Abstract
Modern agriculture requires technology to give precise measures of relevant parameters, such as those associated with pest control. Here, we developed an algorithm model as the basis for a bait spray intervention by monitoring the olive fruit fly Bactrocera oleae (Rossi) with conventional traps covering 24.3 hectares of non-irrigated Baladi olive cultivars in the Hasbaya region. We installed 49 yellow sticky traps with ammonium bicarbonate. The adults, both males and females, were monitored on a weekly basis. The traps and trees were georeferenced, and parameters such as the temperature, relative humidity, tree phenology (BBCH), and fruit load rate were compiled. The results show that the infested fruits were correlated equally with the fruit load rate and the number of adults captured, which in turn were correlated more with the temperature than the relative humidity. The number of males captured was higher than that of females throughout the cultivation period. The first symptoms of the fruits were observed on 22 September, when the BBCH was equal to 85, with an average number of adult captures of less than five when using traps over 7 days.
1. Introduction
The olive tree is considered one of the most important cultivated crops in Lebanon. It represents approximately 43% of the total area cultivated with fruit trees [1]. This crop has been threatened by several pests, especially the olive fruit fly (OFF), Bactrocera oleae Rossi (1790) (Diptera: Tephritidae). This pest is widely distributed in the Mediterranean basin, Middle East, and North Africa and poses a severe economic threat for commercial olive growers, causing economic losses [1,2,3,4]. In fact, in areas where the olive fruit fly is well established, such as in the Mediterranean region, it has been responsible for approximately 30% of crop losses, especially in countries such as Greece, Italy, Spain, and Tunisia, where a large amount of commercial production occurs. If not treated, it is capable of destroying 100% of table cultivars and up to 80% of the oil value [2,3,4]. In Lebanon, the damage losses are not yet reported. The adult females deposit their eggs (50–400 eggs in one lifetime) under the skin of the fruit [5]. The larvae develop and feed on the pulp of the fruit. Infested fruit sometimes drop prematurely. The secondary infestation of bacteria and fungi accumulated in the tunnels created by the larva inside the fruit reduces the oil quality [6,7]. Thus, in years with large fly populations, damaged fruit cannot be processed as table olives, and, in the case of olive oil production, B. oleae directly affects the amount and the quality of the oil by increasing the acidity degree [6,7]. The flies are very mobile and have the ability to seek out cooler areas of the orchard and urban trees. Their mobility and the fact that generations overlap make the treatment of this insect a complicated task [8,9,10]. Moreover, the activity of B. oleae is influenced by several factors, including the latitude, altitude, tree load, irrigation [11], temperature [12], relative humidity [13], variety [14], physicochemical characteristics of the drupe [15], and parasite activity [16]. In Lebanon, chemical spraying is the main option used to control B. oleae [4]. Given its aggressive mode of behavior, the control of the olive fruit fly is highly complex, as conventional chemical control remains ineffective and has a negative impact on the environment and human health, with associated problems of resistance and side effects for beneficial insects [17,18]. There are alternative control measures for this important pest that are more environmentally friendly, such as Spinosad bait spraying, based on the monitoring of the pest population through several types of traps, especially yellow sticky (YS) traps [19,20,21,22]. Thus, the objectives of this study were to evaluate the critical parameters involved in determining when, where, and how to spray, especially the number of adults captured by yellow sticky traps equipped with the attractant ammonium bicarbonate; we also performed observations of the infested fruits, coupled with the collection of climatic data (temperature, relative humidity, etc.) and the phenological characteristics of the tree, especially the fruit load rate.
2. Materials and Methods
2.1. Experimental Site
The site was located in Southern Lebanon in the Hasbaya region. The coordinates were latitude 33°23′53.00′′ N and longitude 35°41′5.70′′ E, with elevation between 525 and 600 m a.s.l. (Goto location: 33.382030, 35.718210) (Figure 1).
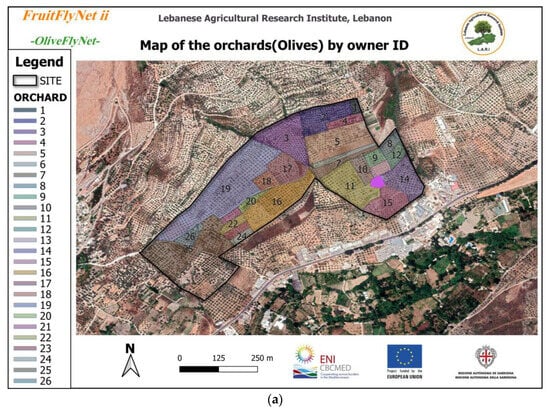
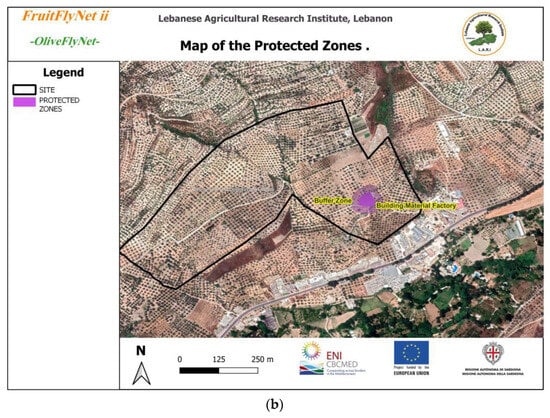
Figure 1.
(a) Area of the experimental site owned by 26 farmers; (b) protected zones in the site composed of cement manufactory.
2.2. Trees and Practices
The site was not flat and was very heterogeneous in terms of topography. It presented large variations in altitude (almost 75 m difference at maximum), showing many hills and valleys. Owned by 26 farmers and with a total area of 24.3 ha, the site was composed of the non-irrigated Baladi cultivar with different clones. This cultivar is characterized by high tolerance to drought and moderate tolerance to OFF. The same agricultural practices were registered despite the variations in tree age, ranging from 20 to 70 years old, and in tree distance, from 5 to 8 m, giving a density of 250 to 350 trees per ha, or 5353 trees in total across 24.3 ha. The tree height ranged between 3 and 7 m, with a canopy diameter between 3 × 3 and 7 × 7.5 m. The fruit load rate of the trees was obtained on 13 August (Figure 2). Pruning was not severe, weeding was almost absent, and spraying was not registered. The addition of animal manure by the farmers in late September was registered.
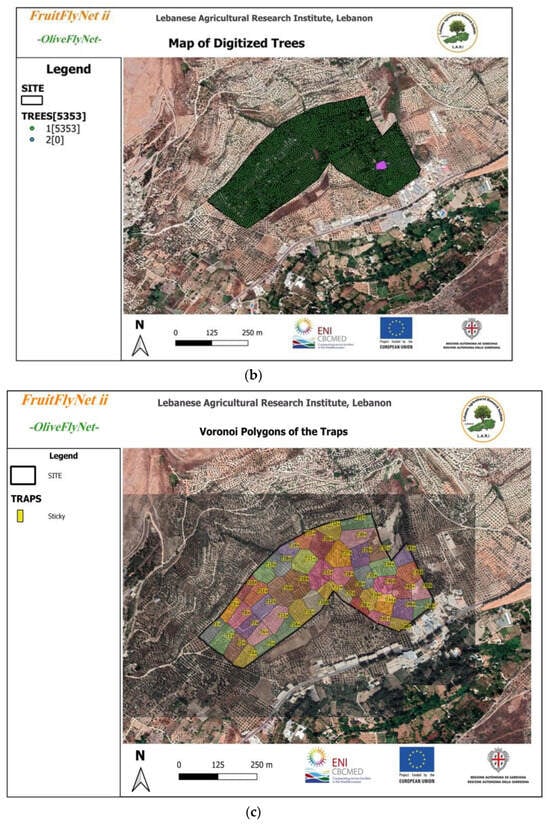

Figure 2.
(a) Output of the OliveFlyNet application interface showing details about information stored in the geodatabase system; (b) tree location map; (c) Voronoi polygons of the trap zones; (d) tree picture.
2.3. Trap Installation
The trap installation was realized on 22 July 2022, and the trap ID added to the tree ID was registered (Figure 3).
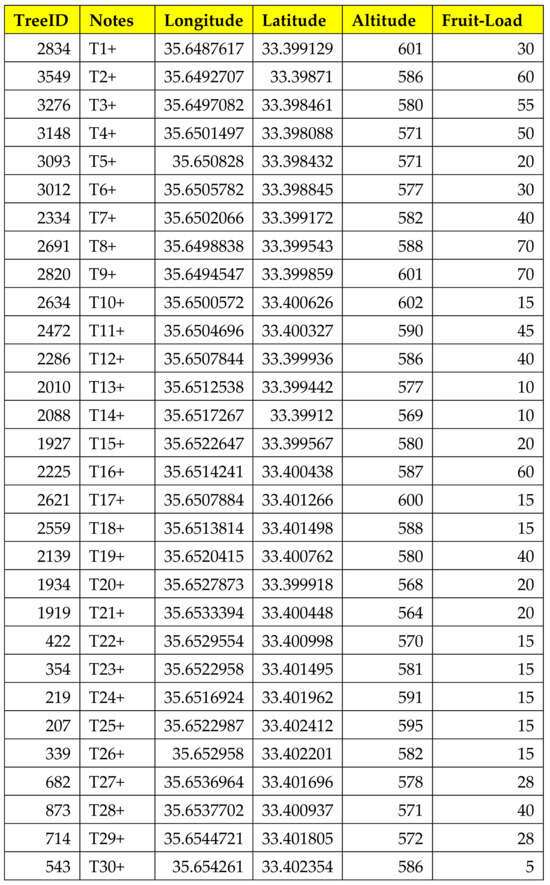
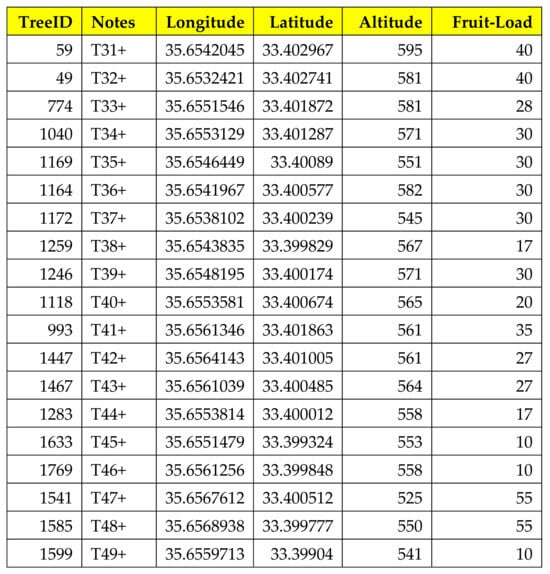
Figure 3.
Output of the OliveFlyNet application interface showing details about information stored in the geodatabase system.
We installed 49 yellow sticky (YS) traps (2 traps/ha) [23,24,25] with ammonium bicarbonate in a small bag (10 g) [26] (Figure 4).

Figure 4.
Yellow sticky traps (2 traps/ha) with ammonium bicarbonate in small bag (10 g).
The traps were numbered from T1+ to T49+. The distance between two traps was 100 m. The size of the yellow panel was 14 × 20 cm, and we used only one side. Traps were placed in the same way, at a 1.8 m height, in the southern part of the canopy. The traps were renewed weekly.
2.4. Meteorological Data Monitoring
Sensors were not applicable. The climatic data were obtained from the LARI weather station, installed 3 km from the site (Figure 5).

Figure 5.
LARI weather station in Hasbaya region.
The Hasbaya AWS of DIAM at LARI uses the Evapotranspiration iMETOS 3.3 by Pessl Instruments (https://metos.at/ accessed on 25 May 2022) and is located at latitude 33.4009732°, longitude 35.6772606°, and altitude 873 m (1 km from the site). It started recording data in mid-December 2013. The iMETOS 3.3 is powered by a rechargeable battery and a solar panel. It is versatile, with the possibility to configure and connect many different sensors—over 600 sensors can be connected through the intelligent sensor bus system. The data logger has a built-in UMTS/CDMA modem for direct communication (GPRS) with the Field Climate Web Platform. The system may store up to 8 MB of logged data. The data are regularly uploaded to the Field Climate Web Platform, with the possibility of a 15 min recording frequency. The reference evapotranspiration is a daily calculated parameter. The Hasbaya AWS is equipped with sensors to measure the following parameters: precipitation (mm), air temperature (°C) (dew point temperature is then estimated), relative humidity (%), wind speed (m/s), solar radiation (W/m2), wind direction (degrees), atmospheric pressure (kPa), reference evapotranspiration (ETo mm by day) (calculated through FAO Penman–Monteith equation). Thus, meteorological data were recorded during the whole experiment, at an interval of 15 min (24/7). The major parameters recorded were the temperature (average, maximum, minimum), the relative humidity (average, maximum, minimum), the wind speed, and the precipitation, which were recorded from the beginning of the experiment, on 22 July, until the end of the experiment, on 10 November.
2.5. Parameters Considered for Spraying
2.5.1. Pest Capture
Bactrocera olea was the pest target in this experiment. Counting of the adults, both males and females, was carried out on a weekly basis. We marked the females with a red circle and the males with a blue circle. The observations began on 29 July and were concluded on 10 November.
2.5.2. BBCH Value
The phenological stage events of the olive were observed and followed in the BBCH (Biologische Bundesanstalt, Bundessortenamt and CHemical industry) centesimal scale adopted by PHENAGRI [27,28,29]. Thus, we reported when we obtained a BBCH A = 75 (50% of its final size), B = 80–85% (fruit becoming light green or yellowish), or C > 85–89% (harvest maturity). The BBCH and the dimensions (W × L, size) of the olive fruits were observed and registered from 29 July to 10 November, with the examination of 6 fruits (total of 250 fruits) per zone trap until 15 September, followed by 12 fruits (total of 588 fruits) per zone trap (588 fruits in total) (Figure 6a).
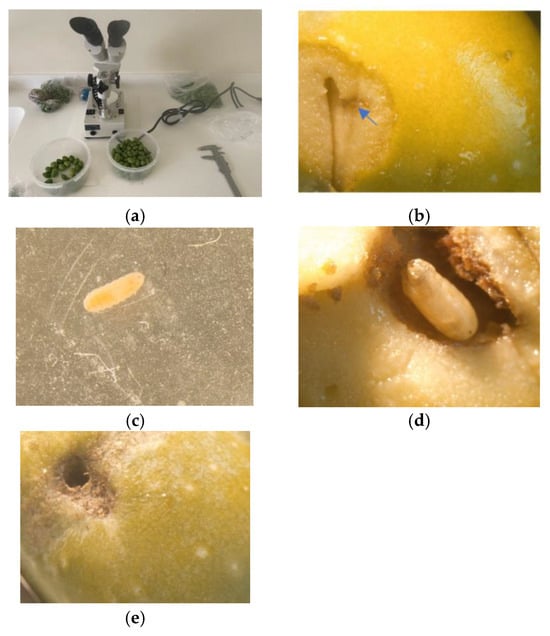
Figure 6.
Fruit observation and dimensions (W × L; size) and rate of fruit infestation. (a) Stereoscope and caliper; (b) egg at the arrow; (c) second-stage larva, L2; (d) pupa in the fruit; (e) empty hole.
2.5.3. Rate of Fruit Infestation, RFI
On a weekly basis and randomly (we crossed the field following the letter W to collect the fruits), we collected and examined 6 fruits per zone trap (we had 49 zone traps) up to September 22 (total of 250 fruits); after this date, we collected 12 fruits per zone trap (total of 588 fruits) to obtain more representative samplings, given that the rate of infestation would be increased, and the fruits were collected in three separate bags related to the three fruit load rates obtained and numbered according to the three zone traps: 1 = 50–75%, 2 = 25–50%, and 3 = 0–25%. The calculation of these rates was in relation to the scale of fruit production [26,30,31,32,33].
Each fruit was examined at the LARI Laboratory at Tel Amara and, if needed, under a stereoscope, to count the infested fruit. A fruit infestation was considered if there was the presence of (1) an egg cone or plenty of eggs, (2) larva at the first second or third stage, (3) pupa (empty or present), or (4) empty hole (Figure 6b–e, respectively).
2.6. Statistical Data Analysis
The mean dimensions and the BBCH of the fruits, referencing date and by trap zone, were treated statistically with the multiple mean comparison test based on ANOVA tests (Tukey tests, p < 0.05; data represented a normal distribution). On the other hand, the rates of fruit infestation, referencing date and by trap zone, were treated statistically, with Kruskal–Wallis tests (p < 0.05), because the data did not follow a normal distribution. Linear regression tests (Pearson correlation) were realized between the fly captures and the temperature and relative humidity registered, respectively. All these analyses were performed using SigmaStat plot software [34].
3. Results
3.1. Monitoring of the Adults
The means followed by high values of the standard deviation were observed during the whole period of the experiment, and they underlined the strong variation in the total number of adults (females marked by red circles and males by blue circles—Figure 7) from trap to trap per date (Figure 8, Figure 9 and Figure 10). This was related to the age of the trees, the variation in the fruit load rate, or the heterogeneous topography of the site, where the climatic conditions (T and RH) had to be registered close to each trap by sensors and not by the weather station, which was located far from the trees. It should be noted that the farmers began their fruit harvesting on 10 October, and it lasted one month.
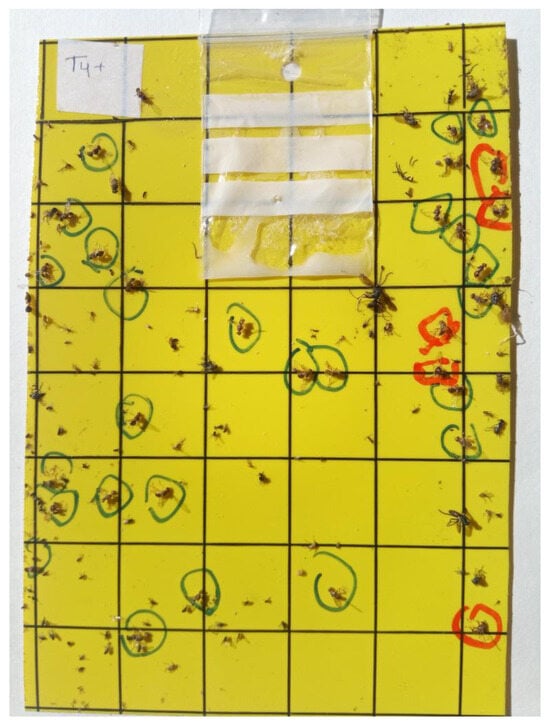
Figure 7.
Sample of yellow sticky trap with ammonium bicarbonate in plastic bag (10 g). Note: We marked the females with a red circle and the males with a blue circle.
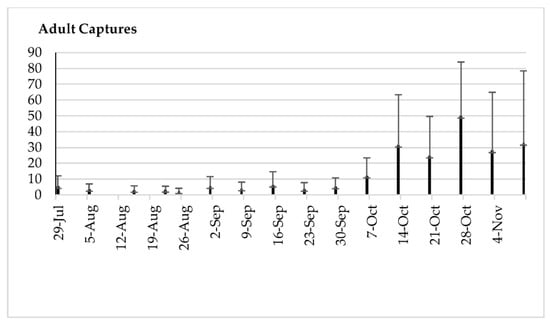
Figure 8.
Means and standard deviations of the adult captures by trap by date (49 yellow sticky traps with ammonium bicarbonate across 24.3 ha).
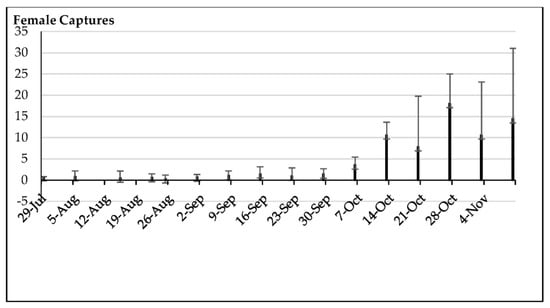
Figure 9.
Means and standard deviations of the female captures by trap by date (49 yellow sticky traps with ammonium bicarbonate across 24.3 ha).
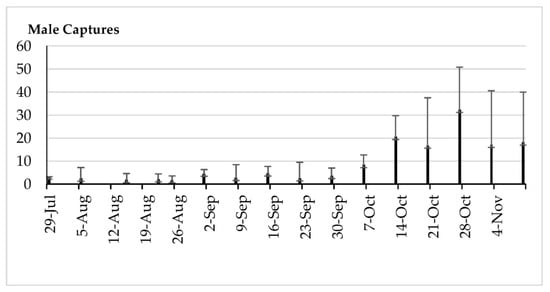
Figure 10.
Means and standard deviations of the male captures by trap by date (49 yellow sticky traps with ammonium bicarbonate across 24.3 ha).
3.2. Adult Abundance
The average number of adults remained low until 22 September, when it reached 4.19 adults/trap/week; then, it increased progressively from 7 October to 10 November, with averages of 12 and 18.4 adults/trap/week, respectively (Figure 8).
The captures were registered in a GIS system. The map samples extracted from the geodatabase system (Figure 11a–c) show the variations, by date, in the flies’ numbers in terms of adults, females, and males, respectively.
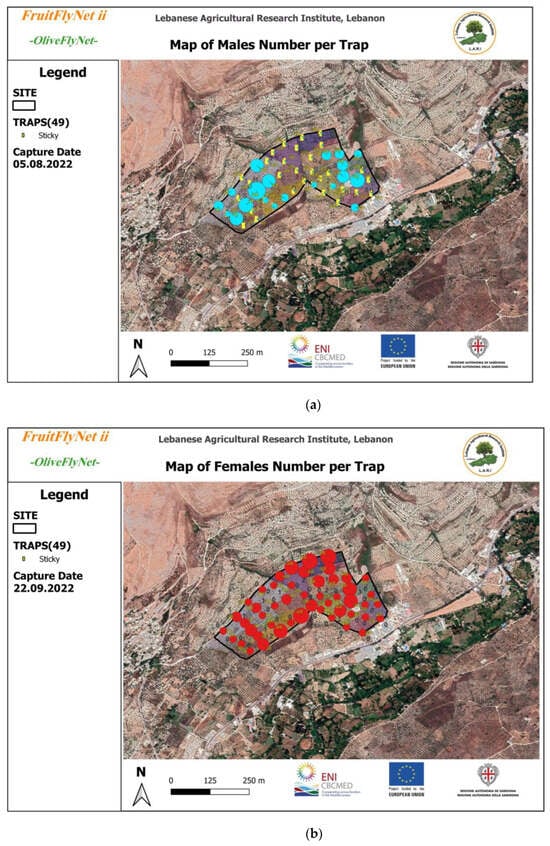
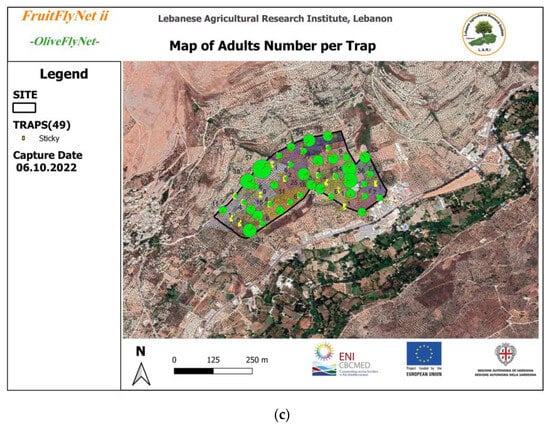
Figure 11.
Bactrocera oleae captures by trap by date in the development of a decision support system (49 yellow sticky traps with ammonium bicarbonate across 24 ha); (a) male captures on 5 August, marked by blue circles; (b) female captures on 22 September, marked by red circles; (c) adult captures on 6 October, marked by green circles. The size of the circles is related to the OFF density of adults, males, and females, respectively.
Thus, the interpolated maps of adult captures (Figure 12) registered on the traps weekly and extracted from the geodatabase also show the huge variation in the captures from trap to trap on the same date.
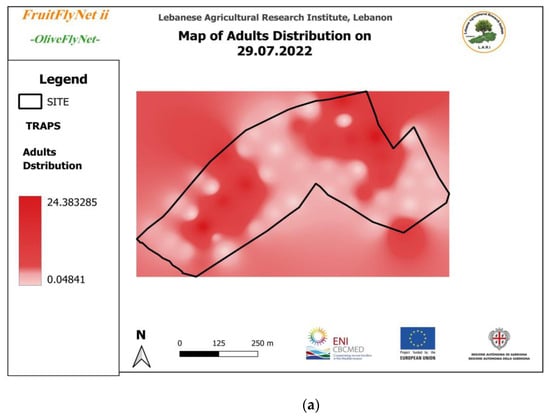
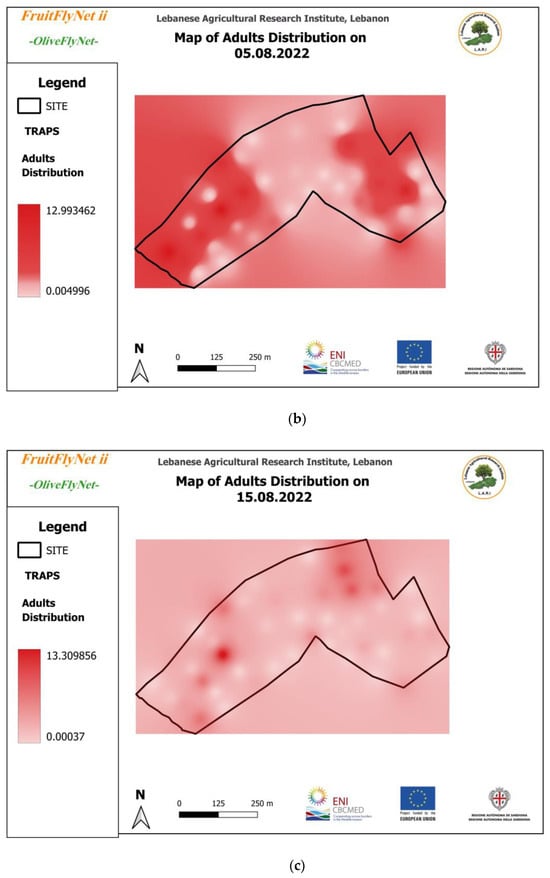
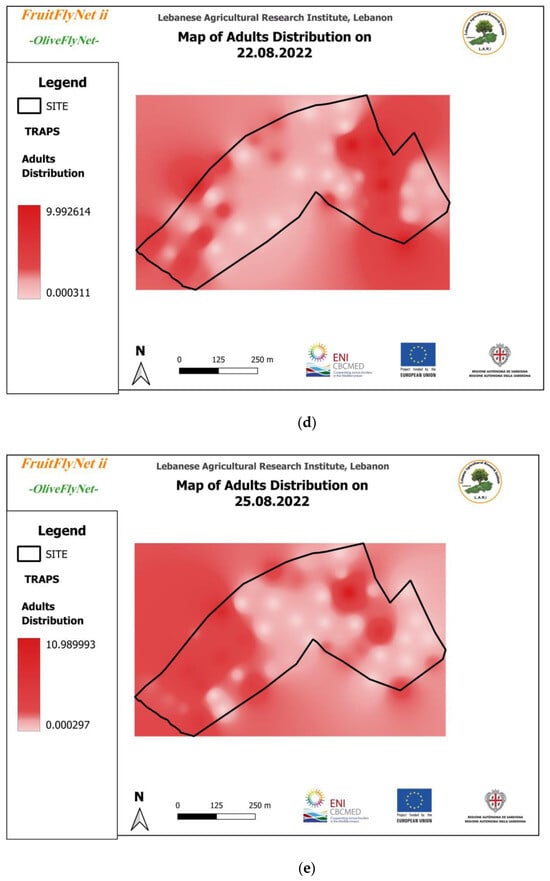
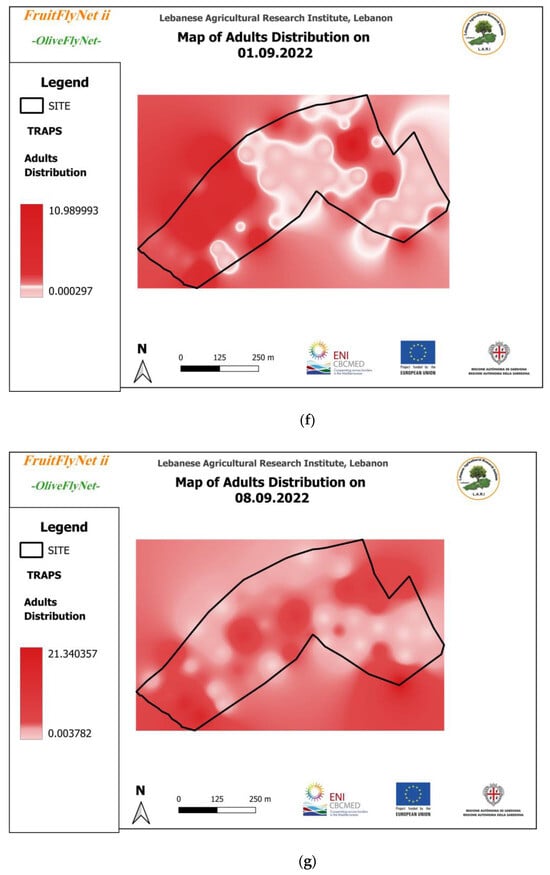
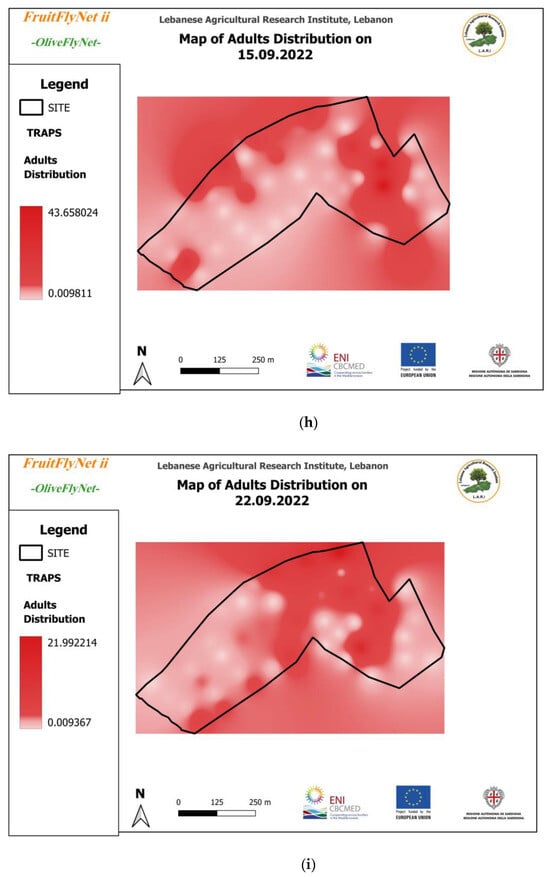
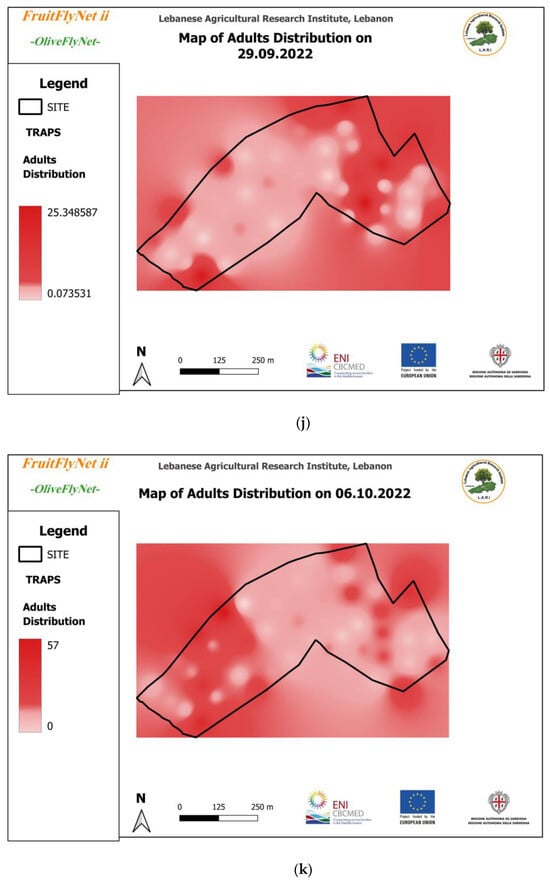
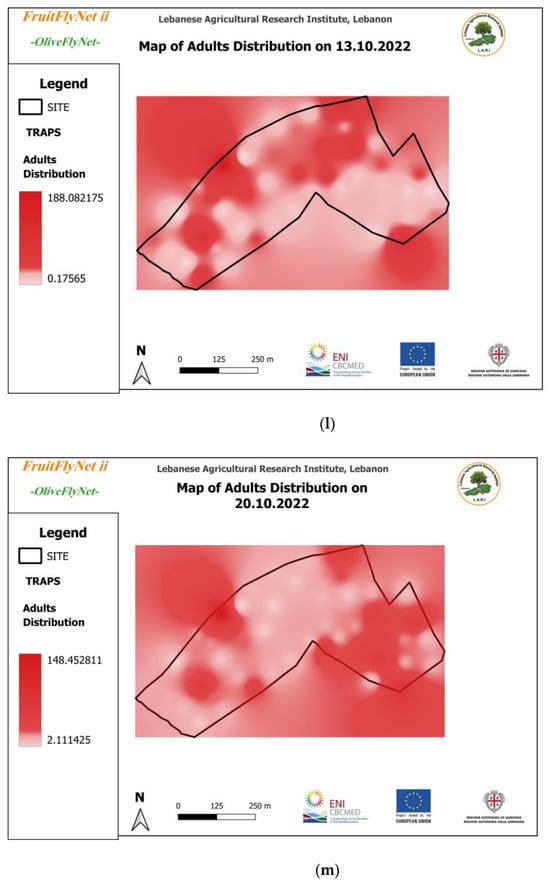
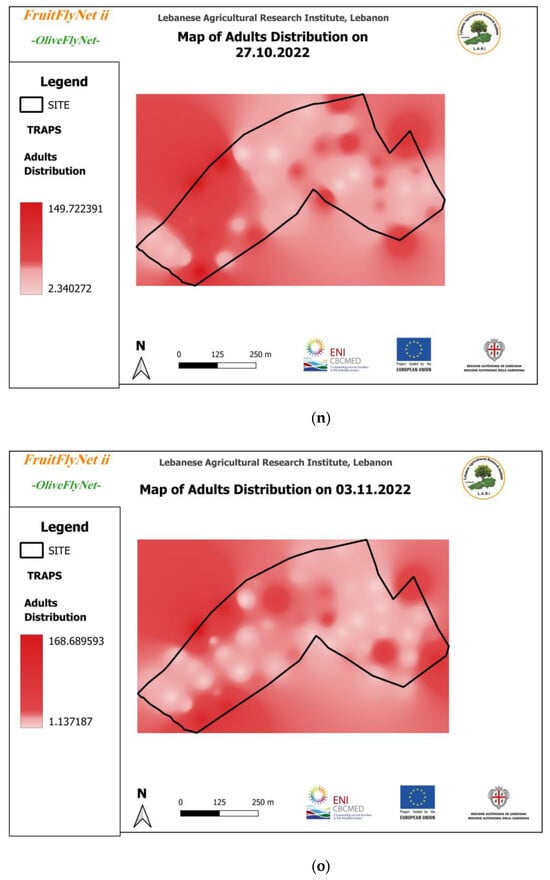
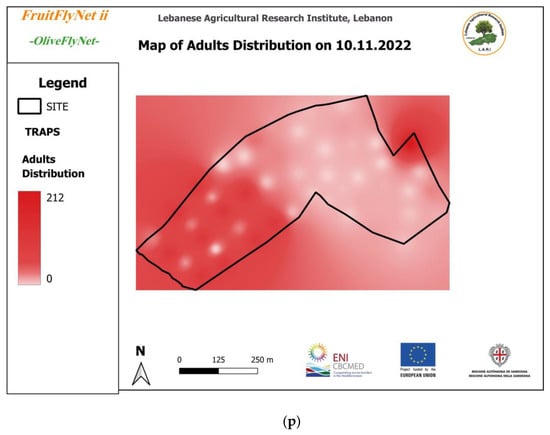
Figure 12.
Interpolated maps of B. oleae adult captures on yellow sticky traps with ammonium bicarbonate, from 29 July to 10 November 2022: (a) 27 July; (b) 5 August; (c) 15 August; (d) 22 August; (e) 25 August; (f) 01 September; (g) 08 September; (h) 15 September; (i) 22 September; (j) 29 September; (k) 6 October; (l) 13 October; (m) 20 October; (n) 27 October; (o) 3 November; (p) 10 November.
These large variations observed in the adult (female and male) captures were also reported in other Mediterranean regions and even in subhumid climates (Essaouira, Morocco; Mezghenna, Algeria and Egypt). In fact, it was observed that the olive fruit fly populations fluctuated over time, from trap to trap, and from one period to another [23,35,36,37,38].
3.3. Spatial and Temporal Distribution of Bactrocera oleae Adults
The temperature and relative humidity were recorded on a daily basis at the LARI weather station, installed at 873 m a.s.l. The minimum, average, and maximum values, for both the registered daily temperature and the relative humidity, were very close to each other, which did not necessarily reflect the real situation near the trap. The season was essentially dry, with almost no rain for the entire period. We noticed two critical periods in which the relative humidity was very low (30–40%). The first was between 15 and 31 August, with an average temperature between 27 and 30 °C, and the second was between 27 September and 2 October, with an average temperature between 23 and 29 °C (Figure 13). In El Fayoum, Egypt (dry region), it was indicated that the relative humidity had a positive effect on the number of olive fruit flies during the 2017 cultivation period when using McPhail traps and yellow traps [38], while many studies have shown the effect of the temperature on the number of flies captured [39,40].
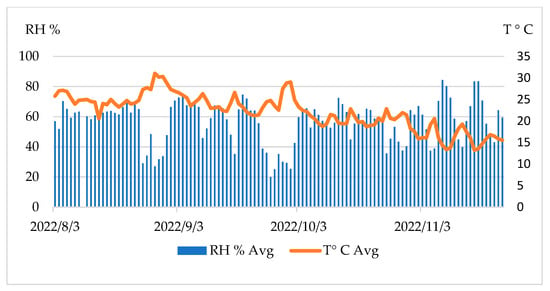
Figure 13.
Average temperature (°C) and relative humidity (%) on a daily basis.
Thus, the density of the fly captures increased from the first week of October. Thereafter, the temperature decreased progressively (from 23 to 13 °C on 10 November), while the relative humidity fluctuated between 40% and 80% (Figure 13). Our statistical analysis shows that the captures were negatively correlated with the temperature, with y = −3.36x + 88.96; r = 0.785 (Pearson correlation) (Figure 14).
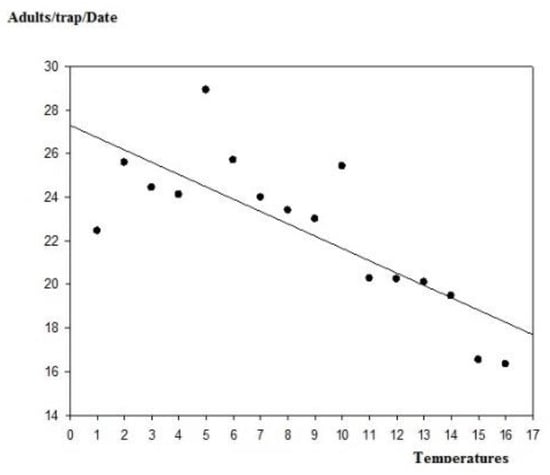
Figure 14.
Linear regression between temperatures registered and the adult captures.
3.4. Sex Ratio
Our observations show that the number of male captures was higher than that of female captures (Figure 15) throughout the whole experiment, with a sex ratio between 0.2:1 and 0.83:1 (F:M) at the end of the cultivation period (Table 1).
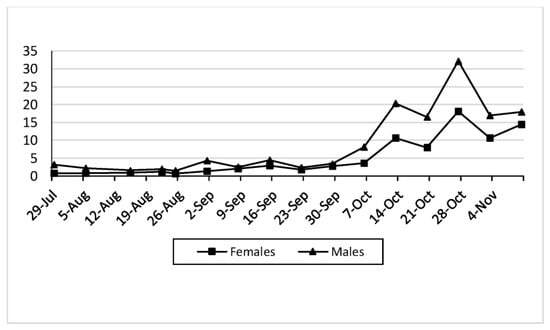
Figure 15.
Representation of the females and males in the whole area (24.3 ha).

Table 1.
Sex ratio of B. oleae during the experimental period (F:M).
However, some studies have reported a sex ratio close to 1:1 [11,41,42], while others [43,44] have observed a slightly higher number of males (52.2% males) than females.
3.5. BBCH and Fruit Dimensions
The olive fruits reached half their size at the beginning of August and were still green until the first week of September, which marked the beginning of the color change (BBCH: 80-81) (Figure 16).
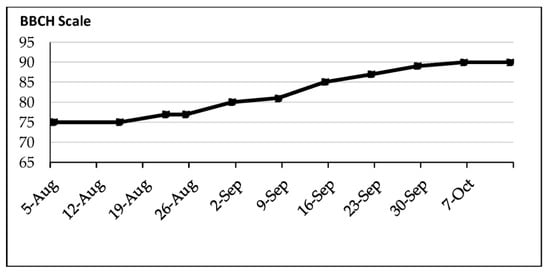
Figure 16.
BBCH scale indicating the phenology of the olive trees.
Progressively, from September 15th, some of the olive fruits started to display a purple color. It is important to note that even at the end of the experiment (10 November), the fruits presented four colors: oily green, green brown, purple, and black purple. The olive fruits declined in size during the drought period, from 22 August to the beginning of September; meanwhile, from the first week of September, they grew in size (Figure 17).
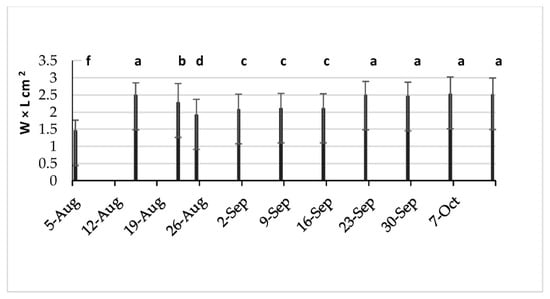
Figure 17.
Size of the olive fruits during the experiment. The histograms followed by different letters are statistically different.
The size gain of the olive fruits was related to the percentage of the fruit load rate (Table 2). Many studies have shown the effect of the fruit load rate, the age, and the variety on the fruit size [31,32,33].

Table 2.
The size of the olive fruits referencing fruit load rate. The numbers, by column, followed by different letters are statistically different.
3.6. Fruit Infestation
From 5 August to 15 September, the olive fruits showed only infertile punctures or “white punctures” (less than 0.1 mm). Thus, these fruits were maintained in the laboratory for one week, and we did not register any hatching larva, eggs, or any other symptoms. Eggs, larvae, pupae, and empty holes due to the OFF attacks started to appear from 22 September (Figure 18). Thus, our observations show that the rate of fruit infestation, RFI, remained equal to zero until 15 September. Then, the first symptoms were registered on 22 September, with RFI 0.4% and 1.5%, in the tree zones with 25–50% and 0–25% fruit load rates, respectively. The RFI remained below 2% until 3 November, when, in the tree zones with a 0–25% fruit load rate, it reached 2.27%. At the end of the experiment (10 November), the RFI reached 4.4% and 3.7% in the tree zones with 25–50% and 0–25% fruit load rates, respectively; the two RFI values registered on this date were biased by the decreased number of the olives due to the end of the fruit harvest. On the other hand, it is important to mention that OFF attacks were absent in the tree zones with a fruit load rate between 50 and 75%. A field evaluation of the susceptibility of mill and table olive varieties to the egg laying of olive flies was performed in Sicily and Spain and the results show that the percentages of fruit infestation increased as the olive fruits increased in diameter [45,46].
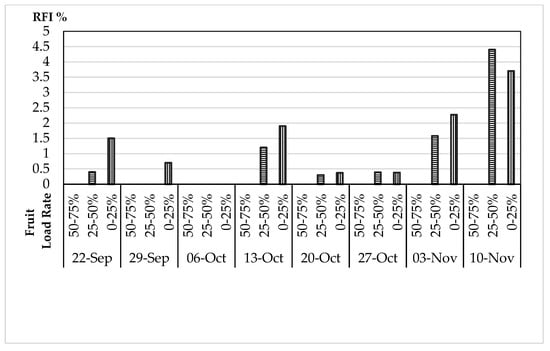
Figure 18.
The rate of fruit infestation RFI % observed during the experiment in relation to the fruit load rate registered (50–75%, 25–50%, or 0–25%).
4. Discussion
Trap captures constitute the major data source for olive fruit fly population analyses. The attractiveness of yellow sticky traps to olive fruit flies was significant in our study but varied over the cultivation period, with stronger attraction effects on the males than the females. Many studies have shown this type of variation, with different effects on the two sexes of wild populations [44,47,48,49,50,51]. The first symptoms were registered on 22 September, where the BBCH was close to that of the harvest period (85–87) and the mean number of adult captures was equal to 4.19 adults (1.84 and 2.35 female and male captures, respectively—Figure 15). In fact, the RFI was more pronounced in the tree zones with a 0–25% fruit load rate (RFI = 1.5%). It should be mentioned that the tolerable fruit damage threshold for table fruits is 2%, and it is 10% for olive oil production in Europe [52]. The increasing number of adult captures observed from the first week of October could be related to the decrease in the temperature values (below 25 °C). The relative humidity showed severe fluctuations in values (from almost 40% to 65% and more) every two weeks. Many studies have described the preference of the olive fruit fly for high humidity, above 50% [39,40,53,54,55], and a moderate temperature below 25°C impacts the number of flies [23,35,36,37,39,40]. The rate of infested fruits observed was significantly higher in the tree zones with a 0–25% fruit load rate than in the other tree zones.
These results highlight the importance of the fruit load rate in relation to the OFF attack. Thus, the number of infested fruits was correlated equally with the fruit load rate and the size of the fruit, as well as with the number of adults captured. Our observations allow us to introduce an algorithm model for bait spraying interventions when we observe the first symptoms, taking into consideration a BBCH threshold equal to at least 85 and a fruit load threshold of more than 25%, with an average number of adult captures of more than one adult per day by trap.
5. Conclusions
The results obtained with the yellow sticky traps, the georeferencing of the olive groves, and the compiled parameters discussed in the article (temperature, relative humidity, tree phenology, and fruit load rate) can be used as input to a geodatabase, which is required to implement a decision support system (DSS) to aid the management of Bactrocera oleae in Lebanon, with the aim of reducing the frequency and volume of insecticide used.
Author Contributions
L.K. and A.E.: conceived and designed the experiment; S.E.R.: conceived the geodatabase; L.K., A.C., E.C., A.Y., G.A., I.J. and A.E.: performed field work; L.K.: analyzed data; L.K.: wrote the paper; M.A., A.C., E.C., A.Y., G.A. and A.E.: revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the EU through the Project FruitFlyNet-ii: STR_B_A.2.1_0043/2020-2023 as part of the ENI CBC Mediterranean Sea Basin Programme 2014–2020. We are grateful to the project for helping us to form, in a concrete way, the DSS for the olive case in Lebanon.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board and then accepted in Appl. Sci. (ISSN 2076-3417) on 10 October 2023.
Informed Consent Statement
Not Applicable.
Data Availability Statement
https://www.mdpi.com/journal/applsci/special_issues/B200I4RKA3 (accessed on 27 July 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-hajj, A.K.; Nemer, N.; Chhadeh, S.; Dandashi, F.; Yosef, H.; Nasrallah, M.; Houssein, M.; Haris, M.; Moussa, Z. Status, Distribution and Parasitism Rate of Olive Fruit Fly (Bactrocera oleae. Rossi) Natural Enemies in Lebanon. J. Agric. Stud. 2017, 6, 246–260. [Google Scholar] [CrossRef][Green Version]
- Nardi, F.; Carapelli, A.; Dallai, R.; Roderick, G.K.; Frati, F. Population structure and colonization history of the olive fly, Bactrocera oleae (Diptera, Tephritidae). Mol. Ecol. 2005, 14, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Ponti, L.; Cossu, Q.A.; Gutierrez, A.P. Climate warming effects on the Olea europaea—Bactrocera oleae system in Mediterranean islands: Sardinia as an example. Glob. Chang. Biol. 2009, 15, 2874–2884. [Google Scholar] [CrossRef]
- Zygouridis, N.E.; Augustinos, A.A.; Zalom, F.G.; Mathiopoulos, K.D. Analysis of olive fly invasion in California based on mi-crosatellite markers. Heredity 2009, 102, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Marchini, D.; Petacchi, R.; Marchi, S. Bactrocera oleae reproductive biology: New evidence on win-tering wild populations in olive groves of Tuscany (Italy). Bull. Insectology 2017, 70, 121–128. [Google Scholar]
- Zalom, F.G.; Van Steenwyk, R.A.; Burrack, H.J. Olive Fruit Fly; Pest Notes; University of California: Riverside, CA, USA, 2009; Volume 74112, 4p. [Google Scholar]
- Caruso, G.; Loni, A.; Raspi, A.; Gucci, R.; Bagnoli, B. Olive fruit fly effects on free acidity and peroxides value of olive oil. In VII International Symposium on Olive Growing; Serman, F.V., Searles, P., Torres, M., Eds.; 2014; Volume 1057, pp. 281–286. ISBN 978-94-62610-47-7. [Google Scholar] [CrossRef]
- Manousis, T.; Moore, N.F. Control of Dacus oleae, a major pest of olives. Int. J. Trop. Insect Sci. 1987, 8, 1–9. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Pantazi-Mazomenou, A.; Stefanou, D. Attract and kill of the olive fruit fly Bactrocera oleae in Greece as a part of an integrated control system. IOBC/WPRS Bull. 2002, 25, 137–146. [Google Scholar]
- Montiel Bueno, A.; Jones, O. Alternative methods for controlling the olive fly, Bactrocera oleae, involving semiochemicals. Bull. OILB/SROP 2002, 25, 147–155. [Google Scholar]
- Neuenschwander, P.; Michelakis, S. Determination of the lower thermal thresholds and day-degree requirements for eggs and larvae of Dacus oleae (Gmel.) (Diptera: Tephritidae) under field conditions in Crete, Greece. Bull. Soc. Entomol. Suisse 1979, 52, 57–74. [Google Scholar]
- Raspi, A.; Canale, A.; Loni, A. Presence of mature eggs in olive fruit fly, Bactrocera oleae (Diptera Tephritidae), at different constant photoperiods and at two temperatures. Bull. Insectology 2005, 58, 125. [Google Scholar]
- Broufas, G.D.; Pappas, M.L.; Koveos, D.S. Effect of relative humidity on longevity, ovarian maturation, and egg production in the olive fruit fly (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2009, 102, 70–75. [Google Scholar] [CrossRef]
- Rizzo, R.; Caleca, V. Resistance to the attack of Bactrocera oleae (Gmelin) of some Sicilian olive cultivars. In Proceedings of the Olivebioteq 2006, Second International Seminar “Biotechnology and Quality of Olive Tree Products around the Mediterranean Basin”, Mazara del Vallo, Italy, 5–10 November 2006; Volume 2, pp. 291–298. [Google Scholar]
- Mraicha, F.; Ksantini, M.; Zouch, O.; Ayadi, M.; Sayadi, S.; Bouaziz, M. Effect of olive fruit fly infestation on the quality of olive oil from Chemlali cultivar during ripening. Food Chem. Toxicol. 2010, 48, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Kapaun, T.; Nadel, H.; Headrick, D.; Vredevoe, L. Biology and parasitism rates of Pteromalus nr. myopitae (Hymenoptera: Pteromalidae), a newly discovered parasitoid of olive fruit fly Bactrocera oleae (Diptera: Tephritidae) in coastal California. Biol. Control 2010, 53, 76–85. [Google Scholar] [CrossRef][Green Version]
- Cardenas, M.; Ruano, F.; Garcia, P.; Pascual, F.; Campos, M. Impact of agricultural management on spider populations in the canopy of olive trees. Biol. Control 2006, 38, 188–195. [Google Scholar] [CrossRef]
- Nobre, T.; Gomes, L.; Rei, F.T. A Re-Evaluation of Olive Fruit Fly Organophosphate-Resistant Ace Alleles in Iberia, and Field Testing Population Effects after in-Practice Dimethoate Use. Insects 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Petacchi, R.; Rizzi, I.; Guidotti, D. The ‘lure and kill’ technique in Bactrocera oleae (Gmel.) control: Effectiveness indices and suitability of the technique in area-wide experimental trials. Int. J. Pest Manag. 2003, 49, 305–311. [Google Scholar] [CrossRef]
- Iannotta, N.; Belfiore, T.; Noce, M.E.; Scalercio, S.; Vizzarri, V. Comparison between Attract and Kill Devices for Bactrocera oleae in an Organic Olive Orchard: Preliminary Data. ISHS Acta Hortic. 2010, 873, 89–94. [Google Scholar] [CrossRef]
- Hamdan, A.J.; Al-Qurna, M. The Seasonal Flight Activity of the Olive Fruit Fly Bactrocera oleae (Gmelin) (Diptera: Tephritidae) in the Central Highlands of West-Bank, Palestine. J. Agric. Sci. 2017, 13, 457–464. [Google Scholar]
- Rizzo, R.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Fofie, N.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four essential oils in protein baits on Bactrocera oleae. Ind. Crop. Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Ordano, M.; Engelhard, I.; Rempoulakis, P.; Nemny-Lavy, E.; Blum, M.; Yasin, S.; Lensky, I.; Papadopoulos, N.; Nestel, D. Olive Fruit Fly (Bactrocera oleae) Population Dynamics in the Eastern Mediterranean: Influence of Exogenous Uncertainty on a Monophagous Frugivorous Insect. PLoS ONE 2015, 10, e0127798. [Google Scholar] [CrossRef]
- FAO/IAEA. Trapping Guidelines for Area-Wide Fruit Fly Programmes, 2nd ed.; Enkerlin, W.R., Reyes-Flores, J., Eds.; Licence: CC BY-NC-SA 3.0 IGO; FAO/IAEA: Rome, Italy, 2018; 65p. [Google Scholar]
- UC IPM Pest Management Guidelines: Olive UC ANR Publication 3452. Agriculture: Olive Pest Management Guidelines. 2014; Olive Fruit Fly. Bactrocera oleae.
- Miranda, M.Á.; Barceló, C.; Valdés, F.; Feliu, J.F.; Nestel, D.; Papadopoulos, N.; Sciarretta, A.; Ruiz, M.; Alorda, B. Developing and Implementation of Decision Support System (DSS) for the Control of Olive Fruit Fly, Bactrocera Oleae, in Mediterranean Olive Orchards. Agronomy 2019, 9, 620. [Google Scholar] [CrossRef]
- Meier, U. BBCH-Monograph. Growth Stages of Plants—Entwicklungsstadien von Pflanzen—Estadios de las Plantas—Développement des Plantes; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997; pp. 94–95. [Google Scholar]
- Sanz-Cortes, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Di Paola, A.; Chiriacò, M.V.; Di Paola, F.; Nieddu, G. A Phenological Model for Olive (Olea europaea L. var europaea) Growing in Italy. Plants 2021, 10, 1115. [Google Scholar] [CrossRef]
- Varikou, K.; Garantonakis, N.; Birouraki, A. Response of olive fruit fly Bactrocera oleae to various attractant combinations, in orchards of Crete. Bull. Insectology 2014, 67, 109–114. [Google Scholar]
- Lavee, S. Alternate bearing in olive initiated by abiotic induction leading to biotic responses. Adv. Hort. Sci. 2015, 29, 213–219. [Google Scholar]
- Beyá-Marshall, V.; Fichet, T. Effect of crop load on the phenological, vegetative and reproductive behavior of the ‘Frantoio’ olive tree (Olea europaea L.). Cienc. Investig. Agrar. 2017, 44, 43–53. [Google Scholar] [CrossRef]
- Wechsler, T.; Bakhshian, O.; Engelen, C.; Dag, A.; Ben-Ari, G.; Samach, A. Determining Reproductive Pa-rameters, which Contribute to Variation in Yield of Olive Trees from Different Cultivars, Irrigation Regimes, Age and Location. Plants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- SigmaPlot 12.3, SigmaPlot Graphing Software from Systat Software. Systat Software: San Jose, CA, USA, 2023.
- Helvaci, M.; Aktaş, M.; Özden, Ö. Occurrence, damage, and population dynamics of the olive fruit fly (Bactrocera oleae Gmelin) in the Turkish Republic of Northern Cyprus. Turk. J. Agric. For. 2018, 42, 453–458. [Google Scholar] [CrossRef]
- Mansour, A.; Kahime, K.; Chemseddine, M.; Boumezzough, A. Study of the Population Dynamics of the Olive Fly, Bac-trocera oleae Rossi. (Diptera, Tephritidae) in the Region of Essaouira. Open J. Ecol. 2015, 5, 174–186. [Google Scholar] [CrossRef]
- Achouche, A.; Abbassi, F.; Benzahra, A.; Djazouli, Z. Study of population dynamics of the olive fruit fly Bactrocera oleae (Diptera, Tephritidae); (rossi, 1790) in the Mezghenna area. Ukr. J. Ecol. 2019, 9, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, A.M.E.; Salem, S.A.; El-Kholy, M.Y.; Abdel-Rahman, R.S.; Abdel-Raheem, M.A. Role of the olive fly, Bac-trocera oleae (rossi) traps in integrated pest management on olive trees under climatic change conditions in Egypt. Plant Arch. 2019, 19 (Suppl. 2), 457–461. [Google Scholar]
- Gonçalves, F.M.; Rodrigues, M.C.; Pereira, J.A.; Thistlewood, H.; Torres, L.M. Natural mortality of immature stages of Bactrocera oleae (Diptera: Tephritidae) in traditional olive groves from north-eastern Portugal. Biocontrol Sci. Technol. 2012, 22, 837–854. [Google Scholar] [CrossRef]
- Marchi, S.; Guidotti, D.; Ricciolini, M.; Petacchi, R. Towards understanding temporal and spatial dynamics of Bactrocera oleae (Rossi) infestations using decade-long agrometeorological time series. Int. J. Biometeorol. 2016, 60, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.; Koukidou, M.; Rempoulakis, P.; Gong, H.-F.; Economopoulos, A.; Vontas, J.; Alphey, L. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Speranza, S.; Bellocchi, G.; Pucci, C. IPM trials on attract-and-kill mixtures against the olive fly Bactrocera oleae (Diptera Tephritidae). Bull. Insectology 2004, 57, 111–115. [Google Scholar]
- Zervas, G. Reproductive Physiology of Dacus oleae (GMELIN) Diptera Tephritidae. Comparison of Wild and Laboratory Reared Flies. Geoponika. 1982. Available online: https://agris.fao.org (accessed on 25 July 2023).
- Katsoyannos, B.I.; Kouloussis, N.A. Captures of the olive fruit fly Bactrocera oleae on spheres of different colours. Entomol. Exp. Appl. 2001, 100, 165–172. [Google Scholar] [CrossRef]
- Rizzo, R.; Virgilio, C.; Alberto, L. Relation of fruit color, elongation, hardness, and volume to the infestation of olive cultivars by the olive fruit fly, Bactrocera oleae. Entomol. Exp. Appl. 2012, 145, 15–22. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Santiago-Álvarez, C.; Cubero-González, S.; Casado-Mármol, G.; Ariza-Fernández, A.; Yousef, M. Field evaluation of the susceptibility of mill and table olive varieties to egg-laying of olive fly. J. Appl. Entomol. 2018, 142, 765–774. [Google Scholar] [CrossRef]
- Zouros, E.; Krimbas, C.B. Frequency of female digamy in a natural population of the olive fruit fly Dacus oleae as found using enzyme polymorphism. Entomol. Exp. Appl. 1970, 13, 1–9. [Google Scholar] [CrossRef]
- Preu, M.; Frieß, J.L.; Breckling, B.; Schröder, W. Case Study 1: Olive Fruit Fly (Bactrocera oleae). In Gene Drives at Tipping Points. Precautionary Technology Assessement and Governance of New Approaches to Genetically Modify Animal and Plant Populations; Von Gleich, A., Schroder, W., Eds.; Springer: Cham, Switzerland, 2019; pp. 79–102. [Google Scholar] [CrossRef]
- Dominici, M.; Pucci, C.; Montanan, G.E. Dacus oleae (Gmel.) ovipositing in olive drupes (Diptera, Tephrytidae). J. Appl. Entomol. 1986, 101, 111–120. [Google Scholar] [CrossRef]
- Gümusay, B.; Ozilbey, U.; Ertem, G.; Oktar, V. Studies on the susceptibility of some important table and oil olive cultivars of Aegean Region to olive fly (Dacus oleae Gmel.) in Turkey. Acta Hortic. 1990, 286, 359–361. [Google Scholar] [CrossRef]
- Neuenschwander, P.; Michelakis, S.; Holloway, P.; Berchtol, W. Factors affecting the susceptibility of fruits of different olive varieties to attack by Dacus oleae (Gmel.) (Dipt., Tephritidae). J. Appl. Entomol. 2009, 100, 174–188. [Google Scholar] [CrossRef]
- Kicenik Devarenne, A.; Vossen, P.M. Monitoring and organic control of olive fruit fly. In Organic Olive Production Manual; Vossen, P.M., Ed.; UC ANR Pub 3505: Oakland, CA, USA, 2007; pp. 47–51. [Google Scholar]
- Fletcher, B.S.; Economopoulos, A.P. Dispersal of normal and irradiated laboratory strains and wild strains of the olive fly Dacus oleae in an olive grove. Entomol. Exp. Appl. 1976, 20, 183–194. [Google Scholar] [CrossRef]
- Broumas, T.; Haniotakis, G.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The efficacy of an improved form of the mass-trapping method, for the control of the olive fruit fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-scale feasibility studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Bueno, A.M.; Jones, O.T. Alternative methods for controlling the olive fly Bactrocera oleae involving semiochemicals. IOBC/WPRS Bull. 2002, 25, 1–11. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).