Abstract
With the increase in bone metabolic diseases owing to the aging of the global population, interest in functional food ingredients for improving bone health is increasing. This study aimed to determine the anti-osteoporosis effect of Benincasa hispida extract (BHE, HR1901-W) and 2-furoic acid in ovariectomy (OVX)-induced osteoporosis in female ICR mice. Thirty-five female ICR mice underwent OVX or sham operation and were randomized into seven groups of five animals as follows: normal, sham, OVX, OVX with genistein (10 mg/kg), 2-furoic acid (20 mg/kg), LBHE (100 mg/kg), and HBHE (200 mg/kg). After an 8-week treatment period, femur and blood samples were collected from mice. Bone density and bone formation markers were significantly recovered in the 2-furoic acid and HBHE supplementation groups compared with those in the OVX group. In addition, bone resorption markers were increased in OVX mice, whereas they were significantly decreased in the OVX + 2-furoic acid and HBHE supplementation groups. This study suggests that BHE supplementation prevents bone resorption and promotes bone formation in OVX mice. These findings indicate that BHE could be used as a promising natural means to prevent OVX-induced osteoporosis and bone metabolic diseases.
1. Introduction
The rapid aging of the global population is expected to cause many social problems, such as health, medical care, and welfare issues, to become visible in the future [1]. From a medical point of view, an increase in the elderly population indicates an increase in geriatric diseases; however, the current social system is not ready for an aging population. As aging progresses, susceptibility to diseases such as muscle dysfunction, vascular aging disorders, diabetes, macular degeneration, Alzheimer’s disease, and osteoporosis increases [2]. In particular, osteoporotic fractures have a serious impact on the patient’s family members and society, as well as causing social and economic losses due to the patient’s movement discomfort after injury. In addition, various fractures caused by osteoporosis can lead to decreased physical activity, muscle weakness, obesity, and metabolic diseases [3].
Bone is a hard tissue calcified with calcium and phosphorus, which plays an important role in supporting and protecting the body. Bones protect vital organs in the body, provide a place for muscles to attach and the bone marrow to make blood, serve as leverage, and store minerals essential for body function [4]. Osteoporosis refers to a condition in which bones that are weakened owing to a decrease in bone mass are prone to fracture even with a light impact [5]. Osteoporosis is mostly insidious and asymptomatic until there are external changes in the body, which are usually not recognized [6]. Osteoporotic fractures appear in various ways in the spine, femur, humerus, and wrist joints [7]. Osteoporosis is a metabolic disease caused by an increase in bone turnover due to an imbalance between bone resorption and bone formation. It is caused by decreased secretion of estrogen, lack of activity, and insufficient calcium intake [8,9,10,11]. In particular, postmenopausal osteoporosis in women is known to be caused by increased bone loss due to estrogen deficiency [12,13]. Estrogen replacement therapy and bone resorption inhibitors, such as calcitonin and bisphosphonates, are commonly used to treat postmenopausal osteoporosis [14]. However, hormone replacement therapy has been reported to require long-term treatment and cause side effects, including coronary artery disease, stroke, thromboembolism, cholecystitis, breast cancer, and endometrial cancer [15,16,17]. Therefore, as society ages, there is a growing demand for disease prevention and the development of health-promoting functional foods using natural products with fewer side effects.
Benincasa hispida (Thunb.) (syn. Benincasa cerifera Savi) is an annual vine belonging to the Cucurbitaceae family, which is grown in Asian countries, such as India, Japan, China, Malaysia, and in the mid-dry regions of the tropical lowlands [18]. B. hispida is a well-known vegetable crop that is generally recognized for its nutritional and medicinal properties, particularly in Asian countries [19,20]. Oval-shaped copper fruit usually weighs less than 10 kg, and fruits and seeds, excluding the skin, are mentioned in the list of food ingredients currently provided in Korea [21]. Oriental medicine has been reported to be effective in improving constipation and cholesterol levels and has anti-obesity effects [22,23]. In addition, B. hispida contains various nutrients, such as volatile oils, flavonoids, phenolic compounds, vegetable proteins, essential minerals, and vitamins. In addition, anti-obesity, anti-inflammatory, anti-oxidant, anti-ulcer, anti-depressant, anti-diarrheal, and anti-pyretic effects have been reported, suggesting that B. hispida is highly useful as a functional food material using natural products [24,25].
Standardization, to control the quality of products and functional raw materials, is an important part of the development of functional foods [26]. Standardization refers to a manufacturing process that can produce consistent quality throughout the manufacturing process (from raw materials to final products), and a standardization method through the analysis of indicator components is commonly used [27]. An indicator component refers to a component determined for quality control among the chemically identified components in the raw materials [28]. In this study, 2-furoic acid was used as an indicator component, and a sample standardized with the concentration of 2-furoic acid in the B. hispida extracts (BHE, HR1901-W) in the range of 0.31 to 0.47 mg/g was used. 2-furoic acid has been reported to be effective in reducing blood sugar, cholesterol, and body fat [29,30,31]. 2-furoic acid has a structure in which a carboxylic acid group is bonded to a five-membered aromatic (2-furyl) group, and the HPLC-PDA analysis method was established in our previous study [32]. In addition, it was confirmed that BHE and 2-furoic acid promote bone formation through upregulation of osteoblast differentiation and downregulation of osteoclast differentiation in MC3T3-E1 cells and RAW 264.7 [33]. In this study, we evaluated the efficacy of standardized BHE and 2-furoic acid in improving bone health in an ovariectomy-induced osteoporosis mouse model.
2. Materials and Methods
2.1. Chemicals and Sample Preparation
Genistein, 2-furoic acid, sodium carboxymethyl cellulose (CMC-Na), and Tween 80 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kits for determination of estradiol, osteoprotegerin (OPG), receptor activator of nuclear factor kappa-Β ligand (RANKL), and deoxypyridinoline (DPD) were purchased from LSBio Inc. (cat# F5297, cat# F23842, cat#F297, cat#F25774, respectively; Seattle, WA, USA). Bone-specific alkaline phosphatase (BALP) was obtained from MyBioSource, Inc. (cat# MBS703336, San Diego, CA, USA).
Benincasa hispida fruit was harvested in September 2021 from Goesan, Chungcheongbuk-do, Korea and identified by Dr. Ju-Hyun Cho at Haram Co. Ltd. (Cheongju, Korea). The harvested B. hispida fruits were extracted in distilled water 10 times the weight of the fruit for 4 h at 100 °C and filtered through a test sieve (45 μm). To obtain B. hispida extract samples, the extracts were concentrated to 10–13 Brix and freeze-dried (certificates of analysis in Supplementary File S1).
2.2. Animal and Experimental Design
To determine the effects of BHE on osteoporosis, five-week-old female ICR mice were provided by Central Lab Animal Inc. (Seoul, Korea); these were maintained under controlled conditions at a temperature of 23 ± 2 °C on a 12/12 h light/dark cycle with free access to food and water (2018S Rodent Diet, Minneapolis, IN, USA) for 1 week. Mice (n = 35) were divided into the following seven groups (n = 5/group), and five groups were subjected to ovariectomy (OVX), whereas one group underwent bilateral surgery (sham): group 1, ICR normal control with vehicle (NC, n = 5); group 2, sham with vehicle (n = 5); group 3, OVX with vehicle (n = 5); group 4, OVX + genistein (10 mg/kg); group 5, OVX + 2-furoic acid (20 mg/kg); group 6, OVX + low concentration of BHE (LBHE, 100 mg/kg); and group 7, OVX + high concentration of BHE (HBHE, 200 mg/kg). For oral administration, all samples were suspended in vehicle (10% (v/v) Tween 80 with 0.2% CMC), and the mice were weighed at 17:00 p.m. every 3 days. Food consumption was measured as the difference between the amount of food served and the remaining amount. The food efficiency ratio (FER) was calculated using the formula: weight gain (g)/food consumed (g). After 8 weeks of administration and 12 h of fasting, femur and blood samples were collected from mice anesthetized with isoflurane. This study was approved by the Institutional Animal Care and Use Committee of Kangwon National University (approval number: KW-210802-2).
2.3. Assessment of Bone Mineral Density
The femurs of the mice were dissected to remove soft tissue and weighed. After fixing with 4% (v/v) paraformaldehyde solution for 24 h, femoral bone mineral content (BMC) and bone mineral density (BMD) were measured using a bone densitometer (PIXImusTM, GE Healthcare, Little Chalfont, UK).
2.4. Histological Assessment of Osteoclast
Fixed femurs were demineralized using ChelatorCalTM (BBC Biochemical, McKinney, TX, USA) for 14 days. After demineralization, paraffin embedding was performed, and the embedded tissue was sectioned to a thickness of 10 μm. For histological examination, the sections were stained with hematoxylin and eosin (H&E) after deparaffinization and dehydration, respectively. All tissues were photographed at 400× magnification using an optical microscope (Nikon, Melville, NY, USA). The osteoclasts were determined based on their morphological characteristics, such as multinucleated cells with eosinophilic cytoplasm near the bone tissues being present along the absorption zone of each long bone. Osteoclasts located at the end of the shaft of the femur were counted, and the average value of each individual present in one femur was calculated.
2.5. Assessment of Serum Biochemical Parameter
Serum was obtained from the blood samples using a microcontainer with a serum separation gel and clot activator (Becton Dickinson, NJ, USA). Collected blood samples were clotted at 24 °C for 30 min and centrifuged at 3000× g for 10 min at 4 °C. The levels of serum estradiol, OPG, RANKL, DPD, and BALP were determined using an ELISA kit in accordance with the manufacturer’s instructions.
2.6. Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM) and were statistically analyzed by analysis of variance and Dunnett’s post hoc test. Statistical package SPSS (version 24.0; IBM Corp., Armonk, NY, USA) was used for all statistical comparisons, and p-value of < 0.05 was considered to indicate statistical significance. Statistical analysis was performed between the sham group and the OVX group (* p < 0.05, ** p < 0.05, and *** p < 0.001 vs. sham group), and between the OVX group and the OVX + sample treatment group (#p < 0.05, ## p < 0.05, and ### p < 0.001 vs. OVX group).
3. Results
3.1. Body Weight and Food Intake
To determine the anti-osteoporosis effect of BHE, mice were subjected to ovariectomy and administered with LBHE (100 mg/kg/day, OVX + LBHE), HBHE (200 mg/kg/day, OVX + HBHE), 2-furoic acid (20 mg/kg/day, OVX + 2-FA), or genistein (10 mg/kg/day, OVX + GEN) for 8 weeks. We compared the anti-osteoporosis effects of BHE and 2-furoic acid with that of genistein as a positive control. Genistein has been reported to provide superior bone protection against osteoporosis [34,35]. In addition, soybean isoflavones containing genistein have been designated as functional ingredients for improving bone health in Korea. As shown in Table 1, there were no significant differences between all groups in initial and final body weight, weight gain, and FER.

Table 1.
Body weight and food intake of mice treated with standardized Benincasa hispida extracts (BHE, HR1901-W).
3.2. Effect of BHE and 2-Furoic Acid on Bone Morphology
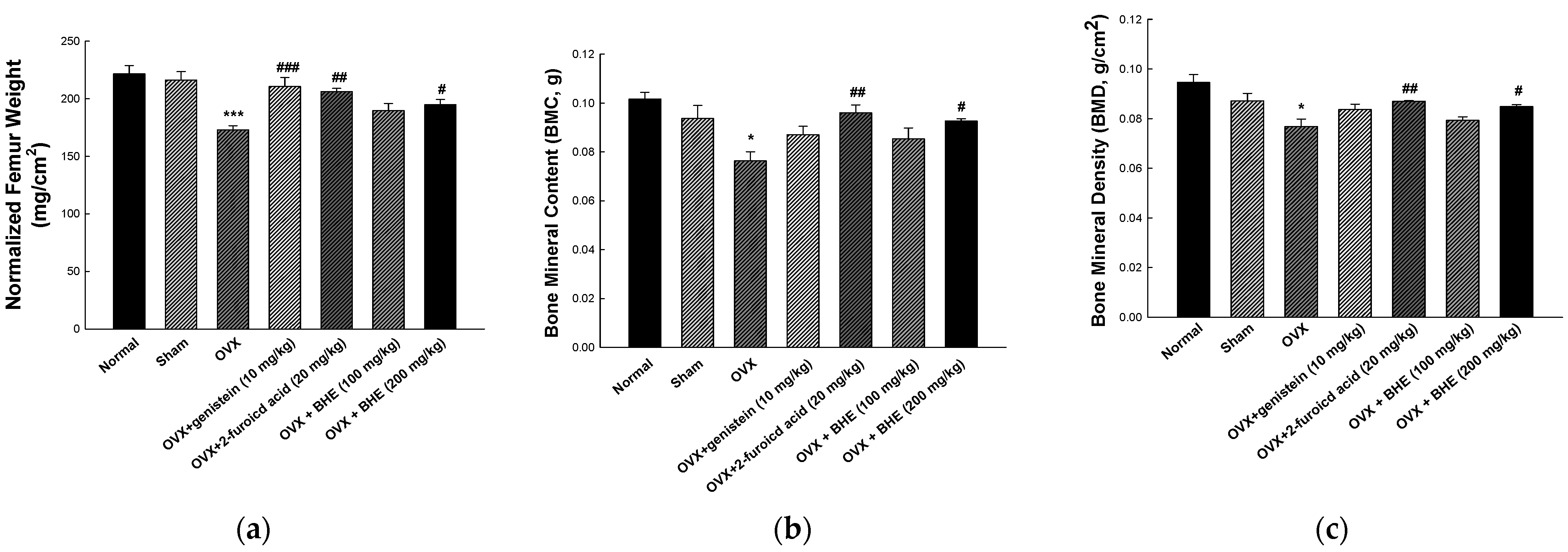
After treatment, normalized femur weight, BMD, and BMC of the femurs extracted from the sacrificed animals were measured, and the results are shown in Figure 1. The normalized femur weights were 216.10 ± 7.42 mg/cm2 in the sham group and 173.00 ± 3.66 mg/cm2 in the OVX group, indicating that the OVX group had significantly lower femur weights than the sham group. Compared with the OVX group, the OVX + LBHE (100 mg/kg) group did not show any difference in femur weights, whereas the OVX + genistein (10 mg/kg), OVX + 2-furoic acid (20 mg/kg), and OVX + HBHE (200 mg/kg) groups showed significant increases in femur weights (Figure 1a). As shown in Figure 1b,c, the BMC and BMD were significantly decreased in the OVX group (0.076 ± 0.004 g and 0.077 ± 0.003 mg/cm2, respectively) compared with the sham group (0.094 ± 0.005 g and 0.087 ± 0.003 mg/cm2, respectively). However, OVX-induced reductions in BMC and BMD were significantly increased in the OVX + 2-furoic acid and OVX + HBHE groups.
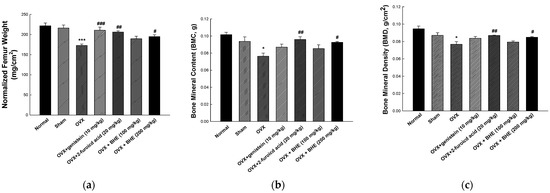
Figure 1.
Effects of Benincasa hispida extracts (BHE, HR1901-W) on femur bone morphologic parameters in ovariectomy-induced osteoporosis in female mice. (a) Normalized femur weight, (b) BMD, and (c) BMC of anesthetized mice at 8 weeks. All values are expressed as the mean ± standard error of the mean (SEM). * p < 0.05, and *** p < 0.001 vs. sham group; # p < 0.05, ## p < 0.05, and ### p < 0.001 vs. OVX group. BMC, bone mineral content; BMD, bone mineral density; OVX, ovariectomy; GEN, genistein; 2-FA, 2-furoic acid.
3.3. Effect of BHE and 2-Furoic Acid on Osteoclast Formation
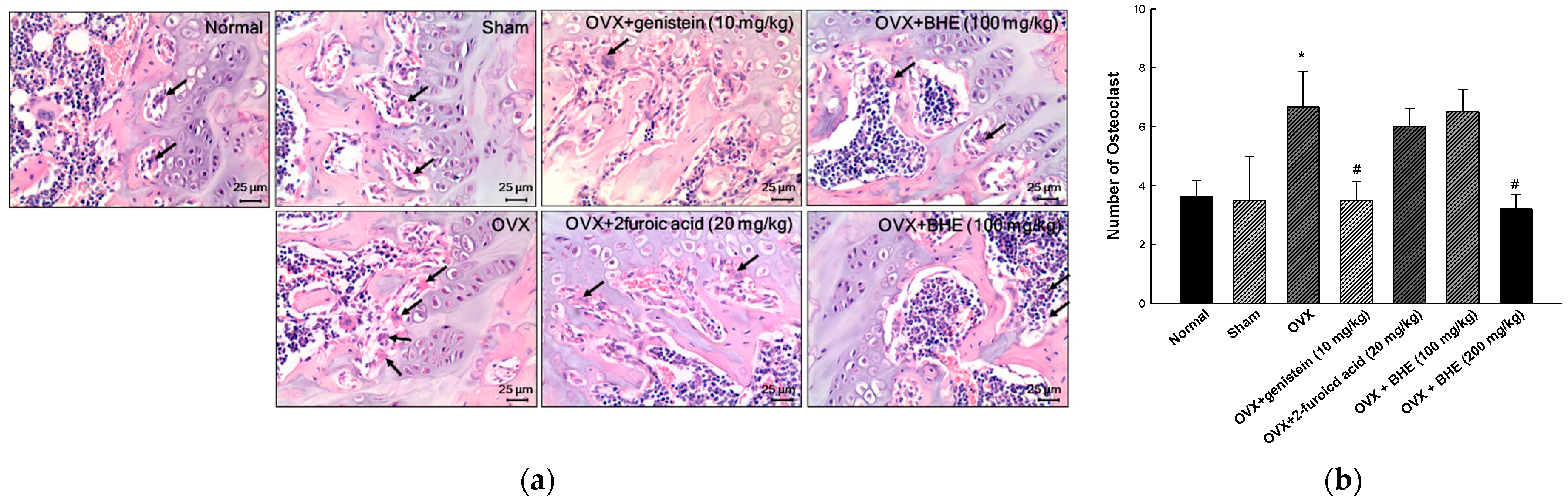
The femoral tissues of OVX-induced osteoporosis mice were investigated for histological changes using H&E staining (Figure 2a). Osteoclasts were identified based on their morphological characteristics, such as multinucleated cells with eosinophilic cytoplasm near the bone tissues, which are present along the absorption zone of each long bone. The osteoclast number for the OVX group (6.67 ± 1.20) was significantly higher than that for the sham group (3.50 ± 1.50). However, the osteoclast number was significantly decreased in the 2-furoic acid (6.00 ± 0.61) and HBHE (3.20 ± 0.49) supplementation groups compared with the OVX group. These results suggested that 2-furoic acid and HBHE inhibited bone resorption by inhibiting osteoclast proliferation in ovariectomy-induced osteoporotic mice.
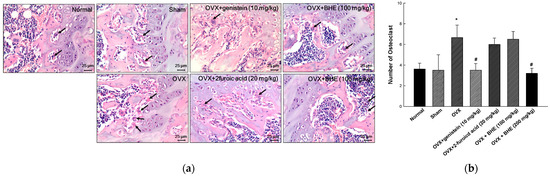
Figure 2.
Effects of Benincasa hispida extracts (BHE, HR1901-W) on osteoclast in histological features of the representative metaphysis of a femur. (a) Giant hyper-nucleated osteoclast (arrowhead) within a histological section (H&E staining) and (b) number of osteoclasts. All values are expressed as the mean ± standard error of the mean (SEM). * p < 0.05 vs. sham group; # p < 0.05 vs. OVX group.
3.4. Effect of BHE and 2-Furoic Acid on Bone Resorption Markers
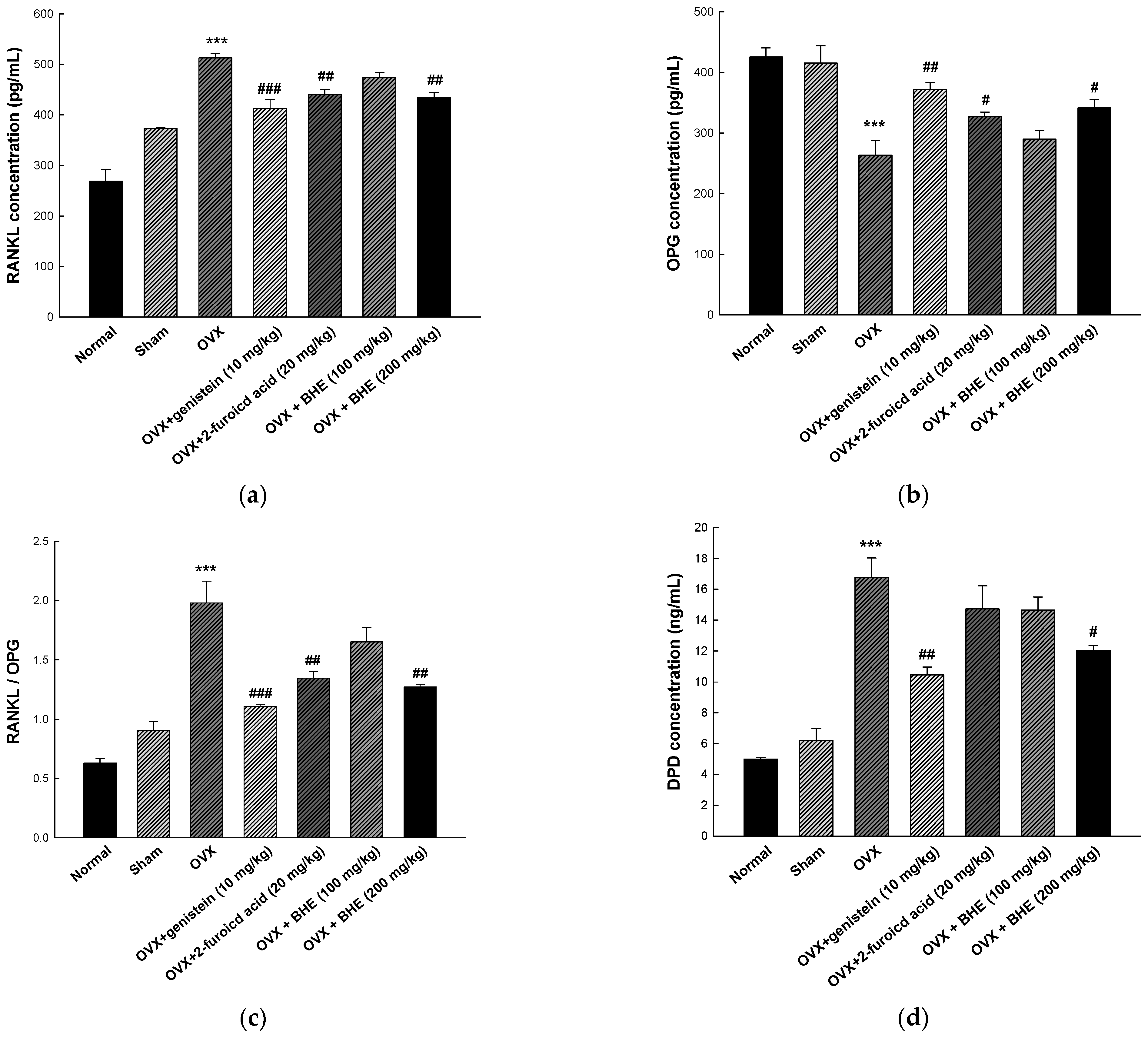
As shown in Figure 3, RANKL, OPG, and DPD were measured as serum markers of bone resorption. RANKL is an osteoclast-stimulating material that allows osteoclasts to perform the bone resorption process, while OPG inhibits bone resorption by blocking the maturation and activation of osteoclasts by RANKL. The RANKL/OPG ratio is an important factor in bone remodeling and directly involved in osteoporosis [36]. Therefore, an increase in the RANKL/OPG ratio may induce metabolic bone diseases, such as osteoporosis and osteosclerosis due to increased osteoclastogenesis, osteoclast activity, and bone resorption. As shown in Figure 3a, the serum RANKL levels were significantly decreased in the 2-furoic acid (440.28 ± 9.57 pg/mL) and HBHE (433.72 ± 10.40 pg/mL) supplementation groups compared with the OVX group (512.55 ± 8.61 pg/mL). Furthermore, as shown Figure 4b, serum OPG levels were increased in the 2-furoic acid (327.29 ± 7.15 pg/mL) and HBHE (341.37 ± 13.71 pg/mL) supplementation groups compared with the OVX group (263.36 ± 23.93 pg/mL). These results induced changes in the RANKL/OPG ratio in favor of bone formation (Figure 3c). DPD is a bone resorption marker released by osteoclasts that breaks down collagen cross-linked with bone during bone resorption [37]. Moreover, as shown Figure 4d, serum DPD levels were decreased in the 2-furoic acid (14.72 ± 1.49 ng/mL) and HBHE (12.04 ± 0.30 ng/mL) supplementation groups compared with the OVX group (16.77 ± 1.25 ng/mL). These results suggest that 2-furoic acid and HBHE induce a reduction in bone resorption markers in ovariectomy-induced osteoporosis mice.
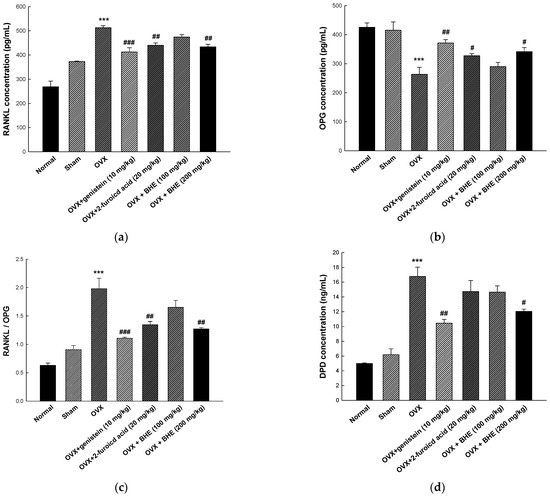
Figure 3.
Effects of Benincasa hispida extracts (BHE, HR1901-W) on bone resorption markers in ovariectomy-induced osteoporosis mice. The concentration of (a) RANKL, (b) OPG, (c) RANKL/OPG ratio, and (d) DPD was determined using enzyme-linked immunosorbent assay (ELISA) kits. All values are expressed as the mean ± standard error of the mean (SEM). *** p < 0.001 vs. sham group; # p < 0.05, ## p < 0.01, and ### p < 0.001 vs. OVX group. RANKL, receptor activator of nuclear factor kappa-Β ligand; OPG, osteoprotegerin; DPD, deoxypyridinoline; OVX, ovariectomy.
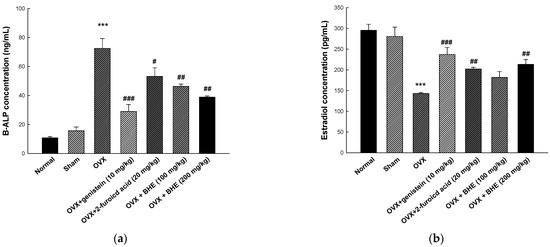
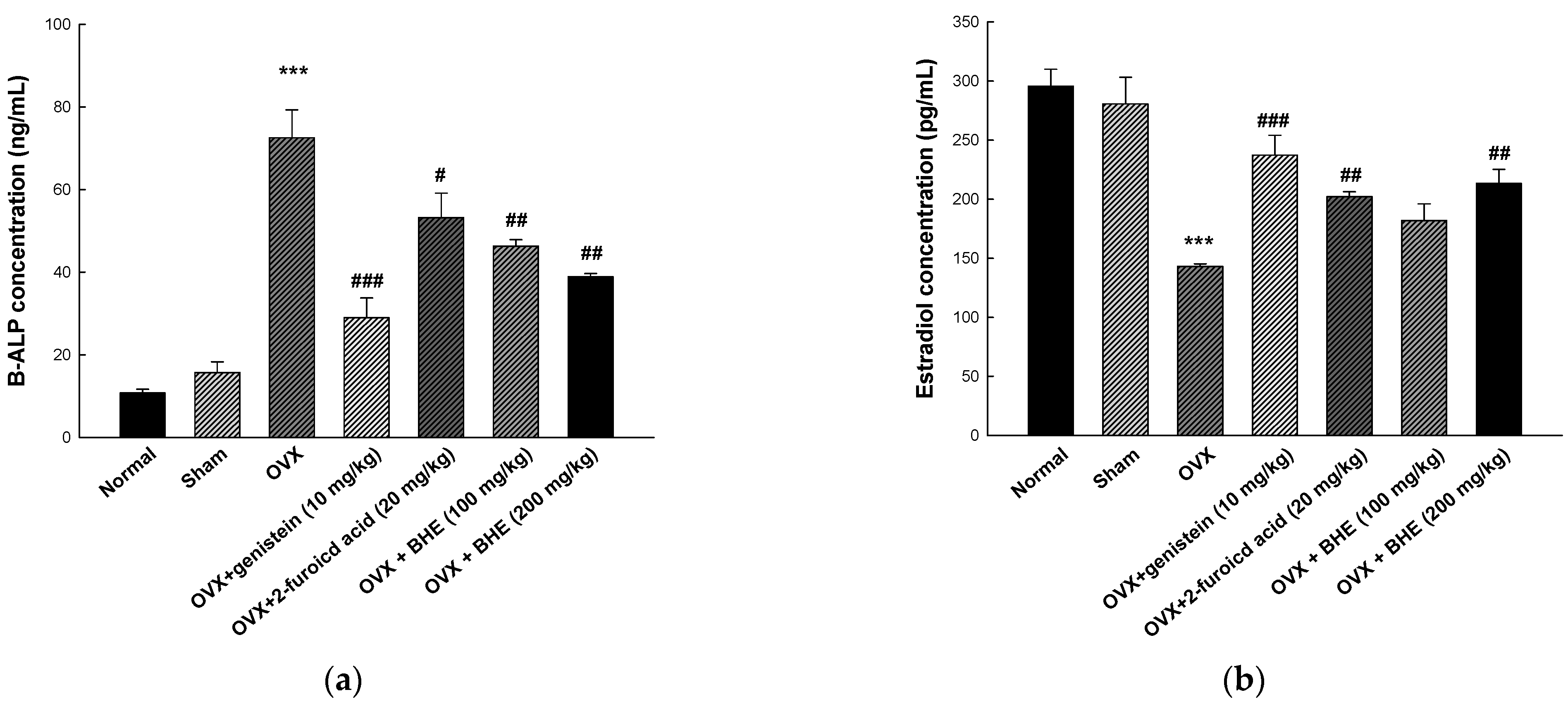
Figure 4.
Effects of Benincasa hispida extracts (BHE, HR1901-W) on bone formation markers in ovariectomy-induced osteoporosis mice. The concentration of (a) B-ALP, and (b) estradiol was determined using enzyme-linked immunosorbent assay (ELISA) kits. All values are expressed as the mean ± standard error of the mean (SEM). *** p < 0.001 vs. sham group; # p < 0.05, ## p < 0.01, and ### p < 0.001 vs. OVX group. BALP, bone-specific alkaline phosphatase; OVX, ovariectomy.
3.5. Effect of BHE and 2-Furoic Acid on Bone Formation Markers
BALP is a membrane-bound osteoblast enzyme involved in bone matrix mineralization and is secreted into circulation during bone resorption [38]. In addition, we measured the serum concentrations of the ovarian hormone estradiol, which was decreased in an ovariectomized menopause model. As shown in Figure 4a, the serum levels of BALP were higher in the ovariectomized mice group (72.52 ± 6.73 ng/mL) than in the sham group (15.71 ± 2.56 ng/mL). However, the BALP levels were significantly decreased in the 2-furoic acid (53.25 ± 5.88 ng/mL) and HBHE (38.89 ± 0.75 ng/mL) supplementation groups compared with the OVX group. As shown Figure 4b, serum estradiol levels were increased in the 2-furoic acid (201.93 ± 4.40 pg/mL) and HBHE (213.28 ± 11.85 pg/mL) supplementation groups compared with the OVX group (143.80 ± 2.22 pg/mL). These results suggest that 2-furoic acid and HBHE induce the recovery of bone formation markers in mice with ovariectomy-induced osteoporosis.
4. Discussion
In this study, the efficacy of BHE in improving bone health was compared using genistein, a phytoestrogen, as a positive control. As shown in Table 1, although there was no significant difference, ovariectomized mice had increased body weight and FER compared with the sham group. Estrogen is an important regulator of lipid metabolism, and decreased estrogen secretion after menopause leads to obesity [39,40]. Weight gain in the ovariectomy model has been reported as a mechanism of action that increases subcutaneous fat and estrogen secretion from adipose tissue [41,42]. In addition, administration of the phytoestrogen genistein has been shown to inhibit weight gain in OVX mice [43,44]. However, in this study, no difference in body weight or FER between the experimental groups of ovariectomized mice was noted. These results are attributed to the differences between the duration and dose of genistein and BHE, and the age at ovariectomy [45,46]. Bone remodeling is a continuous iterative process of creating and resorbing new bone to replace old bone. Osteoblasts play a leading role in the process of creating new bone, whereas osteoclasts play a leading role in the process of resorption, which destroys old bones [47]. Estrogen deficiency induces osteoclast activity and decreases bone mass, leading to osteoporosis. Estrogen deficiency induces bone loss and osteoporosis owing to increased osteoclast activity, and ovariectomy has been reported to be associated with decreased bone formation [48,49]. In agreement with data from the literature, we observed that ovariectomy induced significant reductions in femur weight, BMC, and BMD compared with the sham group (Figure 1). However, supplementation with 2-furoic acid and HBHE (200 mg/kg) inhibited the reduction in femur weight, BMC, and BMD in OVX mice.
Estrogen deficiency increases RANKL, which increases osteoclast activity and decreases osteoclast apoptosis [50]. Binding of RANKL to RANK, a surface receptor of osteoclast progenitor cells, induces osteoclast maturation and bone resorption. In addition, OPG, a soluble decoy receptor produced in osteoblast lineage cells, blocks the binding between RANKL and RANK by binding to RANKL and inhibiting osteoclast formation to prevent excessive bone resorption [36]. Estrogen suppresses RANKL production in osteoblasts and T&B lymphocytes and increases OPG production in osteoblasts [51]. Estrogen deficiency has been reported to change the RANKL/OPG ratio in a direction that favors bone resorption, and ovariectomy is associated with an increase in RANKL and osteoclast numbers and a decrease in OPG production [52,53]. In agreement with data from the literature, we observed that ovariectomy induced significant increases in osteoclast count and RANKL production, and decreased OPG production (Figure 2 and Figure 3a–c). However, 2-furoic acid and HBHE (200 mg/kg) supplementation inhibited the elevation of RANKL and RANKL/OPG ratio and reduced osteoclast number and OPG in OVX mice. During bone resorption, osteoclasts secrete acid and proteolytic enzymes between the cell membrane and the bone matrix. Acid plays a role in dissolving hydroxyapatite, and proteolytic enzymes induce the breakdown of bone matrix proteins, such as collagen [54]. During the breakdown of bone collagen, DPD is released from the bone matrix and is used as a biomarker for bone resorption. Estrogen deficiency has been reported to be associated with an increase in DPD [37], and our results show that ovariectomy reduced DPD production compared with the sham group, which is consistent with previous studies (Figure 3d). However, 2-furoic acid and HBHE (200 mg/kg) supplementation inhibited the reduction in DPD in OVX mice.
ALP is produced in osteoblasts and is stored in vesicles in the cell membrane. The release of ALP stored in the osteoblast membrane is increased in models of estrogen deficiency and osteoporosis [55]. However, since ALP is derived not only from osteoblasts but also from the kidneys and small intestine, only bone-specific ALP activity was measured in this study [56]. In agreement with data from the literature, we observed that ovariectomy induced a significant increase in BALP production (Figure 4a). However, supplementation with 2-furoic acid and HBHE (200 mg/kg) inhibited the increase in BALP levels in OVX mice. In addition, ovariectomy-induced reductions in serum estradiol concentrations increased in mice supplemented with 2-furoic acid and BHE (Figure 4b). Overall, BHE and 2-furoic acid supplementation in OVX menopausal mice appeared to be effective in preventing bone resorption and inducing bone formation. In addition, our previous study reported that BHE promotes the proliferation, differentiation, and mineralization of preosteoblasts and inhibits the differentiation of osteoclast precursors [33]. In addition, no acute toxicity was observed in the group treated with ethanol and aqueous extract of Benincassa hispida at a high dose of 5000 mg/kg in rats [57,58]. Acute and subchronic toxicity studies of Benincasa hispida fruit pulp reported that doses up to 1000 mg/kg were non-toxic and safe for long-term oral consumption [59]. In this study, the anti-osteoporosis effect was confirmed using BHE lower than the toxicity range, and these results suggest that it can be used as a functional food ingredient. Nonetheless, additional biomarker measurements, such as vitamin D absorption, calcium metabolism, and growth hormones must be further studied. In this study, 2-furoic acid at a higher concentration than the 2-furoic acid content of BHE was used, and it needs to be identified as the main active ingredient of BHE that shows an anti-osteoporotic effect. In addition, to further enhance the utilization of BHE as a functional food ingredient related to bone-health promotion, it is necessary to analyze the bioactive compound of BHE and study the molecular mechanism of action, as well as to evaluate the aging model.
5. Conclusions
This study investigated the effects of 2-furoic acid and BHE on bone metabolism in ovariectomized (OVX) menopausal mice. Our results suggest that 2-furoic acid and BHE supplementation contributes to the prevention of BMD reduction, increase in the amount of bone formation markers, and reduction in the amount of bone resorption markers in OVX mice. Therefore, BHE can be used as an effective alternative to prevent postmenopausal osteoporosis. In addition, 2-furocid has the potential to be used as an indicator component and a bioactive compound in the development of functional food materials related to bone health. Although this study evaluated the efficacy of bone-health improvement in the menopause model, it is necessary to conduct a follow-up study to evaluate the aging model and its molecular mechanism of action. Taken together, our findings indicate that BHE regulates bone remodeling and could potentially be used as a functional food ingredient for bone health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13020832/s1, File S1: Certificate of analysis for Benincasa hispida extract (BHE, HR1901-W).
Author Contributions
Conceptualization, S.-I.C. and O.-H.L.; methodology, S.-I.C., X.H. and Y.-E.C.; software, X.M., S.-J.L. and J.-M.Y.; validation, X.M., G.O. and J.-M.Y.; formal analysis, Y.-E.C. and X.H.; investigation, S.-I.C., S.-J.L. and G.O.; resources, X.H., S.-J.L. and J.-M.Y.; data curation, S.-I.C., G.O. and Y.-E.C.; writing—original draft preparation, S.-I.C.; writing—review and editing, J.-H.C. and O.-H.L.; visualization, X.H., X.M. and Y.-E.C.; supervision, J.-H.C. and O.-H.L.; project administration, X.H., S.-J.L. and. J.-M.Y.; funding acquisition, J.-H.C. and O.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Ministry of SMEs and Startups [grant number S3004427].
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Kangwon National University (approval number KW-210802-2 and 13 September 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, Ok-Hwan Lee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hou, X.; Zhang, J.; Li, J.; Wu, P.; Yan, L.; Qian, H. Diagnostic and Therapeutic Roles of Extracellular Vesicles in Aging-Related Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 6742792. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; Van Blitterswijk, C.A.; De Boer, J.; LaPointe, V.L.S. The components of bone and what they can teach us about regeneration. Materials 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.A. Novel insights into the complex architecture of osteoporosis molecular genetics. Ann. N. Y. Acad. Sci. 2019, 1462, 37–52. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the ageing skeleton. Subcell. Biochem. 2019, 91, 453–476. [Google Scholar]
- Johnston, C.B.; Dagar, M. Osteoporosis in older adults. Med. Clin. N. Am. 2020, 104, 873–884. [Google Scholar] [CrossRef]
- Hobson, E.E.; Ralsteo, S.H. Role of genetic factors in the pathophysiology and management of osteoporosis. Clin. Endocrinol. 2001, 54, 1–9. [Google Scholar] [CrossRef]
- Garnero, P.; Delmas, P.D. New developments in biochemical markers for osteoporosis. Calcif. Tissue Int. 1996, 1, S2–S9. [Google Scholar] [CrossRef]
- Johansen, J.S.; Riis, B.J.; Delmas, P.D.; Christiansen, C. Plasma BGP: An indicator of spontaneous bone loss and of the effect of oestrogen treatment in postmenopausal women. Eur. J. Clin. Investig. 1988, 18, 191–195. [Google Scholar] [CrossRef]
- Cauley, J.A.; Gutai, J.P.; Sandier, R.B.; LaPorte, A.E.; Kuller, L.H.; Sashin, D. The relationship of endogenous estrogen to bone density and bone area in normal poetmenopausal women. Am. J. Epldemiol. 1986, 124, 752–761. [Google Scholar] [CrossRef]
- Ohta, H.; Masuzawa, T.; Ikeda, T.; Suda, Y.; Makita, K.; Nozawa, S. Which is more osteoporosis inducing, menopause or oophorectomy? Bone Miner. 1992, 19, 273–285. [Google Scholar] [CrossRef]
- Li, M.; Shen, Y.; Wronski, T.J. Time course of femoral neck osteopenia in ovariectomized rats. Bone 1997, 20, 55–61. [Google Scholar] [CrossRef]
- Valero, M.A.; Loinaz, C.; Larrodera, L.; Leon, M.; Moreno, E.; Hawkins, F. Calcitonin and bisphosphonates treatment in bone loss after liver transplantation. Calcif. Tissue Int. 1995, 57, 15–19. [Google Scholar] [CrossRef]
- Ross, R.K.; Paganini-Hill, A.; Wan, P.C.; Pike, M.C. Effect of hormone replacement therapy on breast cancer risk: Estrogen versus estrogen plus progestin. J. Natl. Cancer Inst. 2000, 92, 328–332. [Google Scholar] [CrossRef]
- Ziel, H.K. Estrogen’s role in endometrial cancer. Obstet. Gynecol. 1982, 60, 509–515. [Google Scholar]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. J. Am. Med. Assoc. 2002, 288, 872–881. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Anwar, F.; Mehmood, T.; Hamid, A.A.; Muhammad, K.; Saari, N. Phenolic compounds, tocochromanols profile and antioxidant properties of winter melon [Benincasa hispida (Thunb.) Cogn.] seed oils. J. Food Meas. Charact. 2019, 13, 940–948. [Google Scholar] [CrossRef]
- Park, G.R.; Lee, J.A. Anti-oxidant, anti-inflammatory and Whitening effect of Benincasa hispida seed extract. J. Converg. Inf. Technol. 2020, 10, 249–256. [Google Scholar]
- Lim, S.J.; Jeong, J.G.; Kim, M.W.; Choi, S.S.; Han, H.K.; Park, J.E. Effects of Benincasa hispida intake on blood glucose and lipid level in streptozotocin induced diabetic rats. Korean J. Nutr. 2003, 36, 335–343. [Google Scholar]
- You, Y.H.; Jun, W.J. Effects of fractions from Benincasa hispida on inhibition of adipogenesis in 3T3-L1 preadipocytes. J. Korean Soc. Food Sci. Nutr. 2012, 41, 895–900. [Google Scholar] [CrossRef]
- Grover, J.K.; Adiga, G.; Vats, V.; Rathi, S.S. Extracts of Benincasa hispida prevent development of experimental ulcers. J. Ethnopharmacol. 2001, 78, 159–164. [Google Scholar] [CrossRef]
- Kim, M.W. Effects of benincasa hispida seed supplementation on glyeogen status and lipid peroxidatin in streptozotecin-induced diabetic rats. J. Nutr. Health 2004, 37, 865–871. [Google Scholar]
- Lim, T.K. Edible medicinal and non-medicinal plants. In Modified Stems, Roots, Bulbs; Springer: Berlin/Heidelberg, Germany, 2012; Volume 11, pp. 285–292. [Google Scholar]
- Gill, N.S.; Dhiman, K.; Bajwa, J.; Sharma, P.; Sood, S. Evaluation of free radical scavenging, anti-inflammatory and analgesic potential of Benincasa hispida seed extract. Int. J. Pharmacol. 2010, 6, 652–657. [Google Scholar] [CrossRef]
- Cho, B.Y.; Park, M.R.; Lee, J.H.; Ra, M.J.; Han, K.C.; Kang, I.J.; Lee, O.H. Standardized Cirsium setidens Nakai ethanolic extract suppresses adipogenesis and regulates lipid metabolisms in 3T3-L1 adipocytes and C57BL/6J mice fed high-fat diets. J. Med. Food 2017, 20, 763–776. [Google Scholar] [CrossRef]
- Jeon, S.Y.; Jeong, E.J.; Baek, J.H.; Cha, Y.J. Analytical method validation of quercetin in Changnyeong onion extract as a functional ingredient for functional health food. J. Korean Soc. Food Sci. Nutr. 2011, 40, 565–569. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, A.-H.; Sun, H.; Yan, G.-L.; Wang, X.-J. Recent advances and effective strategies in the discovery and applications of natural products. RSC Adv. 2018, 8, 812–824. [Google Scholar] [CrossRef]
- Hall, I.H.; Williams, W.L.; Rhyne, K.A.; Knowles, M. The hypolipidemic activity of furoic acid and furylacrylic acid derivatives in rodents. Pharm. Res. 1985, 2, 233–238. [Google Scholar] [CrossRef]
- Hall, I.H.; Wong, O.T.; Reynolds, D.J.; Chang, J.J. Hypolipidemic effects of 2-uroic acid in sprague-dawley rats. Arch. Pharm. 1993, 326, 15–23. [Google Scholar] [CrossRef]
- Sajadi, Z.; Abrishami, M.M.; Chapman, J.M.; Hall, I.H. Synthesis and evaluation of the antitumor properties of esters of 2-furoic acid and 2-furylacrylic acid. J. Pharm. Sci. 1984, 73, 266–267. [Google Scholar] [CrossRef]
- Choi, S.I.; Han, X.; Men, X.; Lee, S.J.; Park, M.H.; Lee, O.H.; Yang, J.M.; Choi, Y.E.; Cho, J.H. Development and validation of an analytical method for 2-furoic acid in Benincasa hispida extracts (HR1901-W). J. Agri. Life Environ. Sci. 2021, 33, 311–320. [Google Scholar]
- Choi, Y.E.; Yang, J.M.; Cho, J.H. Benincasa hispida extract promotes proliferation, differentiation, and mineralization of MC3T3-E1 preosteoblasts and inhibits the differentiation of RAW 246.7 osteoclast precursors. Appl. Sci. 2022, 12, 8849. [Google Scholar] [CrossRef]
- Behloul, N.; Wu, G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013, 698, 31–38. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Z.; Qu, H.; Li, M. Comparative effects of hispidulin, genistein, and icariin with estrogen on bone tissue in ovariectomized rats. Cell Biochem. Biophys. 2014, 70, 485–490. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Tasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Seibel, M.J.; Cosman, F.; Shen, V.; Gordon, S.; Dempster, D.W.; Ratcliffe, A.; Lindsay, R. Urinary hydroxypyridinium crosslinks of collagen as markers of bone resorption and estrogen efficacy in postmenopausal osteoporosis. J. Bone Miner. Res. 1993, 8, 881–889. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- Campbell, S.E.; Febbraio, M.A. Effects of ovarian hormones on exercise metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 515–520. [Google Scholar] [CrossRef]
- Misso, M.L.; Jang, C.; Adams, J.; Tran, J.; Murata, Y.; Bell, R.; Boon, W.C.; Simpson, E.R.; Davis, S.R. Differential expression of factors involved in fat metabolism with age and the menopause transition. Maturitas 2005, 51, 299–306. [Google Scholar] [CrossRef]
- Zoth, N.; Weigt, C.; Laudenbach-Leschowski, U.; Diel, P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J. Steroid Biochem. Mol. Biol. 2010, 122, 100–105. [Google Scholar] [CrossRef]
- Zheng, W.; Rogoschin, J.; Niehoff, A.; Oden, K.; Kulling, S.E.; Xie, M.; Diel, P. Combinatory effects of phytoestrogens and exercise on body fat mass and lipid metabolism in ovariectomized female rats. J. Steroid Biochem. Mol. Biol. 2018, 178, 73–81. [Google Scholar] [CrossRef]
- Shen, H.H.; Huang, S.Y.; Kung, C.W.; Chen, S.Y.; Chen, Y.F.; Cheng, P.Y.; Lam, K.K.; Lee, Y.M. Genistein ameliorated obesity accompanied with adipose tissue browning and attenuation of hepatic lipogenesis in ovariectomized rats with high-fat diet. J. Nutr. Biochem. 2019, 67, 111–122. [Google Scholar] [CrossRef]
- Kim, H.K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.Y.; Zhang, W.; Duan, J.; Hartzel, D.L.; Hamrick, M.W.; Baile, C.A. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J. Nutr. 2006, 136, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y.; Arai, N.; Wang, X.; Wu, J.; Umegaki, K.; Miyaura, C.; Takeda, A.; Ikegami, S. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem. Biophys. Res. Commun. 2000, 274, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, H.S.; Jung, J.I.; Lim, S.M.; Lim, J.H.; Ha, W.H.; Jeon, C.L.; Lee, J.Y.; Kim, E.J. Effect of isoflavone-enriched whole soy milk powder supplementation on bone metabolism in ovariectomized mice. Nutr. Res. Pract. 2018, 12, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef]
- Marie, P.J.; Hott, M.; Modrowski, D.; De Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res. 1993, 8, 607–615. [Google Scholar] [CrossRef]
- Bowman, B.M.; Miller, S.C. Elevated progesterone during pseudopregnancy may prevent bone loss associated with low estrogen. J. Bone Miner. Res. 1996, 11, 15–21. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Female reproductive system and bone. Arch. Biochem. Biophys. 2010, 503, 118–128. [Google Scholar] [CrossRef]
- Hou, J.M.; Xue, Y.; Lin, Q.M. Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Acta Pharmacol. Sin. 2012, 33, 1277–1284. [Google Scholar] [CrossRef]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Halleen, J.M. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif. Tissue Int. 2008, 82, 108–115. [Google Scholar] [CrossRef]
- Mulari, M.; Vääräniemi, J.; Väänänen, H.K. Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc. Res. Tech. 2003, 61, 496–503. [Google Scholar] [CrossRef]
- Noh, D.; Lim, Y.; Lee, H.; Kim, H.; Kwon, O. Soybean-hop alleviates estrogen deficiency-related bone loss and metabolic dysfunction in ovariectomized rats fed a high-fat diet. Molecules 2018, 23, 1205. [Google Scholar] [CrossRef]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Qadrie, Z.L.; Tayebhawisan, N.; Alikhan, M.W.; Samuel, M.; Anandan, R. Antinociceptive and anti-pyretic activity of Benincasa hispida (Thunb) Cogn. in Wistar albino rats. Pak. J. Pharm. Sci. 2009, 22, 287–290. [Google Scholar]
- Jayasree, T.; Kishore, K.K.; Vinay, M.; Vasavi, P.; Dixit, R.; Rajanikanth, M.; Manohar, V.S. Diuretic effect of the chloroform extract of the Benincasa hispida rind (Pericarp) extract in Sprague-Dawley rats. Int. J. Appl. Biol. Pharm. 2011, 2, 94–99. [Google Scholar]
- Shakya, A.; Chaudhary, S.K.; Bhat, H.R.; Ghosh, S.K. Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.) cogniaux fruit extract in rodents. Regul. Toxicol. Pharmacol. 2020, 118, 104785. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).