Abstract
The use of xenogenic cortical bone laminas in Guided Bone Regeneration (GBR) has been well described in the literature over the past decade. These biomaterials present a very low degree of complications due to their nature (porcine or equine collagenated bone) and the fact that when they become exposed, they simply hydrolyze without major complications. One of the problems related to the first generation of these laminas was their extreme rigidity and return elasticity, often forcing clinicians to anchor them with pins and/or screws. A new generation of bone laminas called Flex Cortical Sheet (FCS) have recently been introduced with machine-made thicknesses of 0.2, 0.5, and 0.9 mm and increased flexibility and adaptability to ridge defects. This paper has the goal of presenting a case of vertical and horizontal reconstruction performed by means of a 0.5 mm FCS and showing the workflow necessary to successfully restore a complex situation. After 8 months of healing, the GBR resulted in a horizontal and vertical augmentation of 8 mm and 8 mm, respectively. The radiographic examination at 18 months demonstrated great stability of new bone around implants.
1. Introduction
In the last 30 years, Guided Bone Regeneration (GBR) has become one of the most popular techniques to achieve bone regeneration of horizontal and vertical alveolar ridges, allowing implant rehabilitation [1,2]. The key role of GBR is played by the barrier, which protects a bone graft from non-osteogenic cells and creates a suitable biological environment for osteoprogenitor cells to colonize the bone defect, favoring angiogenesis and bone regeneration [3]. The first-generation barriers were non-resorbable membranes made of PTFE and e-PTFE [4]. Although clinically effective, non-resorbable membranes were also prone to complications, such as membrane exposure, which often led to the impairment of the regenerative procedure [5,6,7]. Recently, a publication describing the management of 80 complications in vertical and horizontal ridge augmentation cases treated with d-PTFE found that 70% of complications appeared 2 months after surgery, 43.75% of complications were in the anterior maxilla, and 20% were in the lower left mandible [8]. These issues, together with the need for a second surgery for their removal, push the research toward the development of resorbable barriers.
Among resorbable barriers, collagen membranes are the most commonly used thanks to their biocompatibility, ease of handling, and active role in recruiting cells into the wound area, thus modulating the overall osteogenic process [9,10]. One of the limitations of collagen membranes is the lack of rigidity and the resorption time, which may not be suitable for large, three-dimensional defects [10].
Due to these limitations, in the last decade, a new class of bioresorbable barriers was developed: the cortical bone laminas. These bone sheets are made of collagen-preserved, partially demineralized cortical bone of porcine [11,12,13] or equine origin (Flex Cortical Sheet—FCS) [14,15,16]. Differently from pure collagen membranes, the thin layer of the remaining hydroxyapatite confers a slight rigidity to the cortical sheet and long-term protection of the bone graft/bone defect (more than 4 months) [14,17]. Multiple formats are available and suitable for horizontal ridge augmentation as well as for vertical ridge augmentation where the anatomy is favorable [11,12,13,18,19]. Another advantage of a cortical bone sheet is that, even in the case of accidental exposure, it is simply hydrolyzed by collagenases while soft tissue granulates on top of the cortical layer without significantly affecting the success of bone regeneration [12]. The bone sheet has also been used successfully in other cranial applications like the reconstruction of the orbital floor and the reparation of the sinus wall combined with zygomatic implants [20,21,22]. Recently, a new generation of FCS has been introduced to the market, with a machine-made thickness of 0.2, 0.5, and 0.9 mm and increased flexibility and adaptability to ridge defects [23].
In most cases, horizontal and vertical ridge augmentation requires a bone graft to achieve the proper volumetric augmentation [2]. Although the autologous bone graft is considered the “gold standard”, there are some drawbacks relative to its use. Indeed, autologous grafts involve the opening of a second surgical site with an increased risk of intra- and post-operative complications, including donor-site pain, abnormal gait (in the case of the iliac crest as the harvesting site), and numbness [24]. Moreover, the supporting clinical evidence for the use of allografts, xenografts, and synthetic biomaterials [2,24,25,26,27] decreased the use of autologous grafts alone.
This case report highlights the clinical findings on the combined use of a new generation of FCS with a collagen-preserved bone substitute of equine origin in the reconstruction of a three-dimensional defect in the lower-posterior mandible. The combination of FCS with fibrine glue represents one of the easiest and more manageable procedures in modern GBR, simplifying graft and membrane stabilization and avoiding the complicated addition of several pins and tacks [17,19].
We can highlight some of the advantages consequent to the use of a FLEX membrane for GBR in comparison to non-resorbable PTFE membranes: (1) ductility, stability, and flexibility; (2) does not develop infection in case of exposure; (3) does require a re-entry procedure for removal, like a PTFE membrane; (4) excellent biological integration; and (5) easy handling, even in the hands of inexperienced clinicians [11,12,13,17,18].
2. Materials and Methods
2.1. Materials
The GBR was performed by means of a 0.5 mm thick collagenated equine bone sheet (Osteoxenon®, Flex Cortical Sheet, Bioteck SpA, Arcugnano, Italy) (FCS) combined with a mix of autogenous bone and xenografts (Osteoxenon®, Mix Granules, Bioteck SpA, Arcugnano, Italy). The autologous grafts were harvested from the lingual aspect of the edentulous ridge exposed during the surgical procedure. The grafts were combined and united with a few drops of fibrin glue (Tisseel®, Baxter, Roma, Italy).
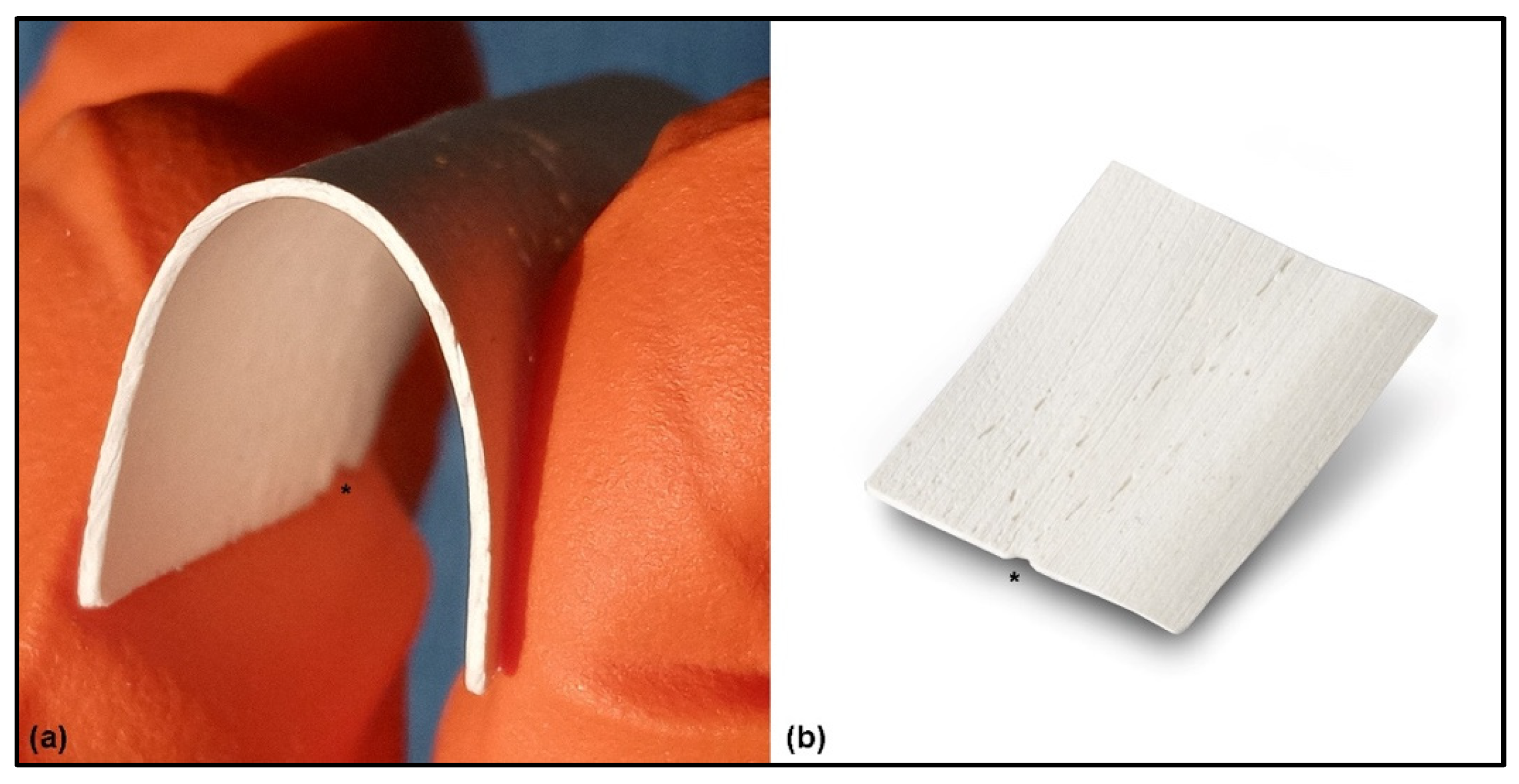
The equine-derived FCS and granules production process removes all the antigenic components from the animal tissue through a specific enzymatic treatment (Zymo-Teck® Process, Bioteck SpA, Arcugnano, Italy). However, the collagen and the mineral phase of the bone are preserved. This allows a physiological interaction with the osteoblasts and osteoclasts of the patient [28], leading to a replacement of the grafting material with new vital bone much faster than with other grafting materials treated with different production processes (i.e., thermal treatments) [27,29]. The FCS is subjected to an additional manufacturing process (according to the manufacturer’s data) to remove part of the mineral components, exposing the bone collagen, and providing flexibility to the sheet once hydrated (Figure 1a). FCS is obtained from the long bones of the equines, therefore the bone fibers run in a parallel way. This fiber trend is indicated by an indentation placed on the side of the FCS. It is important to identify it in order to fold the FCS properly, i.e., by exerting traction forces parallel to the bone fibers to take advantage of their elasticity and prevent the bone sheet from opening due to forces transverse to the fibers themselves (Figure 1b). Finally, the thickness of FCS is obtained by mechanical abrasion of the cortical bone, providing excellent reproducibility [11,12,18].
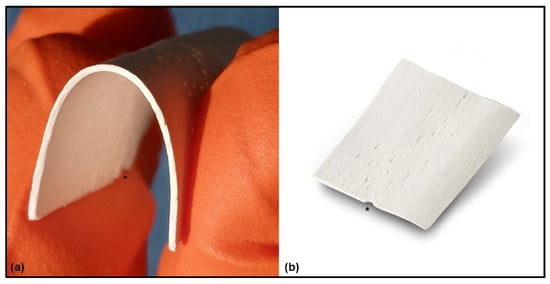
Figure 1.
Flex Cortical Sheet: (a) Once the FCS was hydrated for a few seconds in a sterile saline solution, the sheet became flexible. (b) Appearance of FCS as provided by the manufacturer. A special indentation (*) is placed on one side of the FCS, indicating the direction of the parallel bone fibers and providing an indication to the surgeon about how to fold the FCS, i.e., exert traction forces parallel to the bone fibers.
2.2. Surgical Technique
The ridge defect was reconstructed with the bone graft mix. Fibrin glue plays a key role in this kind of GBR because it increases the stability of the grafts [23]. Following small, consecutive increases of the grafts, the FCS was accurately placed on top of the ridge to create the desired shape.
The FCS is taken from its package, trimmed, and hydrated for a few seconds in a 10 mL sterile saline solution for 10–15 s. Once the proper flexibility is obtained, the FCS is bent, considering the direction of bone fibers as indicated by the small indentation on the side of the FCS itself. Finally, the FCS is positioned to reconstruct the missing anatomy. Once the final size, shape, and position of FCS are reached, the side edges facing the graft are glued with fibrine glue to keep it in position with tight finger pressure for 30 s.
3. Case Presentation
3.1. Clinical History
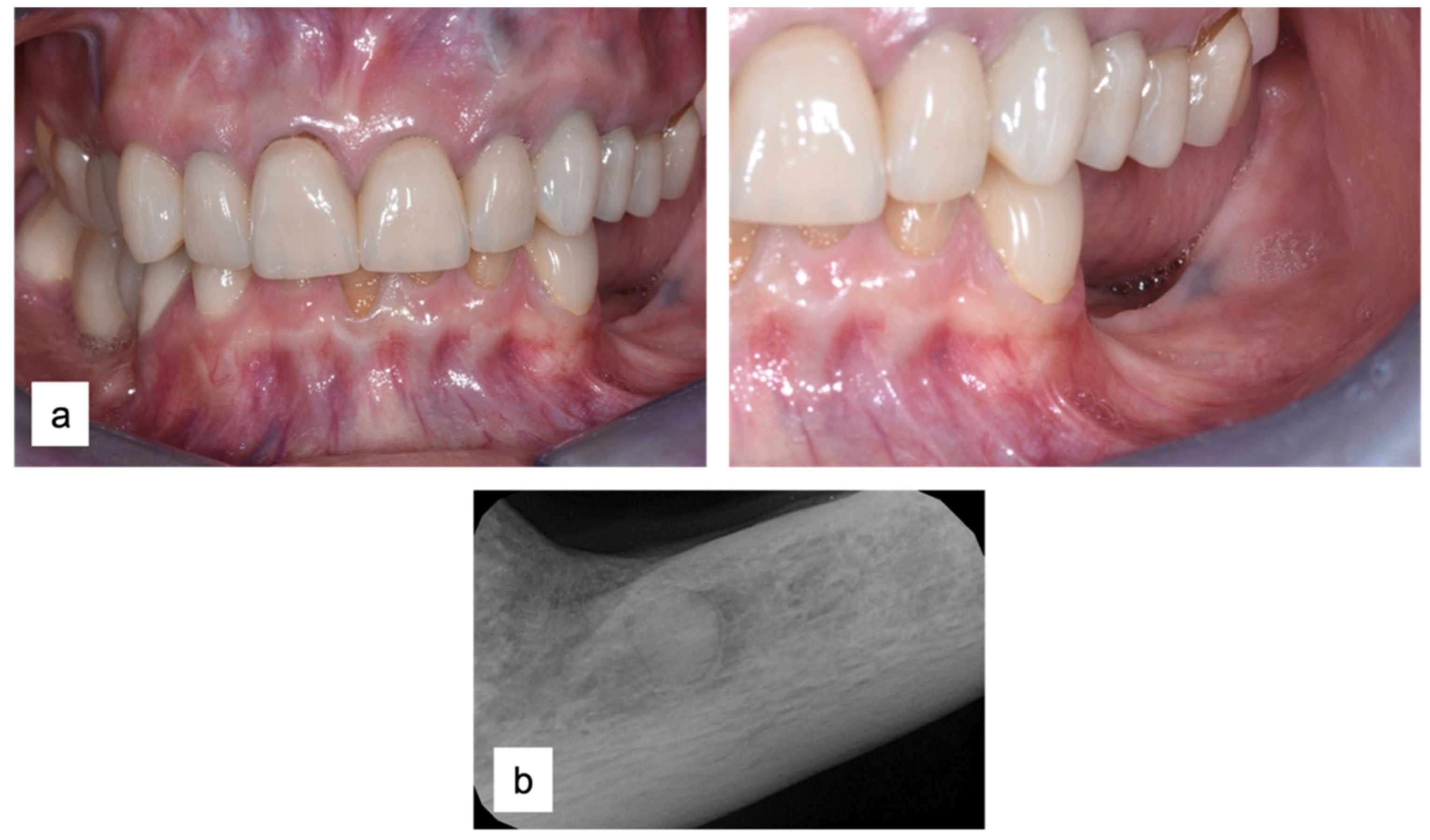
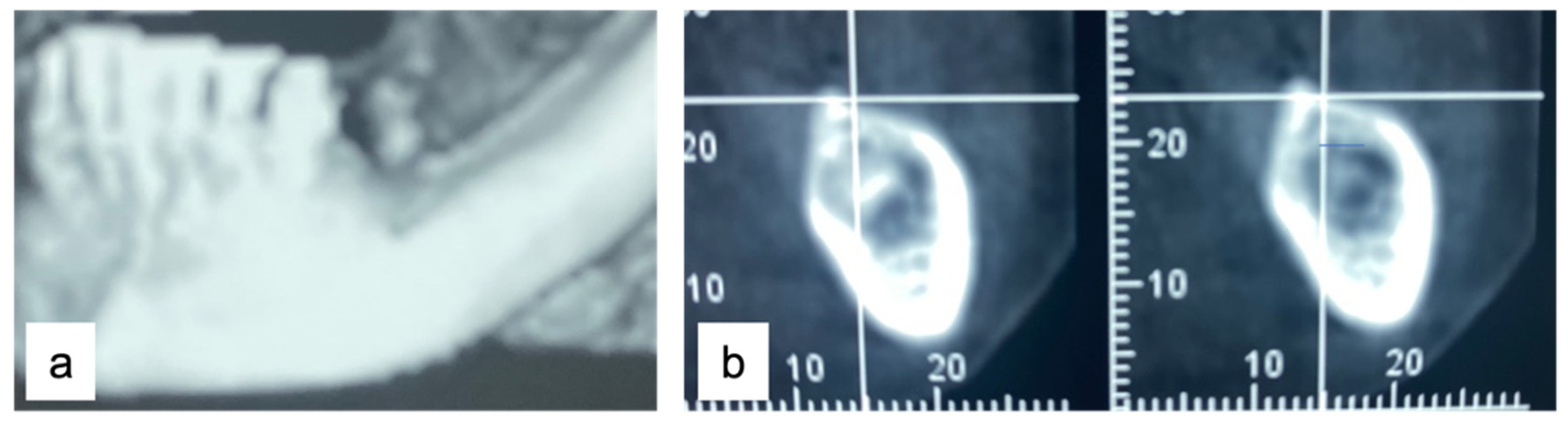
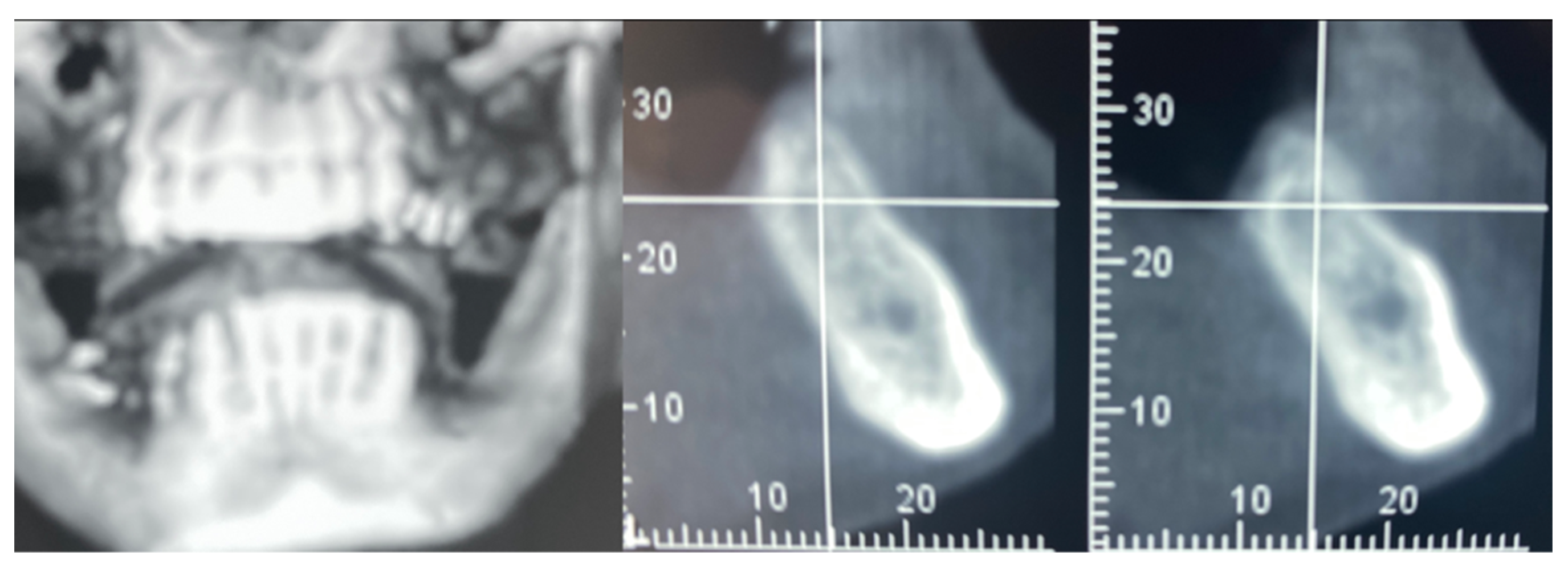
A 61-year-old female patient came to our attention. The clinical evaluation revealed a severe atrophic ridge in the left posterior mandible (Figure 2a). The patient stated that the teeth were extracted 5 years before and the first bicuspid only a month before the consultation. The radiographic evaluation showed the presence of a residual root fragment (Figure 2b). It was also possible to observe the radiolucency of the healing socket of the first bicuspid. The patient was further evaluated with a CBCT to complete the case documentation and data collection. The CBCT showed a severely atrophic ridge with an unfavorable anatomy for dental implant insertion (Figure 3).
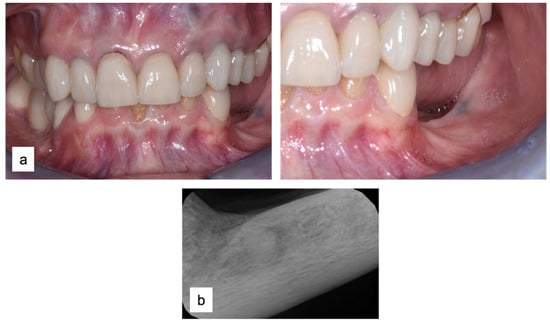
Figure 2.
Consultation visit: (a) clinical evaluation at baseline; (b) periapical radiograph revealed the presence of a residual fragment of the root.
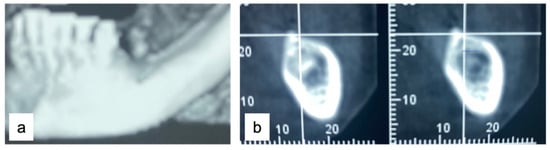
Figure 3.
CBCT at baseline: (a) volumetric reconstruction of the edentulous area; (b) cross-section of the area.
The treatment plan for the edentulous area was formulated in subsequent steps (Table 1). First, mucogingival surgery was required to enhance both the quantity and quality of the soft tissue using a long and thick free gingival graft. This procedure allows for an increase in the quantity of keratinized gingiva and soft tissue thickness in the area, thereby favoring future GBR. After three months, once the soft tissue maturation was complete, the hard tissue regeneration (GBR) of the atrophic ridge was performed. Finally, dental implants were inserted for fixed prosthetic rehabilitation.

Table 1.
Checklist of the therapeutic interventions followed during case management. The time relative to baseline (T0) was reported for each time point (T1, T2, T3, T4, and T5).
The patient did not suffer from any local or systemic contraindications to surgical procedures. The present study was performed in a private clinic, in compliance with the principles of the Declaration of Helsinki on medical protocol and ethics. Written informed consent was obtained for both the clinical procedures and the present study.
3.2. Soft Tissue Augmentation
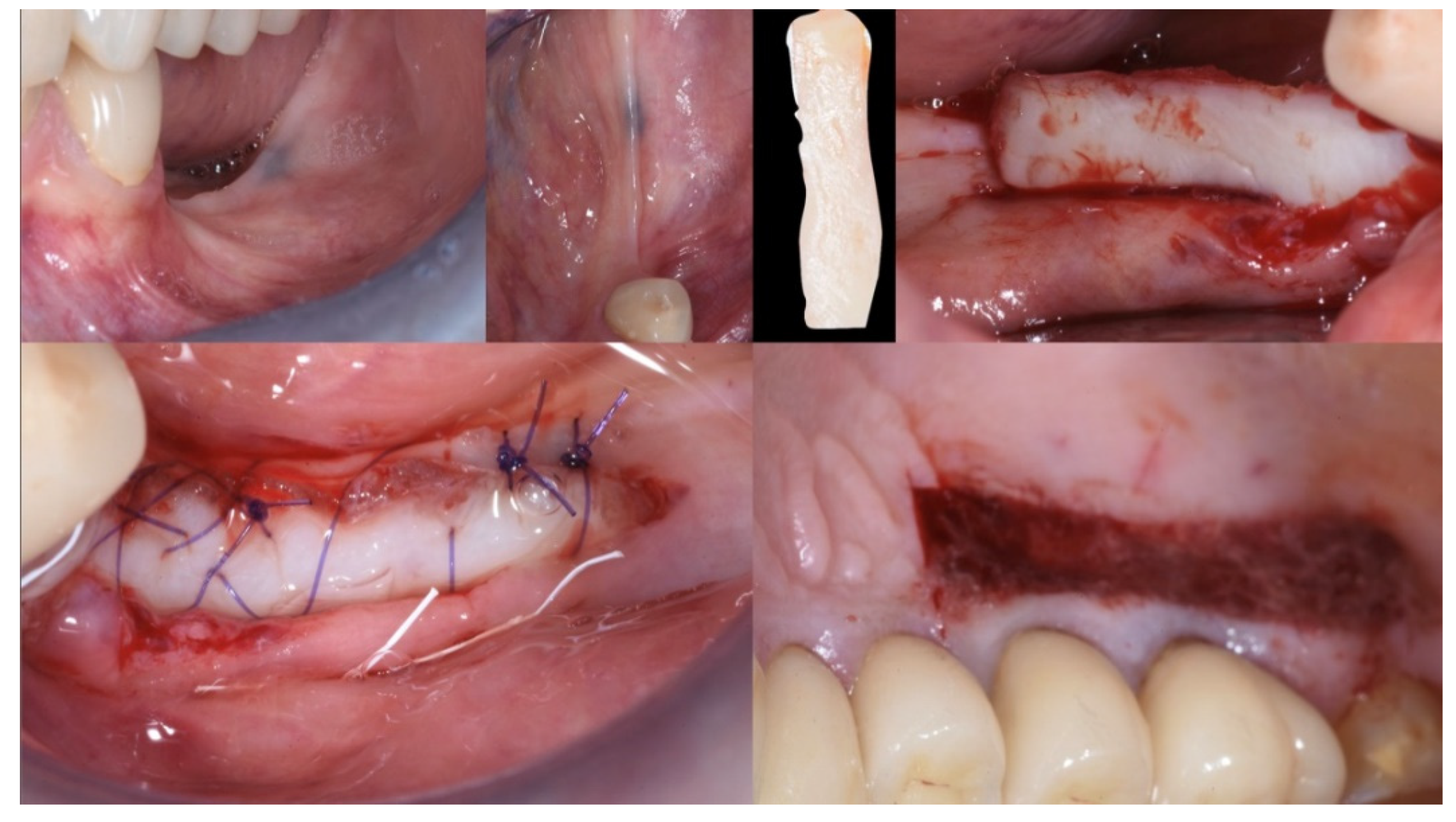
Local anesthesia (articaine 40 mg with adrenaline 1:200,000) was performed both in the donor and recipient sites. The recipient area was prepared with a split-thickness, apically positioned flap anchored to the periosteum with 5.0 PTFE sutures, leaving a bleeding recipient bed for the graft. The free gingival graft (25 mm × 6 mm) was harvested from the distal of the first bicuspid to the distal of the second upper molar. The donor area was protected with a few drops of octyl-butyl cyanoacrylate (Peri-Acryl® 90 HV, Osteophenix, Lecce, Italy). This material protects the wound and has a hemostatic effect. The free gingival graft was sutured at the recipient site with 5.0 polyglactin sutures (Flysorb®, Butterfly, Agrate Brianza, Italy) (Figure 4).
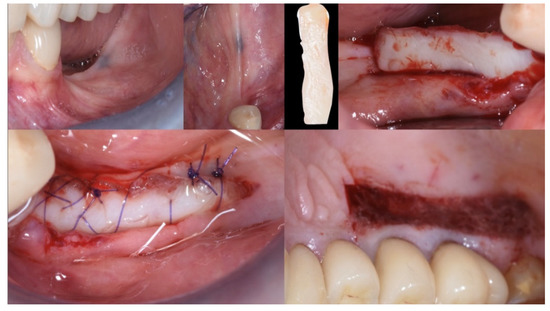
Figure 4.
Mucogingival surgery. A free gingival graft was used to enhance both the quality and quantity of the soft tissue of the edentulous ridge.
Three months after surgery, the graft showed a healthy color and optimal integration into the surrounding tissues. The mucogingival surgery provided good protection for the subsequent hard tissue regeneration (Figure 5).
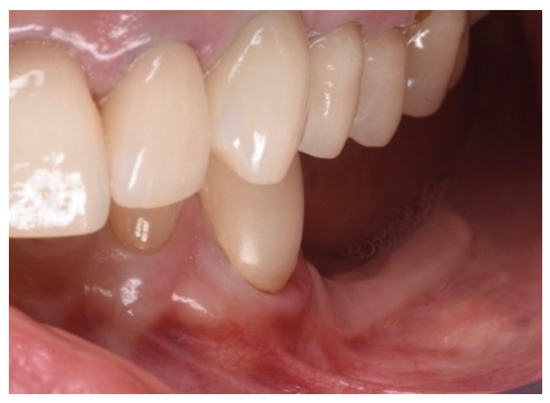
Figure 5.
Soft tissue healing at three months.
3.3. Hard Tissue Augmentation
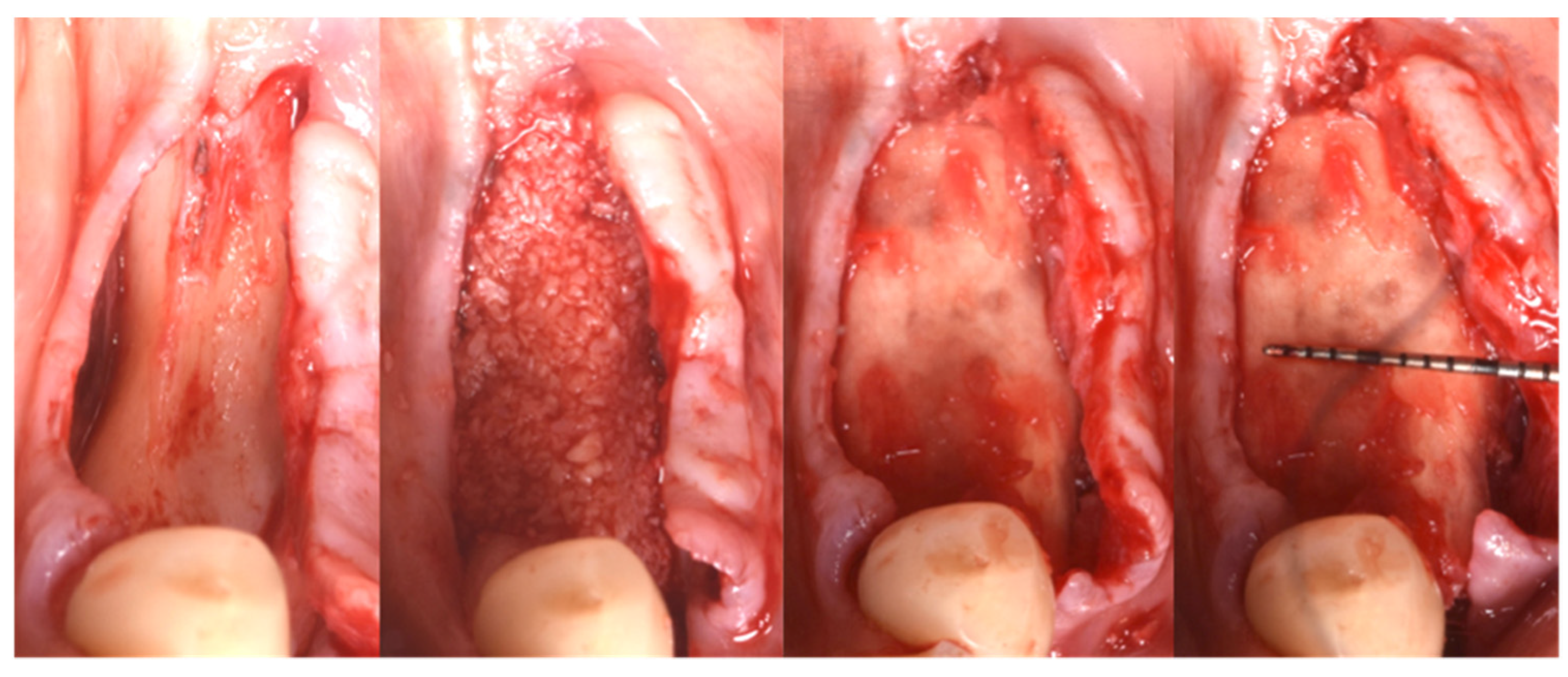
Following local anesthesia (articaine 40 mg with adrenaline 1:200,000), a full-thickness flap was elevated both in the buccal and lingual aspects after a straight incision with a number 12 surgical scalpel. The atrophic ridge was then exposed to allow some autogenous collection from the lingual side. The harvested bone grafts were mixed with the blood clot, the collagenated xenograft, and the fibrin glue (Tisseel®, Baxter, Rome, Italy). The compound was applied as a small consecutive addition to the edentulous ridge, creating a completely new, much larger, and more stable ridge (Figure 6). Passivation of both buccal and lingual flaps was achieved through a soft brushing technique as described by Ronda and Stacchi [30], a very atraumatic way to coronally advance the soft tissue to protect the new volume.
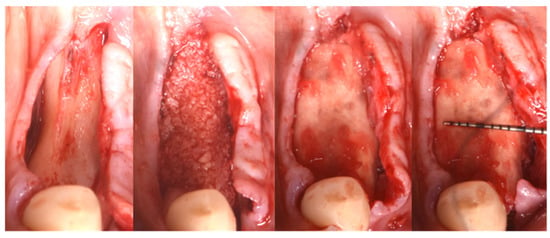
Figure 6.
Guided Bone Regeneration. Flex Cortical Sheet (FCS) was positioned to cover the bone grafts.
The FCS sheet (Osteoxenon®, Flex Cortical Sheet, Bioteck SpA, Arcugnano, Italy) was shaped and trimmed to match the dimension of the regenerated area. The FCS was first tried in place and then glued in its final position to cover the grafts. The final width of the regenerated surgical site was 10 mm (Figure 5).
The mesial and distal papillae of the last tooth in the augmentation area were closed with a double sling 4.0 polyglactin suture (Flysorb®, Butterfly, Agrate Brianza, Italy) [29,31]. One or two horizontal mattresses were placed in the middle of the ridge to put pressure sideways on the new ridge and coronally advance soft tissue. The last is usually a continuous locking mattress, but even a single interrupted suture could work fine.
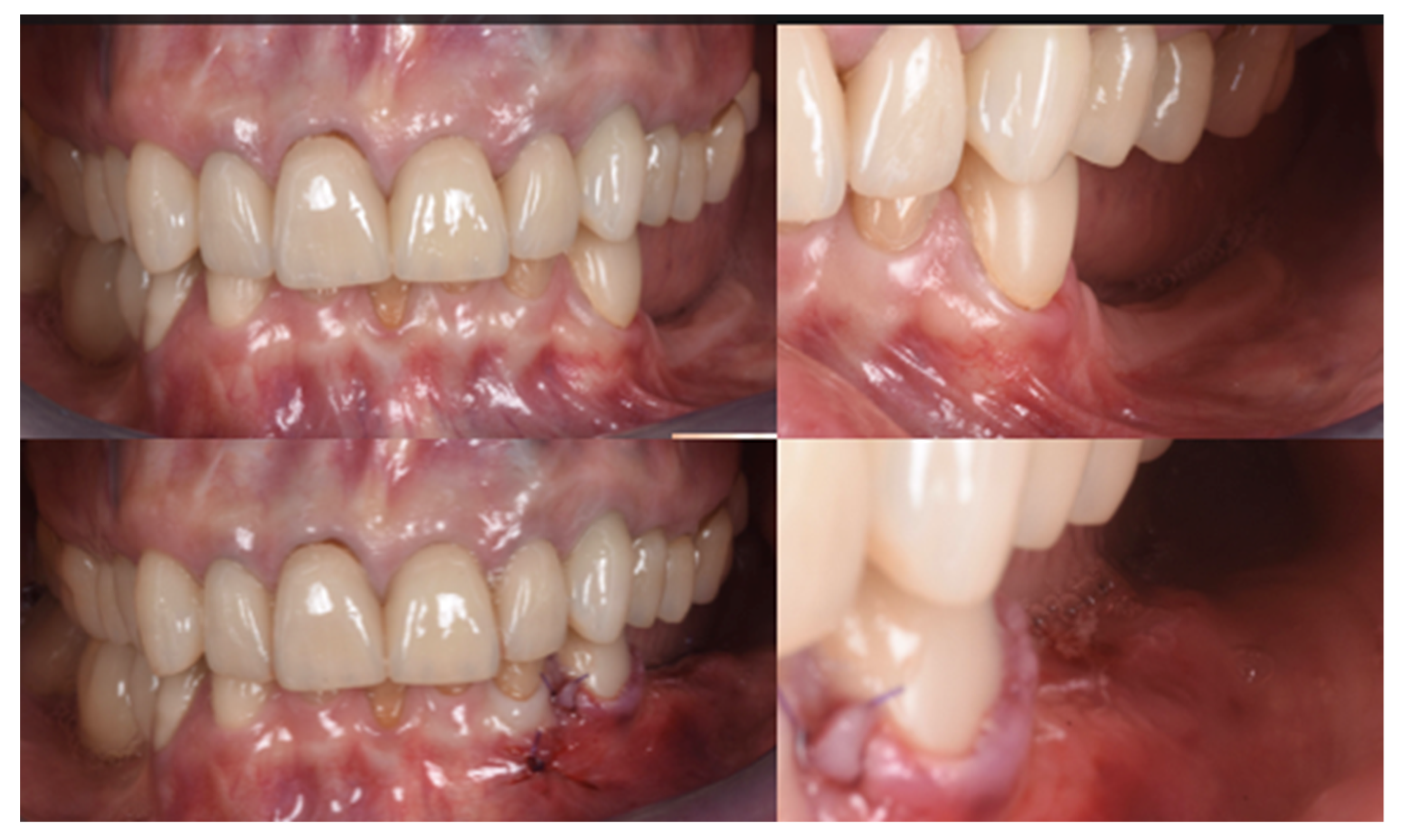
The final assessment showed both vertical and horizontal gains immediately after the surgical procedure (Figure 7). The profile of the atrophic ridge has turned from concave to convex.
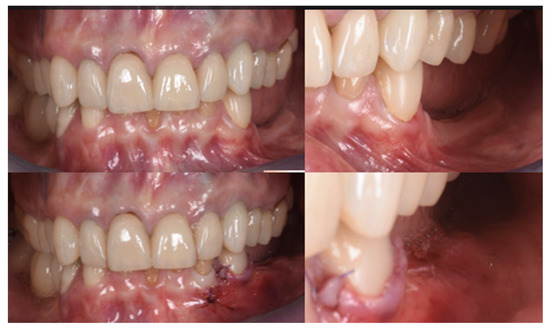
Figure 7.
Comparison between the edentulous ridge before the surgery and immediately after the bone augmentation procedure.
The patient received post-surgical instructions and medications (1 g of amoxicillin twice per day for 6 days and 400 mg of ibuprofen when needed). The patient was also instructed to rinse once a day with 0.12% chlorhexidine before bedtime.
3.4. Implant Insertion
In this case of important bone augmentation, a healing period of 8 months was observed. Indeed, biomaterials, such as FCS, require a longer time than autogenous bone. No complications were experienced during the follow-up. At the end of the healing period, CBCT was performed to plan the implants’ insertion (Figure 8). It was pleasant to observe that the GBR provided the desired volume augmentation for the ridge. The CBCT showed a horizontal and vertical ridge augmentation of 8 mm and 8 mm, respectively.
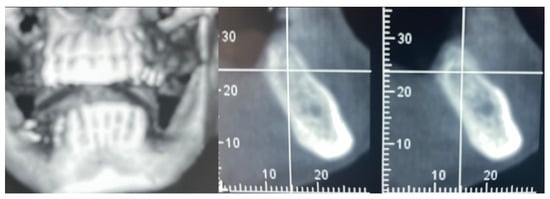
Figure 8.
New volume observed 8 months after surgery.
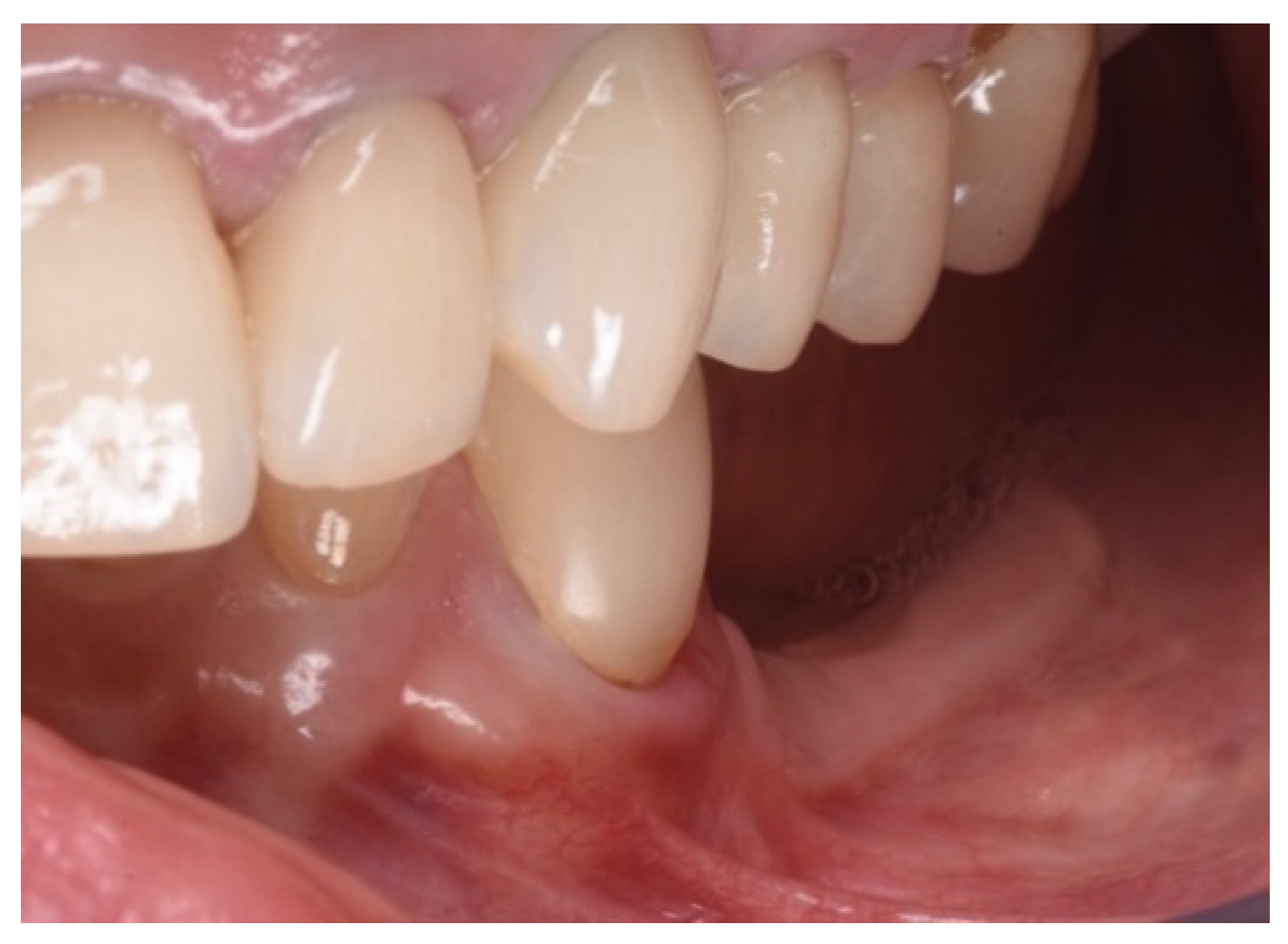
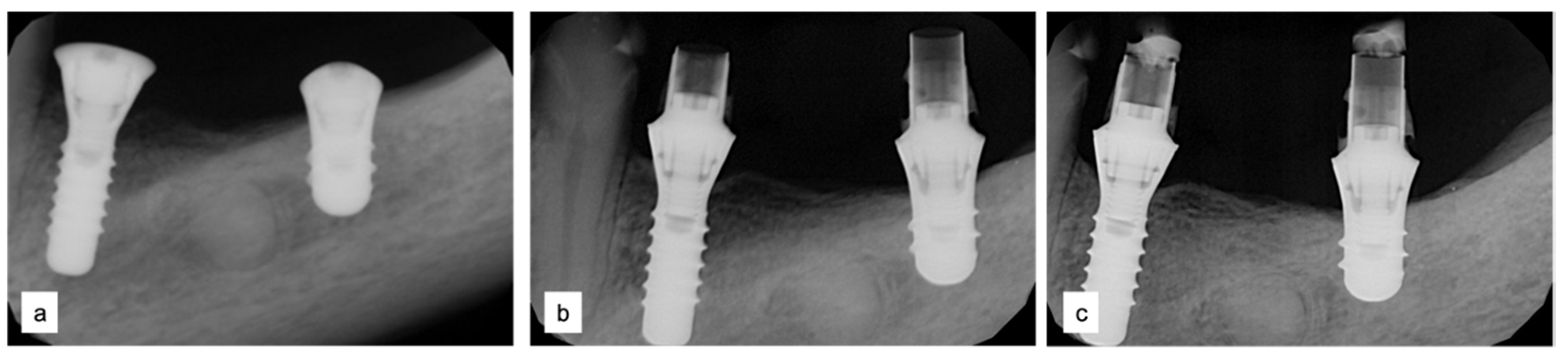
Two implants (Institute Straumann, Basel, Switzerland) of 4 × 12 mm and 5 × 8 mm were inserted in the 3.4 and 3.6 areas, respectively (Figure 9a). The final prosthesis was manufactured with a digital workflow and delivered after three months. The patient was monitored with clinical and radiographic evaluations 12 and 18 months after prosthetic loading. The clinical examination and the digital scans at 12 months of follow-up proved the efficacy of the regenerative approach (Figure 10 and Figure 11). The comparison between 12 (Figure 9b) and 18 month (Figure 9c) radiographs showed stability of the bone crest around the dental implants. Furthermore, a progressive mineralization of the regenerated area was also evident (Figure 9a–c).
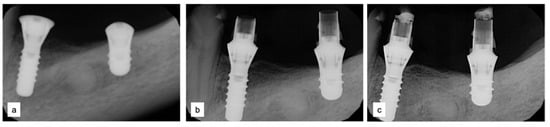
Figure 9.
Periapical radiographs (a) immediately after implant placement (Institute Straumann, Basel, Switzerland), (b) after 12 months, and (c) after 18 months of prosthetic loading.
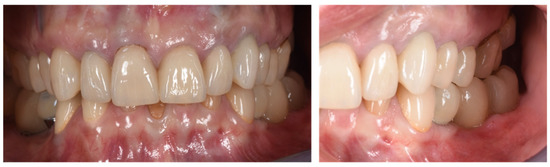
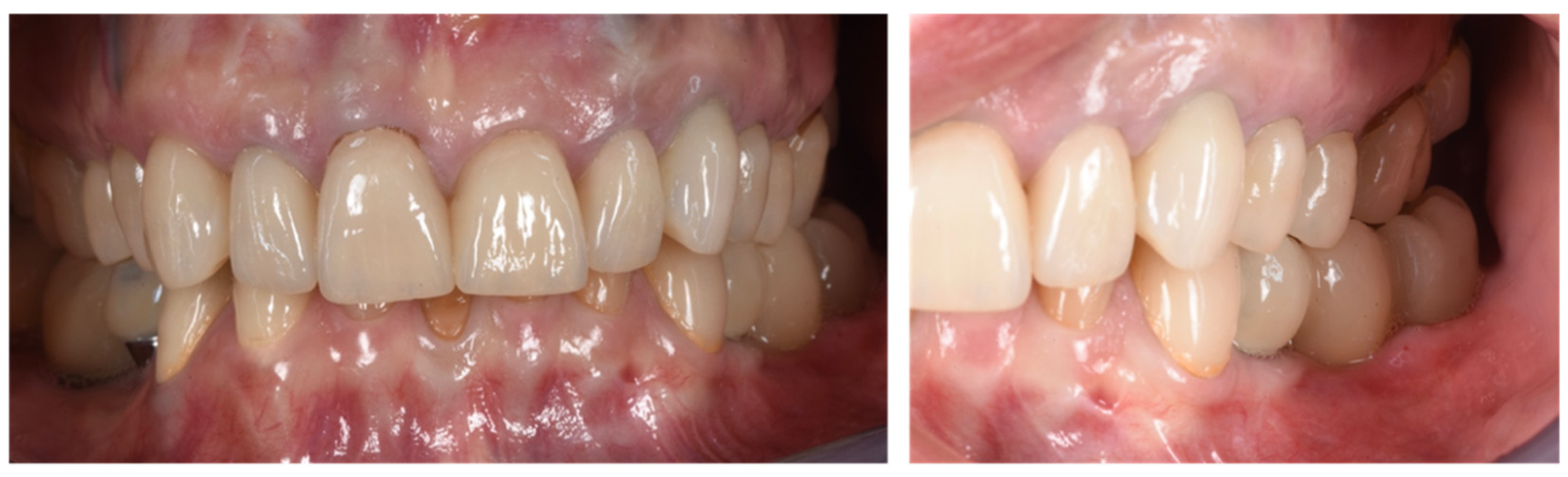
Figure 10.
Clinical evaluation after 18 months of prosthetic loading.
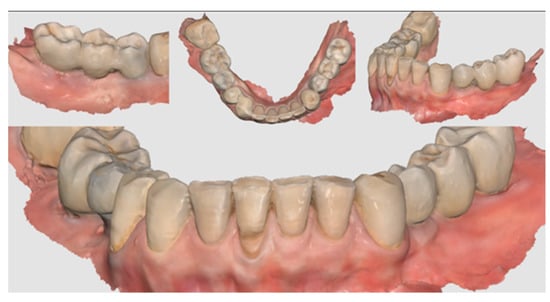
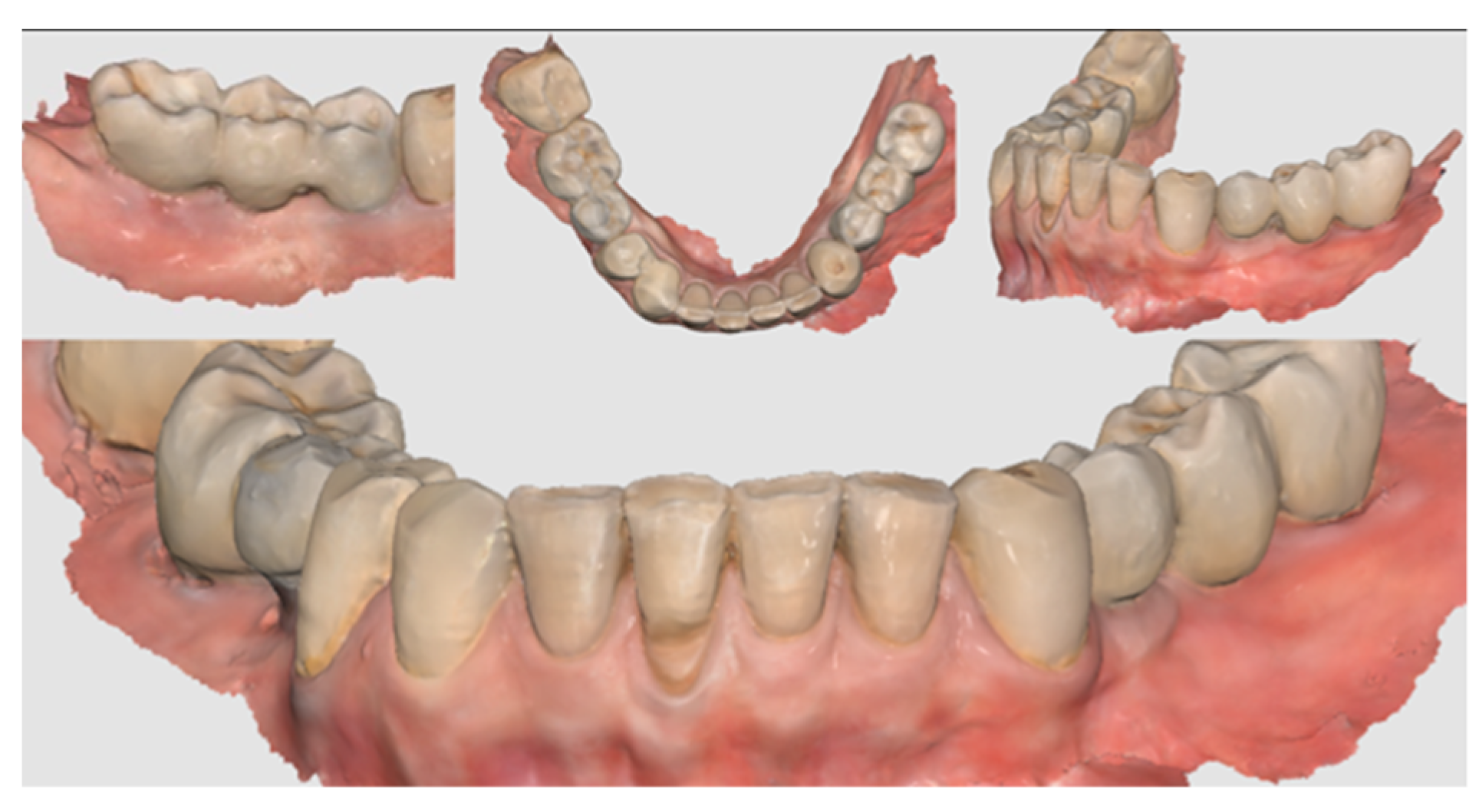
Figure 11.
Scan taken after 18 months of prosthetic loading.
4. Conclusions
Guided Bone Regeneration techniques have evolved in the past thirty years. Today, many different types of barriers and osteoconductive biomaterials can be used, along with surgical techniques using autogenous bone alone or a mix.
Cucchi A. et al. [32,33,34,35] carried out a series of studies comparing the use of dPTFE membranes to titanium meshes, in which the percentages of complications of PTFE membranes varied from 12 to 15%, while conventional titanium meshes or customized CADCAM meshes associated with the use of resorbable membranes showed percentages of complications varying between 4 and 33%.
The cost-benefit ratio of each individual technique should not be underestimated, given the high cost of the techniques described.
Other authors [17,18,36] have demonstrated the efficacy of the use of cortical plates in terms of the results obtained, and a few complications have been reported. With the limitations of case reports but also supported by the cited bibliographic data, we can state that in the case presented, the clinical effectiveness of a second-generation cortical bone sheet (Flex Cortical Sheet) in combination with equine-derived collagen preserved bone granules for GBR was evident. The results showed a horizontal augmentation of 8 mm and a vertical augmentation of 8 mm, both stable, after 18 months of healing. There are five advantages when we compare a bone lamina to other GBR techniques: (1) flexibility and adaptability; (2) when exposed to the oral environment, it tends to hydrolyze, favoring the granulation of soft tissue without developing infection; (3) integrates onsite and/or fully transforms into new bone, and therefore does not require a second stage removal; (4) excellent biological integration; and (5) ease of use even in unexperienced hands. Compared to the first generation of cortical bone laminas, this second generation (Flex Cortical Sheet) displays a more reproducible thickness that guarantees predictable clinical results. Moreover, a Flex Cortical Sheet does not have excessive rigidity or elastic memory, resulting in easier handling and fixation compared to the previous generation. In the present study, the success of the GBR procedure allowed the insertion of a three-unit bridge that satisfied the patient and completed both functional and aesthetic rehabilitation.
In the future, clinical trials comparing FCS with other GBR techniques should be performed to allow for an appropriate comparison.
Author Contributions
Conceptualization, R.R.; methodology, R.R., F.B. and L.M.; software, R.R.; validation, F.B. and L.M.; formal analysis, L.M.; investigation, R.R.; data curation, R.R.; writing—original draft preparation, R.R., L.M., F.B. and E.M.S.; writing—review and editing, R.R., L.M., E.M.S. and F.B.; visualization, L.M.; supervision, F.B.; project administration, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study was conducted in compliance with the principles of the Declaration of Helsinki on medical protocols and ethics. No Ethics Committee approval is required for studies performed in a private setting in Italy.
Informed Consent Statement
Written informed consent was obtained from the patient involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding Authors upon reasonable request.
Acknowledgments
A special thanks to Giorgio Galeano and CDT Maurizio Larosa for the prosthetic restoration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jung, R.E. Bone Augmentation by Means of Barrier Membranes. Periodontology 2000 2003, 33, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Jovanovic, S.A.; Lozada, J.L. Vertical Ridge Augmentation Using Guided Bone Regeneration (GBR) in Three Clinical Scenarios Prior to Implant Placement: A Retrospective Study of 35 Patients 12 to 72 Months after Loading. Int. J. Oral Maxillofac. Implant. 2009, 24, 502–510. [Google Scholar]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical Outcomes of Vertical Bone Augmentation to Enable Dental Implant Placement: A Systematic Review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef]
- Becker, W.; Hujoel, P.; Becker, B.E. Effect of Barrier Membranes and Autologous Bone Grafts on Ridge Width Preservation around Implants. Clin. Implant Dent. Relat. Res. 2002, 4, 143–149. [Google Scholar] [CrossRef]
- Gallo, P.; Díaz-Báez, D. Management Of 80 Complications In Vertical And Horizontal Ridge Augmentation With Nonresorbable Membrane (d-PTFE): A Cross-Sectional Study. Int. J. Oral Maxillofac. Implant. 2019, 34, 927–935. [Google Scholar] [CrossRef]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Is Promoted by the Molecular Events in the Membrane Compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Rossi, R.; Foce, E. Reconstruction of a Horizontal and Vertical Bone Defect Using The Cortical Lamina Technique. Arch. Med. Res. 2019, 7, 1–11. [Google Scholar]
- Rossi, R.; Foce, E.; Scolavino, S. The Cortical Lamina Technique: A New Option for Alveolare Ridge Augmentation, Procedure, Protocol and Case Report. Rev. Dent. Liban. Leban. Dent. Mag. 2017, 52, 35–41. [Google Scholar]
- Rossi, R.; Ghezzi, C.; Tomecek, M. Cortical Lamina: A New Device for the Treatment of Moderate and Severe Tridimensional Bone and Soft Tissue Defects. Int. J. Esthet. Dent. 2020, 15, 454–473. [Google Scholar] [PubMed]
- Di Stefano, D.A.; Piattelli, A.; Zaniol, T.; Iezzi, G. Implant and Prosthetic Success Following Peri-Implant Guided Bone Regeneration in the Esthetic Zone Using an Equine Cortical Bone Membrane and an Equine Enzyme-Treated Bone Graft: A Retrospective Study with 9-Year Follow-Up. Int. J. Oral Maxillofac. Implant. 2020, 35, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Garagiola, U.; Bassi, M.A. Preserving the Bone Profile in Anterior Maxilla Using an Equine Cortical Bone Membrane and an Equine Enzyme-Treated Bone Graft: A Case Report with 5-Year Follow-Up. J. Contemp. Dent. Pract. 2017, 18, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Cazzaniga, A.; Andreasi Bassi, M.; Ludovichetti, M.; Ammirabile, G.; Celletti, R. The Use of Cortical Heterologous Sheets for Sinus Lift Bone Grafting: A Modification of Tulasne’s Technique with 7-Year Follow-Up. Int. J. Immunopathol. Pharmacol. 2013, 26, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Modoni, M.; Monterubbianesi, R.; Dallari, G.; Memè, L. The ‘Guided Tissue Regeneration (GTR) Effect’ of Guided Bone Regeneration (GBR) with the Use of Bone Lamina: A Report of Three Cases with More than 36 Months of Follow-Up. Appl. Sci. 2022, 12, 11247. [Google Scholar] [CrossRef]
- Rossi, R.; Rancitelli, D.; Poli, P.P.; Rasia Dal Polo, M.; Nannmark, U.; Maiorana, C. The Use of a Collagenated Porcine Cortical Lamina in the Reconstruction of Alveolar Ridge Defects. A Clinical and Histological Study. Minerva. Stomatol. 2016, 65, 257–268. [Google Scholar]
- Foti, V.; Savio, D.; Rossi, R. One-Time Cortical Lamina: A New Technique for Horizontal Ridge Augmentation. A Case Series. Br. J. Healthc. Med. Res. 2021, 8, 22–30. [Google Scholar] [CrossRef]
- Hinze, M.; Vrielinck, L.; Thalmair, T.; Wachtel, H.; Bolz, W. Zygomatic Implant Placement in Conjunction with Sinus Bone Grafting: The “Extended Sinus Elevation Technique.” A Case-Cohort Study. Int. J. Oral Maxillofac. Implant. 2013, 28, e376–e385. [Google Scholar] [CrossRef][Green Version]
- Grenga, P.L.; Reale, G.; Cofone, C.; Meduri, A.; Ceruti, P.; Grenga, R. Hess Area Ratio and Diplopia: Evaluation of 30 Patients Undergoing Surgical Repair for Orbital Blow-out Fracture. Ophthalmic. Plast. Reconstr. Surg. 2009, 25, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Rinna, C.; Ungari, C.; Saltarel, A.; Cassoni, A.; Reale, G. Orbital Floor Restoration. J. Craniofac. Surg. 2005, 16, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Tonk, G.; Yadav, P.K.; Agarwal, S.; Jamoh, K. Donor Site Morbidity in Autologous Bone Grafting—A Comparison between Different Techniques of Anterior Iliac Crest Bone Harvesting: A Prospective Study. J. Orthop. Trauma Rehabil. 2022, 29, 22104917221092164. [Google Scholar] [CrossRef]
- Meme, L.; Santarelli, A.; Marzo, G.; Emanuelli, M.; Nocini, P.F.; Bertossi, D.; Putignano, A.; Dioguardi, M.; Lo Muzio, L.; Bambini, F. Novel Hydroxyapatite Biomaterial Covalently Linked to Raloxifene. Int. J. Immunopathol. Pharmacol. 2014, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of Periodontal Tissues: Combinations of Barrier Membranes and Grafting Materials-Biological Foundation and Preclinical Evidence: A Systematic Review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Simion, M.; Dahlin, C.; Rocchietta, I.; Stavropoulos, A.; Sanchez, R.; Karring, T. Vertical Ridge Augmentation with Guided Bone Regeneration in Association with Dental Implants: An Experimental Study in Dogs. Clin. Oral Implant. Res. 2007, 18, 86–94. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Gastaldi, G.; Vinci, R.; Cinci, L.; Pieri, L.; Gherlone, E. Histomorphometric Comparison of Enzyme-Deantigenic Equine Bone and Anorganic Bovine Bone in Sinus Augmentation: A Randomized Clinical Trial with 3-Year Follow-Up. Int. J. Oral Maxillofac. Implant. 2015, 30, 1161–1167. [Google Scholar] [CrossRef]
- Perrotti, V.; Nicholls, B.M.; Piattelli, A. Human Osteoclast Formation and Activity on an Equine Spongy Bone Substitute. Clin. Oral Implant. Res. 2009, 20, 17–23. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Zaniol, T.; Cinci, L.; Pieri, L. Chemical, Clinical and Histomorphometric Comparison between Equine Bone Manufactured through Enzymatic Antigen-Elimination and Bovine Bone Made Non-Antigenic Using a High-Temperature Process in Post-Extractive Socket Grafting. A Comparative Retrospective Clinical Study. Dent. J. 2019, 7, 70. [Google Scholar] [CrossRef]
- Ronda, M.; Stacchi, C. A Novel Approach for the Coronal Advancement of the Buccal Flap. Int. J. Periodontics Restor. Dent. 2015, 35, 795–801. [Google Scholar] [CrossRef]
- Wachtel, H.; Fickl, S.; Zuhr, O.; Hürzeler, M.B. The Double-Sling Suture: A Modified Technique for Primary Wound Closure. Eur. J. Esthet. Dent. 2006, 1, 314–324. [Google Scholar] [PubMed]
- Grassi, A.; Memè, L.; Strappa, E.M. Emanuele Martini and Fabrizio Bambini: Modified Periosteal Inhibition (MPI) Technique for Extraction Sockets: A Case Series Report. Appl. Sci. 2022, 12, 12292. [Google Scholar] [CrossRef]
- Rechtin, M.; Broccoli, N.; Krishan, D.G.; Phero, J.A. Review Use of Tisseel, a fibrin sealant, for particulate graft stabilization. J. Oral Maxillofac. Surg. 2020, 78, e2–e5. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1411–1424. [Google Scholar] [CrossRef]
- Cucchi, A.; Bianchi, A.; Calamai, P.; Rinaldi, L.; Mangano, F.; Vignudelli, E.; Corinaldesi, G. Clinical and volumetric outcomes after vertical ridge augmentation using computer-aided-design/computer-aided manufacturing (CAD/CAM) customized titanium meshes: A pilot study. BMC Oral Health 2020, 20, 219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).