Enrichment of Fermented Milk Drinks with Mespilus germanica and Crataegus azarolus Fruit Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fruit Extracts
2.3. Total Acidity, TA
2.4. Total Soluble Sugars, TSS
2.5. Total Phenolic Content, TPC
2.6. Total Flavonoid Content, TFC
2.7. Antioxidant Properties
2.7.1. Ferric Reducing Antioxidant Power, FRAP
2.7.2. 1,1-Diphenyl-2-picrylhydrazy (DPPH)
2.8. Antidiabetic Properties
2.8.1. Inhibition of α-Glucosidase
2.8.2. Inhibition of α-Amylase
2.9. Production of Enriched Fermeneted Milk Drink
2.9.1. Preparation of Freeze-Dried Extracts (FDEs)
2.9.2. Fermentation Process
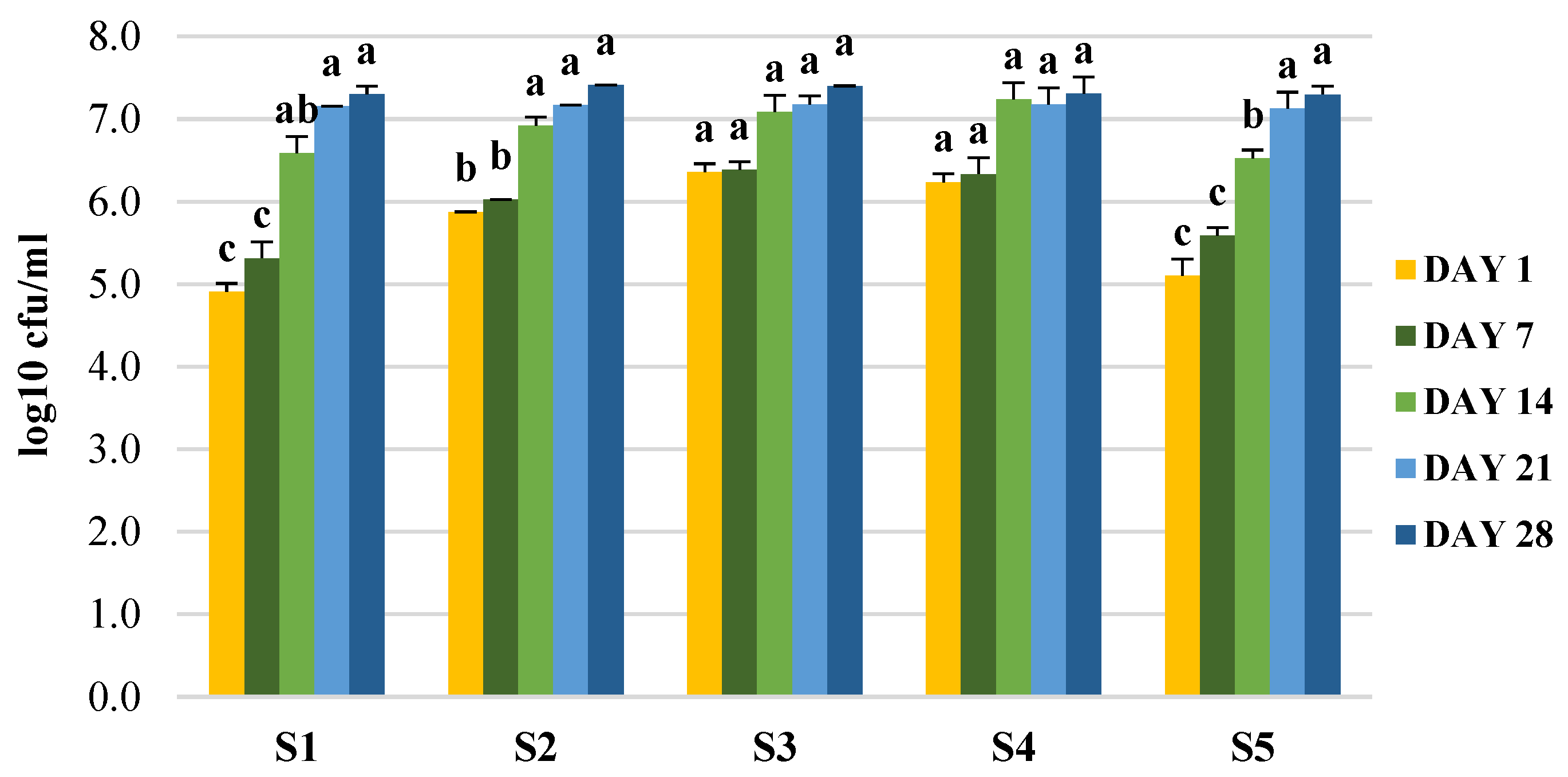
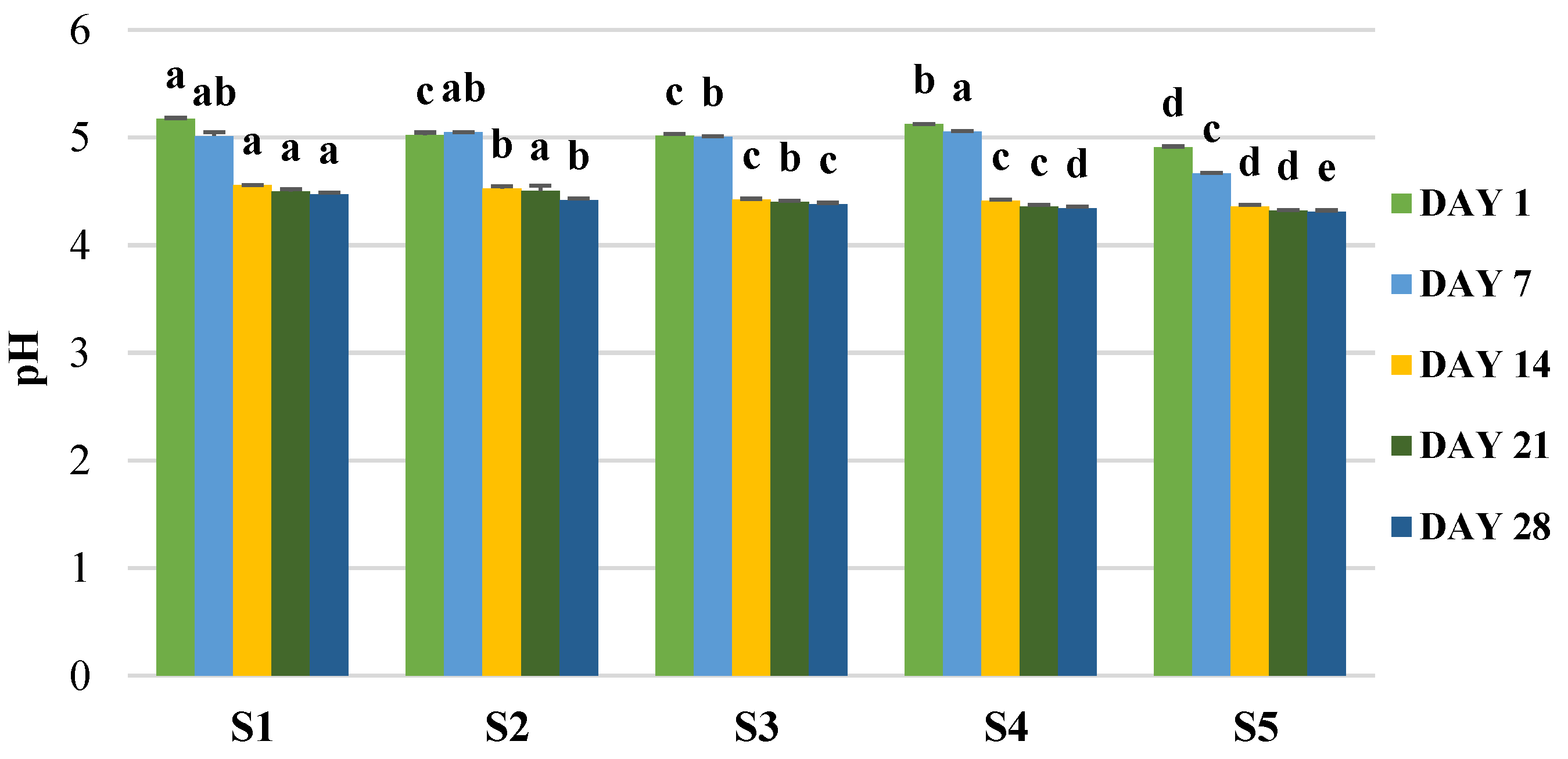
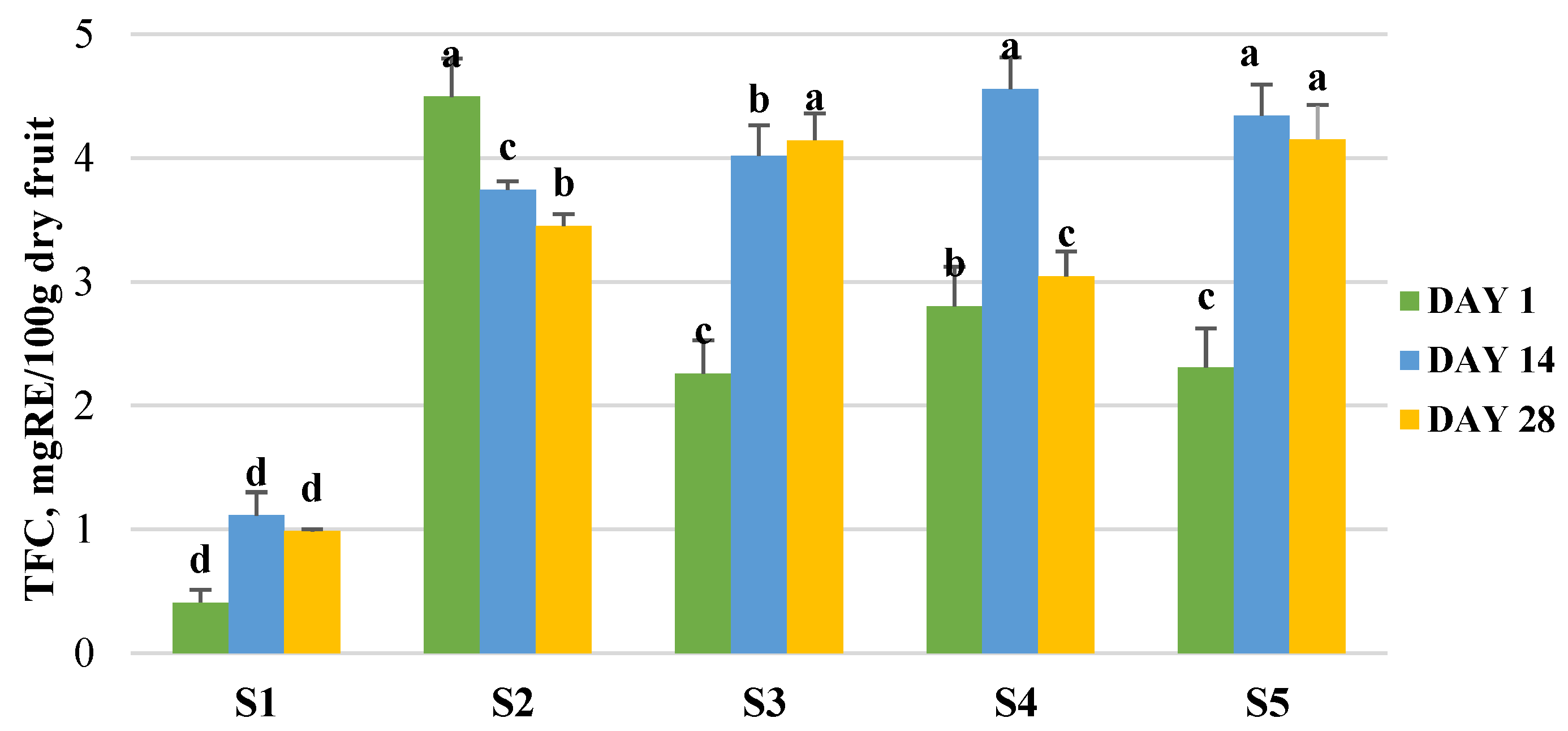
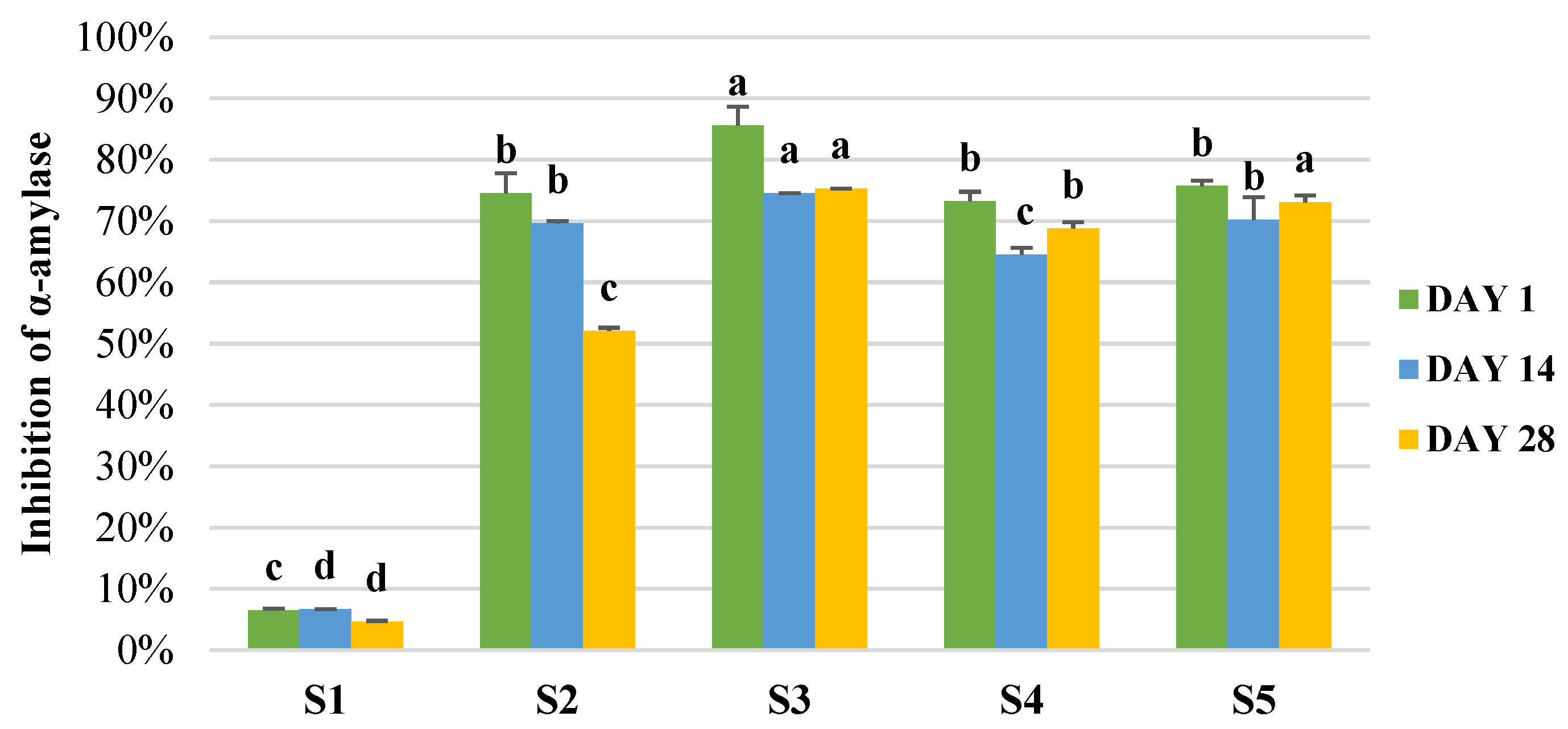
2.10. Effect of the Storage of the Final Products on Microbial and Bioactive Properties
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Fruit Extracts
3.2. Phenolic Composition of Fruit Extracts
3.3. Antioxidant Activities of Fruit Extracts
3.4. Antidiabetic Properties of Fruit Extracts
3.5. Production of Enriched Fermeneted Milk Drink
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Achi, O.K.; Ukwuru, M. Cereal-based fermented foods of Africa as functional foods. Int. J. Microbiol. Appl. 2015, 2, 71–83. [Google Scholar] [CrossRef]
- Dhawi, F.; El-Beltagi, H.S.; Aly, E.; Hamed, A.M. Antioxidant, Antibacterial Activities and Mineral Content of Buffalo Yoghurt Fortified with Fenugreek and Moringa oleifera Seed Flours. Foods 2020, 9, 1157. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Guimarães, J.T.; Capozzi, V.; Russo, P.; Caroprese, M.; Marino, R.; Esmerino, E.A.; Raices, R.S.L.; Silva, M.C.; et al. Novel Milk–Juice Beverage with Fermented Sheep Milk and Strawberry (Fragaria × Ananassa): Nutritional and Functional Characterization. J. Dairy Sci. 2019, 102, 10724–10736. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Bušić, A.; Belščak-Cvitanović, A.; Brnčić, M.; Bosiljkov, T.; Vojvodić, A.; Dujmić, F. Novel Approach for the Development of Functional Goat Milk-Based Beverages Using Medicinal Plant Extracts in Combination with High Intensity Ultrasound Treatment. Food Technol. Biotechnol. 2017, 55, 484–495. [Google Scholar] [CrossRef]
- Habibi Bibalani, G.; Mosazadeh-Sayadmahaleh, F. Medicinal Benefits and Usage of Medlar (Mespilus germanica) in Gilan Province (Roudsar District), Iran. J. Med. Plants Res. 2012, 6, 1155–1159. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Kabala, S.I.; Yagar, H. Assessment of in Vitro Antioxidant and Antidiabetic Capacities of Medlar (Mespilus germanica). Not. Bot. Ho. Agrobot. Cluj-Napoca 2019, 47, 384–389. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Lardos, A.; Heinrich, M. Continuity and Change in Medicinal Plant Use: The Example of Monasteries on Cyprus and Historical Iatrosophia Texts. J. Ethnopharmacol. 2013, 150, 202–214. [Google Scholar] [CrossRef]
- Mraihi, F.; Hidalgo, M.; de Pascual-Teresa, S.; Trabelsi-Ayadi, M.; Chérif, J.K. Wild Grown Red and Yellow Hawthorn Fruits from Tunisia as Source of Antioxidants. Arab. J. Chem. 2015, 8, 570–578. [Google Scholar] [CrossRef]
- Damar, S.; Balaban, M.O.; Sims, C.A. Continuous dense-phase CO2 processing of a coconut water beverage. Int. J. Food Sci. Technol. 2009, 44, 666–673. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food analysis. In Food Science Texts Series, 4th ed.; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; ISBN 978-1-4419-1477-4. [Google Scholar]
- Goulas, V.; Georgiou, E. Utilization of Carob Fruit as Sources of Phenolic Compounds with Antioxidant Potential: Extraction Optimization and Application in Food Models. Foods 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stavrou, K.; Michael, C.; Botsaris, G.; Barbouti, A. The Potential of Sun-Dried Grape Pomace as a Multi-Functional Ingredient for Herbal Infusion: Effects of Brewing Parameters on Composition and Bioactivity. Antioxidants 2021, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, H.B.; Jemia, A.B.; Khlifi, S.; Ahmed, H.B.; Slama, F.B.; Benzarti, A.; Elati, J.; Aouidet, A. Antioxidant Activity and A-Amylase Inhibitory Potential of Rosa canina L. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Graham, K.; Rea, R.; Simpson, P.; Stack, H. Enterococcus Faecalis Milk Fermentates Display Antioxidant Properties and Inhibitory Activity towards Key Enzymes Linked to Hypertension and Hyperglycaemia. J. Funct. Foods 2019, 58, 292–300. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Abd El Ghani, S. Production of Functional Fermented Milk Beverages Supplemented with Pomegranate Peel Extract and Probiotic Lactic Acid Bacteria. J. Food Qual. 2020, 2020, 4710273. [Google Scholar] [CrossRef]
- Silva, M.P.; da Mesquita, M.S.; Fernanda, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Fortification of Yoghurt Drink with Microcapsules Loaded with Lacticaseibacillus Paracasei BGP-1 and Guaraná Seed Extract. Int. Dairy J. 2022, 125, 105230. [Google Scholar] [CrossRef]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. ISO: Geneva, Switzerland, 1998.
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Congiu, F.; Tuberoso, C.I.G.; Amri, M.; Fattouch, S. Comparative Analysis of Tunisian Wild Crataegus azarolus (Yellow Azarole) and Crataegus monogyna (Red Azarole) Leaf, Fruit, and Traditionally Derived Syrup: Phenolic Profiles and Antioxidant and Antimicrobial Activities of the Aqueous-Acetone Extracts. J. Agric. Food Chem. 2013, 61, 12171–12172. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Mićanović, N.; Grozdanić, N.; Kostić, A.Ž.; Gašić, U.; Stanojković, T.; Popović-Djordjević, J.B. Polyphenolic Profile, Antioxidant and Antidiabetic Potential of Medlar (Mespilus germanica L.), Blackthorn (Prunus spinosa L.) and Common Hawthorn (Crataegus monogyna Jacq.) Fruit Extracts from Serbia. Horticulturae 2022, 8, 1053. [Google Scholar] [CrossRef]
- Safari, M.; Ahmady-Asbchin, S. Evaluation of Antioxidant and Antibacterial Activities of Methanolic Extract of Medlar (Mespilus germanica L.) Leaves. Biotechnol. Biotechnol. Equip. 2019, 33, 372–378. [Google Scholar] [CrossRef]
- Akbulut, M.; Ercisli, S.; Jurikova, T.; Mlcek, J.; Gozlekci, S. Phenotypic and Bioactive Diversity on Medlar Fruits (Mespilus germanica L.). Erwerbs-Obstbau 2016, 58, 185–191. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Khaneghah, A.M.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Szołtysik, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Dąbrowska, A.; Bobak, Ł.; Chrzanowska, J. The Effect of Rosa Spinosissima Fruits Extract on Lactic Acid Bacteria Growth and Other Yoghurt Parameters. Foods 2020, 9, 1167. [Google Scholar] [CrossRef] [PubMed]
- Żołnierczyk, A.K.; Ciałek, S.; Styczyńska, M.; Oziembłowski, M. Functional Properties of Fruits of Common Medlar (Mespilus germanica L.) Extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Zhang, T.; Hee, C.; Nee, W.; Bae, H.; Geuk, H.; Petriello, M.C.; Gu, S. Moringa Extract Enhances the Fermentative, Textural, and Bioactive Properties of Yogurt. LWT—Food Sci. Technol. 2019, 101, 276–284. [Google Scholar] [CrossRef]
- CXS 243-2003; Codex Alimentarius. Standard for Fermented Milk. Food and Agriculture Organization of the United Nations: Rome, Italy, 2018.
- Amirdivani, S.; Baba, A.S. Changes in Yogurt Fermentation Characteristics, and Antioxidant Potential and in Vitro Inhibition of Angiotensin-1 Converting Enzyme upon the Inclusion of Peppermint, Dill and Basil. LWT—Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Akan, E.; Yerlikaya, O.; Bayram, O.Y.; Kinik, O. The Effect of Aqueous Extracts of Some Plants on in Vitro Antioxidant and Antidiabetic Activity of Probiotic Yogurt. J. Food Sci. Technol. 2022, 59, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of Fermentation on the Peptide Content, Phenolics and Antioxidant Activity of Defatted Wheat Germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Comparative Antioxidant Activity, Proteolysis and in Vitro α-Amylase and α-Glucosidase Inhibition of Allium Sativum-Yogurts Made from Cow and Camel Milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]


| Fruit Extract | Τ, °C | t, min | Fruit/Water Ratio, w/v | |
|---|---|---|---|---|
| Crataegus azarolus | M1 | 60 | 30 | 1:10 |
| M2 | 60 | 60 | 1:10 | |
| M3 | 60 | 30 | 1:20 | |
| M4 | 60 | 60 | 1:20 | |
| M5 | 80 | 30 | 1:10 | |
| M6 | 80 | 60 | 1:10 | |
| M7 | 80 | 30 | 1:20 | |
| M8 | 80 | 60 | 1:20 | |
| Mespilus germanica | P1 | 60 | 30 | 1:10 |
| P2 | 60 | 60 | 1:10 | |
| P3 | 60 | 30 | 1:20 | |
| P4 | 60 | 60 | 1:20 | |
| P5 | 80 | 30 | 1:10 | |
| P6 | 80 | 60 | 1:10 | |
| P7 | 80 | 30 | 1:20 | |
| P8 | 80 | 60 | 1:20 | |
| Stage | Pressure, Pa | T, °C | Time, s |
|---|---|---|---|
| D01 | 25 | −65 | 550 |
| D02 | 20 | −65 | 600 |
| D03 | 15 | −65 | 800 |
| D04 | 15 | −67 | 999 |
| Fruit Samples | ΤPC, mgGAE/100 g Fruit | TFC, mgRE/100 g Fruit | Antioxidant Activity | ||
|---|---|---|---|---|---|
| FRAP, mmol FeSO4/100 g Fruit | DPPH, % | ||||
| Crataegus azarolus | M1 | 51.52 ± 4.32 d | 82.96 ± 6.22 c | 3998.70 ± 266.39 e | 86.4 ± 0.0 cd |
| M2 | 64.40 ± 14.12 bcd | 24.33 ± 1.22 e | 6770.44 ± 879.03 de | 87.7 ± 1.0 bc | |
| M3 | 53.15 ± 10.63 cd | 56.75 ± 4.54 d | 14744.50 ± 226.27 a | 92.5 ± 2.0 ab | |
| M4 | 69.82 ± 2.24 abcd | 182.10 ± 8.15 a | 13499.62 ± 1658.20 ab | 97.4 ± 0.0 a | |
| M5 | 78.00 ± 0.72 abc | 176.64 ± 5.72 a | 5214.36 ± 546.33 de | 85.0 ± 2.0 cd | |
| M6 | 94.30 ± 15.55 a | 140.25 ± 12.19 b | 5611.56 ± 881.41 de | 81.0 ± 1.0 d | |
| M7 | 78.60 ± 5.77 abc | 209.06 ± 31.47 a | 10767.44 ± 197.17 bc | 87.7 ± 4.0 bc | |
| M8 | 87.58 ± 8.08 ab | 137.62 ± 13.04 b | 8389.47 ± 1257.84 cd | 94.1 ± 1.0 a | |
| Mespilus germanica | P1 | 19.95 ± 4.62 c | 107.16 ± 14.07 b | 5151.23 ± 303.71 b | 91.8 ± 1.0 bc |
| P2 | 35.26 ± 8.51 bc | 130.61 ± 13.55 b | 6314.64 ± 280.30 b | 90.9 ± 1.0 c | |
| P3 | 46.41 ± 9.09 abc | 167.40 ± 30.00 b | 4150.35 ± 618.07 b | 97.3 ± 1.0 a | |
| P4 | 34.59 ± 6.51 bc | 91.01 ± 8.27 b | 12115.88 ± 259.32 a | 97.8 ±0.0 a | |
| P5 | 70.01 ± 13.74 a | 432.18 ± 59.31 a | 5871.71 ± 1056.16 b | 93.5 ± 1.0 b | |
| P6 | 59.10 ± 9.36 ab | 539.40 ± 67.97 a | 2167.40 ± 313.59 b | 92.5 ± 1.0 bc | |
| P7 | 53.52 ± 5.37 ab | 156.68 ± 25.22 b | 12972.12 ± 554.99 a | 97.9 ± 0.0 a | |
| P8 | 65.33 ± 7.62 a | 664.66 ± 113.23 a | 13415.89 ± 873.80 a | 97.8 ± 0.0 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papademas, P.; Ioannou, I.; Aspri, M. Enrichment of Fermented Milk Drinks with Mespilus germanica and Crataegus azarolus Fruit Extracts. Appl. Sci. 2023, 13, 9243. https://doi.org/10.3390/app13169243
Papademas P, Ioannou I, Aspri M. Enrichment of Fermented Milk Drinks with Mespilus germanica and Crataegus azarolus Fruit Extracts. Applied Sciences. 2023; 13(16):9243. https://doi.org/10.3390/app13169243
Chicago/Turabian StylePapademas, Photis, Ioanna Ioannou, and Maria Aspri. 2023. "Enrichment of Fermented Milk Drinks with Mespilus germanica and Crataegus azarolus Fruit Extracts" Applied Sciences 13, no. 16: 9243. https://doi.org/10.3390/app13169243
APA StylePapademas, P., Ioannou, I., & Aspri, M. (2023). Enrichment of Fermented Milk Drinks with Mespilus germanica and Crataegus azarolus Fruit Extracts. Applied Sciences, 13(16), 9243. https://doi.org/10.3390/app13169243








