Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea europaea L., cv. “Koroneiki”)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Soil Sampling and Laboratory Analyses
2.3. Plant Growth
2.4. Leaf Nutrient Concentrations and Total Plant Nutrient Content
2.5. Gas Exchange Measurements and Intrinsic Water Use Efficiency (WUEi)
2.6. Sugar Fractionation
2.7. Statistical Analysis
3. Results
3.1. Nutrient Content of the Two Organic Amendments and Properties of the Soil Mixtures (Substrates)
3.2. Plant Growth
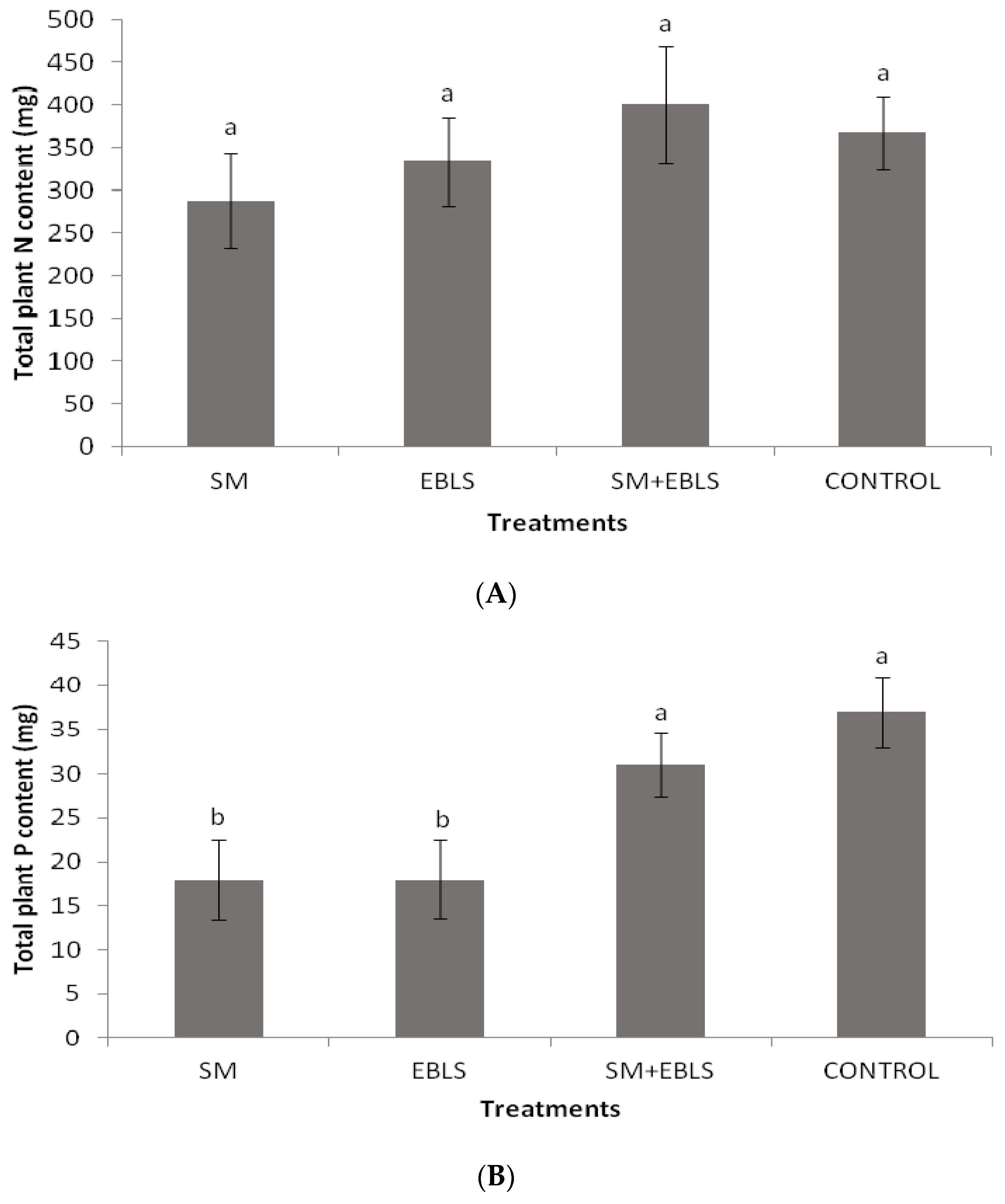
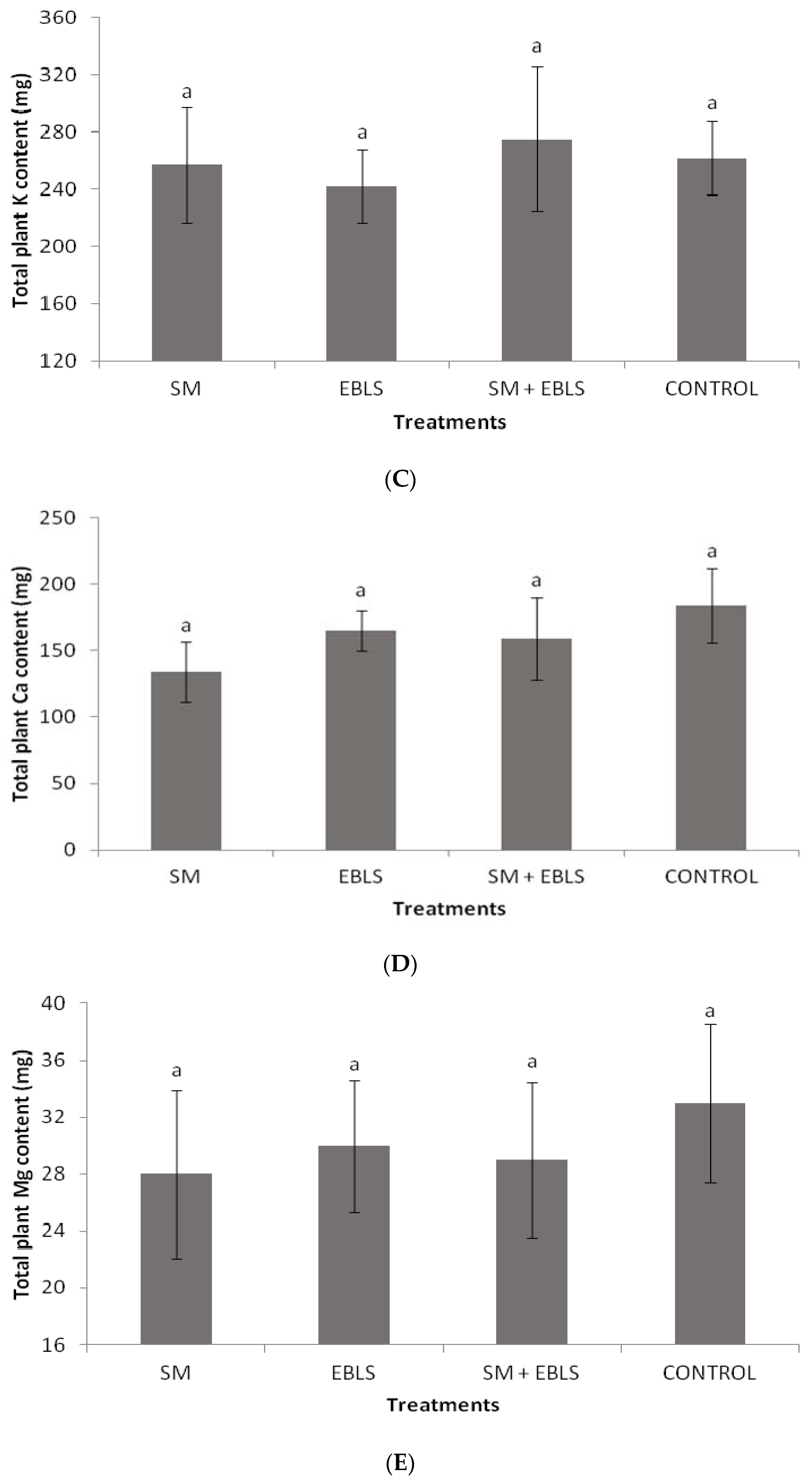
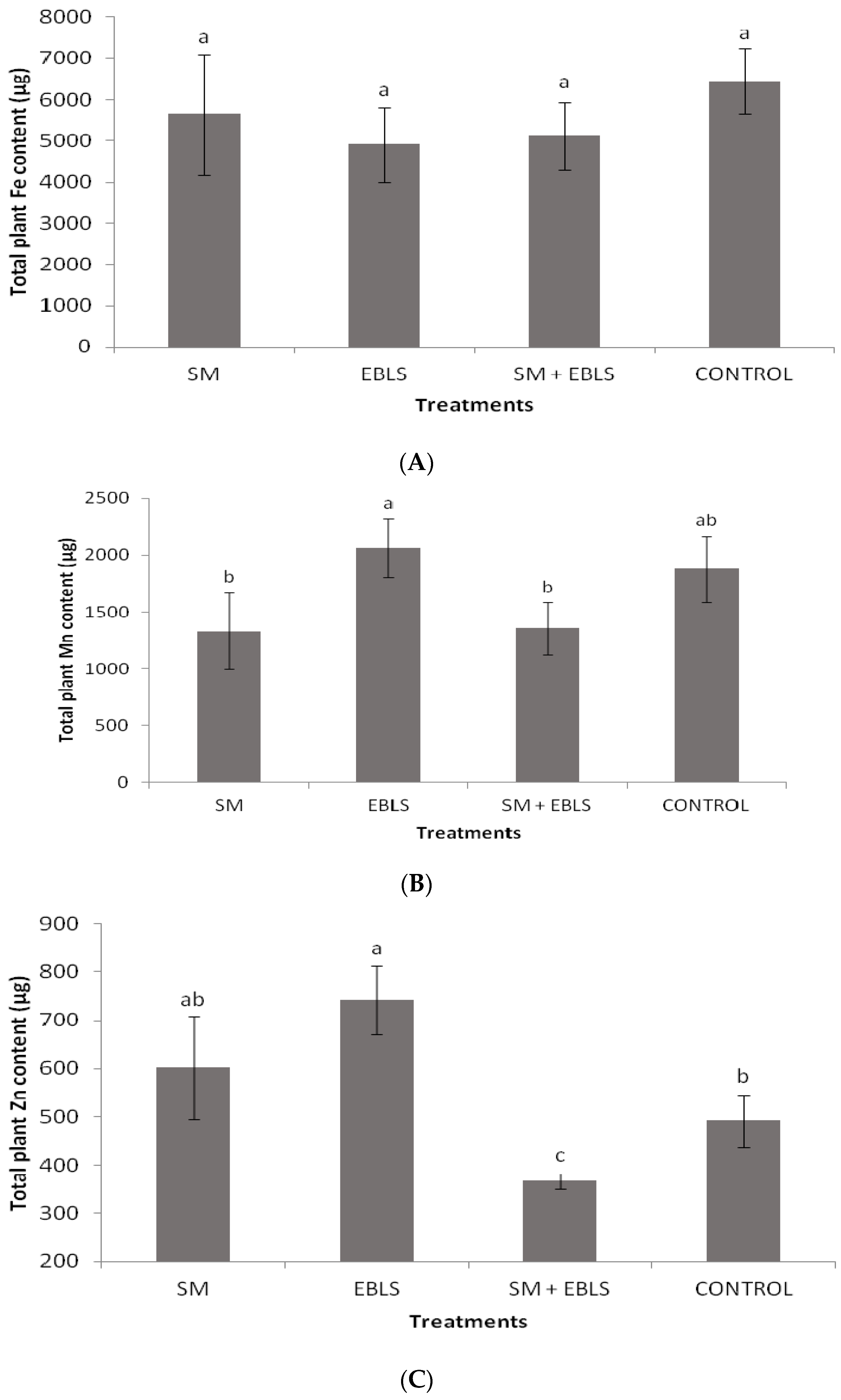
3.3. Foliar Nutrient Concentrations and Total Plant Nutrient Content
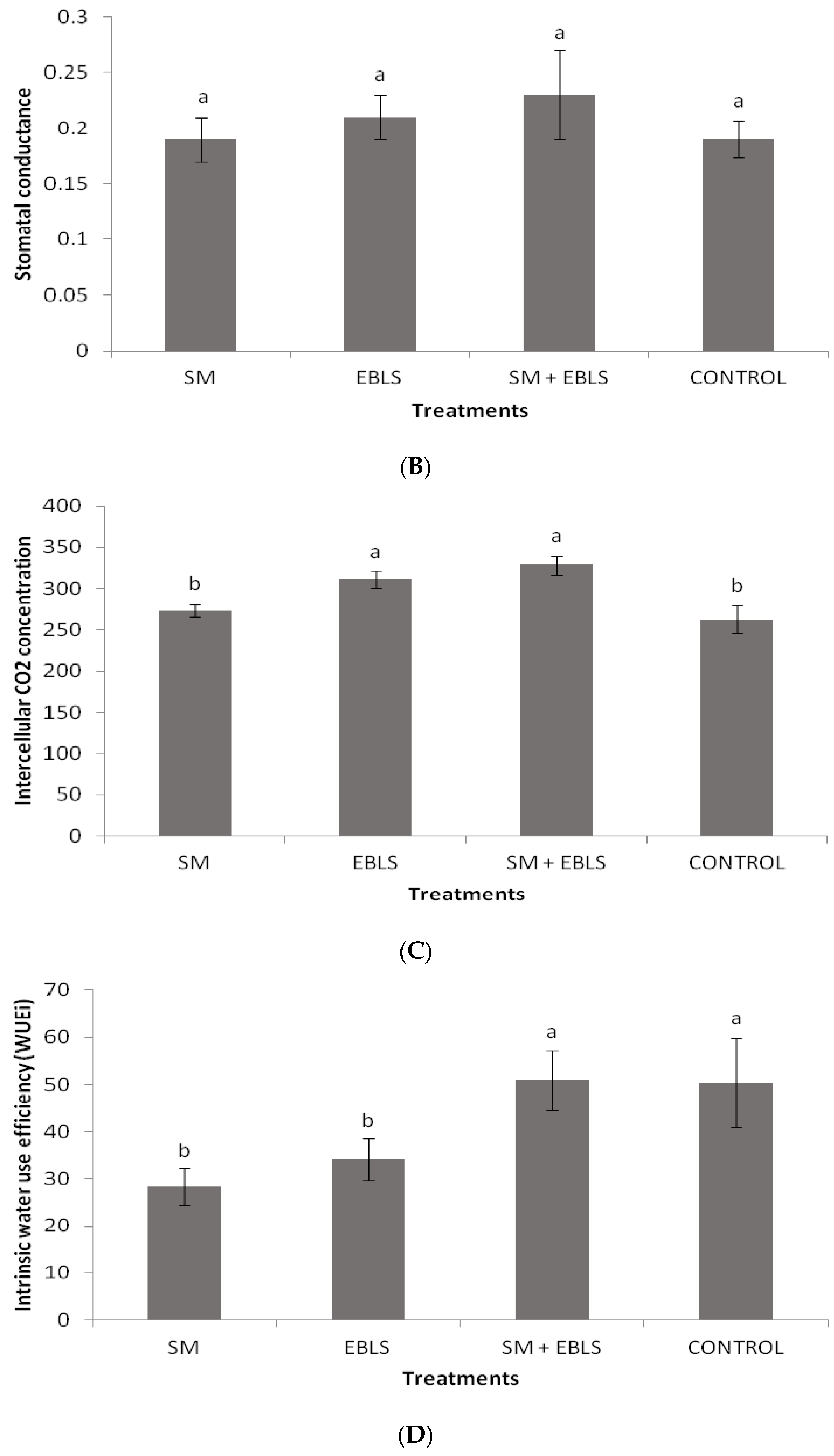
3.4. Gas Exchange Measurements and Intrinsic Water Use Efficiency (WUEi)
3.5. Leaf Sugar Concentrations
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatzistathis, T.; Papaioannou, A.; Gasparatos, D.; Molassiotis, A. From which soil metal fractions Fe, Mn, Zn and Cu are taken up by olive trees (Olea europaea L., cv. ‘Chondrolia Chalkidikis’) in organic groves? J. Environ. Manag. 2017, 203, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosys. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Elfalleh, W.; Belayadi, H.; Haddad, M. Effect of palm waste compost on forage alfalfa growth, yield, seed yield and minerals uptake. Int. J. Recycl. Org. Waste Agricult. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papadakis, I.E.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a controlled release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv. ‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Arrobas, M.; De Almeida, S.F.; Raimundo, S.; Da Silva Domingues, L.; Rodrigues, M.A. Leonardites rich in humic and fulvic acids had little effect on tissue elemental composition and dry matter yield in pot-grown olive cuttings. Soil Syst. 2022, 6, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, H.; Ma, M.; Bu, Y.; Shao, W.; Huang, W.; Ji, Q.; Yao, Y. An apple fruit fermentation (AFF) treatment improves the composition of the rhizosphere microbial community and growth of strawberry (Fragaria x ananassa Duch ‘Benihoppe’) seedlings. PLoS ONE 2016, 18, e0164776. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Q.; Feng, Z.; Liu, Z.; Li, H.; Sun, Y.; Liu, C.; Lai, H. Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 2020, 147, 103426. [Google Scholar] [CrossRef]
- Jindo, K.; Chocano, C.; Melgares de Aguilar, J.; Gonzalez, D.; Hernandez, T.; Garcia, C. Impact of compost application during 5 years on crop production, soil microbial activity, carbon fraction and humification process. Com. Soil Sci. Plant Anal. 2016, 47, 1907–1919. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kopacki, M.; Kiczorowska, B. The response of Šampion trees growing on different rootstocks to applied organic mulches and mycorrhizal substrate in the orchard. Sci. Hortic. 2018, 241, 267–274. [Google Scholar] [CrossRef]
- Leonel, S.; Tecchio, M.A. Cattle manure fertilization increases fig yield. Sci. Agric. 2009, 66, 806–811. [Google Scholar] [CrossRef]
- Baldi, E.; Toselli, M.; Eissenstat, D.M.; Marangoni, B. Organic fertilization leads to increased peach root production and lifespan. Tree Physiol. 2010, 30, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pineiro, A.; Albarran, A.; Rato Nunes, J.M.; Pena, D.; Cabrera, D. Long term impacts of de-oiled two-phase olive mill waste on soil chemical properties, enzyme activities and productivity in an olive grove. Soil Till. Res. 2011, 114, 175–182. [Google Scholar] [CrossRef]
- Garcia-Ruiz, R.; Ochoa, M.V.; Hinojosa, M.B.; Gomez-Munoz, B. Improved soil quality after 16 years of olive mill pomace application in olive oil groves. Agron. Sustain. Dev. 2013, 32, 803–810. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, Y.; Ben-Gal, A.; Dag, A. Sustainable management of olive orchard nutrition: A review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef]
- Rodrigues Prates, A.; Renée Coscione, A.; Carvalho Minhoto Teixeira Filho, M.; Gasparoti Miranda, B.; Arf, O.; Hamilton Abreu-Junior, C.; Carvalho Oliveira, F.; Moreira, A.; Shintate Galindo, F.; Márcia Pereira Sartori, M.; et al. Composted sewage sludge enhances soybean production and agronomic performance in naturally infertile soils (Cerrado region, Brazil). Agronomy 2020, 10, 1677. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic fertilization and tree orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Bargougui, L.; Chaieb, M.; Mekki, A. Physiological and growth responses of young plants of three native olive cultivars to olive waste compost. J. Plant Nutr. 2022, 45, 2478–2498. [Google Scholar] [CrossRef]
- Soares, P.R.; Lopes, M.A.R.; Conceicao, M.A.; Santos, D.V.S.; Oliveira, M.A. Sustainable integration of laying hens with crops in organic farming: A review. Agroecol. Sustain. Food Syst. 2022, 46, 969–1001. [Google Scholar] [CrossRef]
- Therios, I. Mineral Nutrition of Plants; Dedousi Publications: Thessaloniki, Greece, 1996. (In Greek) [Google Scholar]
- El Gammal, O.H.M.; Salama, A.S.M. Effect of Sheep Manure Application Rate and Method on Growth, Fruiting and Fruit Quality of Balady Guava Trees Grown Under Mid-Sinai Conditions. J. Agric. Vet. Sci. 2016, 9, 59–72. [Google Scholar]
- Therios, I. Olives. Crop Production Science in Horticulture; C.A.B. International: Wallingford, UK, 2009. [Google Scholar]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Gee, G.; Bauder, J. Particle-Size Analysis. In Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 539–547. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total Nitrogen. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 595–615. [Google Scholar]
- Olsen, S.; Sommers, L. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Thomas, G.W. Exchangeable Cations Methods of Soil Analysis. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; pp. 159–166. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Hansen, T.H.; De Bang, T.C.; Laursen, K.H.; Pedas, P.; Husted, S.; Schjoerring, J.K. Multielement Plant Tissue Analysis Using ICP Spectrometry. In Plant Mineral Nutrients. Methods in Molecular Biology (Methods and Protocols); Maathuis, F., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 953. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; Division of Agricultural Sciences, University of California: Riverside, CA, USA, 1961; p. 309. [Google Scholar]
- Vemmos, S.N. Carbohydrate content of inflorescent buds of defruited and fruiting pistachio (Pistachia vera L.) branches in relation to biennial bearing. J. Hort. Sci. Biotech. 1999, 74, 94–100. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Ye, G.; Lin, Y.; Kuzyakov, Y.; Liu, D.; Luo, J.; Lindsey, S.; Wang, W.; Fan, J.; Ding, W. Manure over crop residues increases soil organic matter but decreases microbial necromass relative contribution in upland Ultisols: Results of a 27-year field experiment. Soil. Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Nasini, L.; Gigliotti, G.; Balduccini, M.A.; Federici, E.; Cenci, G.; Proietti, P. Effect of solid olive-mill waste amendment on soil fertility and olive (Olea europaea L.) tree activity. Agric. Ecos. Environ. 2013, 164, 292–297. [Google Scholar] [CrossRef]
- Garcia-Orenes, F.; Roldan, A.; Morugan-Coronado, A.; Linares, C.; Cerda, A.; Caravaca, F. Organic fertilization in traditional Mediterranean grapevine orchards mediates changes in soil microbial community structure and enhances soil fertility. Land Degrad. Develop. 2016, 27, 1622–1628. [Google Scholar] [CrossRef]
- Perez-Murcia, M.D.; Bustamante, M.A.; Orden, L.; Rubio, R.; Agullo, E.; Carbonell-Baracchina, A.A.; Moral, R. Use of agri-food composts in almond organic production: Effects on soil and fruit quality. Agronomy 2021, 11, 536. [Google Scholar] [CrossRef]
- Mazeh, M.; Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Tucci, M.; Lodolini, E.A..; Rosati, A.; Famiani, F. Use of an organic fertilizer also having a biostimulant action to promote the growth of young olive trees. Agriculture 2021, 11, 593. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Yang, Y.; Zhao, S.; Zhang, Y.; Wang, X. Effect of composted manure plus chemical fertilizer application on aridity response and productivity of apple trees on the loess plateau, China. Arid Land Res. Manag. 2017, 31, 388–403. [Google Scholar] [CrossRef]
- Zhu, L.D.; Shao, X.H.; Zhang, Y.C.; Zhang, H.; Hou, M.M. Effects of K fertilizer application on photosynthesis and seedling growth of sweet potato under drought stress. J. Food Agric. Environ. 2012, 10, 487–491. [Google Scholar]
- Saykhul, A.; Chatzistathis, T.; Chatzissavvidis, C.; Koundouras, S.; Therios, I.; Dimassi, K. Potassium utilization efficiency of three olive cultivars grown in a hydroponic system. Sci. Hortic. 2013, 162, 55–62. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhang, H.; Jing, Q.; Wu, Y.X.; Xiao, S.Z.; Wang, M.M. Effect of mycorrhizal fungi inoculation and nitrogen fertilization on physiological characteristics, growth and nitrogen and phosphorus uptake of wheat under two distinct water regimes. Agric. Res. Arid Areas 2019, 37, 214–220. [Google Scholar]
- Diekmann, F.; Fischbeck, G. Differences in wheat cultivar response to nitrogen supply. II: Differences in N-metabolism-related traits. J. Agron. Crop Sci. 2005, 191, 362–376. [Google Scholar]
- Braun, H.; Rezende Fontes, P.C.; Da Silva, T.P.; Finger, L.F.; Cecon, P.R.; Sato Ferreira, A.P. Carbohydrates concentration in leaves of potato plants affected by nitrogen fertilization rates. Rev. Ceres Vicosa 2016, 63, 241–248. [Google Scholar] [CrossRef]
- Baghour, M.; Sanchez, E.; Ruiz, J.M.; Romero, L. Metabolism and efficiency of phosphorus utilization during senescence in pepper plants: Response to nitrogenous and potassium fertilization. J. Plant Nutr. 2001, 24, 1731–1743. [Google Scholar] [CrossRef]


| Organic Material | N | P | K | Ca | Mg | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| % D.W. | mg kg−1 D.W. | ||||||||
| SM | 2.63 | 0.16 | 2.99 | 1.25 | 1.32 | 3142 | 283 | 81 | 18 |
| EBLS | 1.25 | 0.08 | 0.31 | 1.11 | 0.36 | 4122 | 1205 | 90 | 16 |
| Treatments | pH | Organic Matter | Kjeldahl N | C/N | Olsen P (mg/100 g Soil) | Ca | Mg | K | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % soil | cmol kg−1 soil | mg kg−1 soil | |||||||||
| Soil + SM | 7.63 | 3.58 | 0.24 | 8.65 | 3.20 | 34.68 | 2.73 | 1.16 | 2.38 | 2.52 | 0.66 |
| Soil + EBLS | 7.67 | 2.95 | 0.20 | 8.56 | 1.88 | 48.89 | 3.20 | 0.62 | 3.08 | 3.30 | 0.92 |
| Soil + SM + EBLS | 7.67 | 5.12 | 0.25 | 11.88 | 2.00 | 55.63 | 3.90 | 1.18 | 2.86 | 3.80 | 1.25 |
| CONTROL soil | 7.68 | 1.55 | 0.17 | 5.29 | 0.74 | 60.95 | 3.35 | 0.53 | 2.70 | 2.48 | 0.45 |
| Main Shoot Length (cm) | Total Biomass (g) | Apical Leaves’ Weight (g) | Basal Leaves’ Weight (g) | Apical Stems’ Weight (g) | Basal Stems’ Weight (g) | Root Weight (g) | Shoot/Root | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | F.W. | D.W. | D.W. | ||
| SM | 121.33 a | 54.08 a | 17.84 a | 8.91 b | 2.99 b | 11.25 a | 4.18 a | 5.03 b | 1.79 b | 11.00 a | 4.60 b | 17.89 a | 4.28 a | 3.09 b |
| EBLS | 114.83 a | 60.55 a | 21.37 a | 13.84 a | 5.21 a | 9.58 a | 3.77 a | 6.78 a | 2.75 a | 11.37 a | 5.12 ab | 18.98 a | 4.51 a | 3.96 a |
| SM + EBLS | 116.58 a | 58.57 a | 20.27 a | 12.61 a | 5.17 a | 10.22 a | 3.86 a | 6.49 a | 2.48 ab | 11.52 a | 4.97 b | 17.73 a | 4.44 a | 3.50 ab |
| CONTROL | 113.50 a | 60.57 a | 21.33 a | 10.29 ab | 4.53 a | 10.16 a | 4.06 a | 6.61 a | 2.98 a | 12.87 a | 6.68 a | 21.03 a | 5.07 a | 3.67 a |
| N | P | K | Ca | Mg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % D.W. | ||||||||||
| Basal leaves | Apical leaves | Basal leaves | Apical leaves | Basal leaves | Apical leaves | Basal leaves | Apical leaves | Basal leaves | Apical leaves | |
| SM | 2.23 ab | 2.31 ab | 0.16 a | 0.15 a | 1.64 a | 1.89 a | 1.33 a | 0.87 a | 0.18 a | 0.15 a |
| EBLS | 1.86 b | 1.87 b | 0.15 a | 0.13 a | 1.31 ab | 1.42 ab | 1.24 a | 0.84 a | 0.15 a | 0.13 a |
| SM + EBLS | 2.50 a | 2.55 a | 0.18 a | 0.17 a | 1.53 ab | 1.63 ab | 1.38 a | 0.88 a | 0.17 a | 0.14 a |
| CONTROL | 1.88 b | 1.81 b | 0.17 a | 0.16 a | 1.19 b | 1.23 b | 1.10 a | 0.77 a | 0.14 a | 0.13 a |
| Fe | Mn | Zn | Cu | |||||
|---|---|---|---|---|---|---|---|---|
| mg kg−1 D.W. | ||||||||
| Basal leaves | Apical leaves | Basal leaves | Apical leaves | Basal leaves | Apical leaves | Basal leaves | Apical leaves | |
| SM | 104 a | 59 a | 55 a | 57 a | 39 a | 42 a | 2.6 b | 3.0 ab |
| EBLS | 79 ab | 68 a | 66 a | 61 a | 35 a | 41 a | 2.0 b | 2.5 b |
| SM + EBLS | 48 b | 70 a | 65 a | 53 a | 24 b | 23 b | 2.8 ab | 3.4 a |
| CONTROL | 104 a | 59 a | 70 a | 60 a | 21 b | 23 b | 3.8 a | 3.7 a |
| Treatments | Suc. | Glu. | Fru. | Man. | Suc./Fru. + Glu. | Non-Translocated Sugars (Fru. + Glu.) | Translocated Sugars (Suc. + Man.) | Translocated/Total Sugars | Translocated/Non-Translocated Sugars | Total Sugars |
|---|---|---|---|---|---|---|---|---|---|---|
| % D.W. | ||||||||||
| SM | 1.74 a | 2.59 b | 0.60 ab | 1.87 b | 0.48 a | 3.19 b | 3.62 ab | 0.38 a | 1.11 ab | 9.70 a |
| EBLS | 1.45 a | 3.01 ab | 0.84 a | 2.13 b | 0.38 a | 3.85 ab | 3.58 ab | 0.38 a | 0.93 ab | 10.07 a |
| SM + EBLS | 1.53 a | 2.91 b | 0.59 b | 2.94 a | 0.44 a | 3.50 b | 4.47 a | 0.45 a | 1.28 a | 10.47 a |
| CONTROL | 0.97 b | 3.63 a | 0.78 a | 2.44 ab | 0.22 b | 4.41 a | 3.40 b | 0.34 a | 0.77 b | 10.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzistathis, T.; Chatzissavvidis, C.; Papaioannou, A.; Papadakis, I.E. Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea europaea L., cv. “Koroneiki”). Appl. Sci. 2023, 13, 1127. https://doi.org/10.3390/app13021127
Chatzistathis T, Chatzissavvidis C, Papaioannou A, Papadakis IE. Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea europaea L., cv. “Koroneiki”). Applied Sciences. 2023; 13(2):1127. https://doi.org/10.3390/app13021127
Chicago/Turabian StyleChatzistathis, Theocharis, Christos Chatzissavvidis, Athanasios Papaioannou, and Ioannis E. Papadakis. 2023. "Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea europaea L., cv. “Koroneiki”)" Applied Sciences 13, no. 2: 1127. https://doi.org/10.3390/app13021127
APA StyleChatzistathis, T., Chatzissavvidis, C., Papaioannou, A., & Papadakis, I. E. (2023). Independent or Combinational Application of Sheep Manure and Litter from Indigenous Field Vegetation of Quercus sp. Influences Nutrient Uptake, Photosynthesis, Intrinsic Water Use Efficiency, and Foliar Sugar Concentrations in Olive Plants (Olea europaea L., cv. “Koroneiki”). Applied Sciences, 13(2), 1127. https://doi.org/10.3390/app13021127









