Photocatalytic Activity of TiNbC-Modified TiO2 during Hydrogen Evolution and CO2 Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining of the TiNbC/TiO2 Heterostructures
2.2. Characterization
2.3. Catalytic Activity Measurements
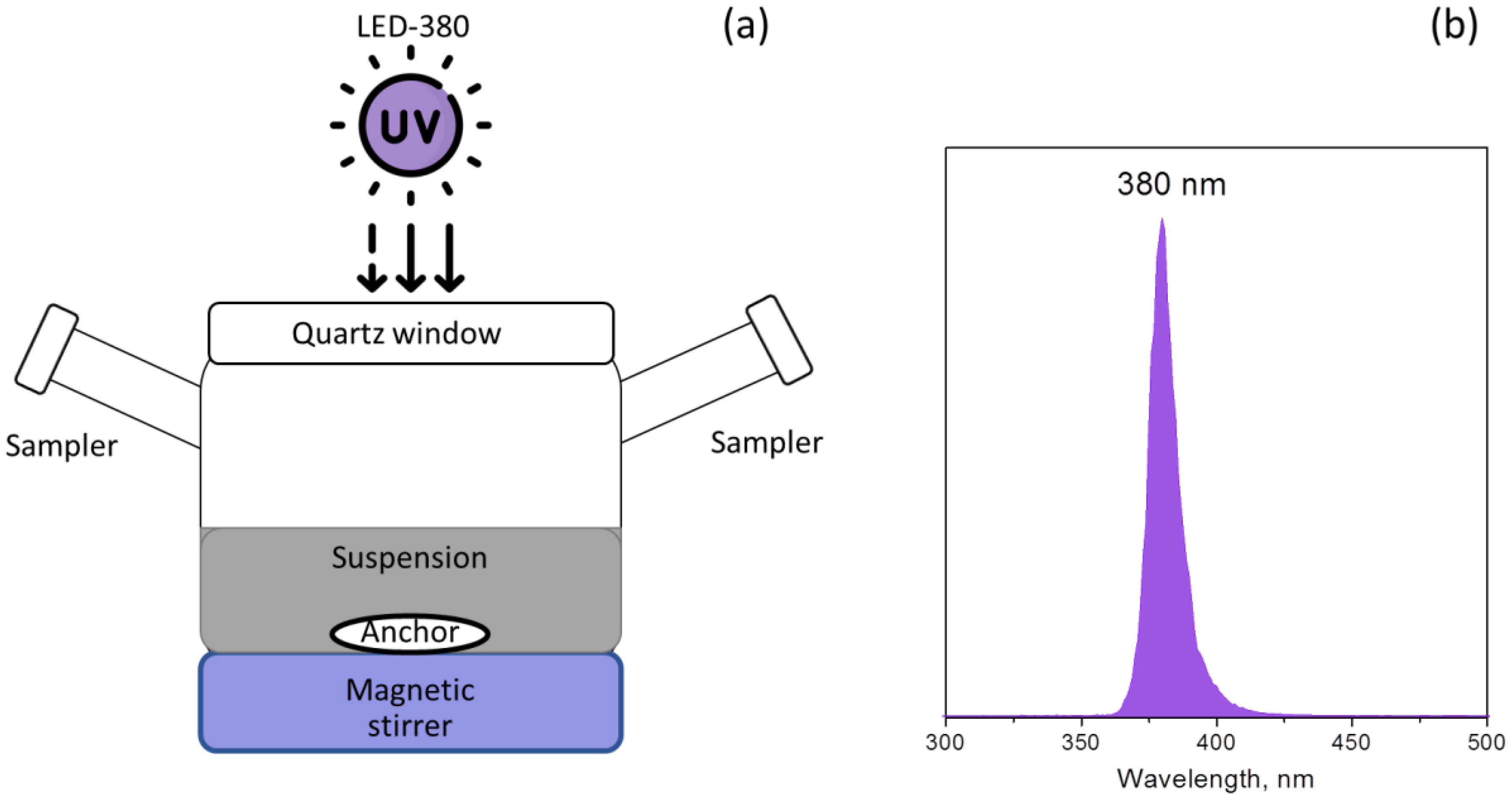
2.3.1. Photocatalytic Hydrogen Evolution
2.3.2. Photocatalytic Reduction of Carbon Dioxide
3. Results and Discussion
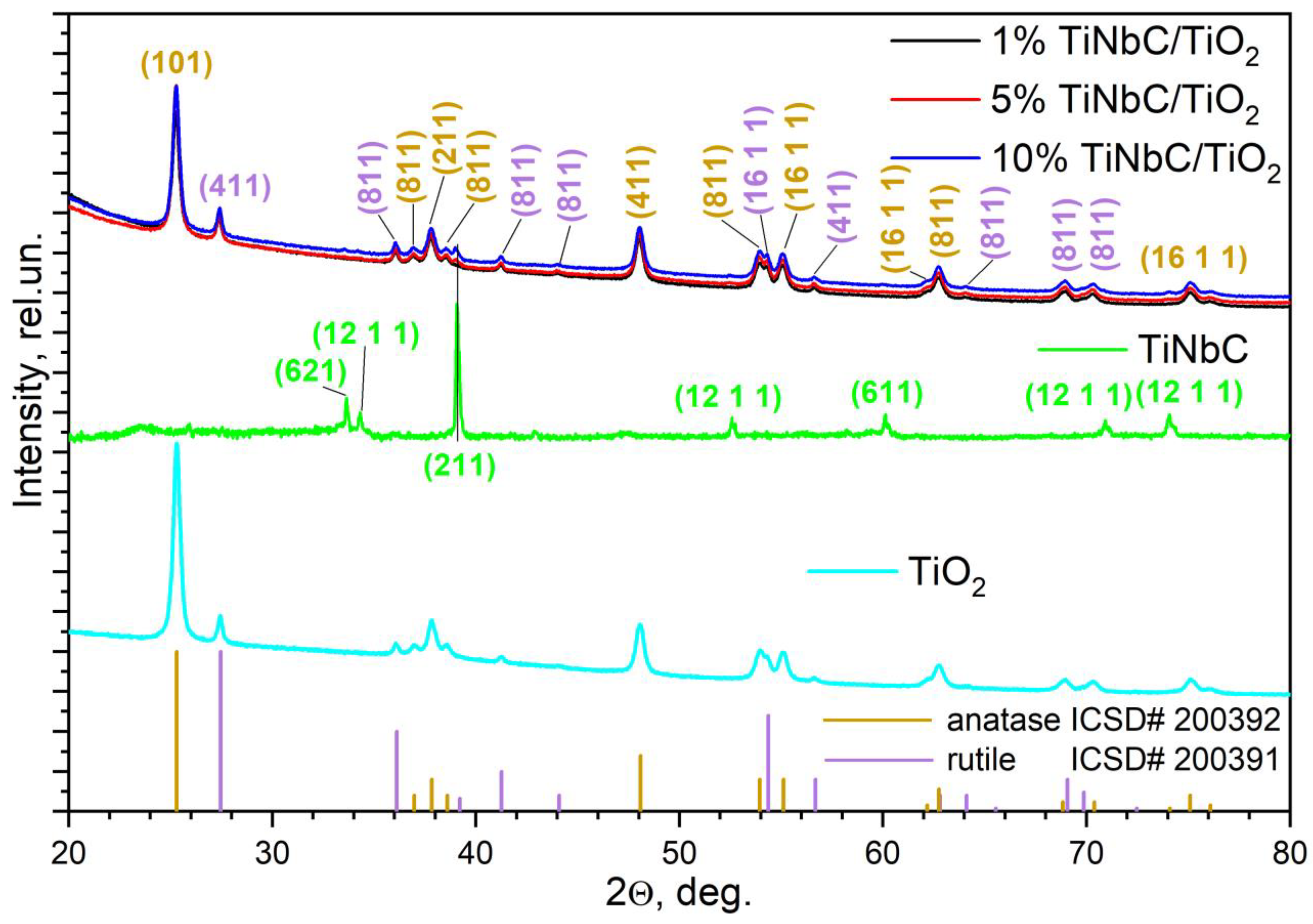
3.1. X-ray Diffraction
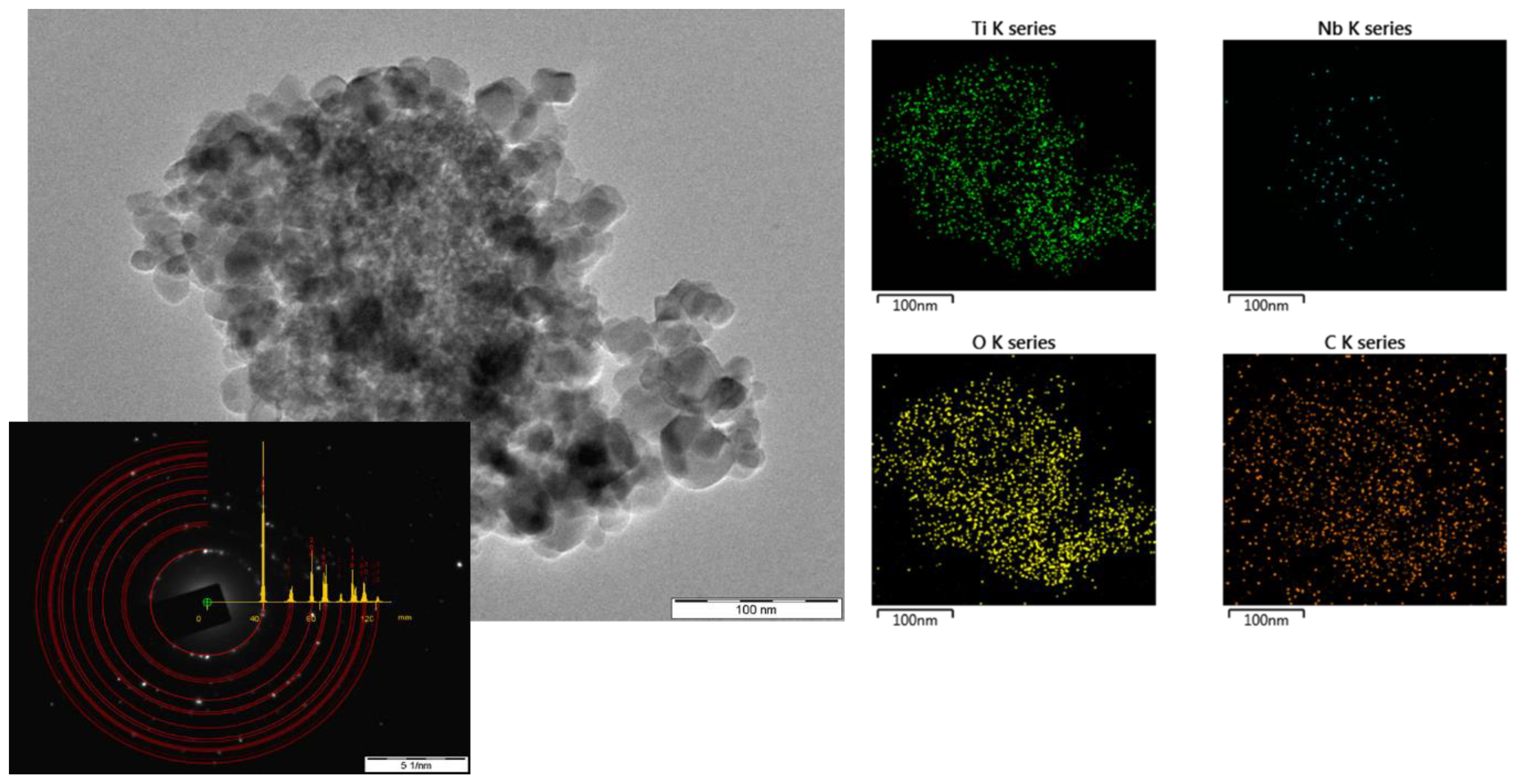
3.2. SEM and TEM Characterizations
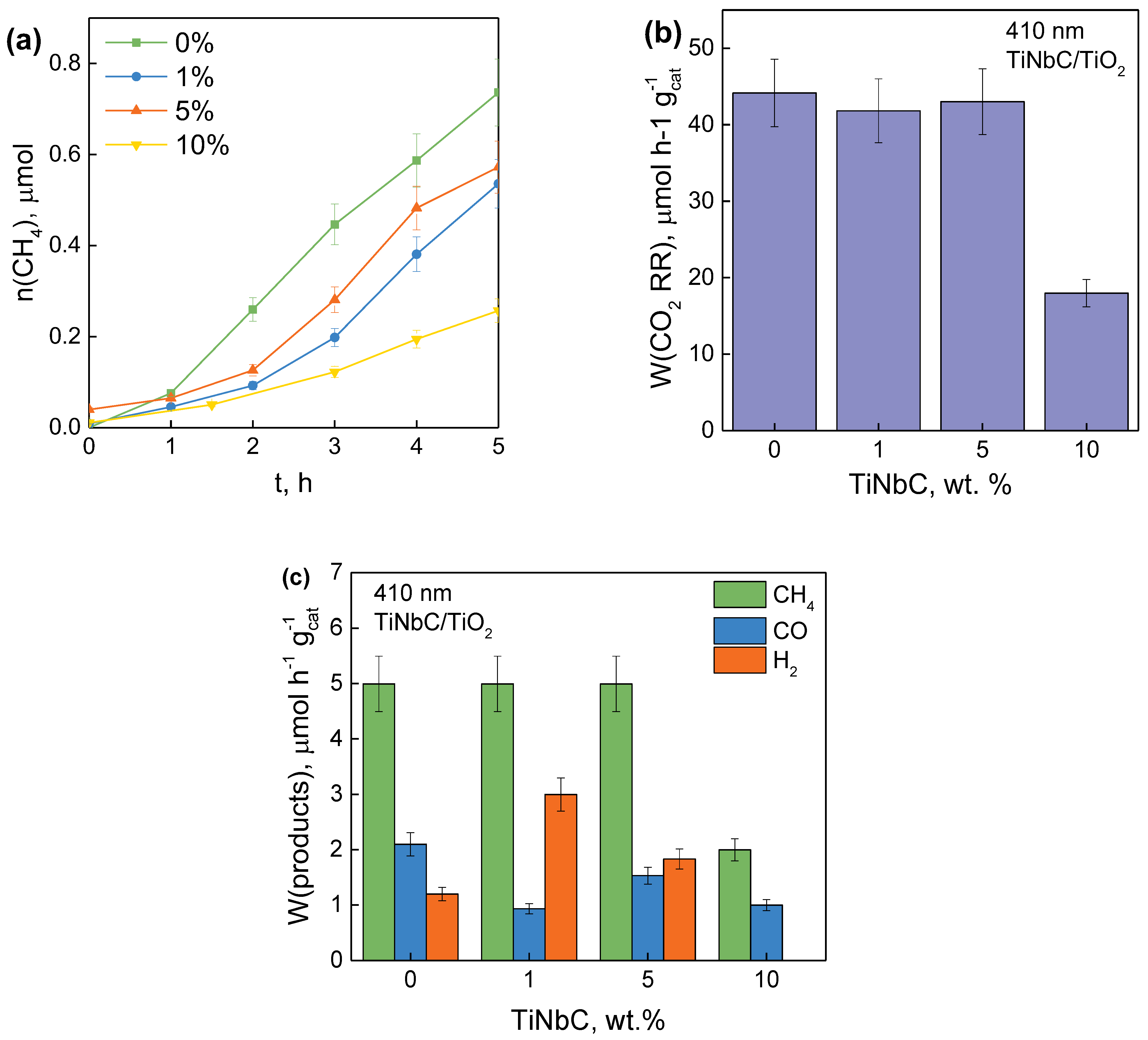
3.3. Study of the Photocatalytic Activity of TiNbC/TiO2 in the Process of CO2 Reduction and Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawla, R.; Singhal, P.; Garg, A.K. Photovoltaic Review of All Generations: Environmental Impact and its Market Potential. Trans. Electr. Electron. Mater. 2020, 21, 456–476. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M.A. Critical Review in Strategies to Improve Photocatalytic Water Splitting towards Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Huang, R.; Li, X.; Gao, W.; Zhang, X.; Liang, S.; Luo, M. Recent Advances in Photocatalytic Nitrogen Fixation: From Active Sites to Ammonia Quantification Methods. RSC Adv. 2021, 11, 14844–14861. [Google Scholar] [CrossRef] [PubMed]
- Kovacič, Ž.; Likozar, B.; Huš, M. Photocatalytic CO2 Reduction: A Review of Ab initio Mechanism, Kinetics, and Multiscale Modeling Simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants using TiO2-based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis: Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Sherryna, A.; Tahir, M. Role of Ti3C2 MXene as Prominent Schottky Barriers in Driving Hydrogen Production through Photoinduced Water Splitting: A Comprehensive Review. ACS Appl. Energy Mater. 2021, 4, 11982–12006. [Google Scholar] [CrossRef]
- Tahir, M.; Sherryna, A.; Mansoor, R.; Khan, A.A.; Tasleem, S.; Tahir, B. Titanium Carbide MXene Nanostructures as Catalysts and Cocatalysts for Photocatalytic Fuel Production: A Review. ACS Appl. Nano Mater. 2022, 5, 18–54. [Google Scholar] [CrossRef]
- Fan, W.K.; Sherryna, A.; Tahir, M. Advances in Titanium Carbide (Ti3C2Tx) MXenes and Their Metal–Organic Framework (MOF)-Based Nanotextures for Solar Energy Applications: A Review. ACS Omega 2022, 7, 38158–38192. [Google Scholar] [CrossRef]
- Murali, G.; Modigunta, J.K.R.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Bury, D.; Jakubczak, M.; Purbayanto, M.A.K.; Wojciechowska, A.; Moszczyńska, D.; Jastrzębska, A.M. Photocatalytic Activity of the Oxidation Stabilized Ti3C2Tx MXene in Decomposing Methylene Blue, Bromocresol Green and Commercial Textile Dye. Small Methods 2023, 1, 2201252. [Google Scholar] [CrossRef] [PubMed]
- Grzegórska, A.; Gajewicz-Skretna, A.; Trykowski, G.; Sikora, K.; Zielińska-Jurek, A. Design and synthesis of TiO2/Ti3C2 composites for highly efficient photocatalytic removal of acetaminophen: The relationships between synthesis parameters, physicochemical properties, and photocatalytic activity. Catal. Today 2023, 413–415, 113980. [Google Scholar] [CrossRef]
- Haneef, T.; Rasool, K.; Iqbal, J.; Nawaz, R.; Mustafa, M.R.U.; Mahmoud, K.A.; Sarkar, T.; Shahzad, A. Recent progress in two dimensional Mxenes for photocatalysis: A critical review. 2D Mater. 2023, 10, 012001. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Ahmaruzzaman, M.; Djellabi, R.; Elimian, E.; Rtimi, S. Advances in 2D MXenes-based materials for water purification and disinfection: Synthesis approaches and photocatalytic mechanistic pathways. J. Environ. Manag. 2022, 324, 116387. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wu, Y.; An, Y.; Cui, B.; Lin, J.; Li, X.; Hu, S. Interface-coupling of CoFe-LDH on MXene as high-performance oxygen evolution catalyst. Mater. Today Energy 2019, 12, 453–462. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Grau, R.R.; Lewandowska-Andralojc, A.; Primo, A.; García, H. Enhancement of the photocatalytic hydrogen production with the exfoliation degree of Nb2C cocatalyst. Int. J. Hydrogen Energy 2023, 48, 20314–20323. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Shuai, T.Y.; Zhan, Q.N.; Xu, H.M.; Zhang, Z.J.; Li, G.R. Recent developments of MXene-based catalysts for hydrogen production by water splitting. Green Chem. 2023, 25, 1749–1789. [Google Scholar] [CrossRef]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent advances of MXenes as electrocatalysts for hydrogen evolution reaction. NPJ 2d Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, T.; Xie, X.; Jiang, W.; Li, J.; Tian, B.; Su, C. Oxygen-functionalized ultrathin Ti3C2Tx MXene for enhanced electrocatalytic hydrogen evolution. Chem. Sus. Chem. 2019, 12, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, N.; Zhou, S.; Zhao, J. MXene nanoribbons as electrocatalysts for the hydrogen evolution reaction with fast kinetics. Phys. Chem. Chem. Phys. 2018, 20, 19390–19397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Huang, L.; Ai, L.; Wang, M.; Jiang, J.; Wang, S. Hierarchical MoS2 nanosheets integrated Ti3C2 MXenes for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 965–976. [Google Scholar] [CrossRef]
- Kuang, P.; He, M.; Zhu, B.; Yu, J.; Fan, K.; Jaroniec, M. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Jia, M.; Lv, X.; Li, X.; Li, R.; Ding, X.; Zheng, Y.-Z.; Tao, X. 1T/2H MoSe2-on-MXene heterostructure as bifunctional electrocatalyst for efficient overall water splitting. Electrochim. Acta 2019, 326, 134976. [Google Scholar] [CrossRef]
- Gandla, D.; Zhang, F.; Tan, D.Q. Advantage of Larger Interlayer Spacing of a Mo2Ti2C3 MXene Free-Standing Film Electrode toward an Excellent Performance Supercapacitor in a Binary Ionic Liquid–Organic Electrolyte. ACS Omega 2022, 7, 7190–7198. [Google Scholar] [CrossRef]
- Li, G.; Zhou, B.; Wang, P.; He, M.; Fang, Z.; Yuan, X.; Wang, W.; Sun, X.; Li, Z. High-Efficiency Oxygen Reduction to Hydrogen Peroxide Catalyzed by Oxidized Mo2TiC2 MXene. Catalysts 2022, 12, 850. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J. Processing of MAX phases: From synthesis to applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Rigby, M.T.P.; Natu, V.; Sokol, M.; Kelly, D.J.; Hopkinson, D.G.; Zou, Y.; Bird, J.R.T.; Evitts, L.J.; Smith, M.; Race, C.P.; et al. Synthesis of new M-layer solid-solution 312 MAX phases (Ta1−xTix)3AlC2 (x ¼ 0.4, 0.62, 0.75, 0.91 or 0.95), and their corresponding MXenes. RSC Adv. 2021, 11, 3110. [Google Scholar] [CrossRef]
- Saraev, A.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Kozlova, E.A. Broadening the Action Spectrum of TiO2-Based Photocatalysts to Visible Region by Substituting Platinum with Copper. Nanomaterials 2022, 12, 1584. [Google Scholar] [CrossRef] [PubMed]
- Syuy, A.; Shtarev, D.; Lembikov, A.; Gurin, M.; Kevorkyants, R.; Tselikov, G.; Arsenin, A.; Volkov, V. Effective method for determining the unit cell parameters of new MXenes. Materials 2022, 15, 8798. [Google Scholar] [CrossRef] [PubMed]
- Tselikov, G.I.; Ermolaev, G.A.; Popov, A.A.; Tikhonowski, G.V.; Panova, D.A.; Taradin, A.S.; Vyshnevyy, A.A.; Syuy, A.V.; Klimentov, S.M.; Novikov, S.M.; et al. Transition metal dichalcogenide nanospheres for high-refractive-index nanophotonics and biomedical theranostics. Proc. Natl. Acad. Sci. USA 2022, 119, e2208830119. [Google Scholar] [CrossRef] [PubMed]
- Tselikov, G.I.; Panova, D.A.; Kazantsev, I.S.; Syuy, A.V.; Tikhonowski, G.V.; Popov, A.A.; Arsenin, A.V.; Volkov, V.S. Laser-fabricated MoS2 nanoparticles as efficient near-infrared photosensitizers. Bull. Russ. Acad. Sci. Phys. 2022, 86, S234–S238. [Google Scholar] [CrossRef]
- Im, J.K.; Sohn, E.J.; Kim, S.; Jang, M.; Son, A.; Zoh, K.-D.; Yoon, Y. Review of MXene-based nanocomposites for photocatalysis. Chemosphere 2021, 270, 129478. [Google Scholar] [CrossRef]



| Sample | Ti, % | Nb, % | C, % | O, % |
|---|---|---|---|---|
| 1% TiNbC/TiO2 | 36.1 ± 0.4 | 1.0 ± 0.2 | 1.2 ± 0.2 | 62.9 ± 0.9 |
| 5% TiNbC/TiO2 | 39.4 ± 0.5 | 3.1 ± 0.3 | 4.1 ± 0.3 | 57.6 ± 0.9 |
| 10% TiNbC/TiO2 | 39.0 ± 0.3 | 4.5 ± 0.1 | 8.2 ± 0.4 | 57.5 ± 0.4 |
| Photocatalyst | Yield, μmol h−1g−1 | W (CO2RR), μmol h−1g−1 | S (CO2RR), % | ||
|---|---|---|---|---|---|
| CH4 | CO | H2 | |||
| TiO2 Evonik P25 | 5.0 | 2.1 | 1.2 | 44.1 | 94.8 |
| 1% TiNbC/TiO2 | 5.0 | 0.9 | 3.0 | 41.8 | 87.5 |
| 5% TiNbC/TiO2 | 5.0 | 1.5 | 1.8 | 43.0 | 92.1 |
| 10% TiNbC/TiO2 | 2.0 | 1.0 | 0 | 18.0 | 100 |
| Photocatalyst | The Rate of H2 Production, μmol/min | Activity in H2, μmol h−1g−1 |
|---|---|---|
| TiO2 Evonik P25 | 0.003 | 4.0 |
| 1% TiNbC/TiO2 | 0.038 | 45.6 |
| 5% TiNbC/TiO2 | 0.047 | 56.4 |
| 10% TiNbC/TiO2 | 0.019 | 22.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syuy, A.V.; Shtarev, D.S.; Kozlova, E.A.; Kurenkova, A.Y.; Zhurenok, A.V.; Shtareva, A.V.; Gurin, M.S.; Tselikov, G.I.; Tikhonowski, G.V.; Arsenin, A.; et al. Photocatalytic Activity of TiNbC-Modified TiO2 during Hydrogen Evolution and CO2 Reduction. Appl. Sci. 2023, 13, 9410. https://doi.org/10.3390/app13169410
Syuy AV, Shtarev DS, Kozlova EA, Kurenkova AY, Zhurenok AV, Shtareva AV, Gurin MS, Tselikov GI, Tikhonowski GV, Arsenin A, et al. Photocatalytic Activity of TiNbC-Modified TiO2 during Hydrogen Evolution and CO2 Reduction. Applied Sciences. 2023; 13(16):9410. https://doi.org/10.3390/app13169410
Chicago/Turabian StyleSyuy, Alexander V., Dmitry S. Shtarev, Ekaterina A. Kozlova, Anna Yu. Kurenkova, Angelina V. Zhurenok, Anna V. Shtareva, Mikhail S. Gurin, Gleb I. Tselikov, Gleb V. Tikhonowski, Aleksey Arsenin, and et al. 2023. "Photocatalytic Activity of TiNbC-Modified TiO2 during Hydrogen Evolution and CO2 Reduction" Applied Sciences 13, no. 16: 9410. https://doi.org/10.3390/app13169410
APA StyleSyuy, A. V., Shtarev, D. S., Kozlova, E. A., Kurenkova, A. Y., Zhurenok, A. V., Shtareva, A. V., Gurin, M. S., Tselikov, G. I., Tikhonowski, G. V., Arsenin, A., & Volkov, V. (2023). Photocatalytic Activity of TiNbC-Modified TiO2 during Hydrogen Evolution and CO2 Reduction. Applied Sciences, 13(16), 9410. https://doi.org/10.3390/app13169410









