Concentration Influence of Complexing Agent on Electrodeposited Zn-Ni Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hull Cell Experiment
2.2. Electrochemical Analysis
2.3. Material Characterization
3. Results and Discussion
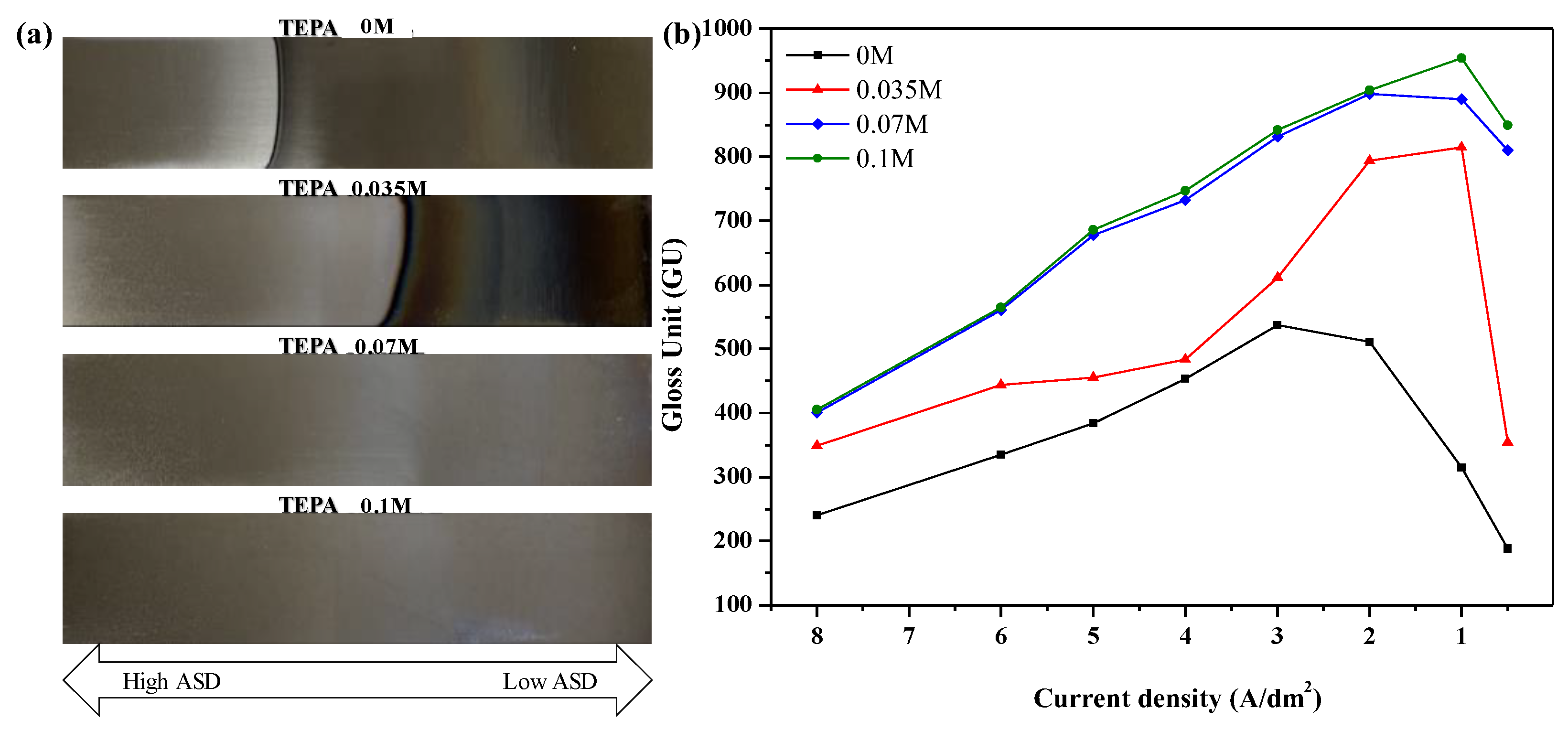
3.1. Appearance of Zn-Ni Alloy Coating
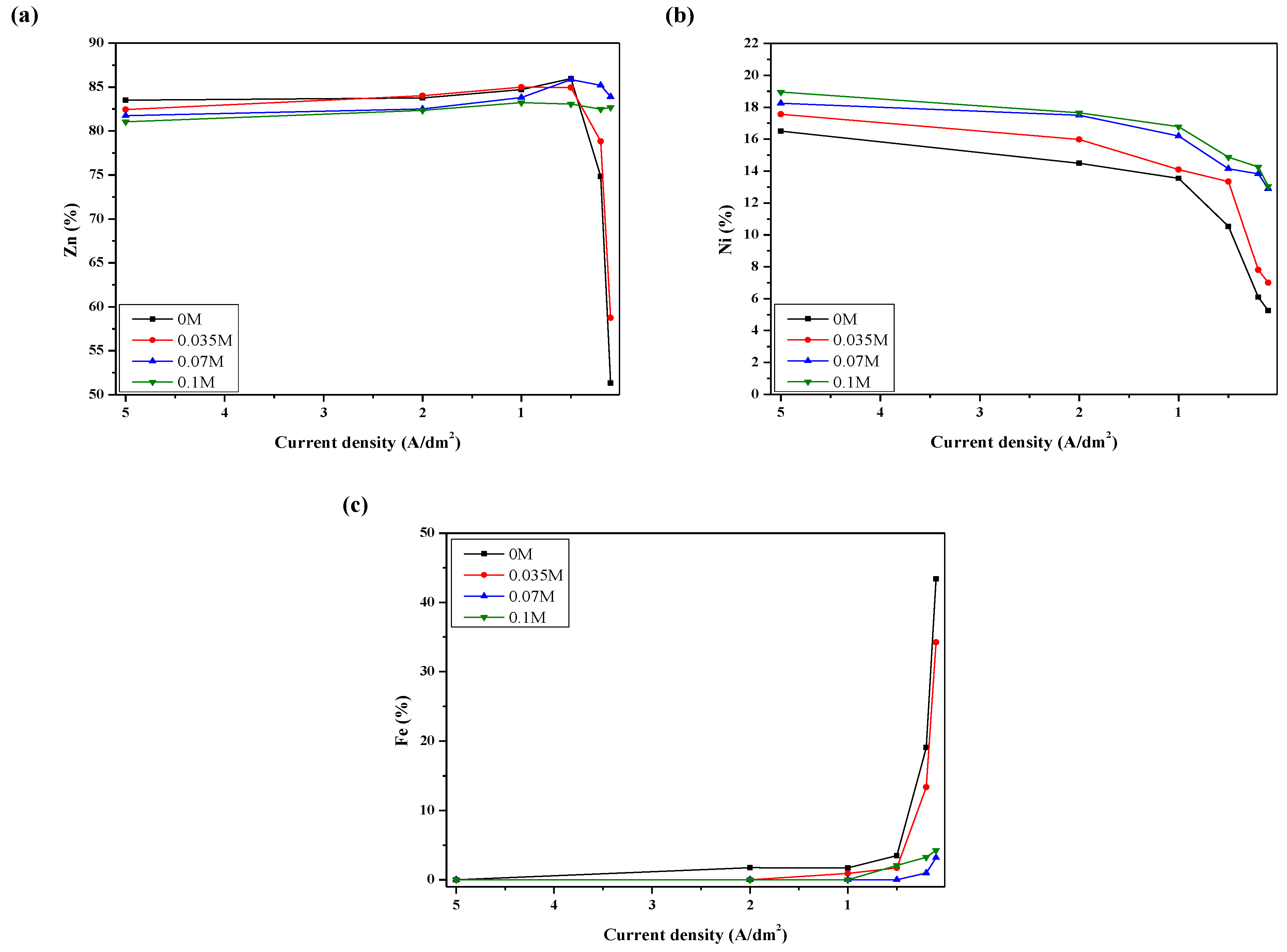
3.2. Microstructure of Zn-Ni Alloy Coating
3.3. Electroplating Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gezerman, A.O.; Çorbacıoglu, B.D. Iron(II) acetylacetonate containing brightener and sulphur containing carrier in nickel plating. Surf. Eng. 2013, 29, 516–521. [Google Scholar] [CrossRef]
- Ma, H.R.; Chen, X.Y.; Li, J.W.; Chang, C.T.; Wang, G.; Li, H.; Wang, X.M.; Li, R.W. Fe-based amorphous coating with high corrosion and wear resistance. Surf. Eng. 2017, 33, 56–62. [Google Scholar] [CrossRef]
- Bajat, J.B.; Kačarević-Popović, Z.; Mišković-Stanković, V.B.; Maksimović, M.D. Corrosion behaviour of epoxy coatings electrodeposited on galvanized steel and steel modified by Zn–Ni alloys. Prog. Org. Coat. 2000, 39, 127–135. [Google Scholar] [CrossRef]
- Andrievski, R.A. Review of thermal stability of nanomaterials. J. Mater. Sci. 2013, 494, 1449–1460. [Google Scholar] [CrossRef]
- Sohi, M.H.; Jalali, M. Study of the corrosion properties of zinc–nickel alloy electrodeposits before and after chromating. J. Mater. Process. Technol. 2003, 138, 63–66. [Google Scholar] [CrossRef]
- El Hajjami, A.; Gigandet, M.P.; De Petris-Wery, M.; Catonne, J.C.; Duprat, J.J.; Thiery, L.; Raulin, F.; Pommier, N.; Starck, B.; Remy, P. Characterization of thin Zn–Ni alloy coatings electrodeposited on low carbon steel. Appl. Surf. Sci. 2007, 254, 480–489. [Google Scholar] [CrossRef]
- Girčiene, O.; Gudavičiute, L.; Juškenas, R.; Ramanauskas, R. Corrosion resistance of phosphated Zn–Ni alloy electrodeposits. Surf. Coat. Technol. 2009, 203, 3072–3077. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Shariat, M.H.; Bahrololoom, M.E. Study of corrosion performance of electrodeposited nanocrystalline Zn–Ni alloy coatings. Corros. Sci. 2012, 59, 81–87. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; Song, H.; An, M. Electrodeposition of nanocrystalline Zn–Ni coatings with single gamma phase from an alkaline bath. Surf. Coat. Technol. 2015, 270, 47–56. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, J.J.; Wang, X.L.; Tu, J.P. Influence of electrodeposition conditions on the microstructure and corrosion resistance of Zn-Ni alloy coatings from a deep eutectic solvent. Surf. Coat. Technol. 2014, 242, 34–41. [Google Scholar] [CrossRef]
- Anwar, S.; Zhang, Y.; Khan, F. Electrochemical behaviour and analysis of Zn and Zn–Ni alloy anti-corrosive coatings deposited from citrate baths. RSC Adv. 2018, 8, 28861–28873. [Google Scholar] [CrossRef] [PubMed]
- Cachet, C.; Saïdani, B.; Wiart, R. The Behavior of Zinc Electrode in Alkaline Electrolytes: I. A Kinetic Analysis of Cathodic Deposition. J. Electrochem. Soc. 1991, 138, 678–687. [Google Scholar] [CrossRef]
- Abibsi, A.; Dennis, J.K.; Short, N.R. The Effect of Plating Variables on Zinc-Nickel Alloy Electrodeposition. Trans. IMF 1991, 69, 145–148. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Ashassi-Sorkhabi, H.; Ghiasvand, H.A.Y. Electrochemical studies of Zn–Ni alloy coatings from non-cyanide alkaline bath containing tartrate as complexing agent. Surf. Coat. Technol. 2008, 202, 2897–2904. [Google Scholar] [CrossRef]
- Bae, S.H.; Oue, S.; Son, I.; Nakano, H. Effect of reaction product of epichlorohydrin and imidazole on the electrodeposition behavior of zn⇓ni alloy from alkaline zincate solution. ISIJ Int. 2021, 61, 2256–2263. [Google Scholar] [CrossRef]
- Bae, S.H.; Oue, S.; Taninouchi, Y.; Son, I.; Nakano, H. Effect of Solution Temperature on Electrodeposition Behavior of Zn–Ni Alloy from Alkaline Zincate Solution. ISIJ Int. 2022, 62, 1522–1531. [Google Scholar] [CrossRef]
- Bae, S.H.; Oue, S.; Taninouchi, Y.K.; Son, I.; Nakano, H. Synergistic Effect of Brightener and Solution Temperature on the Electrodeposition Behavior of Zn-Ni Alloy from Alkaline Zincate Solution. ISIJ Int. 2022, 62, 1918–1929. [Google Scholar] [CrossRef]
- Müller, C.; Sarret, M.; Benballa, M. Complexing agents for a Zn–Ni alkaline bath. J. Electroanal. Chem. 2002, 519, 85–92. [Google Scholar] [CrossRef]
- Bhat, R.S.; Shetty, S.M.; Kumar, N.A. Electroplating of Zn-Ni Alloy Coating on Mild Steel and Its Electrochemical Studies. J. Mater. Eng. Perform. 2021, 30, 8188–8195. [Google Scholar] [CrossRef]
- Moreira, F.L.; Costa, J.M.; de Almeida Neto, A.F. Anticorrosive Zn–Ni alloys: An alternative for the treatment of electroplating industry wastewater. Sustain. Chem. Pharm. 2020, 16, 100263. [Google Scholar] [CrossRef]
- Lei, C.; Alesary, H.F.; Khan, F.; Abbott, A.P.; Ryder, K.S. Gamma-phase Zn-Ni alloy deposition by pulse-electroplating from a modified deep eutectic solution. Surf. Coat. Technol. 2020, 403, 126434. [Google Scholar] [CrossRef]
- Sheu, H.H.; Lee, H.B.; Jian, S.Y.; Hsu, C.Y.; Lee, C.Y. Investigation on the corrosion resistance of trivalent chromium conversion passivate on electroplated Zn–Ni alloy. Surf. Coat. Technol. 2016, 305, 241–248. [Google Scholar] [CrossRef]
- Swathirajan, S. Potentiodynamic and Galvanostatic Stripping Methods for Characterization of Alloy Electrodeposition Process and Product. Electrochem. Soc. 1986, 133, 671. [Google Scholar] [CrossRef]
- Son, B.K.; Choi, J.W.; Jeon, S.B.; Son, I. Zn–Ni Alloy Plating with Trivalent Chromate: Effects of NaF Additive Concentration and Treatment Time on Film Color, Thickness, and Electrochemical Properties. Coatings 2022, 12, 1160. [Google Scholar] [CrossRef]
- Elkhatabi, F.; Benballa, M.; Sarret, M.; Müller, C. Dependence of coating characteristics on deposition potential for electrodeposited Zn–Ni alloys. Electrochim. Acta 1999, 44, 1645–1653. [Google Scholar] [CrossRef]
- Soares, M.E.; Souza, C.A.C.; Kuri, S.E. Corrosion resistance of a Zn–Ni electrodeposited alloy obtained with a controlled electrolyte flow and gelatin additive. Surf. Coat. Technol. 2006, 201, 2953–2959. [Google Scholar] [CrossRef] [Green Version]
- Long, J.M.; Zhang, X.; Pei, H.Z. Effect of Triethanolamine Addition in Alkaline Bath on the Electroplating Behavior, Composition and Corrosion Resistance of Zn-Ni Alloy Coatings. Adv. Mater. Res. 2013, 738, 87–91. [Google Scholar] [CrossRef]
- Gezerman, A.O. Effects of novel additives for zinc-nickel alloy plating. Eur. J. Chem. 2019, 10, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; An, M. Electrochemical Behaviors and Properties of Zn-Ni Alloys Obtained from Alkaline Non-Cyanide Bath Using 5,5′ -Dimethylhydantoin as Complexing Agent. J. Electrochem. Soc. 2015, 162, D412–D422. [Google Scholar] [CrossRef]
- Faid, H.; Galvan, J.C. Morphology and composition of Zn–Ni alloy obtained from sulphate bath containing complexing agent. Trans. IMF 2020, 98, 138–143. [Google Scholar] [CrossRef]
- Liebreich, E. The effects of film formation on the structure of electro-deposited metallic coatings. Trans. Faraday Soc. 1935, 31, 1188. [Google Scholar] [CrossRef]
- Spanjers, C.S.; Dasgupta, A.; Kirkham, M.; Burger, B.A.; Kumar, G.; Janik, M.J.; Rioux, R.M. Determination of Bulk and Surface Atomic Arrangement in Ni-Zn γ-Brass Phase at Different Ni to Zn Ratios. Chem. Mater. 2017, 29, 504–512. [Google Scholar] [CrossRef]
- Hwang, W.S.; Lee, J.J. Effects of Heat Treatment on Hardness of Nanocrystalline Ni-W Electrodeposits. Mater. Sci. Forum 2006, 510–511, 1126–1129. [Google Scholar] [CrossRef]
- Gezerman, A.O. Investigation of some properties of Zinc-Nickel alloy plating with triethanolamine, p-aminobenzenesulfonic acid, and gelatine. Surf. Rev. Lett. 2020, 27, 2050018. [Google Scholar] [CrossRef]
- Dahms, H.; Croll, I.M. The Anomalous Codeposition of Iron-Nickel Alloys. J. Electrochem. Soc. 1965, 112, 771. [Google Scholar] [CrossRef]
- Eliaz, N.; Venkatakrishna, K.; Hegde, A.C. Electroplating and characterization of Zn–Ni, Zn–Co and Zn–Ni–Co alloys. Surf. Coat. Technol. 2010, 205, 1969–1978. [Google Scholar] [CrossRef]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; ISBN 9780470009406. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, B.-K.; Choi, J.-W.; Jeon, S.-B.; Son, I.-J. Concentration Influence of Complexing Agent on Electrodeposited Zn-Ni Alloy. Appl. Sci. 2023, 13, 7887. https://doi.org/10.3390/app13137887
Son B-K, Choi J-W, Jeon S-B, Son I-J. Concentration Influence of Complexing Agent on Electrodeposited Zn-Ni Alloy. Applied Sciences. 2023; 13(13):7887. https://doi.org/10.3390/app13137887
Chicago/Turabian StyleSon, Byung-Ki, Ji-Won Choi, Su-Byung Jeon, and In-Joon Son. 2023. "Concentration Influence of Complexing Agent on Electrodeposited Zn-Ni Alloy" Applied Sciences 13, no. 13: 7887. https://doi.org/10.3390/app13137887
APA StyleSon, B.-K., Choi, J.-W., Jeon, S.-B., & Son, I.-J. (2023). Concentration Influence of Complexing Agent on Electrodeposited Zn-Ni Alloy. Applied Sciences, 13(13), 7887. https://doi.org/10.3390/app13137887






