1. Introduction
Fire in an aircraft oxygen system is a very serious aviation accident that can lead to severe consequences once it occurs. During flight, the oxygen system is one of the most critical pieces of equipment, providing oxygen to passengers and crew members to ensure their safety in high-altitude environments. However, aircraft oxygen systems are prone to fires and can eventually explode, thus posing a great danger to aircraft passengers and crew members. Therefore, the safety of the aircraft oxygen system is an important part of aviation safety and must be highly regarded and strictly regulated.
There have been several accidents caused by aircraft oxygen system fires at home and abroad. In 1984, 1998, and 2003, three P3 airplanes produced by the Lockheed Aircraft Manufacturing Company ignited in the United States; in 2008, a B767-200 cargo aircraft and a B747-400 passenger aircraft produced by the Boeing Company ignited successively in the United States [
1,
2,
3,
4]. In 2008, the aircraft oxygen system of an A319 produced by Eastern Airlines Northwest Branch ignited in China. Since 2016, the oxygen system of one type of aircraft has ignited three times in China [
5,
6,
7,
8]. According to the analysis of the accident wreckage, all three fires occurred at the oxygen nozzle.
The oxygen nozzle is an important piece of equipment that is widely used in the medical, industrial, and aerospace industries, as well as other fields. The main function of the oxygen nozzle is to transfer the oxygen from the cylinder to different components for different needs. In the medical field, oxygen nozzles are widely used in surgical and therapeutic procedures to provide patients with the necessary oxygen supply. In the construction industry, oxygen nozzles are widely used in processes such as welding and cutting, and are sometimes used to provide workers with the necessary oxygen supply. In the aerospace field, oxygen nozzles are used in aircraft and spacecraft to provide passengers and astronauts with the necessary oxygen supply. There are many factors to consider in the design and manufacture of oxygen nozzles, such as safety, durability, and tightness. Therefore, the process requires precision machining and strict quality control. Firstly, safety is one of the most important factors in the design of oxygen nozzles. Oxygen is a highly flammable gas. If the design of an oxygen nozzle is faulty, it can cause serious accidents, such as fires or explosions. Therefore, the design of oxygen nozzles must strictly comply with the relevant safety standards and regulations to ensure safety and reliability. Secondly, durability is also one of the most important factors in the design of oxygen nozzles. Oxygen nozzles are used for long periods of time under high-pressure oxygen environments and therefore require a high level of resistance to wear and corrosion. Thirdly, the tightness of the oxygen nozzle is critical to ensure that it can completely seal in the oxygen to avoid oxygen leakage and waste. High-precision equipment and tools are used to ensure the accuracy of the size and shape of the oxygen nozzle during the machining process. In terms of quality control, oxygen nozzles need to be rigorously inspected and tested to ensure that they meet the relevant safety standards and specifications.
With the rapid development of the civil aviation industry and the increasing length of flights in recent years, research into oxygenated nozzles has been carried out all over the world; in China, Nanjing University and the China Aerospace Science and Industry Corporation have jointly developed an oxygen nozzle for civil aircraft. The oxygen nozzle can be directly connected to the aircraft oxygen system to improve oxygen utilization efficiency. It can increase oxygen concentration in the cabin and reduce overconsumption by adjusting the oxygen flow and outlet angle to reduce aircraft operating costs. In the United States, the Massachusetts Institute of Technology has studied an oxygen nozzle based on nanotechnology that can regulate oxygen flow and concentration by controlling the pore size and length of the nano-channels for more precise control. This technology is used in the research and development of new space shuttles and space probes. In Europe, EADS is working on an oxygen nozzle based on nanomaterials. Due to its topology, the oxygen nozzle optimizes the delivery and dispersion of oxygen in the nanotubes to improve the uniformity of oxygen distribution and utilization in the cabin. Overall, research on aircraft oxygen nozzles is in the starting phase, but oxygen nozzle technology has a wide range of applications. Feng Wenchun and others analyzed the explosion mechanism of the oxygenation process of aircraft oxygen systems at this stage [
9,
10,
11]; Duan Zemin et al. researched the ignition of electrostatic discharge of aircraft oxygen charging valve heads [
12,
13,
14,
15].
Inadequacy in the design of oxygen nozzles has led to many aircraft accidents; therefore, it is necessary to research oxygen nozzles. In this paper, an oxygen nozzle was analyzed considering several aspects, and the oxygen valve was identified as the ignition source. High-pressure oxygen impact tests were carried out, the lifetime of the oxygen valve under high-pressure oxygen was calculated, and F3 was determined as the safer material. This paper provides a reference for the safety design of aircraft oxygen systems.
2. Analysis
2.1. Combustion Analysis
Fire in aircraft oxygen systems is highly dangerous. Fires are necessarily accompanied by the three elements of combustion. In order to identify which substances comprised the three elements of combustion in aircraft oxygen system fires, based on combustion principles and accident experience, a preliminary combustion analysis of the accident was conducted.
Combustion requires three elements: a combustible material, a combustion aid, and heat. Combustion occurs when the three are mixed in the right proportions. Combustible materials can be solids, liquids, or gases. In aircraft oxygen system fires, the combustion aid is oxygen. Oxygen is not a combustible material, but most combustible materials will not burn without oxygen. Heat is a type of energy that is produced by chemical and physical changes. High heat can cause a substance to burn, and the heat generated by combustion can cause a substance to burn continuously until it is exhausted. The three elements of combustion required for ignition are called the fire triangle. According to the analysis of the accident wreckage, the combustion started at the oxygen nozzle.
The oxygen nozzle consists of an oxygen connector, oxygen nozzle, oxygen valve, a sealing gasket, and other components. The oxygen connector is used to connect the oxygen cylinder to the oxygen nozzle, and it is usually made of copper or stainless steel. The oxygen nozzle is used to transfer oxygen from the cylinder to the equipment or appliance requiring oxygen. It is made of copper or aluminum alloy. The oxygen valve is used to control the flow and pressure of oxygen, and is made of stainless steel or copper. The sealing gasket is used to prevent the leakage of oxygen; it is usually made of rubber or ethylene. Under normal oxygen conditions, the temperature required to cause most combustible materials to burn is relatively higher; therefore, an obvious source of ignition is required to provide sufficient heat to reach the ignition point so that combustion can occur for most combustible materials. However, under high concentrations and pressures of oxygen, the ignition point of almost all combustible materials is greatly reduced. Under these conditions, substances that were originally flame-retardant no longer have flame-retardant properties, and they can be ignited at a slightly higher temperature without an obvious ignition source.
In recent years, due to technical innovations in oxygen systems, the oxygen pressure has increased from 15.7 MPa to 20.6 MPa. It is therefore assumed that the increase in oxygen pressure caused these fires.
According to the material parameters of the oxygen nozzle, the ignition point of Nylon 1010 is much lower than that of other materials. Nylon 1010, abbreviated as PA1010, is a common engineering plastic with high strength, wear resistance, and corrosion resistance, and therefore is usually used in the aerospace field. PA1010 is used in aircraft oxygen systems as a highly effective flame-retardant material [
16,
17,
18,
19,
20]. Compared with other component materials, the ignition point of PA1010 is relatively low; thus, the possibility of its combustion is relatively high.
2.2. Energy Spectrum Analysis
In order to determine the location of the origin of the fire and thus verify factors inferring in the combustion analysis, microscopic observation and energy spectrum analysis of the oxygen valve were conducted using scanning electron microscopy and an energy-dispersive spectrometer.
Scanning electron microscopy, abbreviated as SEM, is an observation method combining transmission electron microscopy and optical microscopy. SEM uses a narrowly focused high-energy electron beam to scan the sample, and extracts physical information through the interaction between the beam and the material. The information is collected, amplified, and reimaged to achieve the purpose of characterizing the microscopic morphology of the material.
An energy-dispersive spectrometer, abbreviated as EDS, can be used to analyze the type and content of elements in micro-areas of materials, usually in conjunction with SEM. Each element has its own characteristic wavelength of X-rays. The magnitude of the characteristic wavelength depends on the characteristic energy released during the energy level jump. EDS can be used to perform compositional analysis by using the characteristic energy of the different X-ray photons of different elements.
The composition and ablation of the black adhesions were separately examined on each part of the oxygen nozzle. The locations for which the energy spectrum tests were carried out are shown in
Figure 1. Location 1 is the distal end of the inlet. Location 2 is the proximal end of the inlet. Location 3 is the PA1010 oxygen valve. Location 4 is the middle area of the component. Location 5 is the gas cylinder end. Location 6 is the outlet end. These locations basically cover the inner cavity of the component. The observed images and energy spectrum are shown in
Figure 2. A summary of the results of the energy spectrum analysis are shown in
Table 1.
As can be seen from the results in
Table 1, the Al content at location 3 was 36.01%, which is much higher than that found at the other locations. This indicates that the alloy coating at location 3 was the most severely damaged by the flames in the high-pressure oxygen environment, indicating that location 3 is the area that experienced the most intense combustion in the entire inner cavity. Location 3 has a high probability of being the location of the origin of the fire. The Al contents at locations 1, 2, 4, 5, and 6 were similar and relatively low compared with location 3. The attached ablation products of these locations are similar.
2.3. Material Analysis
2.3.1. Material Selection
Based on the results of the above analysis, the PA1010 oxygen valve was identified as the ignition source preliminarily. The exact cause of the fire was then further explored.
Due to the special properties of oxygen, preventing oxygen system fires and reducing the hazard of fire are the aims of oxygen system safety design. Material selection is a fundamental requirement of oxygen system safety design. Based on the results of the above analysis, PA1010 is no longer considered the best choice for an oxygen valve under high-pressure oxygen conditions. Selecting suitable materials is an important measure to reduce or prevent the risk of fire in aircraft oxygen systems. The materials used in aircraft oxygen systems should be compatible with oxygen.
Based on safety design guidelines for aircraft oxygen systems, there are two fundamental principles for material section. NASA has also given evaluation procedures for materials to test whether materials can be applied in aircraft oxygen systems. In addition, the use of non-metallic materials in oxygen systems should be limited due to their low auto-ignition point, so there are some requirements for non-metallic material properties. These three aspects should be jointly considered to select the best material for the aircraft oxygen system. The procedure for selecting a non-metallic material based on these three aspects is shown in
Figure 3.
2.3.2. Material Comparison
Polytrifluorochloroethylene, abbreviated as F3, is the polymer of trifluoroethylene. F3 has good flame-retardant properties, but it is difficult to process and has higher costs; therefore, it has had limited use in the aviation sector for a long time. The serious damage caused by several recent accidents has made safety a priority in the design of oxygen valves, and F3 is considered a promising material for oxygen valves.
The ignition point of PA1010 is about 500 °C, while the ignition point of F3 is above 670 °C. Therefore, F3 has a higher ignition point than PA1010 and is more difficult to ignite.
The oxygen index of PA1010 is 20, while the oxygen index of F3 is 95. This is due to the higher thermal and chemical stability of F3; it can better resist fire and oxidation. Therefore, F3 is more suitable for applications with high flame-retardant requirements.
The flame expansion rates of PA1010 and F3 are different. The specific values of the flame expansion rate will vary under different conditions. Compared with PA1010, the flame expansion rate of F3 is lower. This is due to the different molecular structures of the two materials. The molecular structure of PA1010 contains nitrogen atoms, which makes its molecular chain loose and easy to burn. The molecular structure of F3 contains fluorine atoms, which makes its molecular chain tighter and difficult to burn.
The differences between PA1010 and F3 are relatively limited in other parameters.
In summary, F3 is more suitable as an oxygen valve material than PA1010.
3. Tests
In order to verify that F3 is more suitable as an oxygen valve material than PA1010, a test platform was constructed, and oxygen impact tests were carried out. The tests were carried out at the National Key Experimental Base of Fire Science.
3.1. Conditions
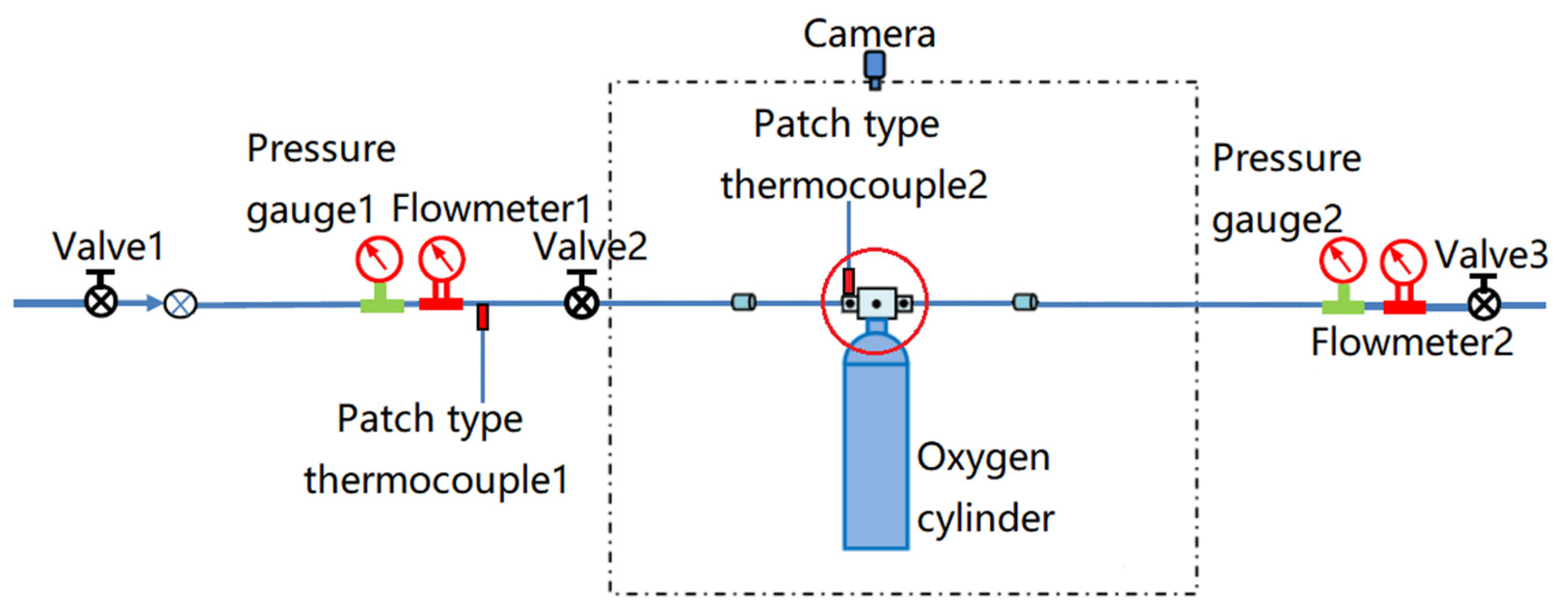
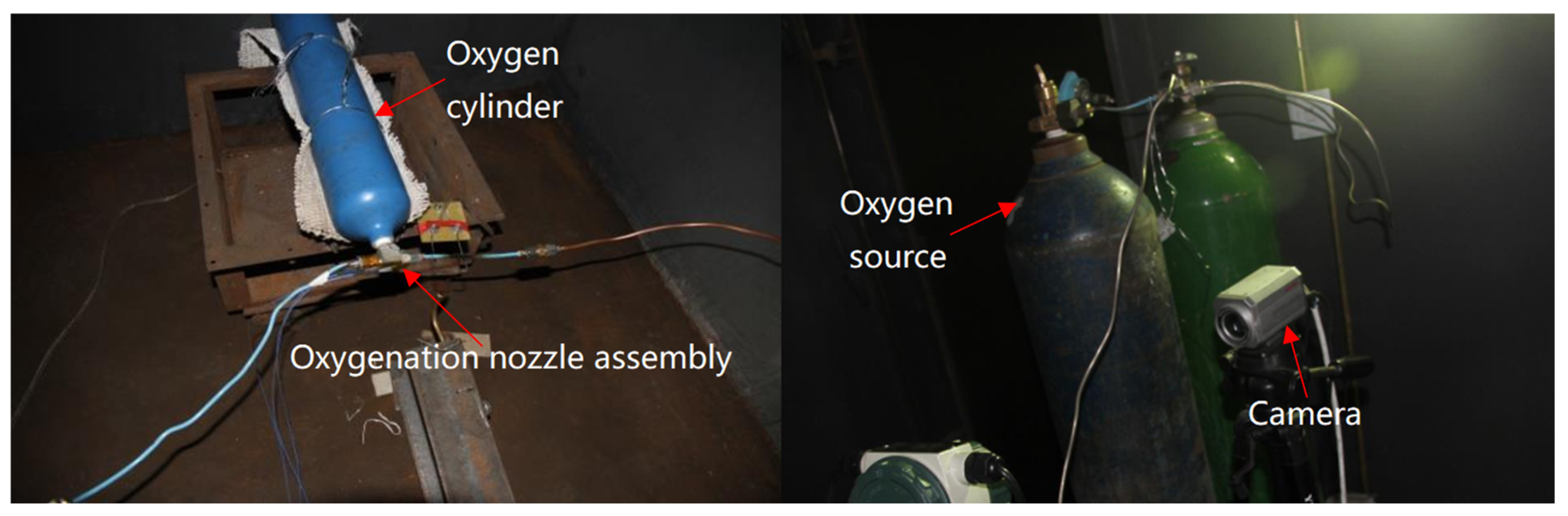
The equipment required for the test included valves, pressure gauges, flow meters, oxygen cylinders, patch-type thermocouples, PA1010 oxygen nozzles, F3 oxygen nozzles, measuring and recording equipment, etc. A pressure gauge was used to determine the oxygen pressure. A contact thermocouple thermometer was used to measure the temperature of the oxygen nozzle valve. A flow meter was used to test the oxygen flow in the oxygen line. The details of the test equipment setup are shown in
Figure 4. The oxygen nozzle is indicated by the red circle in
Figure 4, and it was the main test subject. A simplified diagram of the structure and connection of the oxygen nozzle and oxygen valve is shown in
Figure 5.
3.2. Process
The tests were carried out in a test chamber because of the risk of explosion. The test chamber is shown in
Figure 6; it was welded using extremely thick steel plates to ensure safety during the test. The interior of the test chamber is shown in
Figure 7.
Due to the dangers of oxygen impact tests, the following three steps should be carried out before the test starts: (1) check the pressure of the oxygen source and replace or replenish it appropriately; (2) check the safety conditions of the test site environment and eliminate hidden dangers; (3) check data measurement equipment, camera equipment, and timing equipment. The test equipment was opened after inspection
After inspection, the test equipment was opened according to the specific parameters. The valve was quickly opened, and oxygen was input at a specific pressure within 20 ms. The condition was maintained for 10 s, and then the valve was closed. The operation was performed 5000 times (5000 times is the standard life cycle of an aircraft oxygen valve). The ignition operation was carried out remotely in the control room by the test operators. The number of operations was controlled by the computer system and monitored by a test operator throughout the test process. The test was aborted immediately if a fire occurred during the test, and the number of times the oxygen impact test was aborted was recorded.
The test consisted of four groups, and the specific conditions of each group are shown in
Table 2. According to statistical principles, each group of tests was repeated at least 30 times, and the oxygen valve was replaced after each test.
3.3. Results
3.3.1. Data Statistics
At an oxygen pressure of 15.7 MPa, neither the F3 oxygen valve nor the PA1010 oxygen value ignited after being subjected to 5000 cycles and they maintained a good performance.
At an oxygen pressure of 20.6 MPa, the F3 oxygen valves were not ignited after being subjected to 5000 cycles, and they maintained a good performance; at an oxygen pressure of 20.6 MPa, the PA1010 oxygen valves ignited after 5000 cycles in all 30 tests.
The number of cycles required for the oxygen valve to be ignited in the 30 tests was as follows: 2598, 2388, 2419, 2653, 2730, 2333, 2713, 2590, 2280, 2525, 2387, 2279, 2715, 2278, 2556, 2401, 2393, 2378, 2621, 2444, 2571, 2675, 2557, 2639, 2682, 2707, 2425, 2642, 2687, 2533, and 2691.
3.3.2. Data Processing
Several links were involved in the oxygen impact tests. Each of these links has the potential to produce errors, and the errors can lead to incorrect test data. Considering the serious danger of aircraft oxygen system fires, the test data must be strictly selected to acquire reliable data.
The Grubbs’ criterion is used to eliminate unqualified data. The Grubbs’ criterion belongs to the normal branch of the distribution. According to the nature of normal distribution, if the difference between each measurement and the mean is not too large, and if the difference between a particular test datum and the mean is too large, this test datum is unqualified, and it should be excluded.
According to the Grubbs’ criterion, the difference between a datum and the mean is .
If
can satisfy the relationship
then this datum is unqualified and must be excluded. In the formula
can be derived from
Table 3.
n is the number of data.
a is generally chosen as 0.01 or 0.05; here, 0.01 is chosen.
According to the Grubbs’ criterion, the
and
of the data set can be calculated.
According to the calculations, all the test data are reliable and can be used for the safety design of oxygen valves.
The lifetime of a PA1010 oxygen valve under a high-pressure oxygen environment is about 2532 cycles. In practice, the oxygen valve is usually replaced after being subjected to 2000 cycles to ensure maximum safety.
4. Conclusions
In this paper, the safety design problem of oxygen valves in aircraft oxygen systems under a high-pressure oxygen environment was studied. Based on the results of multiple analyses and oxygen impact tests, the main findings of this study are as follows:
The PA1010 oxygen valve was determined to be the ignition source of three fires of a certain type of aircraft.
The lifetime of the PA1010 oxygen valve under high pressure is about 2532 cycles.
F3 was identified as the superior oxygen valve material.
The study still leaves a lot to be desired. One of the most significant shortcomings is that the cause of the fire of PA1010 oxygen valves needs to be investigated and verified. In addition, it is worth researching further whether there are more suitable materials than F3 for high-pressure oxygen environments.
This paper provides a reference for the safety design of oxygen valves under high-pressure environments and facilitates further research into aircraft oxygen systems.
Author Contributions
Conceptualization, H.Y. and D.J.; methodology, H.Y.; validation, D.J.; formal analysis, H.Y.; investigation, H.Y.; resources, H.Y.; data curation, H.Y.; writing—original draft preparation, H.Y.; writing—review and editing, D.J.; supervision, D.J.; project administration, D.J.; funding acquisition, D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2021 Anhui University Provincial Natural Science Research Project—Key Project (K2210033).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors are grateful to other participants of the project for their cooperation.
Conflicts of Interest
The authors declare that there are no conflict of interest regarding the publication of this paper.
References
- Gregory, F. NASA-STD-8719.15 Safety Standard for Oxygen and Oxygen Systems, Guidelines for Oxygen System Design; Materials Selection, Operation, and Transportation; Office of Safety and Mission Assurance: Washington, DC, USA, 2000.
- Joel, S. Fires in P-3 aircraft oxygen systems. J. ASTM Int. 2006, 3, 13. [Google Scholar]
- Anonymous. Ground Fire Aboard Cargo Airplane, ABX Air Flight 1611, Boeing 767-200, N799AX; Aircraft Accident Summary Report; National Transportation Safety Board: Washington, DC, USA, 2008.
- Anonymous. Oxygen System Fire: Air Accident Investigation Sector; Incident Summary Report, AIFN/0001/2014; Air Accident Investigation Sector of General Civil Aviation Authority: Dubai, United Arab Emirates, 2017. [Google Scholar]
- Li, Y.; Xie, Z.N. Investigation and research on cases of aircraft oxygen system-triggered fire. Saf. Secur. 2014, 25, 44–48. [Google Scholar]
- Liang, H.; Zhuang, S.Q. Analysis of 12.14 oxygen bottle explosion accident. Low Temp. Spec. Gas 2010, 28, 43–46. [Google Scholar]
- Yang, S.X. Analysis of explosion accident during recharging oxygen and safety precautions. Railw. Labour Saf. Sanit. Environ. Preserv. 1990, 1990, 28–30. [Google Scholar]
- Chen, W.H.; Hu, L.T. Analysis of reasons of three oxygen bottle explosion accidents and advice. Deep Cold Technol. 2002, 2002, 48–50. [Google Scholar]
- Feng, W.C.; Dong, P.T.; Yang, J. Combustion mechanism analysis of the aircraft recharging oxygen process. Aeronaut. Sci. Technol. 2014, 25, 44–48. [Google Scholar]
- Feng, W.C. Analysis of aircraft oxygen system fire accident and safety requirement. Aeronaut. Sci. Technol. 2018, 18, 58–63. [Google Scholar]
- Bai, B.; Feng, W.C. Common reason analysis of two aircraft oxygen system fire accident. Aeronaut. Sci. Technol. 2019, 30, 49–52. [Google Scholar]
- Zuo, X. Study on Electrostatic Characteristics of Hyperbaric Oxygen One-Way Valve Head Materials of Aircraft. Master’s Thesis, Hefei University of Technology, Hefei, China, 2018. [Google Scholar]
- Ding, C.D. Experimental Study on Electrostatic Discharge Ignition of Aircraft Oxygen Fill Valve Head. Master’s Thesis, Hefei University of Technology, Hefei, China, 2018. [Google Scholar]
- Bao, X.D. Study on Electrostatic Discharge Ignition of Aircraft Oxygen Filling Valve Head. Master’s Thesis, Hefei University of Technology, Hefei, China, 2021. [Google Scholar]
- Zuo, X.; Duan, Z.M.; Ding, C.D. Contact electrostatic accumulation properties of oxygen filling valve head materials applied to aircraft. Sci. Technol. Eng. 2018, 18, 1–5. [Google Scholar]
- FuKuMoTo, M. Poly-Amide Resin Handbook; Petrochemical Publishing Company: Beijing, China, 1994; pp. 1–40. [Google Scholar]
- Qian, Z.Y.; Guo, B.H.; Shi, J.Z. Chain extension of PA1010 by bisoxazolines. Acta Polym. Sin. 2004, 2004, 506–510. [Google Scholar]
- Wang, J.K.; Zhao, G.S.; Zhou, Y.C. Drawing-induced crystallization behavior and Brill transition of Nylon 1010. Chem. J. Chin. Univ. 2011, 32, 1225–1230. [Google Scholar] [CrossRef]
- Cai, L.H.; Guo, B.H.; Zhang, C. Long-term stress relaxation prediction for Nylon 1010 using time-temperature superposition method. Chem. J. Chin. Univ. 2019, 40, 832–840. [Google Scholar]
- Wu, D.; Zhou, A.; Liu, G. Effect of thermo-oxidative aging on structure and properties of PA1010. Mod. Plast. Process. Appl. 2017, 29, 16–19. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).