Electrochemical Disinfection of Root Canals Bears No Risk of Damaging Periapical Tissues in a Dog Model
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Analysis of Endodontic Treatment
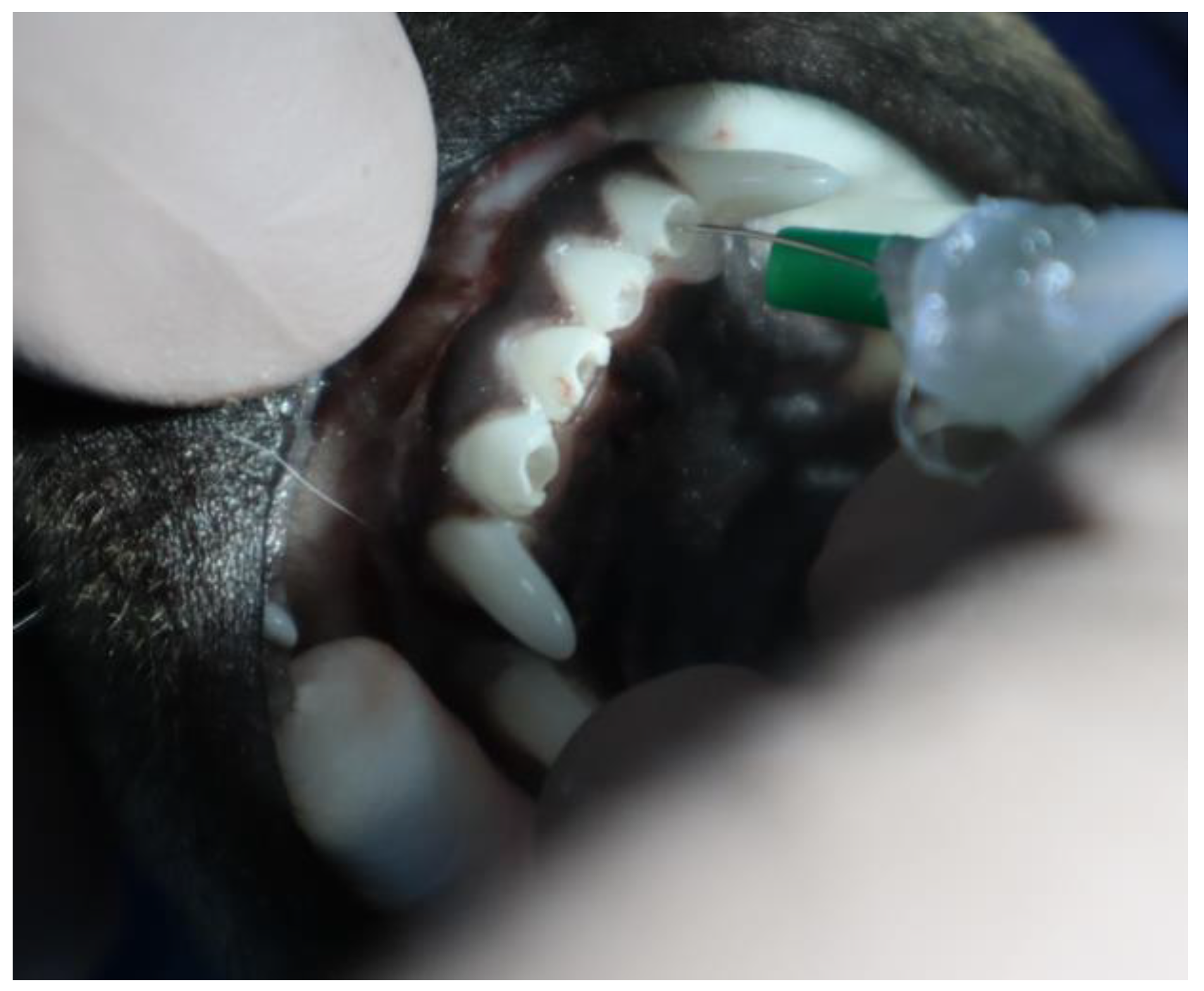
2.2.1. Animal Experiment
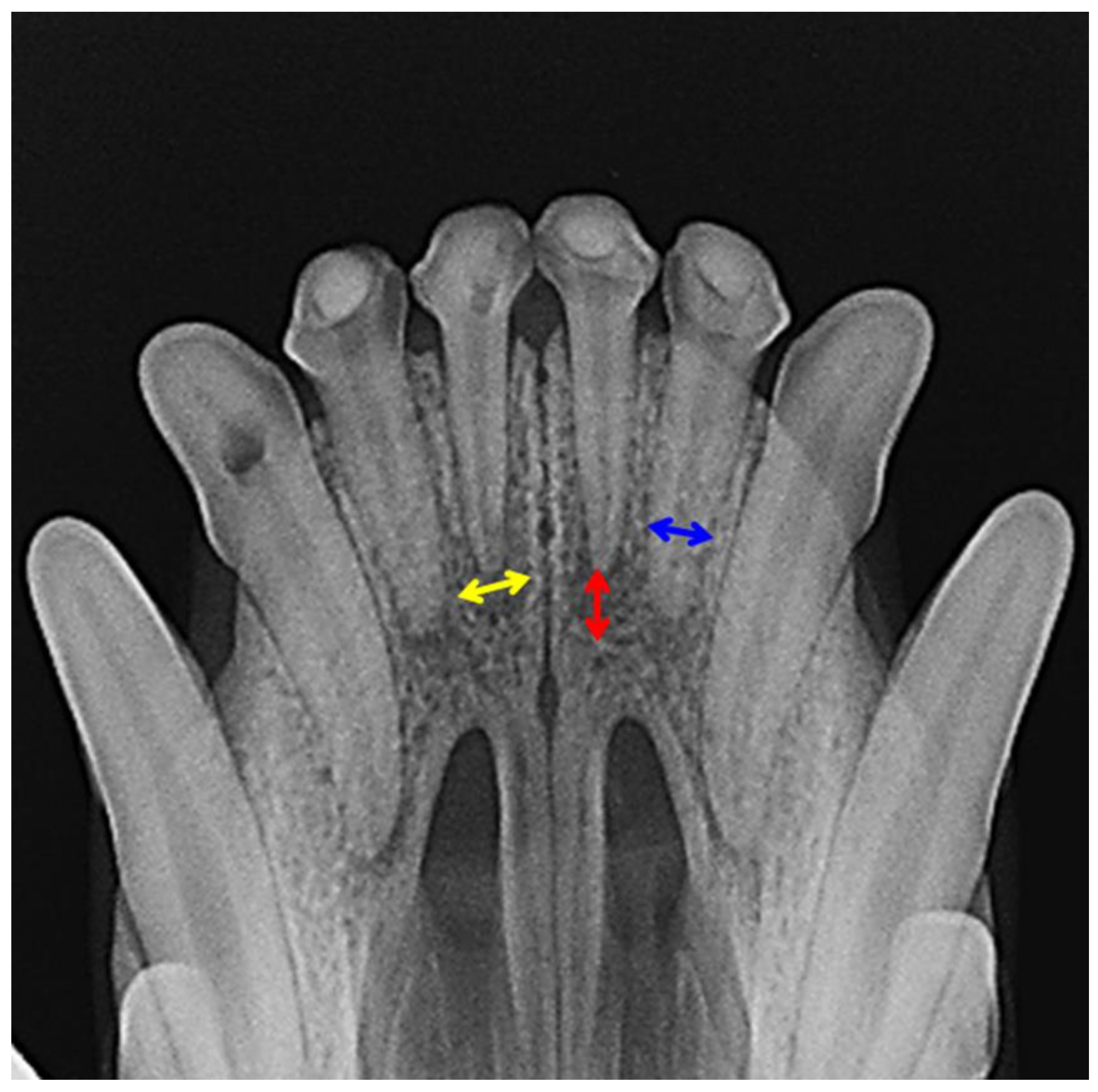
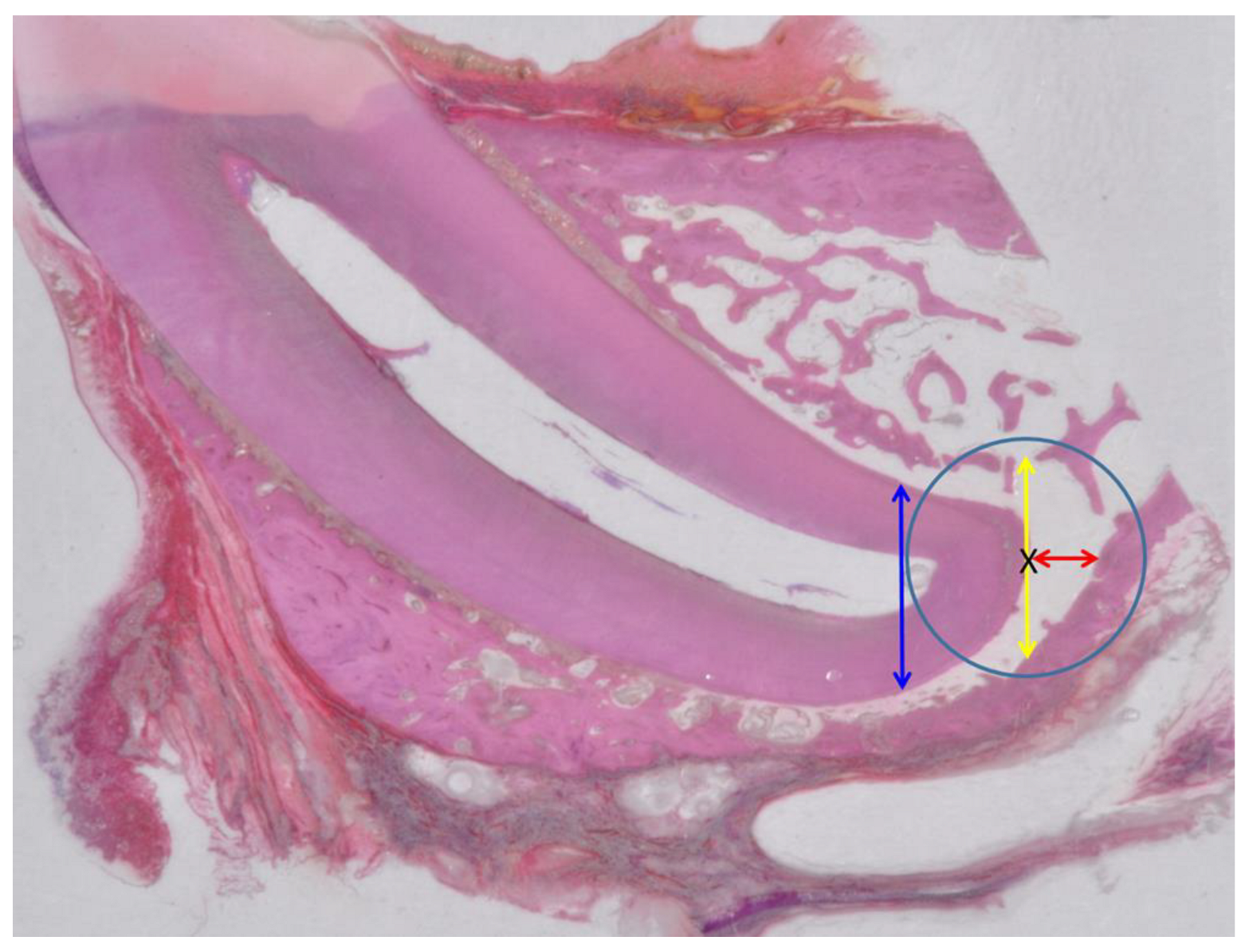
2.2.2. X-ray Analysis and Histology
2.2.3. Statistical Analysis
3. Results
3.1. Risk Assessment Using Cell Lines
3.2. Evaluation of Animal Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandelwal, A.; Janani, K.; Teja, K.; Jose, J.; Battineni, G.; Riccitiello, F.; Valletta, A.; Palanivelu, A.; Spagnuolo, G. Periapical healing following root canal treatment using different endodontic sealers: A systematic review. Biomed. Res. Int. 2022, 2022, 3569281. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, G. Assessment of treatment failure in endodontic therapy. J. Oral Rehabil. 2016, 43, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Niu, L.N.; Zhang, W.; Olsen, M.; De-Deus, G.; Eid, A.A.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Ability of new obturation materials to improve the seal of the root canal system: A review. Acta Biomater. 2014, 10, 1050–1063. [Google Scholar] [CrossRef]
- Sabeti, M.A.; Nekofar, M.; Motahhary, P.; Ghandi, M.; Simon, J.H. Healing of apical periodontitis after endodontic treatment with and without obturation in dogs. J. Endod. 2006, 32, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, F.G.; Endo, M.S.; Gomes, B.P.; Montagner, F.; Tosello, F.B.; Jacinto, R.C. Persistent extraradicular infection in root-filled asymptomatic human tooth: Scanning electron microscopic analysis and microbial investigation after apical microsurgery. J. Endod. 2011, 37, 1696–1700. [Google Scholar] [CrossRef]
- Silva, E.J.; Castro, R.W.; Nejaim, Y.; Silva, A.I.; Haiter-Neto, F.; Silberman, A.; Cohenca, N. Evaluation of root canal configuration of maxillary and mandibular anterior teeth using cone beam computed tomography: An in-vivo study. Quintessence Int. 2016, 47, 19–24. [Google Scholar]
- Lendini, M.; Alemanno, E.; Migliaretti, G.; Berutti, E. The effect of high-frequency electrical pulses on organic tissue in root canals. Int. Endod. J. 2005, 38, 531–538. [Google Scholar] [CrossRef]
- Barbato, L.; Cavalcanti, R.; Rupe, C.; Scartabelli, D.; Serni, L.; Chambrone, L.; Cairo, F. Clinical efficacy of adjunctive methods for the non-surgical treatment of peri-implantitis: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 375. [Google Scholar] [CrossRef]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef]
- Ratka, C.; Weigl, P.; Henrich, D.; Koch, F.; Schlee, M.; Zipprich, H. The effect of in vitro electrolytic cleaning on biofilm-contaminated implant surfaces. J. Clin. Med. 2019, 8, 1397. [Google Scholar] [CrossRef]
- Schlee, M.; Rathe, F.; Brodbeck, U.; Ratka, C.; Weigl, P.; Zipprich, H. Treatment of peri-implantitis-electrolytic cleaning versus mechanical and electrolytic cleaning-A randomized controlled clinical trial six-month results. J. Clin. Med. 2019, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Zipprich, H.; Weigl, P.; Di Gianfilippo, R.; Steigmann, L.; Henrich, D.; Wang, H.L.; Schlee, M.; Ratka, C. Comparison of decontamination efficacy of two electrolyte cleaning methods to diode laser, plasma, and air-abrasive devices. Clin. Oral Investig. 2022, 26, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Virto, L.; Odeh, V.; Garcia-Quismondo, E.; Herrera, D.; Palma, J.; Tamimi, F.; Sanz, M. Electrochemical decontamination of titanium dental implants. An in vitro biofilm model study. Clin. Oral Implant. Res. 2023, 34, 486–497. [Google Scholar] [CrossRef]
- Schlee, M.; Naili, L.; Rathe, F.; Brodbeck, U.; Zipprich, H. Is complete re-osseointegration of an infected dental implant possible? Histologic results of a dog study: A short communication. J. Clin. Med. 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Palarie, V.; Koch, L.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Karl, M. Preclinical testing of boron-doped diamond electrodes for root canal disinfection-A series of preliminary studies. Microorganisms 2022, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Brillas, E. A critical review over the electrochemical disinfection of bacteria in synthetic and real wastewaters using a boron-doped diamond anode. Curr. Opin. Solid. State. Mater. Sci. 2021, 25, 100926. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Kishen, A.; Murray, P.E.; Nekoofar, M.H.; Figueiredo, J.A.P.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; Silva, R.M.; et al. PRIASE 2021 guidelines for reporting animal studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 848–857. [Google Scholar] [CrossRef]
- Koch, M.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Geißdörfer, W.; Dittmar, R.; Grobecker-Karl, T. Pilot study on the use of a laser-structured double diamond electrode (DDE) for biofilm removal from dental implant surfaces. J. Clin. Med. 2020, 9, 3036. [Google Scholar] [CrossRef]
- Borlina, S.C.; de Souza, V.; Holland, R.; Murata, S.S.; Gomes-Filho, J.E.; Dezan, E., Jr.; Marion, J.J.; Neto Ddos, A. Influence of apical foramen widening and sealer on the healing of chronic periapical lesions induced in dogs’ teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 932–940. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. Med. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Meister, T.L.; Brüggemann, Y.; Todt, D.; Conzelmann, C.; Müller, J.A.; Groß, R.; Münch, J.; Krawczyk, A.; Steinmann, J.; Steinmann, J.; et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. 2020, 222, 1289–1292. [Google Scholar] [CrossRef]
- Beus, C.; Safavi, K.; Stratton, J.; Kaufman, B. Comparison of the effect of two endodontic irrigation protocols on the elimination of bacteria from root canal system: A prospective, randomized clinical trial. J. Endod. 2012, 38, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Hülsmann, M.; Siqueira, J.F., Jr. Microorganisms in root canal-treated teeth from a German population. J. Endod. 2008, 34, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Huumonen, S.; Lenander-Lumikari, M.; Sigurdsson, A.; Orstavik, D. Healing of apical periodontitis after endodontic treatment: A comparison between a silicone-based and a zinc oxide-eugenol-based sealer: Apical periodontitis and sealers. Int. Endod. J. 2003, 36, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Zanini, M.; Decerle, N.; Hennequin, M.; Cousson, P.Y. Revisiting Orstavik’s PAI score to produce a reliable and reproducible assessment of the outcomes of endodontic treatments in routine practice. Eur. J. Dent. Educ. 2021, 25, 291–298. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Silva, L.A.; Utrilla, L.S.; Assed, S.; Ether, S.S. Calcium hydroxide root canal sealers--histopathologic evaluation of apical and periapical repair after endodontic treatment. J. Endod. 1997, 23, 428–432. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; Watanabe, S.; Cintra, L.T.; Nery, M.J.; Dezan-Júnior, E.; Queiroz, I.O.; Lodi, C.S.; Basso, M.D. Effect of MTA-based sealer on the healing of periapical lesions. J. Appl. Oral Sci. 2013, 21, 235–242. [Google Scholar] [CrossRef]
- Arslan, H.; Doğanay Yıldız, E.; Topçuoğlu, H.S.; Tepecik, E.; Ayaz, N. Success of maintaining apical patency in teeth with periapical lesion: A randomized clinical study. Quintessence Int. 2019, 50, 686–693. [Google Scholar]
- Bernáth, M.; Szabó, J. Tissue reaction initiated by different sealers. Int. Endod. J. 2003, 36, 256–261. [Google Scholar] [CrossRef]
- Wu, M.K.; Wesselink, P.R.; Walton, R.E. Apical terminus location of root canal treatment procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 89, 99–103. [Google Scholar] [CrossRef]
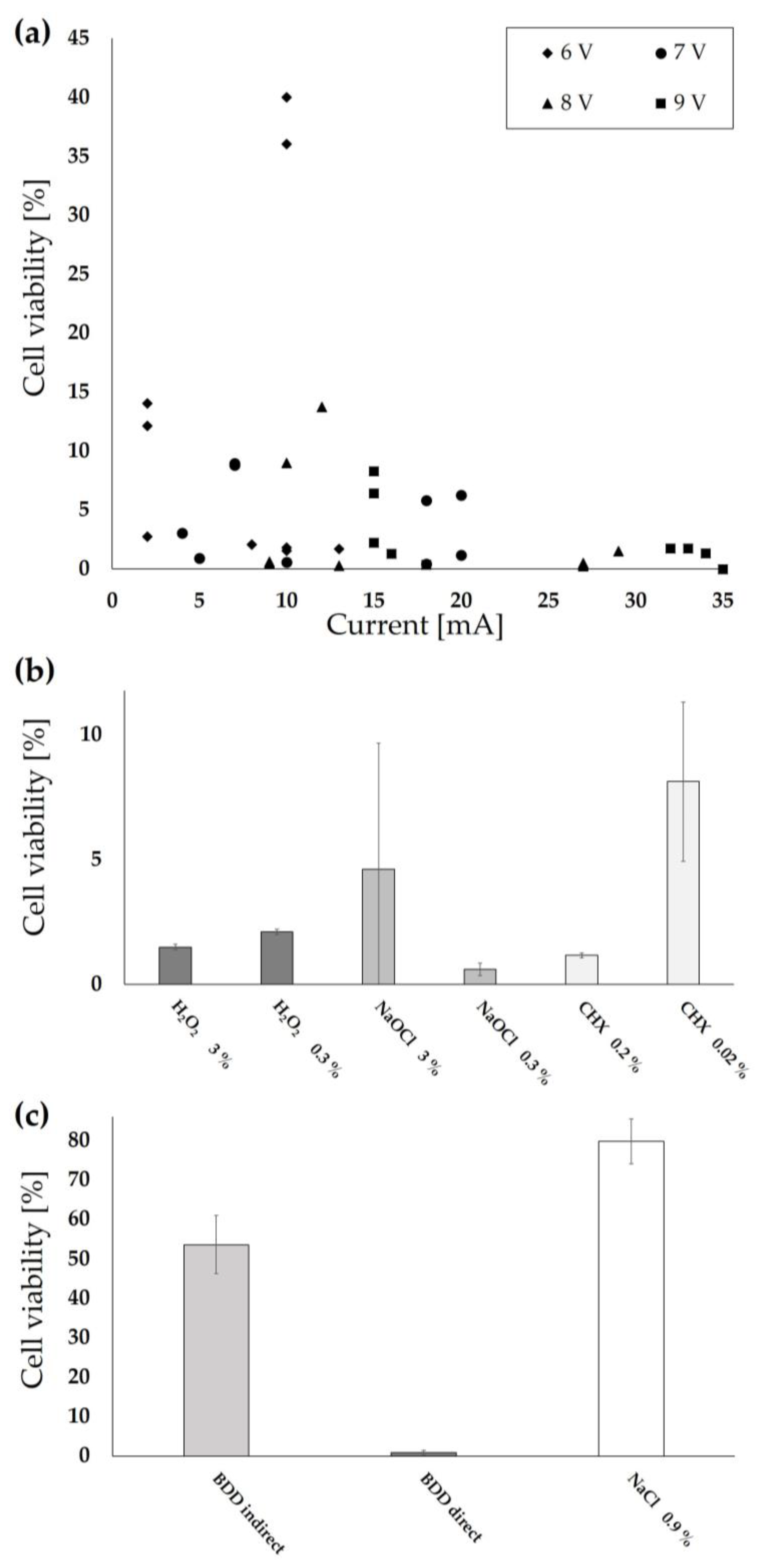
| Group | Treatment |
|---|---|
| Blank | HeLa Cells at confluency treated with 0.5 mL PBS for 2.5 or 5 min |
| Potentiostatic | Application of BDD electrode inside the well plates (direct application) for 2.5 min in 0.5 mL of PBS overlaying the cells |
| Parameters: | |
| 6 V 7 V 8 V 9 V | Direct application at 2–12 mA Direct application at 5–18 mA Direct application at 7–29 mA Direct application at 11–34 mA |
| Amperostatic | Application of BDD electrode in 0.5 mL of PBS for 5 min |
| Direct Indirect | Application at 50 mA for 5 min in 0.5 mL of PBS overlaying the cells Application at 50 mA by adding 0.5 mL pretreated PBS buffer for 5 min |
| NaCl | Application of 0.5 mL of physiologic NaCl solution for 5 min |
| H2O2 | Application of 0.5 mL 3% and 0.3% H2O2 (Otto Fischer GmbH & Co. KG, Saarbrücken, Germany) for 5 min |
| NaOCl | Application of 0.5 mL 3% and 0.3% NaOCl (Heidinger, Stuttgart, Germany) for 5 min |
| CHX | Application of 0.5 mL chlorhexidine digluconate (Chlorhexamed FORTE alkoholfrei 0.2%, GlaxoSmithKline Consumer Healthcare, Munich, Germany) for 5 min |
| Dog | Tooth | [V] | [mA] | [As] | [s] | [mL] | [mL min−1] | [cm2] | [As cm−2] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 5 | 13 | 3.51 | 270 | 6 | 1.33 | 0.0325 | 108 |
| 22 | 5 | 9 | 2.03 | 225 | 5 | 1.33 | 0.0325 | 62.3 | |
| 2 | 12 | 5.5 | 25 | 9 | 360 | 8 | 1.33 | 0.0325 | 277 |
| 22 | 5.5 | 25 | 9 | 360 | 8 | 1.33 | 0.0325 | 277 | |
| 3 | 12 * | 6.6 | 38 | 5.51 | 145 | 3 | 1.33 | 0.0325 | 170 |
| 5.5 | 30 | 6.75 | 225 | 5 | 1.33 | 0.0325 | 207 | ||
| 22 | 5 | 9 | 3.24 | 360 | 8 | 1.33 | 0.0325 | 100 | |
| 4 | 12 | 5.5 | 22 | 7.92 | 360 | 8 | 1.33 | 0.0325 | 244 |
| 22 | 5 | 10 | 3.6 | 360 | 8 | 1.33 | 0.0325 | 111 |
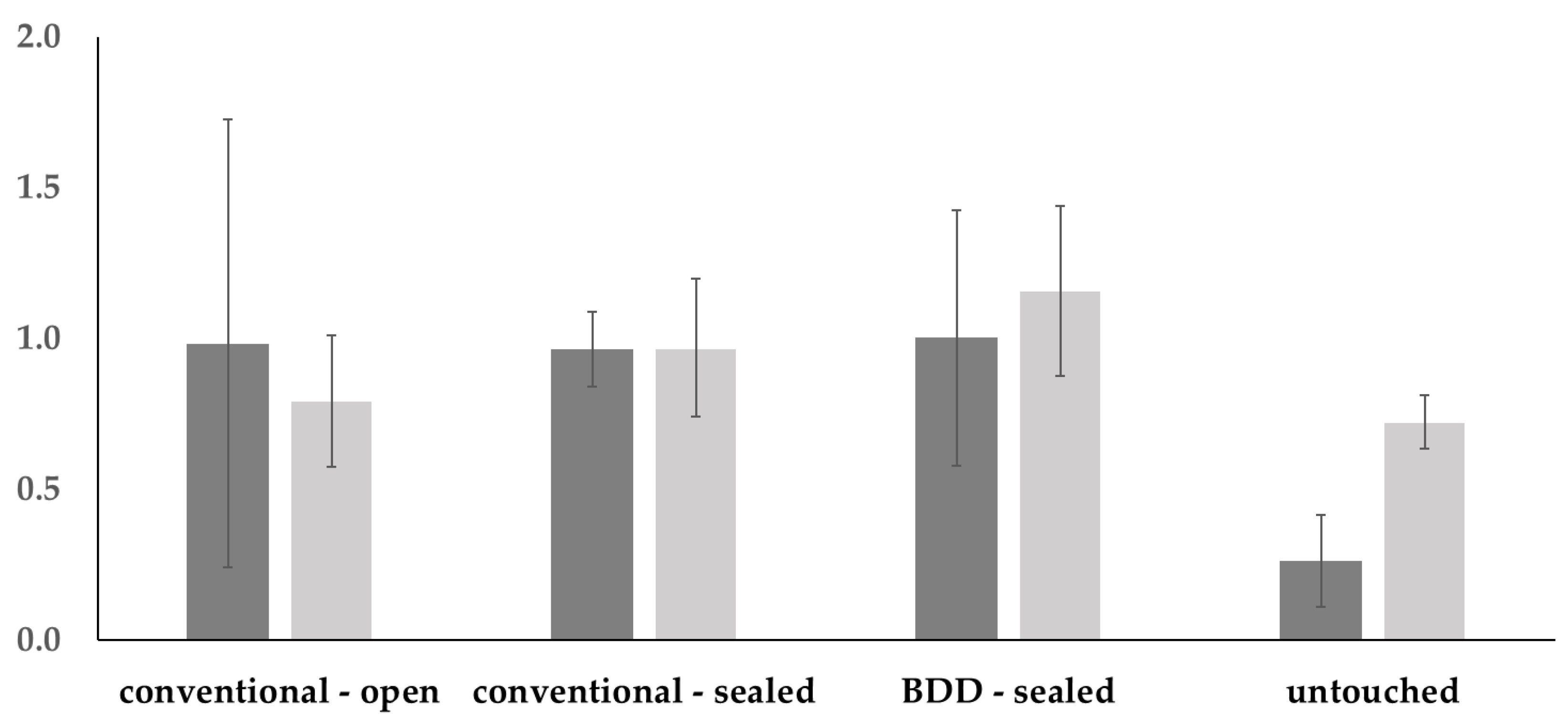
| Vertical radiolucency | Kruskal–Wallis test: p = 0.0427 | |||
| Conventional open | Conventional sealed | BDD sealed | Untouched | |
| Conventional open | 0.922 | 0.963 | 0.281 | |
| Conventional sealed | 0.704 | 0.998 | 0.096 | |
| BDD sealed | 0.208 | 0.878 | 0.110 | |
| Untouched | 0.958 | 0.433 | 0.086 | |
| Horizontal radiolucency | Kruskal–Wallis test: p = 0.0412 | |||
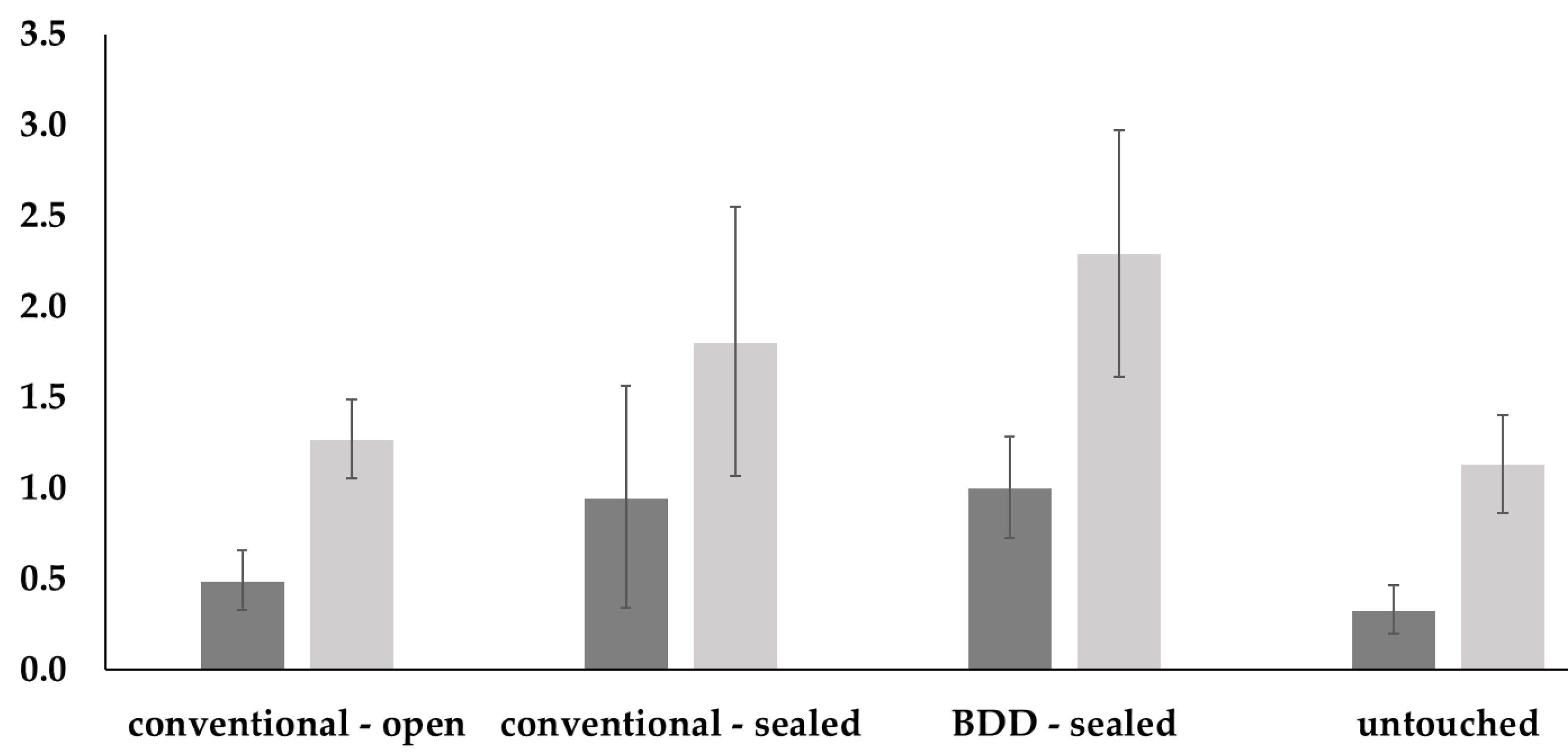
| Vertical radiolucency | Kruskal–Wallis test: p =0.0152 | |||
| Conventional open | Conventional sealed | BDD sealed | Untouched | |
| Conventional open | 0.680 | 0.280 | 0.730 | |
| Conventional sealed | 0.664 | 0.940 | 0.170 | |
| BDD sealed | 0.167 | 0.862 | 0.030 | |
| Untouched | 0.899 | 0.297 | 0.039 | |
| Horizontal radiolucency | Kruskal–Wallis test: p = 0.0178 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, M.; Demmer, E.; Palarie, V.; Burkovski, A.; Karl, M. Electrochemical Disinfection of Root Canals Bears No Risk of Damaging Periapical Tissues in a Dog Model. Appl. Sci. 2023, 13, 8228. https://doi.org/10.3390/app13148228
Koch M, Demmer E, Palarie V, Burkovski A, Karl M. Electrochemical Disinfection of Root Canals Bears No Risk of Damaging Periapical Tissues in a Dog Model. Applied Sciences. 2023; 13(14):8228. https://doi.org/10.3390/app13148228
Chicago/Turabian StyleKoch, Maximilian, Elena Demmer, Victor Palarie, Andreas Burkovski, and Matthias Karl. 2023. "Electrochemical Disinfection of Root Canals Bears No Risk of Damaging Periapical Tissues in a Dog Model" Applied Sciences 13, no. 14: 8228. https://doi.org/10.3390/app13148228
APA StyleKoch, M., Demmer, E., Palarie, V., Burkovski, A., & Karl, M. (2023). Electrochemical Disinfection of Root Canals Bears No Risk of Damaging Periapical Tissues in a Dog Model. Applied Sciences, 13(14), 8228. https://doi.org/10.3390/app13148228








