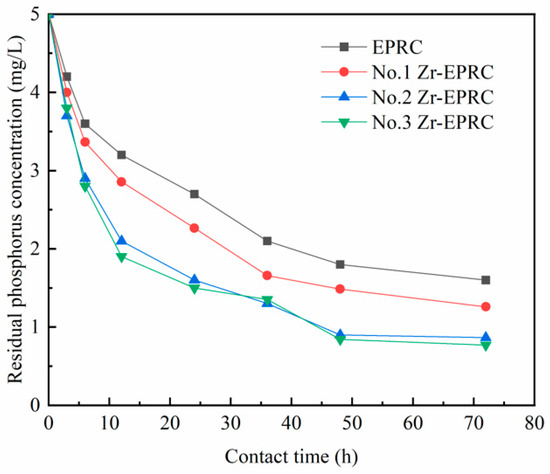
Figure 2.
Comparison of phosphorus removal effect of different modification methods.
Figure 2 reveals that the modification of the substrate to enhance phosphorus removal is more effective when compared to the direct modification of EPRC particles (No. 1 Zr-EPRC particles). Both No. 2 and No. 3 Zr-EPRC particles exhibit significantly better phosphorus removal effects than No. 1 Zr-EPRC particles. This is attributed to the fact that in the case of the direct modification of EPRC particles, zirconium ions can only adhere to the outermost layers of the material, resulting in weaker attachment in terms of quantity and stability compared to substrate modification. Furthermore, when comparing the two groups of particles modified using substrate methods, the adsorption effectiveness of No. 3 is slightly superior to that of No. 2, although the overall difference is not substantial.
Table 5 provides an overview of the state of the four groups of particles following the completion of static phosphorus ion adsorption. After the adsorption process is complete for the original EPRC particles and the No. 1 and No. 3 Zr-EPRC particles, the residual liquid remains clear, the particles remain intact, and there are no anomalies. In contrast, after the adsorption process of No. 2 Zr-EPRC particles is completed, the residual liquid exhibits slight turbidity, and there are indications of detachment on the surface of the adsorbent material, accompanied by slight chalkiness. This phenomenon may be attributed to the high iron content in the steel slag, creating an environment that is conducive to the formation of a zirconium–iron modifier, which significantly increases the roughness of the substrate’s surface. This, in turn, may not be conducive to subsequent cement adhesion. However, increasing the amount of cement blending could potentially lead to a reduction in the adsorption effectiveness.

Table 5.
State of each group of materials after adsorption of phosphorus ions.
In summary, among the three modification methods, both No. 2 and No. 3 Zr-EPRC particles exhibit superior modification effects compared to the No. 1 particles. However, it is worth noting that the No. 2 Zr-EPRC particles face greater challenges during the granulation process. Therefore, the approach of separately modifying the substrate and then granulating it, as demonstrated in the No. 3 particles, was selected for the preparation of Zr-EPRC particles. This method will serve as the focus of subsequent experiments and investigations.
3.1.2. Determination of Optimum Loads
As evident from Figure 3 and Figure 4, the influence of zirconium on the modification of fly ash and steel slag is highly significant. As the mass ratio of zirconium chloride octahydrate increases gradually, the adsorption rate and capacity of both modified steel slag and modified fly ash initially exhibit a rapid increase, reaching their peak values at mass ratios of 0.4 and 0.6, respectively. This phenomenon can be attributed to the greater loading of zirconium ions in the form of active zirconium oxides and hydroxides onto the surface and pores of the adsorbent materials as the zirconium content rises. Consequently, this results in an increased number of phosphorus adsorption sites, leading to a rapid enhancement in both the adsorption rate and capacity.
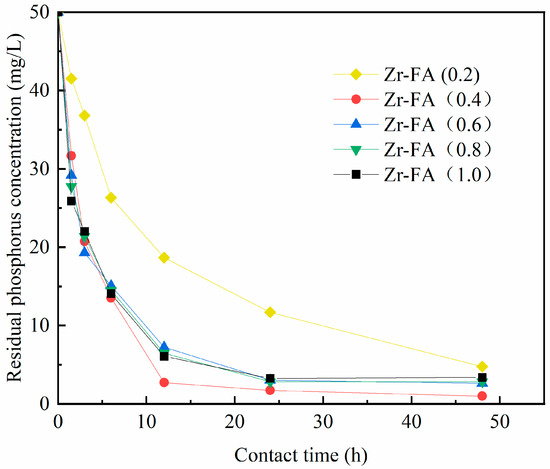
Figure 3.
Effects of different loadings on phosphorus removal effect of fly ash.
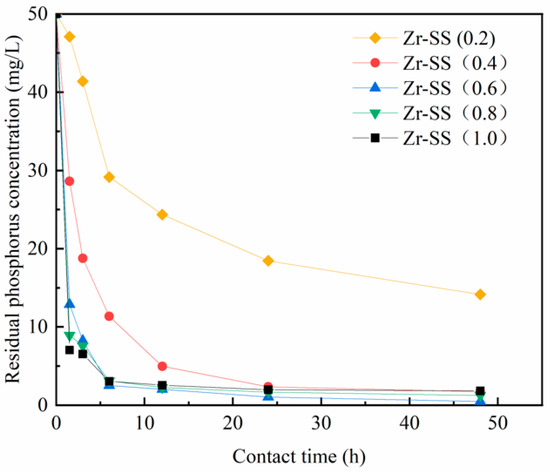
Figure 4.
Effects of different loadings on phosphorus removal effect of steel slag.
However, with a further increase in the mass ratio of zirconium chloride octahydrate, a surplus of activated zirconium oxides and hydroxides begins to block the pores of the adsorbent material, impeding the diffusion of phosphorus within these pores. Consequently, this leads to a reduction in the overall adsorption capacity. Therefore, it is judicious to conduct the modification of fly ash and steel slag with zirconium chloride octahydrate at mass ratios of 0.4 and 0.6. This approach achieves a balanced loading of zirconium onto the adsorbent substrate, thereby enhancing the pore structure, improving the adsorption efficiency, and minimizing material wastage.
3.1.3. Selection of Optimum Loading pH
Figure 5 and Figure 6 illustrate that the phosphorus removal efficiency of both modified steel slag and modified fly ash experiences an initial enhancement with an increase in the loading solution’s pH. The optimal adsorption effect is observed when the loading pH reaches approximately 10. Beyond a pH of 10, the adsorption capacity of the modified materials gradually decreases.
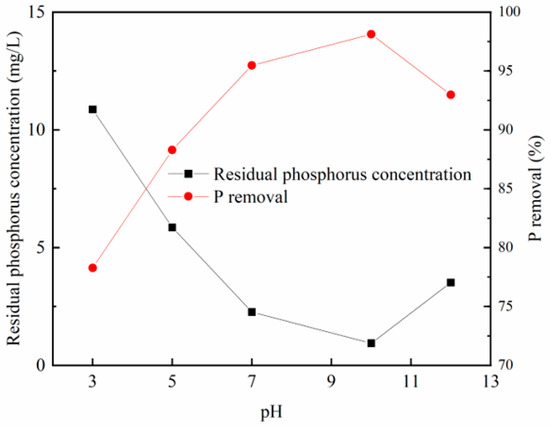
Figure 5.
Effects of different loading pH on phosphorus removal effect of steel slag.
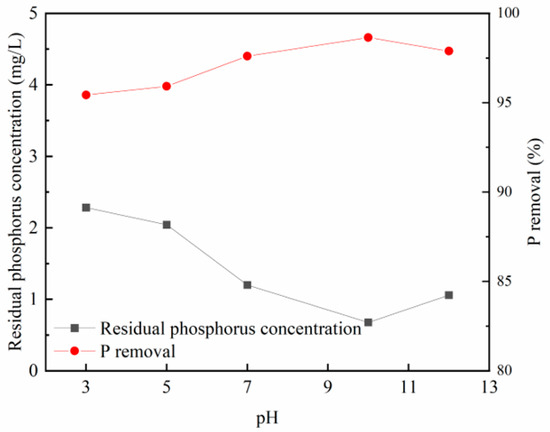
Figure 6.
Effects of different loading pH on phosphorus removal effect of fly ash.
This behavior can be attributed to the predominance of zirconium in the form of zirconium oxides or hydroxides on the adsorbent material, making it more susceptible to ligand exchange with phosphate ions. The increasing alkalinity of the modified solution leads to the transformation of zirconium oxide in the solution into zirconium oxychloride octahydrate hydroxide or hydrated zirconium oxide, facilitating phosphate adsorption in the subsequent static experiments, resulting in an improved phosphorus removal effect.
However, as the solution’s alkalinity continues to rise, the adsorbent material’s pore size also increases, hindering effective zirconium attachment and reducing the specific surface area. Consequently, this weakening of the adsorption capacity occurs. Therefore, it is advisable to prepare the adsorbent material at a pH value of approximately 10.
3.1.4. Determination of Optimum Load Time
Figure 7 and Figure 8 show that the equilibrium adsorption capacity of both modified steel slag and fly ash gradually increases during the initial loading period. When the loading time reaches 8 h, the loaded adsorption capacity of modified steel slag reaches 49.03 mg/L. Beyond this point, further increases in the loading time have minimal impacts on the adsorption capacity. By the time the loading duration reaches 12 h, the adsorption effect of steel slag and fly ash stabilizes, with no significant changes in the adsorption capacity.
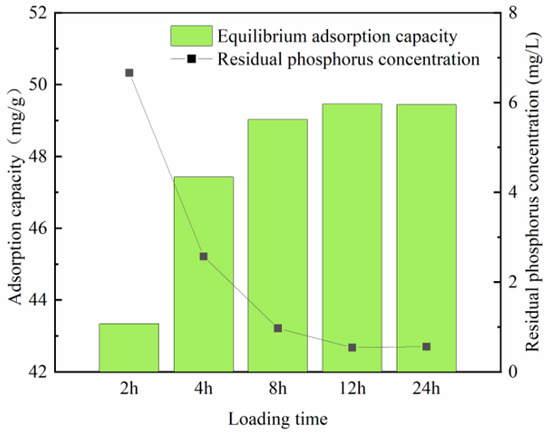
Figure 7.
Phosphorus adsorption in steel slag under different loading times.
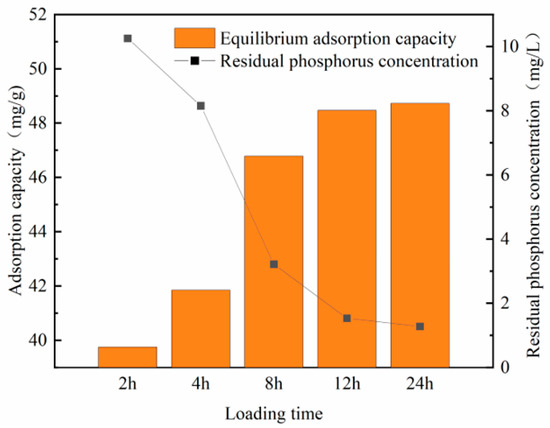
Figure 8.
Phosphorus adsorption via fly ash under different loading times.
This phenomenon arises from the process of zirconium being loaded onto the adsorbent material through condensation reactions. Initially, a surplus of active sites exists on the material’s surface, allowing for the rapid loading of active zirconium onto both the material’s surface and within its pore structure. However, as time progresses, the number of available active sites on the material becomes saturated, leading to a plateau in the adsorption effect. Consequently, the optimal loading time for the material is determined to be 12 h.
3.2. Characterization of Zr-EPRC
3.2.1. Scanning Electron Microscopy (SEM)
Through an SEM observation (Figure 9a–d), we found that the surface of the Zr-EPRC material is rough and uneven, providing a large surface area. The internal structure appeared fragmented and loose, with irregularly stacked coral-shaped protrusions, increasing the contact area between the material and the adsorbate. Additionally, the material exhibited abundant pore structures, offering ample space for adsorption and enhancing its adsorption capacity, thereby creating favorable conditions for efficient phosphorus removal.
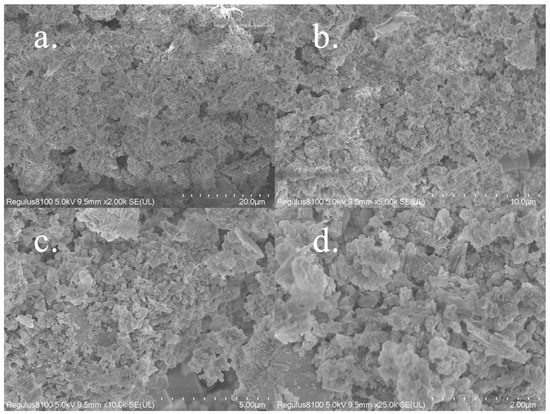
Figure 9.
SEM images of Zr-EPRC. The magnifications from (a–d) are 2000, 5000, 10,000, and 25,000.
3.2.2. Energy-Dispersive X-ray Spectroscopy (EDS)
The EDS analysis results (Figure 10) indicated that zirconium accounted for 20.4% of the elements in the material, confirming the successful loading of zirconium onto the material. Apart from zirconium, numerous other metallic elements such as Ca, Mg, and Fe were detected, indicating their roles in the adsorption of phosphate ions.
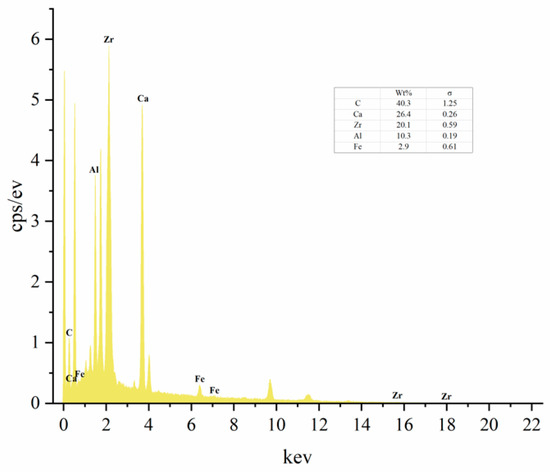
Figure 10.
EDS spectrum analysis graph of Zr-EPRC.
3.2.3. Brunauer–Emmett–Teller (BET) Analysis
A BET analysis revealed that the specific surface area of Zr-EPRC was 56.01 m³/g, with a pore volume of 0.092 cm³H/g and an average pore diameter of 7.290 nm. The adsorption–desorption isotherms and pore size distribution (Figure 11 and Figure 12) further confirmed the irregular mesoporous nature of the material, characterized by an H3-type hysteresis loop in the adsorption isotherm.
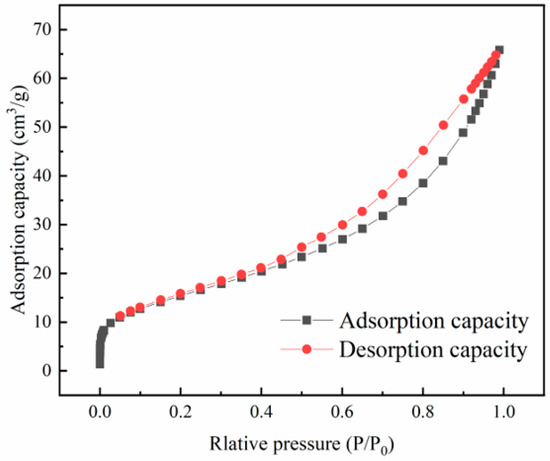
Figure 11.
Zr-EPRC adsorption–desorption isotherm curve.
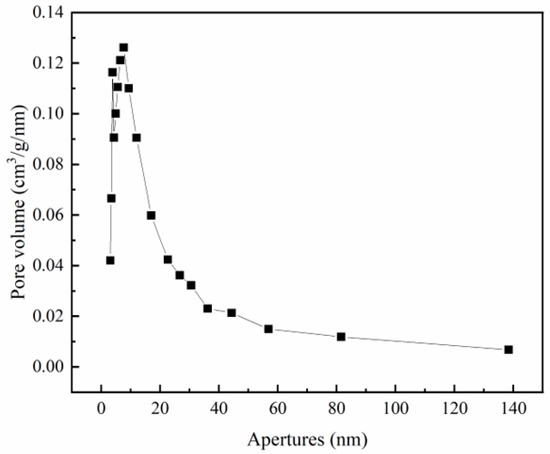
Figure 12.
Zr-EPRC pore size distribution chart.
3.2.4. Comparison with EPRC
Table 6 presents the BET characterization results for Zr-EPRC particles before and after modification, alongside the previous findings for the EPRC particles. The specific surface area, pore volume, average pore diameter, and adsorption capacity are crucial parameters indicating the material’s adsorption efficiency. By comparing the data, it becomes apparent that the modification with zirconium oxychloride octahydrate significantly enhances the Zr-EPRC particles’ surface area and pore size. Specifically, the specific surface area of Zr-EPRC is 12.43 times greater than that of the original EPRC, and the average pore diameter was triple that of the original. The adsorption capacity of Zr-EPRC was 12.550 mg/g under the experimental conditions at 25 °C, which was four times higher than that of EPRC. These considerable improvements underscore the enhanced phosphorus adsorption capacity of Zr-EPRC particles, demonstrating their promising potential for practical applications in phosphate removal.

Table 6.
Comparison of catalytic efficiency of Zr-EPRC and EPRC.
3.3. Characteristics of Zr-EPRC Particles for Phosphorus Removal via Static Adsorption
3.3.1. Isothermal Adsorption Experiments
In the isothermal adsorption experiments, both Langmuir and Freundlich isotherm models were used to simulate the adsorption of phosphate using Zr-EPRC particles at different temperatures. The fitting results are presented in Figure 13 and Figure 14, and the relevant data are summarized in Table 7.
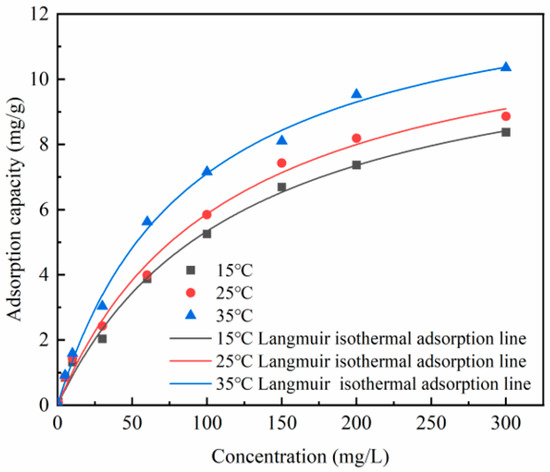
Figure 13.
Langmuir adsorption model fit for Zr-EPRC particles.
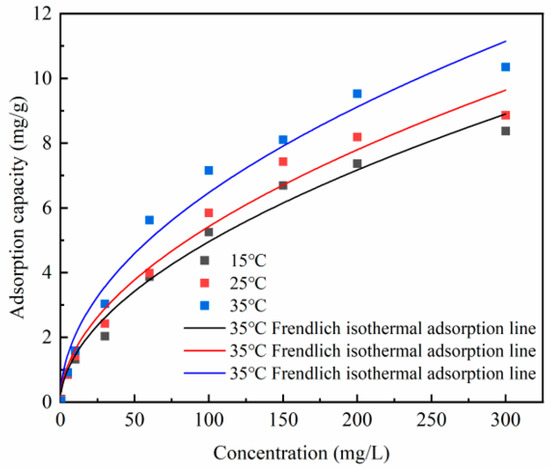
Figure 14.
Freundlich adsorption model fit for Zr-EPRC particles.

Table 7.
Adsorption isotherms fitted to Zr-EPRC particles.
As shown in Figure 13 and Figure 14, the adsorption capacity of the Zr-EPRC particles gradually increased as the concentration of phosphate in the solution increased, ultimately reaching a stable value. Notably, temperature played a pivotal role in influencing the adsorption isotherm. At the same concentration, the adsorption capacity of the Zr-EPRC particles for phosphorus increased significantly with the rising temperature.
The Langmuir model was applied to fit the isotherm data for the Zr-EPRC particles, and the fitting results are depicted in Figure 13. The correlation coefficients (R2) for the Langmuir model, as summarized in Table 7, were consistently higher than 0.990 at different temperatures. This indicates that the Langmuir adsorption model provides a more accurate description of the adsorption process of phosphate via Zr-EPRC particles. The application of Freundlich’s formula to fit the isothermal adsorption data is illustrated in Figure 14. While the isothermal data can also be adequately fitted using the Freundlich model, the R2 values for Freundlich (RF2) were slightly lower than the R2 values for Langmuir (RL2), suggesting that the Langmuir isothermal adsorption model offers a more precise description of the adsorption process. Consequently, it can be inferred that the adsorption of phosphate via Zr-EPRC particles follows a monolayer adsorption mechanism dominated by chemisorption.
Table 7 summarizes the values of Qm and KL at different temperatures, along with the corresponding values of KF and 1/n. It is evident that Qm increases significantly with the rising temperature, further confirming the positive impact of temperature on phosphate adsorption. This aligns with the observations made in the adsorption kinetics experiments. Moreover, the consistently observed 1/n values below 1 at all tested temperatures suggest the presence of a chemical affinity between the Zr-EPRC particles and phosphate, which is in accordance with previous findings.
In summary, the Langmuir adsorption model accurately describes the monolayer adsorption behavior of phosphate via Zr-EPRC particles, with an increasing adsorption capacity at higher temperatures, suggesting chemisorption as the dominant mechanism.
3.3.2. Adsorption Kinetics
- Adsorption kinetics at different initial concentrations
Figure 15 illustrates the adsorption kinetics of phosphate via Zr-EPRC particles at different initial concentrations. Initially, the adsorption rate is rapid, gradually slowing down with time until reaching adsorption equilibrium. This behavior is attributed to the ample availability of adsorption sites on the adsorbent at the start of the process, resulting in fast adsorption rates. As time progresses, the adsorption sites become gradually depleted, leading to a slower adsorption rate until equilibrium is attained. Additionally, it is evident from the data in the figure that higher initial phosphorus concentrations in the water result in greater unit adsorption amounts in the Zr-EPRC particles. This can be attributed to the increased number of phosphate ions in the water at higher concentrations, facilitating more frequent interactions between the adsorbent and adsorbate. Furthermore, an elevated phosphorus concentration in the solution enhances the concentration gradient between the material and the solution, promoting the adsorption reaction in the direction of adsorption.
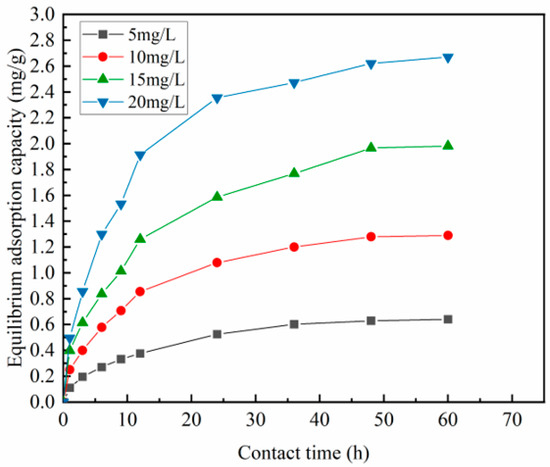
Figure 15.
Adsorption of phosphorus by Zr-EPRC at various concentrations.
The adsorption of phosphorus by Zr-EPRC particles was plotted with time as the X-axis, and the adsorption capacity was plotted as the Y-axis for each time period. The data were fitted using both quasi-primary and quasi-secondary kinetic models. The results are presented in Figure 16 and detailed in Table 8.
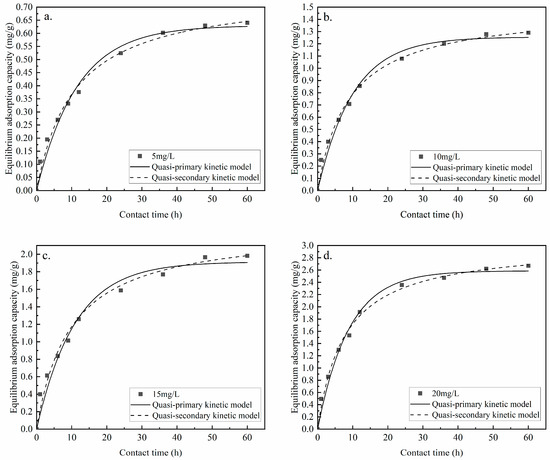
Figure 16.
Adsorption kinetics fitting at different concentrations. (a) Zr-EPRC adsorption at a phosphorus concentration of 5 mg/g; (b) Zr-EPRC adsorption at a phosphorus concentration of 10 mg/g; (c) Zr-EPRC adsorption at a phosphorus concentration of 15 mg/g; (d) Zr-EPRC adsorption at a phosphorus concentration of 20 mg/g.

Table 8.
Adsorption kinetics fitting results at different concentrations.
Figure 16 and Table 8 showcase the fitting results of both kinetic models. The correlation coefficients for both the quasi-primary and quasi-secondary kinetic equations are above 0.97, demonstrating good regressivity for both models. These models effectively describe the adsorption performance of phosphorus by Zr-EPRC particles. However, the correlation coefficients for the quasi-secondary kinetic model, represented as R22, are slightly higher than those for the quasi-primary kinetics, denoted as R12, indicating that the quasi-secondary adsorption model better represents this adsorption process. Consequently, the adsorption of phosphorus by Zr-EPRC particles is characterized as chemisorption. The experimental results align with this conclusion as the equilibrium adsorption capacity of Zr-EPRC particles increases with the rising initial concentrations of the phosphorus solution. Furthermore, the rate constant, k2, decreases because, at higher concentrations, the constant number of adsorption sites leads to increased competition among the adsorbate molecules. Consequently, there is a decrease in the probability of individual molecules binding to the adsorption sites, resulting in a decrease in the equilibrium constant of the adsorption reaction, k2.
- Adsorption kinetics at different initial temperatures
As depicted in Figure 17, the adsorption of phosphorus by Zr-EPRC particles exhibits an increasing trend with the rising temperature. This behavior is attributed to several factors. First, higher temperatures result in enhanced molecular thermal motion for both the adsorbent and adsorbate, leading to intensified interaction forces. This, in turn, facilitates the reaction between active sites on the adsorbent and the adsorbate. Additionally, an increased temperature promotes the diffusion of adsorbate molecules, ultimately increasing the adsorption capacity of the adsorbent.
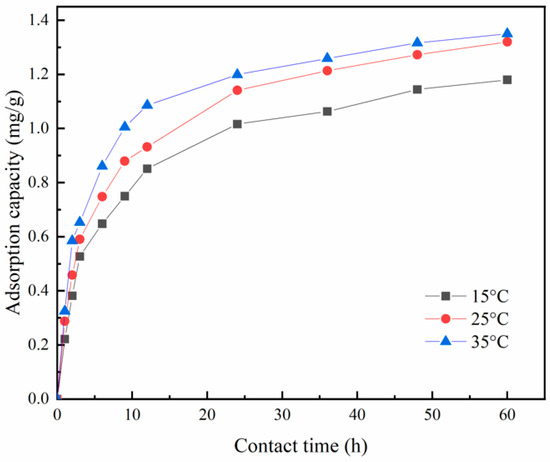
Figure 17.
Adsorption of phosphorus by Zr-EPRC at various temperatures.
For each time period, the adsorption of phosphorus by Zr-EPRC particles was plotted with time as the X-axis, and the adsorption capacity was plotted as the Y-axis. Subsequently, the data were fitted using both the quasi-primary and quasi-secondary kinetic models. The fitting results are detailed in Figure 18 and Table 9.
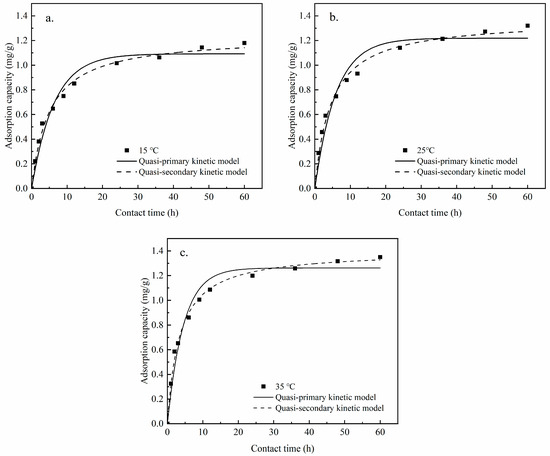
Figure 18.
Adsorption kinetics fitting at different temperatures. (a) Zr-EPRC adsorption at phosphorus concentration at 15 °C; (b) Zr-EPRC adsorption at phosphorus concentration at 25 °C; (c) Zr-EPRC adsorption at phosphorus concentration at 35 °C.

Table 9.
Adsorption kinetics fitting results at different temperatures.
From the fitting analyses, it is evident that the correlation coefficients of the quasi-secondary kinetic model for all three different temperature conditions (denoted as R22) were higher than those of the quasi-primary kinetic model. Therefore, the quasi-secondary kinetic model more accurately describes the kinetic process of phosphorus adsorption by Zr-EPRC particles compared to the quasi-primary kinetic model, with the correlation coefficients exceeding 0.99. Additionally, when comparing the equilibrium adsorption capacity at the three temperatures, it is apparent that the equilibrium adsorption capacity increases with the rising temperature. This observation aligns with the experimental phenomenon and indicates that elevated temperatures contribute to higher adsorption capacities of the particles.
Furthermore, the rate constant K2 remains relatively stable within the tested temperature range, suggesting that the phosphorus adsorption rate of the material is insensitive to changes in temperature. This implies that increasing the temperature has no significant impact on the adsorption rate of the material. Consequently, in practical applications for treating eutrophic water bodies, the material can maintain a relatively stable phosphorus removal effect, and the influence of seasonal temperature variations can be disregarded.
3.3.3. Single Impact Factor Adsorption Experiments
- Dosing
Table 10 presents the relationship between the dosage of Zr-EPRC particles and the phosphorus removal efficiency. The experimental results indicate that a higher dosage leads to a better phosphorus removal performance. However, as the dosage increases, the unit adsorption capacity decreases, resulting in reduced material utilization efficiency. This phenomenon can be explained by the fact that, at a fixed phosphate concentration, the total amount of phosphate remains constant, but a higher dosage provides more phosphorus adsorption sites and increases the contact opportunities between phosphate ions and active sites, thereby enhancing the phosphorus removal effect. Nevertheless, as the dosage increases, the available phosphate ions for adsorption on each site decrease, leading to a decrease in the material utilization efficiency.

Table 10.
Effect of dosage on phosphorus removal effect of Zr-EPRC particles.
In the case of low initial dosages, increasing the dosage significantly improves the phosphorus removal effect. However, as the dosage reaches a certain level, the enhancement effect on phosphorus removal diminishes because the wastewater’s phosphorus concentration becomes lower. As shown in the figure, when 1.5 g of Zr-EPRC particles were used, the phosphate removal rate exceeded 90%, and the remaining phosphorus concentration was 0.304 mg/L. Therefore, the optimal dosage for treating 5 mg/L of phosphorus-containing wastewater was determined to be 1.5 g/200 mL. This dosage is much lower than that of the original EPRC (3.5 g/250 mL), highlighting the superior phosphorus removal performance of Zr-EPRC. It is important to note that the optimum dosage obtained in the experiment serves as a reference and may need an adjustment based on the specific composition and concentration of phosphorus-containing water in practical applications.
- pH
Figure 19 illustrates the impact of pH on the adsorption efficiency of Zr-EPRC. It is evident that the adsorption of phosphate increases as the pH decreases. The adsorption efficiency experiences a slight reduction within the pH range of 7 but then decreases significantly when the pH exceeds 7. The findings from the pH single-factor adsorption experiment appear to contradict the optimal pH obtained from the substrate’s modification experiment. The apparent discrepancy arises from the distinction between the optimal pH during substrate modification and the pH during the subsequent adsorption stage. The modification experiment identifies the optimal pH for substrate modification, which may differ from the ambient pH observed during the adsorption experiment. This observation can be attributed to two factors:
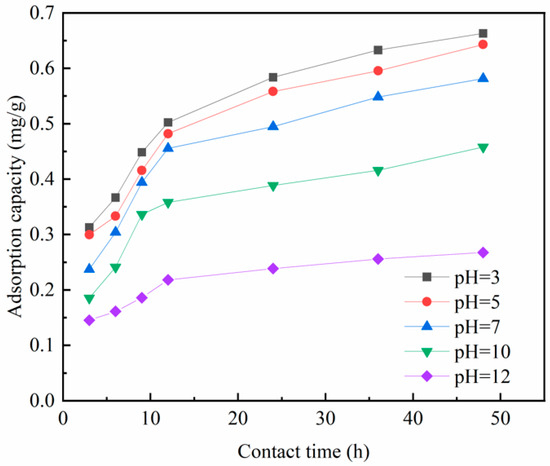
Figure 19.
Effect of pH on phosphorus removal effect of Zr-EPRC particles.
Phosphate Speciation: Under acidic conditions, phosphorus mainly exists in the form of H2PO3−, while under alkaline conditions, it primarily exists as HPO32− or PO33−. H2PO3− has a lower free energy of adsorption and is more prone to adsorption reactions compared to HPO32− and PO33−.
Surface Reactivity: The adsorption of zirconium metal for phosphorus removal occurs through the exchange of ligands between the hydroxyl groups on the surface of zirconium oxide and phosphate in water. The reactivity of the hydroxyl groups on the surface of hydrated zirconium oxide depends on the solution’s pH. Acidic conditions favor the protonation of the hydrated zirconium oxide surface and the attraction of phosphate through electrostatic forces. However, under higher pH conditions, the deprotonation of hydrated zirconium oxide reduces the reactivity. Additionally, the increased OH− ions in the solution can compete with phosphate for active sites, further weakening the adsorption capacity of the adsorbent.
In practical water treatment processes, it is advisable to avoid treating highly alkaline wastewater directly. Instead, the alkalinity of the wastewater can be adjusted via pH modification prior to treatment. This ensures the effective removal of phosphorus from the wastewater.
- Co-existing anions
Figure 20 demonstrates that Zr-EPRC particles exhibit a strong resistance to interference from co-existing anions, maintaining effective adsorption capacity for phosphorus removal even in the presence of competing ions. When analyzing the impact of each interfering ion on the adsorption capacity, the following observations can be made:
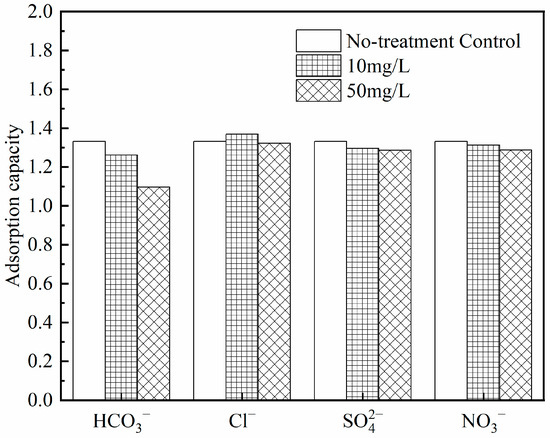
Figure 20.
Effect of coexisting ions on phosphorus removal effect of Zr-EPRC particles.
NO3−, SO42−, and HCO3− have some inhibitory effects on the adsorption and phosphorus removal capabilities of Zr-EPRC particles. Among these, the inhibitory effect of HCO3− is relatively pronounced. This is because the introduction of HCO3− into the solution raises its pH, resulting in an increase in the OH− concentration. This decrease in the surface protonation of hydrated zirconia hampers the effective adsorption of phosphorus. Adjusting the solution pH to neutral prior to the adsorption experiments resulted in only a 3.3% decrease in the adsorption capacity compared to the blank group, confirming that HCO3− primarily affects Zr-EPRC particles through pH alteration.
Low concentrations of Cl− appear to enhance the adsorption of phosphorus by Zr-EPRC, while high concentrations of Cl− inhibit this process. The change in the adsorption capacity could be attributed to the increased ion concentration on the material’s surface upon adding chloride ions, reducing the ζ-potential on the material’s surface and promoting adsorption. However, as the concentration of chloride ions increases, their competitive strength with phosphoric acid as an anion also increases, leading to the inhibition of adsorption.
In summary, the inhibitory effect of anions on Zr-EPRC particles is limited, and these particles display excellent ion selectivity and resistance to interference during practical applications.
4. Conclusions
This study comprehensively evaluated the potential of Zr-EPRC particles as an effective solution for phosphorus removal in wastewater treatment. Through systematic experimentation and analysis, several key findings and conclusions have been drawn, shedding light on the suitability and optimization of Zr-EPRC particles in practical applications.
Modification Methods Comparison: The investigation into different modification approaches for EPRC particles unveiled that each method improved phosphorus removal compared to the unmodified EPRC. However, direct modification yielded limited enhancement, co-modification raised concerns of surface chalking, and separate substrate modification resulted in Zr-EPRC particles characterized by both stable performance and simplified manufacturing processes. Consequently, the separate substrate modification approach emerged as the most promising method.
Optimal Preparation Conditions: Crucial insights were gained concerning the optimal conditions for Zr-EPRC particle synthesis. These conditions included a precise mass ratio of zirconium chloride octahydrate to fly ash and steel slag (0.4 and 0.6, respectively), a pH of 10, and a loading time of 12 h at a constant temperature.
Adsorption Isotherms and Kinetics: This study determined that the Langmuir model better described the adsorption isotherms for phosphorus in Zr-EPRC particles. This indicated a significant enhancement in the adsorption capacity compared to the original EPRC material. Additionally, the quasi-secondary kinetic model provided a more accurate representation of the adsorption kinetics.
Dosage and pH Impact: The results revealed a positive correlation between the effectiveness of phosphorus removal by Zr-EPRC particles and the dosage. A lower ambient pH for adsorption conditions was identified as favorable for phosphorus removal due to the prevalence of the H2PO3− form. The ambient pH for adsorption is different from the loading solution pH. The ambient pH for adsorption affects phosphate speciation and surface reactivity. The loading solution pH mainly affects the form of zirconium present on the adsorbent material and the pore size of the material.
Resistance to Interference: Zr-EPRC particles exhibited robust resistance to interference from co-existing ions in water. This characteristic underscores their potential for effective deployment in complex sewage treatment scenarios.
The study introduces a novel approach by utilizing zirconium oxychloride octahydrate-modified materials to prepare Zr-EPRC, contributing to an enhanced adsorption and phosphorus removal efficiency. The observed increase in both the surface area and average pore size in Zr-EPRC, compared to the previous EPRC results, signifies a substantial improvement. Specifically, Zr-EPRC exhibits a surface area that is 12.39 times that of the original EPRC and an average pore size that is three times larger. These significant enhancements underscore the material’s promising capability for efficient phosphorus removal through adsorption. This noteworthy advancement adds valuable insights to the existing literature on enhanced phosphorus removal materials.
In summary, this investigation underscores the substantial promise of Zr-EPRC particles, particularly when prepared via separate substrate modification, for addressing phosphorus pollution in wastewater treatment. Their exceptional adsorption characteristics, stability, and resilience to interference make them a highly viable solution for a wide range of practical wastewater treatment applications. This study offers valuable insights for optimizing the implementation of Zr-EPRC particles in real-world wastewater treatment processes, highlighting their potential significance in addressing phosphorus-related environmental challenges.
Author Contributions
Conceptualization, Y.L. and J.S.; methodology, Y.L. and J.S.; software, J.S.; validation, Y.L. and J.S.; formal analysis, J.S.; investigation, J.S.; resources, Y.L.; data curation, J.S.; writing—original draft preparation, J.S.; writing—review and editing, Y.L. and J.S.; visualization, Y.L. and J.S.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Moal, M.; Gascuel-Odoux, C.; Menesguen, A.; Souchon, Y.; Etrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Shang, J.H.; Zhang, C.; Zhang, W.L.; Niu, L.H.; Wang, L.F.; Zhang, H.J. The role of freshwater eutrophication in greenhouse gas emissions: A review. Sci. Total Environ. 2021, 768, 144582. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.X.; Dong, J.F.; Yang, H.; Van Zwieten, L.; Lu, H.; Alshameri, A.; Zhan, Z.H.; Chen, X.; Jiang, X.D.; et al. A Critical Review of Methods for Analyzing Freshwater Eutrophication. Water 2021, 13, 225. [Google Scholar] [CrossRef]
- Gilbert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Carstensen, J.; Conley, D.J.; Dromph, K.; Fleming-Lehtinen, V.; Gustafsson, B.G.; Josefson, A.B.; Norkko, A.; Villnas, A.; Murray, C. Long-term temporal and spatial trends in eutrophication status of the Baltic Sea. Biol. Rev. Camb. Philos. Soc. 2017, 92, 135–149. [Google Scholar] [CrossRef]
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R. Phosphorus control is critical to mitigating eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef]
- Edmondson, W.T.; Anderson, G.C.; Peterson, D.R. Artificial eutrophication of lake washington. Limnol. Oceanogr. 1956, 1, 47–53. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, W.; Wang, X.; Couture, R.-M.; Larssen, T.; Zhao, Y.; Li, J.; Liang, H.; Liu, X.; Bu, X. Decline in Chinese lake phosphorus concentration accompanied by shift in sources since 2006. Nat. Geosci. 2017, 10, 507–511. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gaoa, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Weiss, C.M. Relation of phosphates to eutrophication. J. Am. Water Work. Assoc. 1969, 61, 387–391. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Zhang, C.C.; Guisasola, A.; Baeza, J.A. A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal. Water Res. 2022, 212, 118102. [Google Scholar] [CrossRef]
- Li, Y.J.; He, X.M.; Hu, H.M.; Zhang, T.T.; Qu, J.; Zhang, Q.W. Enhanced phosphate removal from wastewater by using in situ generated fresh trivalent Fe composition through the interaction of Fe(II) on CaCO3. J. Environ. Manag. 2018, 221, 38–44. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)(2) treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Mikhak, A.; Sohrabi, A.; Kassaee, M.Z.; Feizian, M.; Disfani, M.N. Removal of Nitrate and Phosphate from Water by Clinoptilolite-Supported Iron Hydroxide Nanoparticle. Arab. J. Sci. Eng. 2017, 42, 2433–2439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.M.; Singh, R.P. Enhanced Phosphorus Removal from Wastewater Using RSPRC and a Novel Reactor. Appl. Sci. 2020, 10, 3629. [Google Scholar] [CrossRef]
- Chmielewská, E.; Hodossyová, R. Removal of phosphate from aqueous solutions. Kinetics, thermodynamics and isotherm calculations. Fresenius Environ. Bull. 2013, 22, 598–603. [Google Scholar]
- Lakshmi, V.; Resmi, V.G.; Raju, A.; Deepa, J.P.; Rajan, T.P.D.; Pavithran, C.; Pai, B.C. Concentration dependent pore morphological tuning of kaolin clay foams using sodium dodecyl sulfate as foaming agent. Ceram. Int. 2015, 41, 14263–14269. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.Y.; He, Y.L.; Zhang, B.; Kirumba, G.; Xie, J. Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. J. Colloid Interface Sci. 2013, 402, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Krishna, R.H.; Swamy, A. Physico-Chemical Key Parameters, Langmuir and Freundlich isotherm and Lagergren Rate Constant Studies on the removal of divalent nickel from the aqueous solutions onto powder of calcined brick. Int. J. Eng. Res. Dev 2012, 4, 29–38. [Google Scholar]
- Chung, H.-K.; Kim, W.-H.; Park, J.; Cho, J.; Jeong, T.-Y.; Park, P.-K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).