Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro
Abstract
:1. Introduction
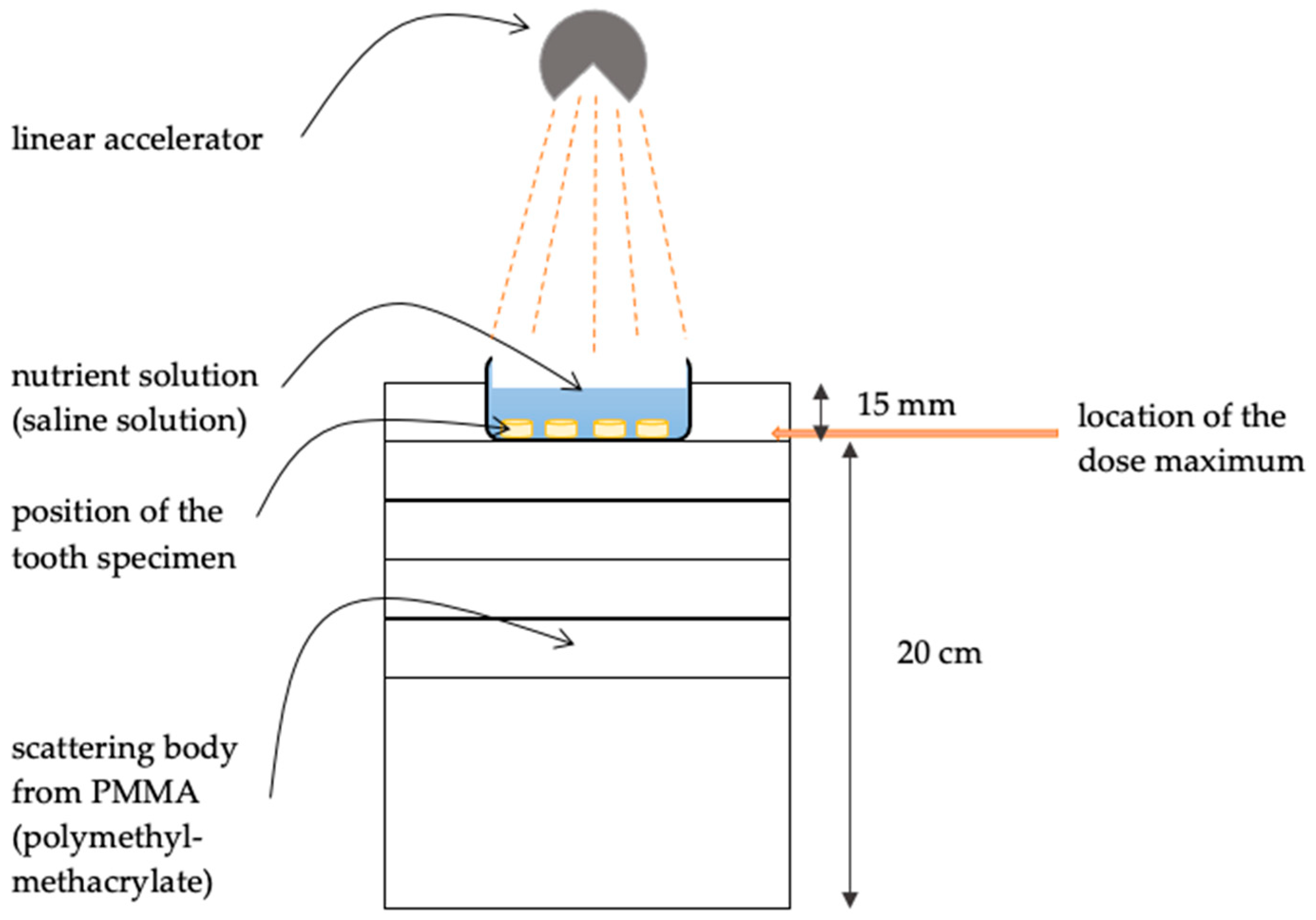
2. Materials and Methods
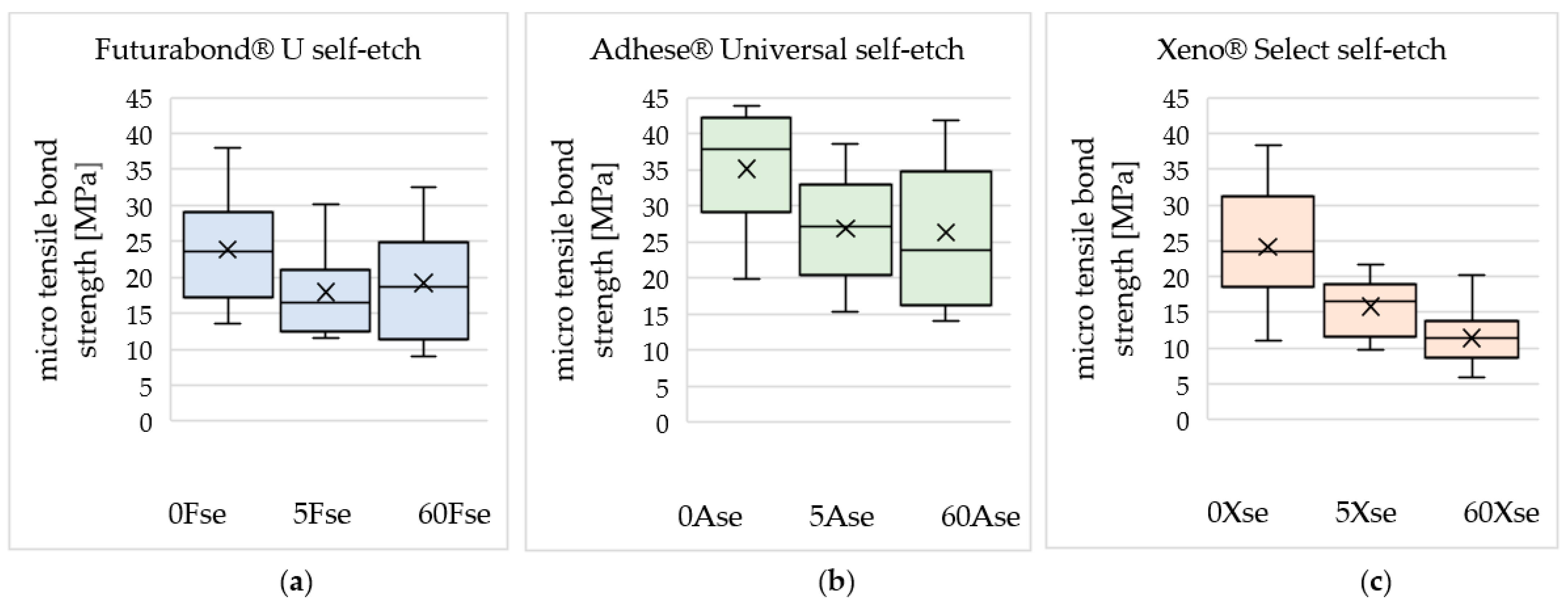
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Cancer Today. Cancer Fact Sheets. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 28 March 2023).
- Robert Koch Institute; Association of Population-based Cancer Registries in Germany. Cancer in Germany 2017/2018, 13th ed.; Robert Koch Institute: Berlin, Germany, 2022; pp. 32–33. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Bourhis, J.; Etessami, A.; Lusinchi, A. New trends in radiotherapy for head and neck cancer. Ann. Oncol. 2005, 16 (Suppl. S2), ii255–ii257. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, J.J.; Goldenberg, D. Oral complications of radiotherapy. Lancet Oncol. 2006, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Hinkelbein, W.; Hellwig, E.; Meyer-Lückel, H. Radiation-related damage to dentition. Lancet Oncol. 2006, 7, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mesia, R.; Iglesias, L.; Lambea, J.; Martinez-Trufero, J.; Soria, A.; Taberna, M.; Trigo, J.; Chaves, M.; Garcia-Castano, A.; Cruz, J. SEOM clinical guidelines for the treatment of head and neck cancer (2020). Clin. Transl. Oncol. 2021, 23, 913–921. [Google Scholar] [CrossRef]
- Ray-Chaudhuri, A.; Shah, K.; Porter, R.J. The oral management of patients who have received radiotherapy to the head and neck region. Br. Dent. J. 2013, 214, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gernhardt, C.R.; Koravu, T.; Gerlach, R.; Schaller, H.G. The influence of dentin adhesives on the demineralization of irradiated and non-irradiated human root dentin. Oper. Dent. 2004, 29, 454–461. [Google Scholar]
- Hey, J.; Seidel, J.; Schweyen, R.; Paelecke-Habermann, Y.; Vordermark, D.; Gernhardt, C.; Kuhnt, T. The influence of parotid gland sparing on radiation damages of dental hard tissues. Clin. Oral Investig. 2013, 17, 1619–1625. [Google Scholar] [CrossRef]
- Kuhnt, T.; Jirsak, N.; Muller, A.C.; Pelz, T.; Gernhardt, C.; Schaller, H.G.; Janich, M.; Gerlach, R.; Dunst, J. Quantitative and qualitative investigations of salivary gland function in dependence on irradiation dose and volume for reduction of xerostomia in patients with head-and-neck cancer. Strahlenther. Onkol. 2005, 181, 520–528. [Google Scholar] [CrossRef]
- Fränzel, W.; Gerlach, R.; Hein, H.J.; Schaller, H.G. Effect of tumor therapeutic irradiation on the mechanical properties of teeth tissue. Z. Med. Phys. 2006, 16, 148–154. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Palma-Dibb, R.G.; Paula-Silva, F.W.; Oliveira, H.F.; Nelson-Filho, P.; Silva, L.A.; Queiroz, A.M. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J. Dent. 2014, 42, 986–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, R.; Xu, C.; Liu, Y.; Gorski, J.P.; Wang, Y.; Walker, M.P. Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch. Oral Biol. 2015, 60, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Daood, U.; Bourgi, R.; Cuevas-Suarez, C.E.; Devoto, W.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderon, J.E.; Radwanski, M.; Orsini, G.; et al. Effect of Collagen Crosslinkers on Dentin Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis. Cells 2022, 11, 2417. [Google Scholar] [CrossRef] [PubMed]
- Eusufzai, S.Z.; Barman, A.; Jamayet, N.B.; Ahmad, W.; Mahdi, S.S.; Sheikh, Z.; Daood, U. Effects of Riboflavin Collagen Crosslinker on Dentin Adhesive Bonding Efficiency: A Systematic Review and Meta-Analysis. Materials 2023, 16, 1701. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, H. Ionizing irradiation affects the microtensile resin dentin bond strength under simulated clinical conditions. J. Conserv. Dent. 2013, 16, 148–151. [Google Scholar] [CrossRef]
- Gernhardt, C.R.; Nguyen, A.D.; Michaelis, M.; Puetz, N. Clinical Outcome of Class I and II Restorations with and without an Intermediary Layer of a Flowable Composite after 24 Months: A Prospective, Randomized, Split-Mouth-Designed, Controlled and Single-Blinded Clinical Trial. Appl. Sci. 2023, 13, 4224. [Google Scholar] [CrossRef]
- Badr, C.; Spagnuolo, G.; Amenta, F.; Khairallah, C.; Mahdi, S.S.; Daher, E.; Battineni, G.; Baba, N.Z.; Zogheib, T.; Qasim, S.S.B.; et al. A Two-Year Comparative Evaluation of Clinical Performance of a Nanohybrid Composite Resin to a Flowable Composite Resin. J. Funct. Biomater. 2021, 12, 51. [Google Scholar] [CrossRef]
- De Moor, R.J.G.; Stassen, I.G.; van ’t Veldt, Y.; Torbeyns, D.; Hommez, G.M.G. Two-year clinical performance of glass ionomer and resin composite restorations in xerostomic head- and neck-irradiated cancer patients. Clin. Oral Investig. 2011, 15, 31–38. [Google Scholar] [CrossRef]
- McComb, D.; Erickson, R.L.; Maxymiw, W.G.; Wood, R.E. A clinical comparison of glass ionomer, resin-modified glass ionomer and resin composite restorations in the treatment of cervical caries in xerostomic head and neck radiation patients. Oper. Dent. 2002, 27, 430–437. [Google Scholar]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Sezinando, A.; Monteiro, P.C. Laboratory bonding ability of a multi-purpose dentin adhesive. Am. J. Dent. 2012, 25, 153–158. [Google Scholar] [PubMed]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. B. Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Yoshida, Y.; Hirata, I.; Snauwaert, J.; De Munck, J.; Okazaki, M.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Influence of the chemical structure of functional monomers on their adhesive performance. J. Dent. Res. 2008, 87, 757–761. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Tian, F.C.; Niu, L.N.; Ochala, K.; Chen, C.; Fu, B.P.; Wang, X.Y.; Pashley, D.H.; Tay, F.R. Defying ageing: An expectation for dentine bonding with universal adhesives? J. Dent. 2016, 45, 43–52. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef]
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine--Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H. Immediate bonding properties of universal adhesives to dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Villat, C.; Abouelleil, H.; Gustin, M.P.; Grosgogeat, B. Tensile Bond Strengths of Two Adhesives on Irradiated and Nonirradiated Human Dentin. Biomed. Res. Int. 2015, 2015, 798972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galetti, R.; Santos-Silva, A.R.; Antunes, A.N.; Alves Fde, A.; Lopes, M.A.; de Goes, M.F. Radiotherapy does not impair dentin adhesive properties in head and neck cancer patients. Clin. Oral Investig. 2014, 18, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Gernhardt, C.R.; Kielbassa, A.M.; Hahn, P.; Schaller, H.G. Tensile bond strengths of four different dentin adhesives on irradiated and non-irradiated human dentin in vitro. J. Oral Rehabil. 2001, 28, 814–820. [Google Scholar] [CrossRef]
- Rodrigues, R.B.; Soares, C.J.; Junior, P.C.S.; Lara, V.C.; Arana-Chavez, V.E.; Novais, V.R. Influence of radiotherapy on the dentin properties and bond strength. Clin. Oral Investig. 2018, 22, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kobler, A.; Schaller, H.G.; Gernhardt, C.R. Effects of the desensitizing agents Gluma and Hyposen on the tensile bond strength of dentin adhesives. Am. J. Dent. 2008, 21, 388–392. [Google Scholar]
- Awwad, H.K.; Lotayef, M.; Shouman, T.; Begg, A.C.; Wilson, G.; Bentzen, S.M.; Abd El-Moneim, H.; Eissa, S. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: Influence of proliferation. Br. J. Cancer 2002, 86, 517–523. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Beetz, I.; Schendera, A.; Hellwig, E. Irradiation effects on microhardness of fluoridated and non-fluoridated bovine dentin. Eur. J. Oral Sci. 1997, 105, 444–447. [Google Scholar] [CrossRef]
- McLean, D.E.; Meyers, E.J.; Guillory, V.L.; Vandewalle, K.S. Enamel Bond Strength of New Universal Adhesive Bonding Agents. Oper. Dent. 2015, 40, 410–417. [Google Scholar] [CrossRef]
- Pouyanfar, H.; Tabaii, E.S.; Aghazadeh, S.; Nobari, S.; Imani, M.M. Microtensile Bond Strength of Composite to Enamel Using Universal Adhesive with/without Acid Etching Compared to Etch and Rinse and Self-Etch Bonding Agents. Open. Access. Maced. J. Med. Sci. 2018, 6, 2186–2192. [Google Scholar] [CrossRef] [Green Version]
- Elkaffas, A.A.; Hamama, H.H.H.; Mahmoud, S.H. Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis. Restor. Dent. Endod. 2018, 43, e29. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled Nano-layering at the Adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minagi, S.; et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Yoshioka, M.; Snauwaert, J.; Abe, Y.; Lambrechts, P.; Vanherle, G.; Okazaki, M. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J. Dent. Res. 2001, 80, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J. Dent. Res. 2016, 95, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.R.; Zanatta, R.F.; Silva, T.J.; Huhtala, M.F.; Borges, A.B. Influence of previous acid etching on bond strength of universal adhesives to enamel and dentin. Gen. Dent. 2017, 65, e17–e21. [Google Scholar]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withers, H. The four R’s of radiotherapy. Adv. Radiat. Biol. 1975, 5, 261. [Google Scholar]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maître, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Fu, K.K.; Pajak, T.F.; Trotti, A.; Jones, C.U.; Spencer, S.A.; Phillips, T.L.; Garden, A.S.; Ridge, J.A.; Cooper, J.S.; Ang, K.K. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Benson, R.; Julka, P.K.; Rath, G.K. Altered fractionation radiotherapy in head and neck squamous cell carcinoma. J. Egypt. Natl. Cancer Inst. 2016, 28, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortfeld, T. IMRT: A review and preview. Phys. Med. Biol. 2006, 51, R363–R379. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arid, J.; Palma-Dibb, R.G.; de Oliveira, H.F.; Nelson-Filho, P.; de Carvalho, F.K.; da Silva, L.A.B.; de Siqueira Mellara, T.; da Silva, R.A.B.; Faraoni, J.J.; de Queiroz, A.M. Radiotherapy impairs adhesive bonding in permanent teeth. Support. Care Cancer 2020, 28, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Grötz, K.; Duschner, H.; Kutzner, J.; Thelen, M.; Wagner, W. Neue Erkenntnisse zur Ätiologie der sogenannten Strahlenkaries. Nachweis direkter radiogener Veränderungen an der Schmelz-Dentin-Grenze. Strahlenther. Onkol. 1997, 173, 668–676. (In German) [Google Scholar] [CrossRef]
- Lu, H.; Zhao, Q.; Guo, J.; Zeng, B.; Yu, X.; Yu, D.; Zhao, W. Direct radiation-induced effects on dental hard tissue. Radiat. Oncol. 2019, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.M.; Troconis, C.C.; Palmier, N.R.; Gomes-Silva, W.; Paglioni, M.D.; Araújo, A.L.; Arboleda, L.P.; Filho, A.J.; González-Arriagada, W.A.; Goes, M.F.; et al. The impact of head and neck radiotherapy on the dentine-enamel junction: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e96–e105. [Google Scholar] [CrossRef]
- Pioch, T.; Golfels, D.; Staehle, H.J. An experimental study of the stability of irradiated teeth in the region of the dentinoenamel junction. Endod. Dent. Traumatol. 1992, 8, 241–244. [Google Scholar] [CrossRef]
- al-Nawas, B.; Grötz, K.A.; Rose, E.; Duschner, H.; Kann, P.; Wagner, W. Using ultrasound transmission velocity to analyse the mechanical properties of teeth after in vitro, in situ, and in vivo irradiation. Clin. Oral Investig. 2000, 4, 168–172. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Wang, Y.; Thiagarajan, G.; Gorski, J.P.; Reed Edwards, R.; McGuire, J.D.; Walker, M.P. Oral cancer radiotherapy affects enamel microhardness and associated indentation pattern morphology. Clin. Oral Investig. 2018, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieshout, H.F.; Bots, C.P. The effect of radiotherapy on dental hard tissue--a systematic review. Clin. Oral Investig. 2014, 18, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Schweyen, R.; Hey, J.; Fränzel, W.; Vordermark, D.; Hildebrandt, G.; Kuhnt, T. Radiation-related caries: Etiology and possible preventive strategies. What should the radiotherapist know? Strahlenther. Onkol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Wrbas, K.T.; Schulte-Mönting, J.; Hellwig, E. Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch. Oral Biol. 1999, 44, 243–251. [Google Scholar] [CrossRef]
- Springer, I.N.; Niehoff, P.; Warnke, P.H.; Böcek, G.; Kovács, G.; Suhr, M.; Wiltfang, J.; Açil, Y. Radiation caries--radiogenic destruction of dental collagen. Oral Oncol. 2005, 41, 723–728. [Google Scholar] [CrossRef]
- Frank, R.M.; Herdly, J.; Philippe, E. Acquired dental defects and salivary gland lesions after irradiation for carcinoma. J. Am. Dent. Assoc. 1965, 70, 868–883. [Google Scholar] [CrossRef]
- Hong, C.H.L.; Hu, S.; Haverman, T.; Stokman, M.; Napeñas, J.J.; Braber, J.B.; Gerber, E.; Geuke, M.; Vardas, E.; Waltimo, T.; et al. A systematic review of dental disease management in cancer patients. Support. Care Cancer 2018, 26, 155–174. [Google Scholar] [CrossRef]
- Clinical Guideline “Evidence-Based Management Strategies for Oral Complication from Cancer Treatment”. Available online: http://www.isoo.world/continuing-education.html#guidelines (accessed on 22 September 2020).
- Haveman, C.W.; Summitt, J.B.; Burgess, J.O.; Carlson, K. Three restorative materials and topical fluoride gel used in xerostomic patients: A clinical comparison. J. Am. Dent. Assoc. 2003, 134, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.B.; Retief, D.H.; Bradley, E.L. Fluoride release from and tensile bond strength of Ketac-Fil and Ketac-Silver to enamel and dentin. Dent. Mater. 1986, 2, 241–245. [Google Scholar] [CrossRef]
- Hicks, J.; Garcia-Godoy, F.; Donly, K.; Flaitz, C. Fluoride-releasing restorative materials and secondary caries. Dent. Clin. N. Am. 2002, 46, 247–276. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Krämer, N.; Schmidt, M.; Lücker, S.; Domann, E.; Frankenberger, R. Glass ionomer cement inhibits secondary caries in an in vitro biofilm model. Clin. Oral Investig. 2018, 22, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Horiot, J.C.; Schraub, S.; Bone, M.C.; Bain, Y.; Ramadier, J.; Chaplain, G.; Nabid, N.; Thevenot, B.; Bransfield, D. Dental preservation in patients irradiated for head and neck tumours: A 10-year experience with topical fluoride and a randomized trial between two fluoridation methods. Radiother. Oncol. 1983, 1, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mjör, I.A. The reasons for replacement and the age of failed restorations in general dental practice. Acta Odontol. Scand. 1997, 55, 58–63. [Google Scholar] [CrossRef]
- da Cunha, S.R.; Ramos, P.A.; Haddad, C.M.; da Silva, J.L.; Fregnani, E.R.; Aranha, A.C. Effects of Different Radiation Doses on the Bond Strengths of Two Different Adhesive Systems to Enamel and Dentin. J. Adhes. Dent. 2016, 18, 151–156. [Google Scholar] [CrossRef]
- Ugurlu, M. Effect of the double application of universal adhesives on the dentine bond strength after radiotherapy. Aust. Dent. J. 2020, 65, 181–188. [Google Scholar] [CrossRef]

| 0 Gy | 5 Gy | 60 Gy | |
|---|---|---|---|
| Futurabond® U | 0 Fse 1 | 5 Fse | 60 Fse |
| AdheSE® Universal | 0 Ase | 5 Ase | 60 Ase |
| Xeno® Select | 0 Xse | 5 Xse | 60 Xse |
| Adhesive | Batch Number | pH | Main Components * | |
|---|---|---|---|---|
| F | Futurabond® U, Voco | LOT 1527116 | 2.3 | BIS-GMA 1 HEMA 2 HEDMA 3 Acidic adhesive monomer Urethanedimethacrylate Catalyst |
| A | AdheSE® Universal, Ivoclar Vivadent | LOT U54013 | 2.5–3 | HEMA 2 BIS-GMA 1 Ethanol D3MA 4 MDP 5 MCAP 6 Camphorquinone |
| X | Xeno® Select, Dentsply Sirona | LOT 1602000694 | <2 | Bifunctional acrylates Tert-butyl alcohol Functionalized phosphoric acid ester (ethyl 2-[5-dihydrogen phosphoryl-5,2-dioxapentyl]acrylate) Acidic acrylates 4-dimetylaminobenzonitril |
| Futurabond® U | AdheSE® Universal | Xeno® Select | |
|---|---|---|---|
| 0 Gy | 23.87 | 35.10 | 24.17 |
| ±7.49 | ±8.41 | ±8.36 | |
| 5 Gy | 17.95 | 26.86 | 15.78 |
| ±5.95 | ±7.41 | ±3.89 | |
| 60 Gy | 19.21 | 26.30 | 11.42 |
| ±7.34 | ±10.07 | ±3.86 | |
| Decrease in mean microtensile bond strength after irradiation with 60 Gy compared to 0 Gy | −19.5% | −25.1% | −52.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broscheit, S.; Vordermark, D.; Gerlach, R.; Gernhardt, C.R. Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro. Appl. Sci. 2023, 13, 7873. https://doi.org/10.3390/app13137873
Broscheit S, Vordermark D, Gerlach R, Gernhardt CR. Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro. Applied Sciences. 2023; 13(13):7873. https://doi.org/10.3390/app13137873
Chicago/Turabian StyleBroscheit, Sina, Dirk Vordermark, Reinhard Gerlach, and Christian Ralf Gernhardt. 2023. "Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro" Applied Sciences 13, no. 13: 7873. https://doi.org/10.3390/app13137873
APA StyleBroscheit, S., Vordermark, D., Gerlach, R., & Gernhardt, C. R. (2023). Impact of Preceded Tumor Therapeutic Irradiation on the Microtensile Bond Strength of Universal Adhesives Applied in Self-Etch Mode to Human Dentin In Vitro. Applied Sciences, 13(13), 7873. https://doi.org/10.3390/app13137873







