Abstract
Biologically active compounds present in the diet can interact with biological membranes (such as cell membranes), changing their properties. Their mutual interactions can influence their respective activities. In this study, we analyzed the interactions of oleanolic acid and phenolic compounds such as apigenin, rutin, resveratrol and ferulic acid with phosphatidylcholine membranes. Spectroscopic methods (fluorescence spectroscopy, dynamic light scattering) and machine learning were applied. The results of structural studies were compared with the antioxidant activity of the investigated substances in lipid membranes. In liposomes loaded with oleanolic acid, the pro-oxidant activity of resveratrol arises from changes in membrane structure, leading to an increased exposure of its hydrophilic region to external radicals. A similar mechanism may be involved in the pro-oxidant action of oleanolic acid. By contrast, apigenin, rutin and ferulic acid are present at the membrane surface. Their presence in this region protects the bilayer from radicals generated in the aqueous phase. Lower antioxidant activity observed in the case of ferulic aid is probably related to weaker interactions of this compound with the membrane, compared to the investigated flavonoids. Appropriate machine learning models for predicting oleanolic acid and phenolic compounds have been developed for the future application of intelligent predictive systems to optimizing manufacturing processes involving liposomes. The most effective regression model turned out to be the MLP 1:1-100-50-50-6:1, identifying resveratrol with a determination index of 0.83.
1. Introduction
Food ingredients play a key role in interactions with biological membranes, which contain a variety of components such as lipids, proteins, and more. As a result, various food ingredients can affect lipid membranes, changing their physical properties and affecting biochemical reactions. One example is the biological activity of oleanolic acid (3β-hydroxy-olean-12-en-28-oic acid) (OA), contained in many plants [1,2], which is classified as a plant secondary metabolite [3]. OA shows biological activity, which manifests itself as antioxidant, anti-inflammatory, antidiabetic, antitumor and antimicrobial effects [1,4]. Not only nutrients, but also secondary plant metabolites influence interactions with biological membranes. These metabolites are often found in plants and have potential biological activity that affects a variety of processes in organisms. An example is pentacyclic triterpenes, which by interacting with bacterial membranes can increase the effectiveness of antibiotics such as ampicillin [3]. Other examples are two pentacyclic triterpene oleanolic acid and ursolic acid, which show a moderate fluidity-modulating effect in liquid-crystalline liposomes made from dipalmitoyl phosphatidylcholine (DPPC), and a distinct condensing effect in liquid-crystalline and crystalline membranes. These effects were comparable to those observed in the presence of cholesterol [5].
Other secondary metabolites that are widespread in the plant kingdom are polyphenols. Also in this case, some manifestations of biological activity and pro-health effects are related to interaction with lipid cell membranes, as reported for green tea catechins [6,7]. Hydrophobic interactions between polyphenols and lipid membranes are also observed [8]. Planar aromatic moieties of phenolic compounds enable van der Waals forces, promoting a hydrophobic model of interactions. Flavonoids containing hydroxyl, galloyl and gallate groups affect the mainly hydrophilic domain of bilayers, forming hydrogen bonds. These compounds cause lipid aggregation, resulting in a reduction of membrane surface area and an increase of membrane rigidity. Trans-stilbenes, characterized by an open benzyl ring, penetrate more deeply into hydrophobic parts of the membrane, thereby increasing membrane area. Consequently, the membrane becomes more fluid and undergoes fluctuations [9]. Ferulic acid interacts electrostatically with phospholipids, zwitterionic polar groups within liposomal membranes. The affinity of ferulic acid to liposomes depends on their lipid composition. In simplified models composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), there is an increase in membrane rigidity. Conversely, no alterations in fluidity were observed in rigid membranes containing cholesterol and sphingomyelin [10].
Food ingredients, as well as secondary plant metabolites, can interact with biological membranes, which can have significant consequences for various biological functions of organisms. Understanding these interactions could lead to the discovery of new mechanisms of action of nutrients and plant metabolites, affecting cell signaling pathways and metabolism in organisms. The rigid planar multicyclic structure and hydrophilic 3β-hydroxyl group of oleanolic acid confers a structural feature close to cholesterol. As cholesterol is an essential component of lipid membranes in animal cells, we can assume that oleanolic acid will also exert a significant impact on the structure and biophysical properties of lipid membranes. Although this effect has been determined in liposome models, to date there has been no study of how the simultaneous presence of OA and phenolic compounds affect the properties of lipid membranes. The combination of OA and phenolic compounds is a characteristic feature of the Mediterranean diet. Oleanolic acid is typical of the Oleaceae family; while capers are rich in rutin, apigenin is found in oregano, resveratrol occurs in red wine and ferulic acid is present in thyme [11].
Today, artificial intelligence is becoming an alternative solution to support many decision-making problems [12,13,14]. The effective collection of huge amounts of data, the detection of patterns [15], the prediction of research aspects and the optimization of processes in food, among others, are possible with the help of artificial intelligence [16]. These activities are aimed at optimizing existing processes at a company in order to increase efficiency and competitiveness in the market. The effectiveness and popularization of machine and deep learning also affects the analysis of research results, the identification of new drugs and the support of clinical studies [17,18,19,20]. However, few studies have been conducted to predict the size of liposomes in solution [21,22]. The application of machine learning, by Python, to predict oleanolic acid and phenolic compound concentrations based on liposome size has not yet been encountered in the literature.
In view of the above, it seems reasonable to use modern methods that have an impact when predicting these data. The innovation in the research reported here was the use of an artificial neural network in the regression problem (Multilayer Perceptron by regression) to predict the concentration of oleanolic acid and phenolic compounds based on the size of liposome particles in solution. The use of a regression model based on the size of liposomes makes it possible to determine the optimal conditions between the properties of liposomes and their chemical activity.
Designation of the appropriate type of MLP model by regression, based on an adequate coefficient of determination between different concentrations of oleanolic acid and phenolic compounds, will translate into optimization of the processes of production, storage and transport of liposomes.
The aim of this study was to evaluate the effects of the co-presence of oleanolic acid and phenolic compounds (apigenin, rutin, resveratrol, ferulic acid) on the properties of model liposome membranes by analyzing interactions with the membrane surface and hydrophobic core. In addition, the effects of these compounds on the process of lipid peroxidation in the membrane were examined, which may indeed support a better understanding of their activity in the presence of oleanolic acid as well as predicting these interactions using artificial intelligence.
2. Materials and Methods
2.1. Chemicals and Reagents
The following reagents were used in the experiments: L-a-phosphatidylcholine (PC) from egg yolk, Type XVI-E, ≥99% (TLC), Sigma Aldrich, Steinheim, Germany; NBD-PE (N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt), Thermo Fisher Scientific, Waltham, MA, USA; DPH (1,6-diphenyl-1,3,5-hexatriene), 98%, Sigma Aldrich, Steinheim, Germany; oleanolic acid (OA, >98%, Aktin); apigenin (AP, >95%, Fluorochem); rutin (RT, ≥95%, Glentham); resveratrol (RS, >98%, Fluorochem); trans-ferulic acid (FA, 99%, Sigma-Aldrich); methanol (99.8%), chloroform (98.5%), n-hexane (99% HPLC-grade), POCh, Gliwice, Poland. All chemicals were of analytical grade.
2.2. Preparation of Liposomes
Phosphatidylcholine liposomes containing OA and OA with addition of phenolic compounds (apigenin, rutin, resveratrol, ferulic acid) were prepared. Briefly, 0.2 mL of phosphatidylcholine (PC) solution in chloroform at a concentration of 1.6 mg/mL was pipetted into a 25 mL conical flask. Next, to 0.333 mL of NBD-PE in methanol (11.4 μmol/L) or DPH in n-hexane (1 mmol), appropriate quantities of oleanolic acid in methanol (1 mmol) and phenolic compounds in methanol (1 mmol) were added. The solvents were then evaporated using a rotary evaporator working at a water-bath temperature equal to 30 °C. The obtained film was hydrated with 4 mL of 0.01 mol/L phosphate buffer (pH 7.4). A prepared suspension of liposomes was extruded applying a LiposoFast-Basic LF-1 extruder (Avestin Europe GmbH, Mannheim, Germany) equipped with a 400 nm diameter membrane. The final concentration of reagents in liposome containing OA was as follows: PC—0.08 mg/mL, NBD-PE and DPH—0.95 μmol/L, OA—0 to 100 μmol/L. Samples with OA and phenolic compounds contained: PC—0.08 mg/mL, NBD-PE and DPH—0.95 μmol/L, OA—20 μmol/L and phenolic compounds (apigenin, rutin, resveratrol, ferulic acid)—0 to 100 μmol/L.
2.3. Steady-State Fluorescence and Anisotropy Fluorescence Measurements
Fluorescence probes NBD-PE and DPH (1,6-diphenyl-1,3,5-hexatriene) were embedded into a phosphatidylcholine membrane. Steady-state fluorescence emission was recorded at 22 °C using the F-7100 fluorescence spectrometer (Hitachi, Mito, Japan) equipped with a quartz cuvette. The excitation wavelength for samples containing NBD-PE was set at 463 nm and the emission was in the range from 473 nm to 620 nm. In the case of DPH excitation, the wavelength was 350 nm, and the emission range was from 375 nm to 550 nm. Fluorescence was measured at a 90° angle. The fluorescence emission anisotropy was measured at 536 nm (for NBD-PE) and 434 nm (for DPH), with the excitation wavelength at 463 nm and 350 nm, respectively. An appropriate instrumental procedure for anisotropy calculation using the F-7100 fluorescence spectrometer (Hitachi, Mito, Japan) was applied.
2.4. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
The liposomes’ size (hydrodynamic diameter) was determined using dynamic light scattering (DLS), and electrophoretic light scattering (ELS) was employed to determine the zeta potential of the membranes. Both measurements were performed with a Zetasizer Nano ZS-90 (Malvern Instruments, Malvern, UK) at 20 °C. The hydrodynamic diameter of liposomes was expressed as a size distribution by intensity. Zeta potential was calculated using Zetasizer Nano ZS-90 software ver. 7.3 from the Henry equation, with f(Ka) equal to 1.5 (Smoluchowski approximation).
2.5. Antioxidant Activity Assay
The antioxidant activity of oleanolic acid and phenolic compounds within PC membranes was tested at 25 °C in the presence of the hydrophilic free radical generator AAPH (2,2′-azobis(2-amidinopropane)) after 10 min of incubation in the dark. Liposomes were labeled with DPH, and fluorescence of the probe was measured at excitation wavelength 350 nm and emission wavelength 434 nm. DPH is able to reveal the oxidative events in liposomal membranes as a result of lipid oxidation and the probe’s own oxidation. In such case DPH fluorescence emission decreases due to changes in membrane fluidity as a result of lipid peroxidation and disruption of DPH [23]. Antioxidant activity was calculated on the basis of DPH initial (Fo) and post-incubation (F) fluorescence intensity. The lower Fo/F value was recorded, the higher antioxidant activity of the analyzed compound.
2.6. Statistical Analysis
Analysis of variance (ANOVA) was employed using Statistica 13.0 (StatSoft, Inc., Tulsa, OK, USA) software. The experiments and analyses were conducted in triplicate.
2.7. Machine Learning
The research involved a machine learning process using the Python environment (Figure 1). Currently, Python has become the most popular and also the most effective tool for designing and executing machine learning models. In this study, Multilayer Perceptron (MLP) networks were designed and performed in a regression problem using Pandas and scikit-learn libraries. The machine learning process proceeded according to predetermined stages.
Figure 1.
MLP by Python generation procedure based on machine learning.
In the initial stage, numerical data were prepared. The dataset contained information on input and output variables. The input (quantitative) variable in the set determined the diameter of liposomes (liposome size is the average of three independent measurement repetitions of size distribution by intensity) in acid and phenolic compounds. The output (quantitative) variables informed the probability of solution concentration at a given liposome size. The probabilities of occurrence/non-occurrence of acid and phenolic compound concentrations were determined numerically via binary, that is, as a value of 1 and 0 for each output variable specifying the concentration in solution: 0, 5, 10, 20, 50, 100. As a result of machine learning data preparation, five learning sets were created. The next step required determining the topology of the artificial neural network. Considering the research problem specified, a regression model was used. In the Python environment, the responsible regression models are the Linear Regression model and the Multilayer perceptron model (MLP Regression). Initial work was done using linear regression. Unfortunately, adequate models were not determined using the Linear Regression algorithm because they did not obtain the optimal coefficient of determination indicating a correct fit to the model. The second algorithm, i.e., the Multilayer perceptron model, was selected, which satisfied the model fit criterion. The structures of each neural network was characterized with one neuron in the input layer, three hidden layers (200 neurons in the first layer, 100 neurons in the second layer and 50 neurons in the third layer) and six neurons in the output layer. As part of the machine learning stage, the adam algorithm was used [14,24,25]. In this research stage, an optimal MLP model by regression was obtained, which was able to effectively identify oleanolic acid and phenolic compounds based on the size of liposomes.
3. Results and Discussion
3.1. Interactions of Oleanolic Acid and Polyphenols with Lipid Membranes—Steady-State Fluorescence Measurements
Lipid membranes consist of a surface hydrophilic part and a hydrophobic core. Interactions between biologically active substances and these two regions can affect the biological properties of the membrane in different ways. Therefore, it is important to determine the localization of individual substances in different parts of the bilayer. In this study, fluorescent probes NBD-PE and DPH (diphenylhexatriene) were used to localize oleanolic acid and polyphenols in different parts of the bilayer. In the NBD-PE fluorescence probe, a moiety responsible for fluorescence emission is located on the surface of the lipid membrane. Consequently, it demonstrates interactions of compounds with the bilayer hydrophilic surface. Fluorescence quenching is caused by the interactions of the probe with water molecules. In turn, DPH is a hydrophobic molecule that localizes in the acyl chain region of a lipid membrane. In the presence of oleanolic acid, a decrease of NBD-PE fluorescence intensity at 534 nm was recorded at a triterpene concentration equal to 100 μmol (Figure 2a). This means that OA at that concentration interacts with the bilayer and causes membrane structural changes leading to increased surface exposure to water molecules. Observed changes in DPH fluorescence emissions at 434 nm (Figure 3a), in the presence of oleanolic acid (at concentrations ranging from 5 to 100 μmol), indicate the location of these molecules in the hydrophobic core of the bilayer. Interactions of OA with the lipid bilayer have already been observed by Han and coworkers [5]. It exhibits a moderate fluidity-modulating effect in liquid-crystalline liposomes made from dipalmitoyl phosphatidylcholine (DPPC) and a distinct condensing effect in liquid-crystalline and crystalline membranes. These effects are comparable to those observed in the presence of cholesterol. DPPC is membrane-substituted with saturated palmitic acid residues. In our study, natural phosphatidylcholine containing palmitic, oleic, linoleic and stearic acid residues was used. Such a membrane is liquid-crystalline at room temperature, i.e., under the conditions applied in this experiment.
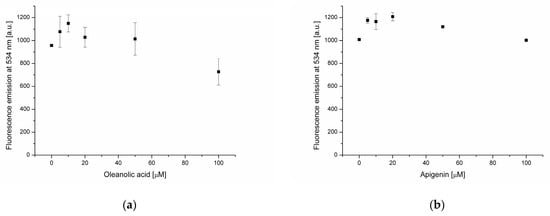
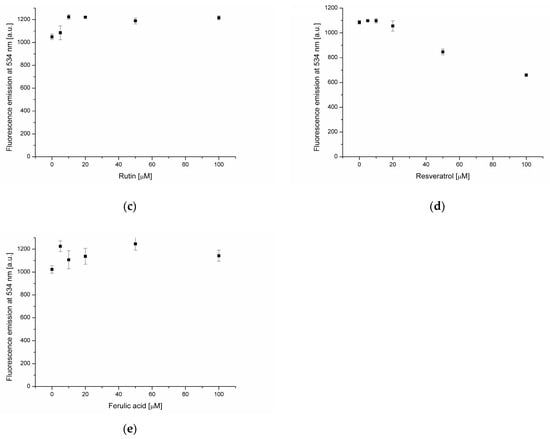
Figure 2.
Fluorescence intensity of NBD-PE probe in lipid membrane in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
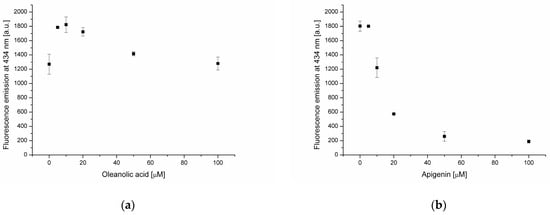
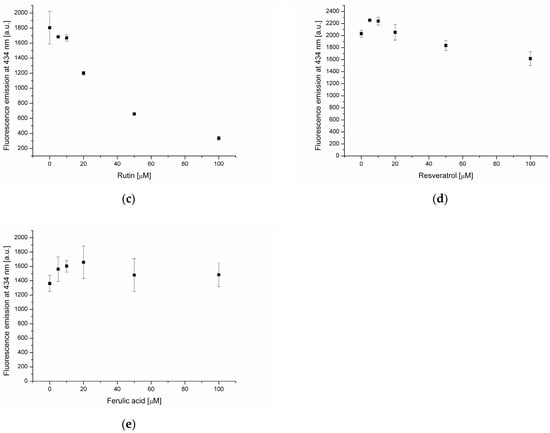
Figure 3.
Fluorescence intensity of DPH probe in lipid membrane in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
Subsequent experiments focused on the interaction of selected phenolic compounds (apigenin (AP), rutin (RT), resveratrol (RS) and ferulic acid (FA)) with phosphatidylcholine membranes containing 20 μmol of OA. The increase of NBD-PE fluorescence intensity was recorded in the case of AP, RT and FA (Figure 2b,c,e). The observed changes are associated with the accumulation of polyphenols on the membrane surface, which subsequently hampers the quenching of probe fluorescence by water molecules. Such a mechanism of NBD-PE fluorescence decrease, resulting from the formation of hydrogen bonds with water molecules, was proposed by Kittipongpittaya and collaborators [26]. The mildest inhibitory effect was observed in the case of ferulic acid, while the converse effect was noted for resveratrol (Figure 2d). In the presence of resveratrol, a decrease of about 40% in NBD-PE fluorescence intensity at 100 μmol was found. Fluorescence-quenching was observed at RS concentrations higher than 20 μmol, indicating changes in membrane structure leading to increased surface exposure to the aqueous environment. Further details concerning the interactions of phenolic compounds with the lipid membrane are revealed through measurements of the hydrophobic probe DPH fluorescence emission. At resveratrol concentrations of 5–20 μmol, an increase in DPH emissions were recorded (Figure 3d), which indicates the embedding of polyphenol into the membrane’s hydrophobic core. In turn, at RS concentrations ranging from 20 to 100 μmol, a decrease of DPH fluorescence was found. This correlates well with NBD-PE emission-quenching at RS concentrations above 20 μmol. The incorporation of RS into the hydrophobic core of the membrane likely “loosens” its structure and increases exposure to the surrounding water, which quenches NBD-PE emission and creates a less favorable environment for DPH fluorescence.
A previous study reported that flavonoids have the ability to quench DPH emission [27]. In the case of apigenin and rutin (Figure 3b,c) a strong decrease of DPH fluorescence in the presence of these two flavonoids was recorded. This phenomenon is due to the incorporation of AP and RT into the hydrophobic core of the membrane, where the probe is present. No statistically significant changes in DPH fluorescence were found in the presence of ferulic acid, indicating its absence in the hydrophobic membrane region (Figure 3e).
Fluorescent probes’ emission measurements revealed significant differences in the effects of the analyzed substances on the phosphatidylcholine lipid membrane. OA is located in the hydrophobic core of the bilayer, and at higher concentration (100 μmol), causes changes in the membrane structure leading to increased exposure of its surface to the aqueous environment. In the phosphatidylcholine membrane enriched with oleanolic acid (20 μmol), AP and RT are accumulated at the bilayer surface as well as in the hydrophobic core. In turn, RS is located in the hydrophobic membrane region, and at concentrations above 20 μmol, changes in the bilayer structure under the influence of this compound increase exposure of the hydrophilic membrane region to the water environment. The weakest interactions with the bilayer were found in the case of FA. It is located at the membrane surface and is not present in the hydrophobic core, as indicated by the DPH probe measurements.
3.2. Influence of Oleanolic Acid and Polyphenols on Lipid Membranes Structure—Fluorescence Anisotropy Measurements
NBD-PE and DPH fluorescence anisotropy measurements revealed changes in the lipid membranes’ structure in the presence of the analyzed substances. DPH is a probe situated in the hydrophobic bilayer region parallel to the acyl chains [28]. Its fluorescence anisotropy measurements are used to monitor acyl chains’ rotational diffusion. Reduction of the probe’s rotational mobility results in the increase of the anisotropy value [29]. In turn, the moiety responsible for NBD-PE fluorescence is located in hydrophilic part of bilayer. A slight increase of NBD-PE fluorescence anisotropy was recorded in the case of a bilayer containing 20 μmol of OA and 100 μmol of resveratrol simultaneously (Figure 4d). This confirms the structural changes observed in the hydrophilic part of the membrane during steady-state fluorescence measurements. In the other cases, there was no statistically significant effect of OA and polyphenols on the NBD-PE emission anisotropy (Figure 4a–c,e). A slight increase in DPH fluorescence anisotropy was observed in the samples containing 100 μmol OA as well as OA (20 μmol) and apigenin (20–100 μmol) simultaneously (Figure 5a,b). This indicates a decrease in membrane fluidity in the presence of the substances used. No statistically significant changes in DPH fluorescence anisotropy were observed in the other samples (Figure 5c–e). Han and co-authors [5] discovered that OA reduces the flexing motion of fatty acyl chain regions in DPPC (dipalmitoyl phosphatidylcholine), being in liquid-crystalline phase. This phenomenon leads to a decrease of bilayer fluidity [5]. A similar effect was observed for cholesterol, whereby the modulating influence of OA is weaker. In our experiment, the phosphatidylcholine membrane was in the liquid-crystalline phase at room temperature, indicating that our results are similar to those of Han and co-workers. Tsuchiya and co-workers [30] found that apigenin at the concentration 0.625–10 μmol decreases the fluidity of phosphatidylcholine membranes (acyl chains 18:1/16:0 ratio equal to 1) containing 20 mol% of cholesterol. In the case of our study, a bilayer was incorporated with 20 μmol of OA, which may affect its structure, similarly to cholesterol. The hardening effect of apigenin has also been confirmed for plasma membranes of L929 cells [31].
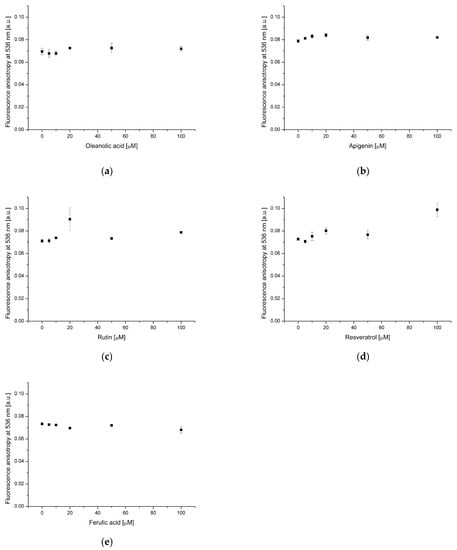
Figure 4.
Fluorescence anisotropy of NBD-PE probe in lipid membrane in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
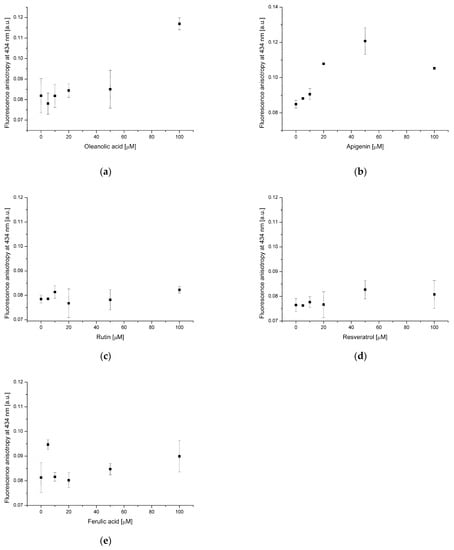
Figure 5.
Fluorescence anisotropy of DPH probe in lipid membrane in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
3.3. Influence of Oleanolic Acid and Polyphenols on Phosphatidylcholine Liposomes Hydrodynamic Diameter—Dynamic Light Scattering (DLS) Measurements
DLS measurements reveal the hydrodynamic diameter of liposomes. This parameter can be influenced by various factors, such as the aggregation or the appearance of substances that change the hydrophilic-hydrophobic balance. In the presence of OA, the decrease of a membrane’s hydrodynamic diameter from about 245 nm (PC membrane without OA) to 175 nm (100 μmol OA) was observed (Figure 6a). Song and co-workers [32] found that sterols structurally similar to OA increase the size of liposomes made from egg yolk phosphatidylcholine. In their research, the mean vesicle size was 82 nm, and had increased to about 110 nm, 175 nm and 325 nm after addition of 40% of cholesterol, β-sitosterol and stigmasterol, respectively. However, in up to 20% of sterols no significant differences were observed. The authors highlight that the planar and non-bulky structure of the cholesterol molecule is less likely than β-sitosterol and stigmasterol to cause steric hindrance, which results in the formation of smaller liposomes. In our experiment, at 100 μmol OA the concentration of this compound was 57% of PC content. Thus, with a comparable ratio of OA to membrane, the obtained effect was a reduced hydrodynamic diameter of liposomes, quite different from that observed in the presence of cholesterol, β-sitosterol and stigmasterol. From NBD-PE fluorescence measurements, it is evident that 100 μmol OA located in the hydrophobic core of the membrane causes structural changes leading to increased exposure of the bilayer surface to the aqueous environment. Hence, it can be assumed that OA causes the rearrangement of phospholipid packing in the bilayer, which leads to a change in the membrane curvature and the formation of liposomes of smaller size.
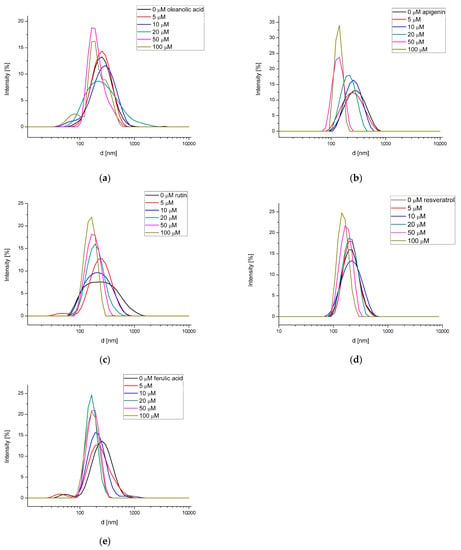
Figure 6.
Distribution of PC liposomes hydrodynamic diameter in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
In subsequent experiments, the impacts of phenolic compounds on phosphatidylcholine membranes enriched with 20 μmol of OA were investigated. In the case of apigenin (Figure 6b), the decrease of the liposomes’ hydrodynamic diameter from about 275 nm to about 140 nm at 100 μmol of AP was found. Banerjee and co-workers [33,34] prepared distearoyl phosphatidylcholine (DSPC) liposomes containing apigenin (DSPC:apignin ratio—2.5:1). They found, in two experiments, that the hydrodynamic diameter of membranes loaded with AP was slightly higher (103.2–104.3 nm) in comparison with empty DSPC (91.84–94.07 nm). The increased diameter of membranes loaded with AP suggests the formation of hydrogen bonds between flavonoids and the hydrophilic part of the bilayer [35,36], as confirmed in studies with dipalmitoyl phosphatidylcholine (DPPC) membranes. However, it is also possible that hydrophobic interactions between apigenin and the phospholipid membrane are involved [33]; the location of AP in the hydrophobic region of the membrane was confirmed by DPH fluorescence measurements in our experiment. The decrease of the liposomes’ hydrodynamic diameter in the presence of AP in our study is probably due to the effect of OA on the structure of the bilayer, thus modifying the action of apigenin.
As with apigenin, the presence of rutin also caused a reduction of phosphatidylcholine liposome size from about 220 nm to about 155 nm at a flavonoid concentration of 100 μmol (Figure 6c). According to Halevas and co-workers [36], the addition of rutin to liposomes composed of egg phosphatidylcholine/cholesterol, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/1,2-di-(9Z-octadecenoyl)-sn-glycero-3-phosphoetha-nolamine (DOPE) and 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA)/DOPE caused an increase of hydrodynamic diameter from 107 nm, 114 nm and 112 nm to 147.2 nm, 129 nm and 139 nm, respectively. On the contrary, liposomes containing egg yolk L-α-phosphatidylcholine/cholesterol/ceramide-3/oleic acid have sizes between 136.55–517.49 nm, and after the addition of rutin this decreases to between 131.48–152.76 nm. Thus, the effect of rutin on liposomes size depends on membrane composition. In the case of rutin (as for apigenin), the effect of the flavonoid on the hydrodynamic diameter of the liposome is probably determined by the presence of OA in its structure, which can affect the hydrophilic-lipophilic balance of the system.
Similar to flavonoids, loading liposomes with resveratrol led to a reduction in hydrodynamic diameter from about 205 nm to about 150 nm (Figure 6d). A slight reduction in the hydrodynamic diameter of membranes composed from soybean phosphatidylcholine after the addition of resveratrol was found by Isailovic and co-workers [37]. This held true for multilamellar vesicles and liposomes obtained by extrusion, but not for the sonication technique. This observation indicates a different effect of RS on the liposome’s size compared to flavonoids. It seems that the decrease in the diameter of phospholipid membranes is not determined by the presence of OA.
Ferulic acid added to membranes containing 20 μmol of OA caused liposome size reduction from about 250 nm (empty liposomes) to about 170 nm (liposomes loaded with FA; Figure 6e). Ara and co-authors [38] observed a similar phenomenon in liposomes composed of egg phosphatidylcholine. Empty membranes (20 μmol) had a hydrodynamic diameter of 112.5 nm, and after adding ferulic acid (0.2 μmol), this value decreased slightly to 109.3 nm. It can be concluded from the above that the reduction of liposome size by FA is independent of the presence of OA.
3.4. Influence of Oleanolic Acid and Polyphenols on Phosphatidylcholine Liposomes Zeta Potential
Zeta potential measurements indicate how electrical charge varies on the membrane surface. Using this data, it is possible to extract information about changes in the membrane’s biophysical properties, as well as indirectly about the presence of the analyzed molecules on the liposomes’ surface. The zeta potential of a phosphatidylcholine membrane at pH 7.4 is negative and amounts to −49.6 mV (Figure 7a). After the addition of OA (5–100 μmol), zeta potential decreases to about −40 mV (−39.5 mV to −43.9 mV, depending on the concentration).
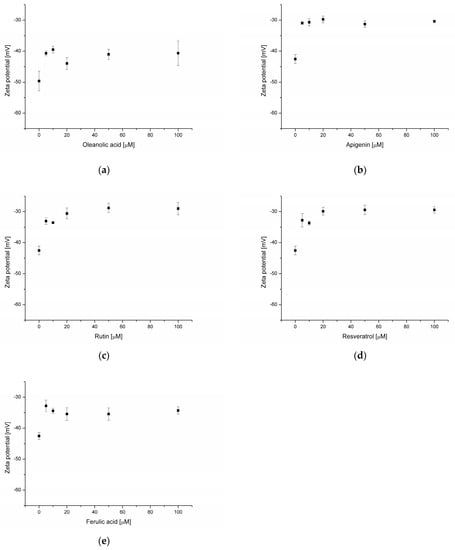
Figure 7.
Zeta potential of PC liposomes in the presence of oleanolic acid (a), and oleanolic acid mixed with apigenin (b), rutin (c), resveratrol (d) and ferulic acid (e).
Further experiments considered the effect of phenolic compounds on the zeta potential of an OA (20 μmol)-enriched membrane. The addition of AP (Figure 7b) resulted in a lowering of zeta potential from −42.5 mV (empty liposome) to about −30 mV (regardless of concentration in the range 5–100 μmol of flavonoid). A similar phenomenon was recorded in the presence of rutin (decrease of potential zeta from −42.5 mV to −29 mV at an RT concentration of 100 μmol; Figure 7c). An almost equal potential lowering (from −42.5 mV to −29.5 mV) was found at a resveratrol concentration of 100 μmol (Figure 7d). The smallest decrease of this value was observed in samples containing FA; at a ferulic acid concentration of 100 μmol, zeta potential was equal to −34.3 mV (Figure 7e). According to Song and co-workers [31], the zeta potential of egg yolk phosphatidylcholine liposomes in water was about—33 mV. After the addition of cholesterol, stigmasterol and β-sitosterol, it increased to about −34 mV, −37 mv and −38 mV, respectively. The authors claim that zeta potential is related to hydrogen bonding between sterols and phospholipids. Hydrogen bond strength favors the presence of electronegative atoms [39]. OA considered in our experiment influences zeta potential differently than structurally similar cholesterol, stigmasterol and β-sitosterol. As shown in fluorescence measurements, OA is located in the hydrophobic core of the membrane, which does not promote the formation of hydrogen bonds. On the other hand, the lowering of zeta potential observed in the presence of OA may indicate its partial presence on the membrane surface.
In DSPC liposomes, apigenin slightly (from −4.2 mV to −3.8 mV) reduces zeta potential compared to empty membranes [34]. In the case of RT, the influence of flavonoids on liposome zeta potential is dependent on bilayer composition. In liposomes made from egg phosphatidylcholine and cholesterol, the addition of rutin slightly increased this negative value from −17 mV to −20 mV. For DOPC/DOPE membranes, a decrease of zeta potential from −32 mV to −21 mV in the presence of rutin was observed. In turn, RT changed the potential value in DOPE/DOPA membranes, shifting it from positive (42 mV) to negative (−46 mV) [37]. Isailovic and co-workers [36] found that resveratrol did not affect soybean phosphatidylcholine liposomes’ surface charge. In the current study, the observed decrease in zeta potential after the addition of RS may indicate that OA modifies the effect of resveratrol on the phosphatidylcholine membrane surface charge. In membranes composed of egg phosphatidylcholine, it was observed that ferulic acid caused a decrease of zeta potential from −10,5 mV to 7.37 mV [38]. The data presented above indicate that changes of zeta potential in liposomes can be affected by various factors, such as the type of lipids that compose the bilayer and the presence of additional substances (cholesterol, plant sterols, triterpenoids and phenolic compounds). Isailovic and co-workers [37] claim that the value of the zeta potential helps identify the dominant compound on a particle’s surface. In our study, this allows us to supplement the data provided by spectroscopic measurements. On the basis of zeta potential decrease in the case of all substances analyzed by us, we can conclude that, at least in some part, they interact with the membrane surface.
3.5. Antioxidant Activity of Phenolic Compounds on Phosphatidylcholine Membranes
In order to check the antioxidant activity of phenolic compounds (AP, RT, RS, FA), we monitored the peroxidation of phosphatidylcholine membranes induced by hydrophilic free radical generator AAPH, employing a DPH probe. DPH operates as a probe to reveal the oxidative events within the membrane, both due to lipid oxidation as well as its own oxidation. In both cases it is expected that the DPH fluorescence intensity decreases, due to a variation of the membrane structure (fluidity) as a result of lipid peroxidation and disruption of DPH molecules. The antioxidant activity of phenolic compounds in liposomes was tested at 25 °C in the presence of AAPH after 10 min of incubation. The initial probe fluorescence intensity (Fo) was divided by the intensity after incubation with radicals at specified antioxidant concentrations. The lower the DPH Fo/F value is, the higher the antioxidant activity of the compound was recorded. As a result of free radical action on phosphatidylcholine liposomes in the absence of OA, DPH fluorescence decreased to about 62% of the initial value (Figure 8). After the addition of 5–100 μmol OA, a reduction of fluorescence by about half was observed. This demonstrates the pro-oxidant effect of oleanolic acid in phosphatidylcholine membranes. In subsequent experiments, the impact of phenolic compounds on the peroxidation of liposomes containing 20 μmol OA was analyzed. For apigenin and rutin (100 μmol), a decrease of Fo/F value to 1.31 and 1.23, respectively, versus 1.85 and 1.92 in control samples, was observed. This indicates a pronounced antioxidant effect that was dependent on the flavonoid concentration. A weaker antioxidant activity was observed for ferulic acid: in this case, at 100 μmol a reduction of Fo/F value to 1.44 was noted, compared to 2.16 in the control. On the contrary, for resveratrol, there was a pro-oxidant effect that manifested itself most clearly at concentrations of 50 μmol and 100 μmol, causing an increase of Fo/F to 1.94 and 2.13, respectively, compared to 1.69 in the absence of RS (Figure 8).
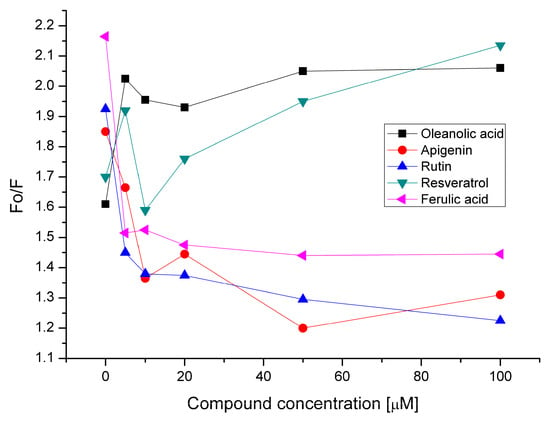
Figure 8.
DPH fluorescence intensity changes during AAPH-induced phosphatidylcholine membranes peroxidation in the presence of oleanolic acid and oleanolic acid mixed with apigenin, rutin, resveratrol and ferulic acid.
The antioxidant activity of phenolic compounds is widely recognized. Zhang and co-workers [40] investigated the antioxidant activity of polyphenols added during preparation of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) liposomes and incubated with membranes after their hydration. As the agent that induces peroxidation, AAPH was used. Lipid peroxidation inhibition capacity (LPIC), expressed as Trolox equivalent, was equal to 0.36, 3.62 and 2.23 after the addition of, respectively, apigenin, rutin and ferulic acid to DOPC before membrane hydration. When polyphenols were added after liposome hydration, LPIC values changed and amounted to 2.38, 4.68 and 4.28, respectively [40]. The observed differences are due to the presence of phenolic compounds in the aqueous phase in the second variant of the experiment. This allowed them to directly scavenge free radicals generated from water-soluble AAPH outside the membrane. Resveratrol (10–20 μmol) is able to reduce the peroxidation rate of phosphatidylcholine liposomes in the presence of AAPH [41]. In this range of concentrations in our experiment, the anti- or pro-oxidant effect of RS was not pronounced. However, a clear pro-oxidation impact was noted at 50–100 μmol. In an ORAC (Oxygen Radical Absorption Capacity) assay, resveratrol showed antioxidant activity up to 35 μmol (8 μg/mL), and in DPPH (2,2-diphenyl-l-picrylhydrazyl) radical-scavenging activity tests up to 280 μmol (64 μg/mL) [42]. The pro-oxidant effect of resveratrol observed in the current study is probably not a consequence of its ability to scavenge free radicals, but of its effect on the membrane structure. As indicated by fluorescence measurements, RS is located in the hydrophobic core of the membrane, but at concentrations above 20 μmol, changes the structure of the bilayer cause increased exposure of its hydrophilic region to external aqueous media. Thus, the availability of peroxidation-sensitive regions of the bilayer for free radicals generated from AAPH in water is increased, resulting in a pro-oxidant effect. A similar mechanism may be involved in the pro-oxidant action induced by the presence of OA in the membrane. On the contrary, AP, RT and FA are located at the membrane surface, as shown by fluorescence measurements. Their presence in this region protects the membrane from radicals generated from AAPH in the aqueous phase. Lower antioxidant activity observed in the case of FA is probably related to weaker interaction of this compound with the membrane, compared to the flavonoids tested. The higher antioxidant activity of AP observed in this study, relative to the results achieved by Zhang et al. [40], may be due to the modification of its efficiency by OA present in liposomes.
3.6. Machine Learning
In this study, we conducted an analysis to predict the effectiveness of identifying compounds with varying concentrations. The comparative analysis of regression models helps illustrate how the compounds in question affect their identification efficiency by determining the size of liposomes. Nowadays, concentration-dependent identification of substances is a key aspect in the pharmaceutical industry [43], especially when they can be a potential threat to the environment [44,45]. Analytical methods and artificial intelligence are becoming effective solutions for identifying compounds and drugs [19,46]. Ocampo et al. [21] conducted the first liposome size prediction activities (output variables), obtaining an optimal determination rate [21]. Chen et al. conducted a similar study on liposome prediction using machine learning methods. They also demonstrated optimal models characterized by a high coefficient of determination for the test set at 0.83, considering, in addition to liposome size, polydispersity index, zeta potential and encapsulation [47]. In this research, the concentration prediction of compounds was focused on considering only liposome size (input variables), which was analyzed so effectively by Ocampo et al. [21].
In the results, optimal neural models were obtained for the regression problem posed. Mean square error (MSE), mean absolute error (MAE) and coefficient of determination (R2) were determined for each model to determine performance, insight into accuracy and how the size of liposomes fit due to the acid concentration of each compound. MSE and MAE allow the determination of the efficiency of predicting compound concentration due to liposome size. In the case of the coefficient of determination, a model fit with the highest possible score is sought [48]. Table 1 shows five adequate models that had the lowest MSE and MAE values. In Table 1, the best model was MLP 1:1-100-50-50-6:1, which predicted the efficiency of identifying the compound resveratrol (RS). The selected MLP 1:1-100-50-50-6:1 model achieved the lowest MSE value of 0.0148 for the test set. This means that this model showed high performance in identifying acid concentration to factual data informing the size of liposomes. As with the MSE, the MAE value seeks to obtain the lowest egg to determine the optimal neural network model. In Table 1, the lowest MAE value was achieved by the MLP 1:1-100-50-20-6:1 network associated with oleanolic acid (OA). The regression models (optimal R2 min. 0.7) resulted in MLP 1:1-100-50-50-6:1, MLP 1:1-100-50-20-6:1, MLP 1:1-100-50-50-6:1 and MLP 1:1-200-100-50-50-6:1 networks. This means that four models fit the data well, namely the models with resveratrol (RS), oleanolic acid (OA), ferulic acid (FA) and rutin (RT), compared to the model identifying apigenin (AP) (R2 = 0.5563). The highest rate of determination was achieved by the network identifying resveratrol (RS), for which an R2 of 0.8247 was achieved in the test set.

Table 1.
Results of compound concentration prediction based on liposome size for: resveratrol (RS), oleanolic acid (OA), ferulic acid (FA), rutin (RT) and apigenin (AP).
4. Conclusions
Fluorescence measurements showed differences in the effects of the analyzed substances on the phosphatidylcholine lipid membrane. Oleanolic acid located in the hydrophobic core of the bilayer caused changes in the membrane structure, leading to increased exposure of its surface to the aqueous environment. In the PC membrane enriched with oleanolic acid (20 μmol), apigenin and rutin were accumulated at the bilayer surface as well as in the hydrophobic core. In turn, RS was located in the hydrophobic membrane region, and at concentrations above 20 μmol, changes in the bilayer structure occurred under the influence of this compound and an increased exposure of the hydrophilic membrane region to the water environment was observed. The weakest interactions with the bilayer were found in the case of ferulic acid. It was located at the membrane surface and is not present in the hydrophobic core.
Oleanolic acid causes rearrangement of phospholipid packing in the bilayer, which leads to a change in the membrane curvature and formation of liposomes of smaller size. This phenomenon probably influences changes of the liposomes’ hydrodynamic diameter in the presence of apigenin and rutin. For apigenin, a decrease of the liposomes’ hydrodynamic diameter from about 275 nm to about 140 nm at 100 μmol of flavonoid was found. In the case of rutin (100 μmol), a size decrease from about 220 nm to about 155 nm was recorded. In turn, zeta potential measurements showed that, at least in some part, all the substances analyzed interact with the membrane surface. This led to potential decreases, which in the case of rutin was reduced to −29 mV.
The results of structural studies were compared with the antioxidant activity of the investigated substances in the lipid membrane. In liposomes loaded with oleanolic acid, the pro-oxidant activity of resveratrol observed in the present study is likely not due to its ability to scavenge free radicals. Instead, it arises from its impact on the membrane structure, leading to an augmented exposure of the hydrophilic region to the surrounding aqueous environment. Thus, the availability of peroxidation-sensitive parts of the bilayer for free radicals generated from AAPH in water is increased, resulting in a pro-oxidant effect. A similar mechanism may be involved in the pro-oxidant action induced by the presence of oleanolic acid in the membrane. On the contrary, apigenin, rutin and ferulic acid are present at the membrane surface, as shown by fluorescence measurements. Their presence in this region protects the bilayer from radicals generated from AAPH in the aqueous phase. Lower antioxidant activity observed in the case of ferulic acid is probably related to weaker interaction of this compound with the membrane, compared to the flavonoids tested. This is illustrated by the Fo/F value, which at a concentration of 100 μmol is equal to 1.44 for ferulic acid, compared to 1.31 and 1.23 for apigenin and rutin, respectively. The data presented above suggest that the antioxidant/pro-oxidative properties of the investigated phenolic compounds in the lipid membrane containing oleanolic acid result mainly from their interaction with the bilayer and changes in its structure caused by their presence. In this case, the ability to scavenge free radicals is of less importance, as demonstrated for resveratrol. The recognized mechanism allows for a better understanding of the biological activity of apigenin, rutin, resveratrol and ferulic acid in the presence of oleanolic acid in the context of their interactions with biological membranes. In the future, structural studies of the investigated phenolic compounds occurring in a natural form in plants are planned.
This research developed models to predict concentrations for selected compounds based on liposome size. The most effective regression model turned out to be a 1:1-100-50-50-6:1 MLP network identifying resveratrol, for which the determination index for the test set was 0.83. This means that the Multilayer perceptron model explains about 83% of the variation in the data in the test set. The high R2 value means that the Multilayer perceptron model also reflects well the relationship between liposome size and the concentration of this compound. In addition, resveratrol also achieved the lowest MSE, which means the model’s ability to generalize these data is very good. In the future, we plan to perform additional studies considering a larger dataset to confirm the accuracy and applicability of the models in practice.
Author Contributions
Conceptualization, K.D., K.P. and S.M.R.; methodology, K.D. and K.P.; software K.D. and K.P.; validation, K.D. and K.P.; formal analysis, K.D. and K.P.; investigation, K.D., K.P., D.D. and E.B.; resources, K.D., K.P. and S.M.R.; data curation, K.D. and K.P.; writing—original draft preparation, K.D. and K.P.; writing—review and editing, K.D., K.P. and S.M.R.; visualization, K.D. and K.P.; supervision, K.D. and K.P.; project administration, K.D. and K.P.; funding acquisition, K.D., K.P. and S.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the Associate Laboratories LAQV-REQUIMTE (UIDB/50006/2020 and UIDP/50006/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, S.; Zhu, F.; Xu, L. Exploring the Underlying Mechanism of Oleanolic Acid Treating Glioma by Transcriptome and Molecular Docking. Biomed. Pharmacother. 2022, 154, 113586. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Catteau, L.; Boukricha, L.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.-P. Effect of Ursolic and Oleanolic Acids on Lipid Membranes: Studies on MRSA and Models of Membranes. Antibiotics 2021, 10, 1381. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Ko, Y.I.; Park, S.J.; Jin, I.J.; Kim, Y.M. Oleanolic Acid and Ursolic Acid Stabilize Liposomal Membranes. Lipids 1997, 32, 769–773. [Google Scholar] [CrossRef]
- Naparlo, K.; Bartosz, G.; Stefaniuk, I.; Cieniek, B.; Soszynski, M.; Sadowska-Bartosz, I. Interaction of Catechins with Human Erythrocytes. Molecules 2020, 25, 1456. [Google Scholar] [CrossRef] [PubMed]
- Naftalin, R.J.; Afzal, I.; Cunningham, P.; Halai, M.; Ross, C.; Salleh, N.; Milligan, S.R. Interactions of Androgens, Green Tea Catechins and the Antiandrogen Flutamide with the External Glucose-Binding Site of the Human Erythrocyte Glucose Transporter GLUT1. Br. J. Pharmacol. 2003, 140, 487–499. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-Dependent Interactions of Polyphenols with a Biomimetic Membrane System. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. The Biophysical Interaction of Ferulic Acid with Liposomes as Biological Membrane Model: The Effect of the Lipid Bilayer Composition. J. Mol. Liq. 2021, 324, 114689. [Google Scholar] [CrossRef]
- Rabbani, M.; Pezeshki, A.; Ahmadi, R.; Mohammadi, M.; Tabibiazar, M.; Ahmadzadeh Nobari Azar, F.; Ghorbani, M. Phytosomal Nanocarriers for Encapsulation and Delivery of Resveratrol-Preparation, Characterization, and Application in Mayonnaise. LWT 2021, 151, 112093. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, J.; Han, L.; Guo, S.; Cui, Q. Application of Machine Vision System in Food Detection. Front. Nutr. 2022, 9, 888245. [Google Scholar] [CrossRef] [PubMed]
- Przybył, K.; Wawrzyniak, J.; Koszela, K.; Adamski, F.; Gawrysiak-Witulska, M. Application of Deep and Machine Learning Using Image Analysis to Detect Fungal Contamination of Rapeseed. Sensors 2020, 20, 7305. [Google Scholar] [CrossRef]
- Przybył, K.; Koszela, K.; Adamski, F.; Samborska, K.; Walkowiak, K.; Polarczyk, M. Deep and Machine Learning Using SEM, FTIR, and Texture Analysis to Detect Polysaccharide in Raspberry Powders. Sensors 2021, 21, 5823. [Google Scholar] [CrossRef]
- Przybył, K.; Samborska, K.; Koszela, K.; Masewicz, L.; Pawlak, T. Artificial Neural Networks in the Evaluation of the Influence of the Type and Content of Carrier on Selected Quality Parameters of Spray Dried Raspberry Powders. Measurement 2021, 186, 110014. [Google Scholar] [CrossRef]
- Przybył, K.; Duda, A.; Koszela, K.; Stangierski, J.; Polarczyk, M.; Gierz, Ł. Classification of Dried Strawberry by the Analysis of the Acoustic Sound with Artificial Neural Networks. Sensors 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-Generation Machine Learning for Biological Networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef]
- Carracedo-Reboredo, P.; Liñares-Blanco, J.; Rodríguez-Fernández, N.; Cedrón, F.; Novoa, F.J.; Carballal, A.; Maojo, V.; Pazos, A.; Fernandez-Lozano, C. A Review on Machine Learning Approaches and Trends in Drug Discovery. Comput. Struct. Biotechnol. J. 2021, 19, 4538–4558. [Google Scholar] [CrossRef]
- Dara, S.; Dhamercherla, S.; Jadav, S.S.; Babu, C.M.; Ahsan, M.J. Machine Learning in Drug Discovery: A Review. Artif. Intell. Rev. 2022, 55, 1947–1999. [Google Scholar] [CrossRef]
- Weissler, E.H.; Naumann, T.; Andersson, T.; Ranganath, R.; Elemento, O.; Luo, Y.; Freitag, D.F.; Benoit, J.; Hughes, M.C.; Khan, F.; et al. The Role of Machine Learning in Clinical Research: Transforming the Future of Evidence Generation. Trials 2021, 22, 537. [Google Scholar] [CrossRef]
- Ocampo, I.; López, R.R.; Camacho-León, S.; Nerguizian, V.; Stiharu, I. Comparative Evaluation of Artificial Neural Networks and Data Analysis in Predicting Liposome Size in a Periodic Disturbance Micromixer. Micromachines 2021, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Shabanzadeh, P.; Senu, N.; Shameli, K.; Ismail, F.; Zamanian, A.; Mohagheghtabar, M. Prediction of Silver Nanoparticles’ Diameter in Montmorillonite/Chitosan Bionanocomposites by Using Artificial Neural Networks. Res. Chem. Intermed. 2015, 41, 3275–3287. [Google Scholar] [CrossRef]
- Balducci, V.; Incerpi, S.; Stano, P.; Tofani, D. Antioxidant Activity of Hydroxytyrosyl Esters Studied in Liposome Models. Biochim. Biophys. Acta Biomembr. 2018, 1860, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Kingma, D.P.; Ba, J.L. Adam: A Method for Stochastic Optimization. 3rd International Conference on Learning Representations, ICLR 2015—Conference Track Proceedings. arXiv 2014, arXiv:1412.6980. [Google Scholar] [CrossRef]
- Patterson, J.; Gibson, A. Deep Learning A Practitioner’s Approach; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2017. [Google Scholar]
- Kittipongpittaya, K.; Panya, A.; Cui, L.; McClements, D.J.; Decker, E.A. Association Colloids Formed by Multiple Surface Active Minor Components and Their Effect on Lipid Oxidation in Bulk Oil. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1955–1965. [Google Scholar] [CrossRef]
- Schoefer, L.; Braune, A.; Blaut, M. A Fluorescence Quenching Test for the Detection of Flavonoid Transformation. FEMS Microbiol. Lett. 2006, 204, 277–280. [Google Scholar] [CrossRef]
- Zhao, H.; Lappalainen, P. A Simple Guide to Biochemical Approaches for Analyzing Protein-Lipid Interactions. Mol. Biol. Cell 2012, 23, 2823–2830. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. Heat-Induced Changes in Lupin Seed γ-Conglutin Structure Promote Its Interaction with Model Phospholipid Membranes. Food Chem. 2022, 374, 131533. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Nagayama, M.; Tanaka, T.; Furusawa, M.; Kashimata, M.; Takeuchi, H. Membrane-Rigidifying Effects of Anti-Cancer Dietary Factors. BioFactors 2002, 16, 45–56. [Google Scholar] [CrossRef]
- Lenne-Gouverneur, A.F.; Lobstein, A.; Haan-Archipoff, G.; Duportail, G.; Anton, R.; Kuhry, J.G. Interactions of the Monomeric and Dimeric Flavones Apigenin and Amentoflavone with the Plasma Membrane of L929 Cells; A Fluorescence Study. Mol. Membr. Biol. 1999, 16, 157–165. [Google Scholar] [CrossRef]
- Song, F.; Chen, J.; Zheng, A.; Tian, S. Effect of Sterols on Liposomes: Membrane Characteristics and Physicochemical Changes during Storage. LWT 2022, 164, 113558. [Google Scholar] [CrossRef]
- Banerjee, K.; Banerjee, S.; Mandal, M. Enhanced Chemotherapeutic Efficacy of Apigenin Liposomes in Colorectal Cancer Based on Flavone-Membrane Interactions. J. Colloid Interface Sci. 2017, 491, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Banerjee, S.; Das, S.; Mandal, M. Probing the Potential of Apigenin Liposomes in Enhancing Bacterial Membrane Perturbation and Integrity Loss. J. Colloid Interface Sci. 2015, 453, 48–59. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlȩga, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. FTIR, 1H NMR and EPR Spectroscopy Studies on the Interaction of Flavone Apigenin with Dipalmitoylphosphatidylcholine Liposomes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 518–527. [Google Scholar] [CrossRef]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A. Flavonoid-Liposomes Formulations: Physico-Chemical Characteristics, Biological Activities and Therapeutic Applications. Eur. J. Med. Chem. Rep. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Dordević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol Loaded Liposomes Produced by Different Techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Ara, T.; Ono, S.; Hasan, M.; Ozono, M.; Kogure, K. Protective Effects of Liposomes Encapsulating Ferulic Acid against CCl4-Induced Oxidative Liver Damage in Vivo Rat Model. J. Clin. Biochem. Nutr. 2023, 72, 46–53. [Google Scholar] [CrossRef]
- Tai, K.; He, X.; Yuan, X.; Meng, K.; Gao, Y.; Yuan, F. A Comparison of Physicochemical and Functional Properties of Icaritin-Loaded Liposomes Based on Different Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 218–231. [Google Scholar] [CrossRef]
- Zhang, J.; Stanley, R.A.; Melton, L.D. Lipid Peroxidation Inhibition Capacity Assay for Antioxidants Based on Liposomal Membranes. Mol. Nutr. Food Res. 2006, 50, 714–724. [Google Scholar] [CrossRef]
- Tadolini, B.; Juliano, C.; Piu, L.; Franconi, F.; Cabrini, L. Resveratrol Inhibition of Lipid Peroxidation. Free Radic. Res. 2000, 33, 105–114. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical Techniques in Pharmaceutical Analysis: A Review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Lodén, M.; Ungerth, L.; Serup, J. Changes in European Legislation Make It Timely to Introduce a Transparent Market Surveillance System for Cosmetics. Acta Derm. Venereol. 2007, 87, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Küster, A.; Adler, N. Pharmaceuticals in the Environment: Scientific Evidence of Risks and Its Regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130587. [Google Scholar] [CrossRef] [PubMed]
- Bonfilio, R.; de Araújo, M.B.; Salgado, H.R.N. Recent Applications of Analytical Techniques for Quantitative Pharmaceutical Analysis: A Review. WSEAS Trans. Biol. Biomed. 2010, 7, 316–338. [Google Scholar]
- Chen, L. Stock Price Prediction Using Adaptive Time Series Forecasting and Machine Learning Algorithms; University of California: Los Angeles, CA, USA, 2020. [Google Scholar]
- Bashir, D.; Montañez, G.D.; Sehra, S.; Segura, P.S.; Lauw, J. An Information-Theoretic Perspective on Overfitting and Underfitting. In Lecture Notes in Computer Science, Proceedings of the Australasian Joint Conference on Artificial Intelligence, Canberra, ACT, Australia, 29–30 November 2020; Springer: Cham, Switzerland, 2020; Volume 12576. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).