Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review
Abstract
1. Introduction
2. Topics and Results
2.1. Biomechanical Parameters, Healing, and Therapeutic Effects of Stretching
2.2. Animal, Mathematical, and Computational Models of Stretching
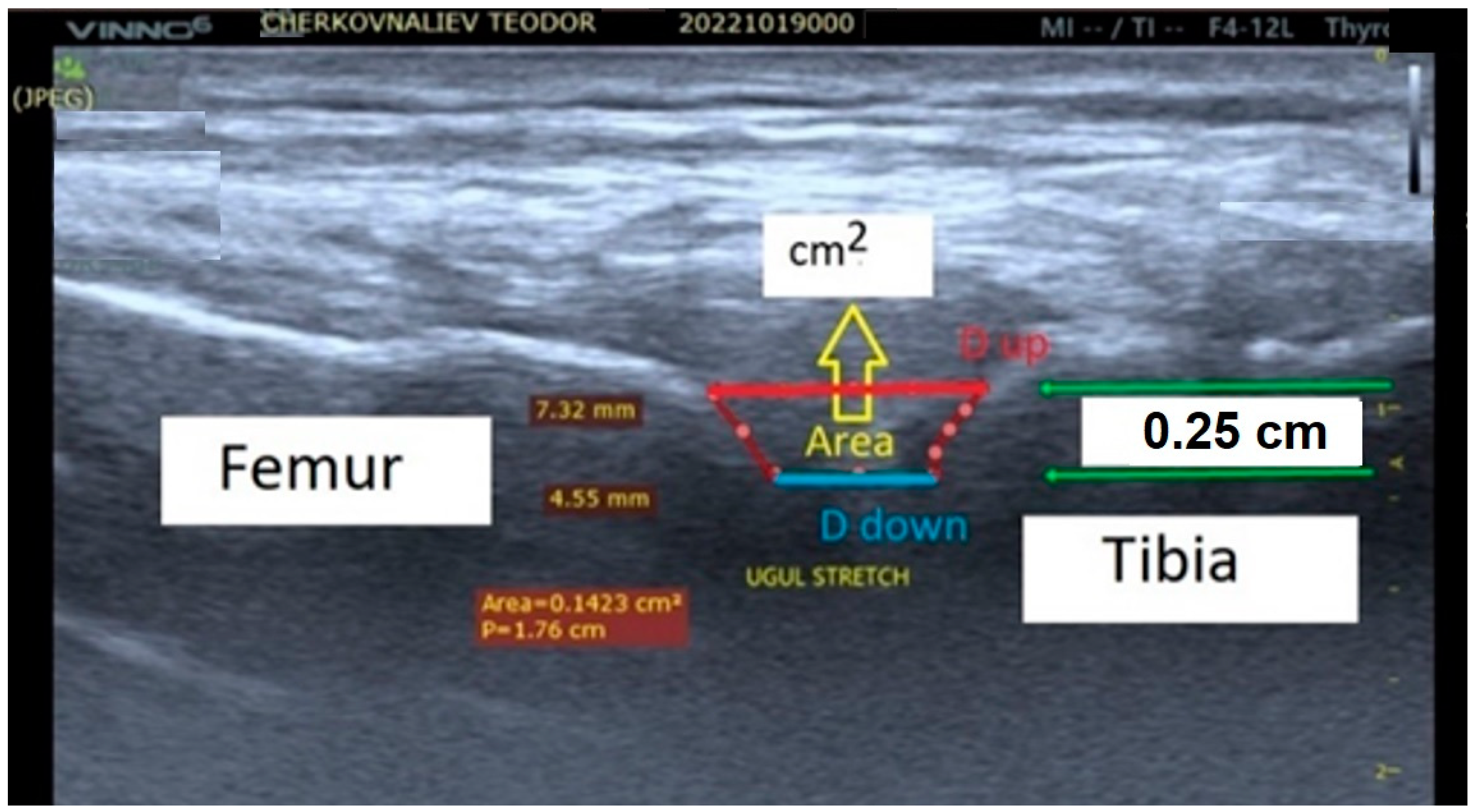
2.3. Biomechanical Effects of Static and Active Isometric Stretching Applied to the Human Knee Joint
2.4. Biomechanical and Biological (Cellular and Molecular) Mechanisms of Stretching
2.5. Stretching Is an Integral Component of Mind–Body Exercises Such as Yoga (Mainly Hatha Yoga), Tai Chi, and Gingong
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weerapong, P.; Hume, P.A.; Kolt, G.S. Stretching: Mechanisms and benefits for sport performance and injury prevention. Phys. Ther. Rev. 2004, 9, 189–206. [Google Scholar] [CrossRef]
- Taylor, D.C.; Dalton, J.D., Jr.; Seaber, A.V.; Garrett, W.E., Jr. Viscoelastic properties of muscle-tendon units: The biomechanical effects of stretching. Am. J. Sports Med. 1990, 18, 300–309. [Google Scholar] [CrossRef]
- Ferber, R.; Osternig, L.R.; Gravelle, D.C. Effect of PNF stretch techniques on knee flexor muscle EMG activity in older adults. J. Electromyogr. Kinesiol. 2002, 12, 391–397. [Google Scholar] [CrossRef]
- Freitas, S.R.; Mendes, B.; Le Sant, G.; Andrade, R.J.; Nordez, A.; Milanovic, Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand. J. Med. Sci. Sports 2018, 28, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Arntz, F.; Markov, A.; Behm, D.G.; Behrens, M.; Negra, Y.; Nakamura, M.; Chaabene, H. Chronic Effects of Static Stretching Exercises on Muscle Strength and Power in Healthy Individuals Across the Lifespan: A Systematic Review with Multi-level Meta-analysis. Sports Med. 2023, 53, 723–745. [Google Scholar] [CrossRef]
- Knudson, D. The biomechanics of stretching. J. Exerc. Sci. Physiother. 2006, 2, 3–12. [Google Scholar]
- Wang, L.; Cui, J.B.; Xie, H.M.; Zuo, X.Q.; He, J.L.; Jia, Z.S.; Zhang, L.N. Effects of Different Static Progressive Stretching Durations on Range of Motion, Myofibroblasts, and Collagen in a Posttraumatic Knee Contracture Rat Model. Phys. Ther. 2022, 102, pzab300. [Google Scholar] [CrossRef] [PubMed]
- Salsich, G.B.; Mueller, M.J.; Sahrmann, S.A. Passive ankle stiffness in subjects with diabetes and peripheral neuropathy versus an age-matched comparison group. Phys. Ther. 2000, 80, 352–362. [Google Scholar] [CrossRef]
- Sacco, I.C.; Sartor, C.D. From treatment to preventive actions: Improving function in patients with diabetic polyneuropathy. Diabetes/Metab. Res. Rev. 2016, 32, 206–212. [Google Scholar] [CrossRef]
- Williams, D.B.; Brunt, D.; Tanenberg, R.J. Diabetic neuropathy is related to joint stiffness during late stance phase. J. Appl. Biomech. 2007, 23, 251–260. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.; Zhang, Q.B.; Zhou, Y.; Zhang, R.; Wang, F. Possible mechanism of static progressive stretching combined with extracorporeal shock wave therapy in reducing knee joint contracture in rats based on MAPK/ERK pathway. Biomol. Biomed. 2023, 23, 277–286. [Google Scholar] [CrossRef]
- Nakamura, M.; Konrad, A.; Kasahara, K.; Yoshida, R.; Murakami, Y.; Sato, S.; Wilke, J. The Combined Effect of Static Stretching and Foam Rolling With or Without Vibration on the Range of Motion, Muscle Performance, and Tissue Hardness of the Knee Extensor. J. Strength Cond. Res. 2022, 37, 322–327. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Cini, A.; Sbruzzi, G.; Lima, C.S. Influence of static stretching on hamstring flexibility in healthy young adults: Systematic review and meta-analysis. Physiother. Theory Pract. 2016, 32, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, T.; Sato, S.; Yahata, K.; Yoshida, R.; Takeuchi, K.; Nakamura, M. Effects of stretching intensity on range of motion and muscle stiffness: A narrative review. J. Bodyw. Mov. Ther. 2022, 32, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.; Langevin, H.M. Stretching impacts inflammation resolution in connective tissue. J. Cell. Physiol. 2016, 231, 1621–1627. [Google Scholar] [CrossRef]
- Król, M.; Kupnicka, P.; Bosiacki, M.; Chlubek, D. Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review. Int. J. Mol. Sci. 2022, 23, 10127. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chang, N.J.; Wu, W.L.; Guo, L.Y.; Chu, I.H. Acute effects of foam rolling, static stretching, and dynamic stretching during warm-ups on muscular flexibility and strength in young adults. J. Sport Rehabil. 2017, 26, 469–477. [Google Scholar] [CrossRef]
- Cipriani, D.J.; Terry, M.E.; Haines, M.A.; Tabibnia, A.P.; Lyssanova, O. Effect of stretch frequency and sex on the rate of gain and rate of loss in muscle flexibility during a hamstring-stretching program: A randomized single-blind longitudinal study. J. Strength Cond. Res. 2012, 26, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, CD010802. [Google Scholar] [CrossRef]
- Taylor, D. Physical activity is medicine for older adults. Postgrad. Med. J. 2014, 90, 26–32. [Google Scholar] [CrossRef]
- Copeland, J. Stretching: Mechanisms and benefits for sport performance and injury prevention. New Zealand J. Physiother. 2005, 33, 68–69. [Google Scholar]
- Kataura, S.; Suzuki, S.; Matsuo, S.; Hatano, G.; Iwata, M.; Yokoi, K.; Tsuchida, W.; Banno, Y.; Asai, Y. Acute effects of the different intensity of static stretching on flexibility and isometric muscle force. J. Strength Cond. Res. 2017, 31, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Gad, M.; Tilp, M.J.S.J. Effect of PNF stretching training on the properties of human muscle and tendon structures. Scand. J. Med. Sci. Sports 2015, 25, 346–355. [Google Scholar] [CrossRef]
- Hotta, K.; Behnke, B.J.; Arjmandi, B.; Ghosh, P.; Chen, B.; Brooks, R.; Maraj, J.J.; Elam, M.; Maher, P.; Kurien, D.; et al. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J. Physiol. 2018, 596, 1903–1917. [Google Scholar] [CrossRef]
- Guissard, N.; Duchateau, J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2004, 29, 248–255. [Google Scholar] [CrossRef]
- Middag, T.R.; Harmer, P. Active-isolated stretching is not more effective than static stretching for increasing hamstring ROM. Med. Sci. Sports Exerc. 2002, 34, S151. [Google Scholar] [CrossRef]
- Stoichev, S.; Ivanov, I.; Ranchev, S.; Jotov, I. A review of the biomechanics of synovial joints with emphasize to static stretching exercise. Ser. Biomech. 2021, 35, 3–20. [Google Scholar]
- Ranchev, S.; Ivanov, I.; Iotov, I.; Stoytchev, S. On the biomechanical processes in human knee joint during active isometric stretching. Ser. Biomech. 2019, 33, 56–61. [Google Scholar]
- Davies, D.V. Synovial membrane and synovial fluid of joints. Lancet 1946, 248, 815–819. [Google Scholar] [CrossRef]
- Bryanton, M.; Bilodeau, M. The role of thigh muscular efforts in limiting sit-to-stand capacity in healthy young and older adults. Aging Clin. Exp. Res. 2017, 29, 1211–1219. [Google Scholar] [CrossRef]
- Hill, K.J.; Robinson, K.P.; Cuchna, J.W.; Hoch, M.C. Immediate effects of proprioceptive neuromuscular facilitation stretching programs compared with passive stretching programs for hamstring flexibility: A critically appraised topic. J. Sport Rehabil. 2017, 26, 567–572. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, C.L.; Chang, N.J. Acute effects of dynamic stretching followed by vibration foam rolling on sports performance of badminton athletes. J. Sports Sci. Med. 2020, 19, 420. [Google Scholar] [PubMed]
- Kokkonen, J.; Nelson, A.G.; Eldredge, C.; Winchester, J.B. Chronic static stretching improves exercise performance. Med. Sci. Sports Exerc. 2007, 39, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Konrad, A.; Nakamura, M.; Paternoster, F.K.; Tilp, M.; Behm, D.G. A comparison of a single bout of stretching or foam rolling on range of motion in healthy adults. Eur. J. Appl. Physiol. 2022, 122, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Ando, A.; Chimoto, E.; Tsuchiya, M.; Takahashi, I.; Sasano, Y.; Onoda, Y.; Suda, H.; Itoi, E. Expression of collagen types I and II on articular cartilage in a rat knee contracture model. Connect. Tissue Res. 2010, 51, 22–30. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Zhang, M.; Germscheid, N.M.; Wang, C.; Hart, D.A. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop. 2008, 79, 116–125. [Google Scholar] [CrossRef]
- Tokuda, K.; Yamanaka, Y.; Kosugi, K.; Nishimura, H.; Okada, Y.; Tsukamoto, M.; Tajima, T.; Suzuki, H.; Kawasaki, M.; Uchida, S.; et al. Development of a novel knee contracture mouse model by immobilization using external fixation. Connect. Tissue Res. 2022, 63, 169–182. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhou, Y.; Zhong, H.Z.; Liu, Y. Effect of stretching combined with ultrashort wave diathermy on joint function and its possible mechanism in a rabbit knee contracture model. Am. J. Phys. Med. Rehabil. 2018, 97, 357–363. [Google Scholar] [CrossRef]
- Stoytchev, S.; Nikolov, S. Effects of flow-dependent and flow-independent viscoelastic mechanisms on the stress relaxation of articular cartilage. Ser. Biomech. 2023, 37, 43–50. [Google Scholar] [CrossRef]
- McNair, P.; Stanley, S. Effect of passive stretching and jogging on the series muscle stiffness and range of motion of the ankle joint. Br. J. Sports Med. 1996, 30, 313–318. [Google Scholar] [CrossRef]
- Magnusson, S. Passive properties of human skeletal muscle during stretch manoeuvres. MedSci. Sports Exerc. 1998, 8, 65–77. [Google Scholar]
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Sorensen, H.; Kjaer, M. A mechanism for altered flexibility in human skeletal muscle. J. Physiol. 1996, 497, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.; Simonsen, E.; Dyhre-Poulsen, P.; Aagaard, P.; Mohr, T.; Kjaer, M. Viscoelastic stressrelaxation during static stretch in human skeletal muscle in the absence of EMG activity. MedSci. Sports Exerc. 1996, 6, 323–328. [Google Scholar]
- McNair, P.J.; Dombroski, E.W.; Hewson, D.J.; Stanley, S.N. Stretching at the ankle joint: Viscoelastic responses to holds and continuous passive motion. Med. Sci. Sports Exerc. 2001, 33, 354–358. [Google Scholar] [CrossRef]
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Gleim, G.; McHugh, M.; Kjaer, M. Viscoelastic response to repeated static stretching in the human hamstring muscle. Scand. J. MedSci. Sports 1995, 5, 342–347. [Google Scholar] [CrossRef]
- Magnusson, S.; Aagaard, P.; Larsson, B.; Kjaer, M. Passive energy absorption by human muscle-tendon unit is unaffected by increase in intramuscular temperature. J. Appl. Physiol. 2000, 88, 1215–1220. [Google Scholar] [CrossRef]
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Kjaer, M. Biomechanical responses to repeated stretches in human hamstring muscle in vivo. Am. J. Sports Med. 1996, 24, 622–628. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Is passive stiffness in human muscles related to the elasticity of tendon structures? Eur. J. Appl. Physiol. 2001, 85, 226–232. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Effects of resistance and stretching training programs on the viscoelastic properties of human tendon structures in vivo. J. Physiol. 2002, 538, 219–226. [Google Scholar] [CrossRef]
- Cotofana, S.; Eckstein, F.; Wirth, W.; Souza, R.B.; Li, X.; Wyman, B.; Graverand, M.-P.H.-L.; Link, T.; Majumdar, S. In vivo measures of cartilage deformation: Patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur. Radiol. 2011, 21, 1127–1135. [Google Scholar] [CrossRef]
- Herberhold, C.; Faber, S.; Stammberger, T.; Steinlechner, M.; Putz, R.; Englmeier, K.H.; Reiser, M.; Eckstein, F. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J. Biomech. 1999, 32, 1287–1295. [Google Scholar] [CrossRef]
- Ranchev, S.; Ivanov, I.M.; Yotov, I.; Stoytchev, S. Studies on paradox in the work of musculoskeletal system in isometric stretching. J. Appl. Sports Sci. 2020, 2, 80–90. [Google Scholar] [CrossRef]
- Behm, D.G.; Alizadeh, S.; Daneshjoo, A.; Konrad, A. Potential Effects of Dynamic Stretching on Injury Incidence of Athletes: A Narrative Review of Risk Factors. Sports Med. 2023, 53, 1359–1373. [Google Scholar] [CrossRef]
- Kuntz, A.B.; Chopp-Hurley, J.N.; Brenneman, E.C.; Karampatos, S.; Wiebenga, E.G.; Adachi, J.D.; Noseworthy, M.; Maly, M.R. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS ONE 2018, 13, e0195653. [Google Scholar] [CrossRef]
- Trindade, T.B.; de Medeiros, J.A.; Dantas, P.M.S.; de Oliveira Neto, L.; Schwade, D.; de Brito Vieira, W.H.; Oliveira-Dantas, F.F. A comparison of muscle electromyographic activity during different angles of the back and front squat. Isokinet. Exerc. Sci. 2020, 28, 1–8. [Google Scholar] [CrossRef]
- Raikova, R.; Ivanov, I.; Hristov, O.; Markova, N.; Trenev, L.; Angelova, S. Detailed investigation of the knee biomechanics during posture maintenance applying different static loading on the spine. Int. J. Bioautom. 2023, 27, 83. [Google Scholar] [CrossRef]
- Abusharkh, H.A.; Reynolds, O.M.; Mendenhall, J.; Gozen, B.A.; Tingstad, E.; Idone, V.; Abu-Lail, N.; Van Wie, B.J. Combining stretching and gallic acid to decrease inflammation indices and promote extracellular matrix production in osteoarthritic human articular chondrocytes. Exp. Cell Res. 2021, 408, 112841. [Google Scholar] [CrossRef]
- Madhavan, S.; Anghelina, M.; Rath-Deschner, B.; Wypasek, E.; John, A.; Deschner, J.; Piesco, N.; Agarwal, S. Biomechanical signals exert sustained attenuation of proinflammatory gene induction in articular chondrocytes. Osteoarthr. Cartil. 2006, 14, 1023–1032. [Google Scholar] [CrossRef][Green Version]
- Bouffard, N.A.; Cutroneo, K.R.; Badger, G.J.; White, S.L.; Buttolph, T.R.; Ehrlich, H.P.; Stevens-Tuttle, D.; Langevin, H.M. Tissue stretch decreases soluble TGF-β1 and type-1 procollagen in mouse subcutaneous connective tissue: Evidence from ex vivo and in vivo models. J. Cell. Physiol. 2008, 214, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Berrueta, L.; Urso, K.; Olenich, S.; Muskaj, I.; Badger, G.J.; Aliprantis, A.; Lafyatis, R.; Langevin, H.M. Stretching reduces skin thickness and improves subcutaneous tissue mobility in a murine model of systemic sclerosis. Front. Immunol. 2017, 8, 124. [Google Scholar] [CrossRef]
- Syedain, Z.H.; Tranquillo, R.T. TGF-β1 diminishes collagen production during long-term cyclic stretching of engineered connective tissue: Implication of decreased ERK signaling. J. Biomech. 2011, 44, 848–855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: A systematic review. Appl. Physiol. Nutr. Metab. 2016, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.O.; Medeiros, D.M.; Minotto, B.B.; Lima, C.S. Comparison between static stretching and proprioceptive neuromuscular facilitation on hamstring flexibility: Systematic review and meta-analysis. Eur. J. Physiother. 2018, 20, 12–19. [Google Scholar] [CrossRef]
- Patel, N.K.; Newstead, A.H.; Ferrer, R.L. The effects of yoga on physical functioning and health related quality of life in older adults: A systematic review and meta-analysis. J. Altern. Complement. Med. 2012, 18, 902–917. [Google Scholar] [CrossRef]
- Gothe, N.P.; Kramer, A.F.; McAuley, E. The effects of an 8-week Hatha yoga intervention on executive function in older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vergara, D.; Grabowska, W.; Yeh, G.Y.; Khalsa, S.B.; Schreiber, K.L.; Huang, C.A.; Zavacki, A.; Wayne, P.M. A systematic review of in vivo stretching regimens on inflammation and its relevance to translational yoga research. PLoS ONE 2022, 17, e0269300. [Google Scholar]
- Gothe, N.P.; McAuley, E. Yoga is as good as stretching–strengthening exercises in improving functional fitness outcomes: Results from a randomized controlled trial. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 406–411. [Google Scholar] [CrossRef]
- Pizza, F.X.; Koh, T.J.; McGregor, S.J.; Brooks, S.V. Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. J. Appl. Physiol. 2002, 92, 1873–1878. [Google Scholar] [CrossRef]
- Chu, S.Y.; Chou, C.H.; Huang, H.D.; Yen, M.H.; Hong, H.C.; Chao, P.H.; Wang, Y.-H.; Chen, P.-Y.; Nian, S.-X.; Chen, Y.-R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Danhauer, S.C.; Addington, E.L.; Cohen, L.; Sohl, S.J.; Van Puymbroeck, M.; Albinati, N.K.; Culos-Reed, S.N. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer 2019, 125, 1979–1989. [Google Scholar] [CrossRef]
- Sumi, K.; Ashida, K.; Nakazato, K. Repeated stretch–shortening contraction of the triceps surae attenuates muscle atrophy and liver dysfunction in a rat model of inflammation. Exp. Physiol. 2019, 105, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Eda, N.; Ito, H.; Shimizu, K.; Suzuki, S.; Lee, E.; Akama, T. Yoga stretching for improving salivary immune function and mental stress in middle-aged and older adults. J. Women Aging 2018, 30, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Collet, J.P.; Lau, J. The effect of Tai Chi on health outcomes in patients with chronic conditions: A systematic review. Arch. Intern. Med. 2004, 164, 493–501. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef]
- Ghasemi, E.; Khademi-Kalantari, K.; Khalkhali-Zavieh, M.; Rezasoltani, A.; Ghasemi, M.; Baghban, A.A.; Ghasemi, M. The effect of functional stretching exercises on functional outcomes in spastic stroke patients: A randomized controlled clinical trial. J. Bodyw. Mov. Ther. 2018, 22, 1004–1012. [Google Scholar] [CrossRef]
- Park, S.H. Effects of passive static stretching on blood glucose levels in patients with type 2 diabetes mellitus. J. Phys. Ther. Sci. 2015, 27, 1463–1465. [Google Scholar] [CrossRef]
- Ferretti, M.; Madhavan, S.; Deschner, J.; Rath-Deschner, B.; Wypasek, E.; Agarwal, S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C1610–C1615. [Google Scholar] [CrossRef] [PubMed]
- Berrueta, L.; Bergholz, J.; Munoz, D.; Muskaj, I.; Badger, G.J.; Shukla, A.; Kim, H.J.; Zhao, J.J.; Langevin, H.M. Stretching reduces tumor growth in a mouse breast cancer model. Sci. Rep. 2018, 8, 7864. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Ahamad, A.; Fatima, A.; Ahmad, I.; Malhotra, D.; Al Muslem, W.H.; Abdulaziz, S.; Nuhmani, S. Immediate and Long-Term Effectiveness of Proprioceptive Neuromuscular Facilitation and Static Stretching on Joint Range of Motion, Flexibility, and Electromyographic Activity of Knee Muscles in Older Adults. J. Clin. Med. 2023, 12, 2610. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Passini, F.S.; Silván, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Araújo, V.L.; Leal, Â.M.; Serrão, P.R.; Perea, J.P.; Santune, A.H.; Sacco, I.; Aranha, G.A.; Fernandes, R.A.S.; Salvini, T.F.; et al. Diabetes and peripheral neuropathy are related to higher passive torque and stiffness of the knee and ankle joints. Kinesiology 2022, 54, 92–104. [Google Scholar] [CrossRef]
- Smith, J.R.; Walker, J.M. Knee and elbow range of motion in healthy older individuals. Phys. Occup. Ther. Geriatr. 1983, 2, 31–38. [Google Scholar] [CrossRef]
- Page, P. Current concepts in muscle stretching for exercise and rehabilitation. Int. J. Sports Phys. Ther. 2012, 7, 109. [Google Scholar] [PubMed]
- Behm, D.G.; Aragão-Santos, J.C.; Korooshfard, N.; Anvar, S.H. Alternative Flexibility Training. Int. J. Sports Phys. Ther. 2023, 18, 285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvetkova, E.; Koytchev, E.; Ivanov, I.; Ranchev, S.; Antonov, A. Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review. Appl. Sci. 2023, 13, 8596. https://doi.org/10.3390/app13158596
Zvetkova E, Koytchev E, Ivanov I, Ranchev S, Antonov A. Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review. Applied Sciences. 2023; 13(15):8596. https://doi.org/10.3390/app13158596
Chicago/Turabian StyleZvetkova, Elissaveta, Eugeni Koytchev, Ivan Ivanov, Sergey Ranchev, and Antonio Antonov. 2023. "Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review" Applied Sciences 13, no. 15: 8596. https://doi.org/10.3390/app13158596
APA StyleZvetkova, E., Koytchev, E., Ivanov, I., Ranchev, S., & Antonov, A. (2023). Biomechanical, Healing and Therapeutic Effects of Stretching: A Comprehensive Review. Applied Sciences, 13(15), 8596. https://doi.org/10.3390/app13158596






