1. Introduction
The development of compatible materials and inks and their use in appropriate printing methods represents an emerging methodology to produce novel, large area, highly flexible printed electronic devices [
1]. To date, printed electronics demand new classes of materials and multiple types of inks that are highly attractive for potential applications, including photovoltaics, batteries, antennas, sensors, biosensors, etc. [
2,
3,
4,
5,
6,
7,
8]. The production of conductive inks suitable for flexography, gravure, screen, or inkjet printing on several substrates is of high importance for the industry. Thus far, Ag nano-ink is the most popular conductive ink used in functional printing due to its excellent electrical conductivity and strong antioxidant characteristics [
9]. The main drawbacks to the use of nanosilver are the high cost of the raw material and the electromigration that deteriorates its potential uses. Copper is also often used for conductive inks due to its high conductivity; however, copper suffers from oxidation [
10].
Recently, carbon-based nanocomposites have attracted much interest in printing technology for the development of novel, low-cost, stable, and effective conductive inks. In this regard, various carbon nanoallotropes such as carbon nanotubes, graphene, and their derivatives have been examined, affording a plethora of potential products [
10,
11,
12,
13]. Graphene is a single-atom thick sheet of carbon atoms arrayed in a honeycomb pattern and the world’s thinnest, strongest, and stiffest material [
14]. Because of its remarkable inherent carrier mobility and unique carrier transport properties, graphene is expected to be an excellent conductive pigment [
15,
16,
17]. On the other hand, graphene is nowadays produced at a low cost from an abundant and natural raw material such as graphite with effective and scalable procedures. The most widely used graphene precursors are reduced graphene oxide (rGO) and the few layered graphene nanosheets (FLG) that are produced by the liquid-phase exfoliation of graphite in several liquids or water solutions [
18]. The latter has much higher electrical conductivity, but it is produced with a much lower yield compared to rGO, which is produced from the treatment of graphite with strong oxidants and the reduction of the as-formed graphene oxide (GO). Furthermore, FLGs are only rarely dispersed in specific solvents, and thus their incorporation into industrial inks has a lot of difficulties, such as low yield and inhomogeneity.
rGO has been used as an alternative conductive pigment. The dispersibility of rGO is also very low, similar to FLG. In several cases, reduction of GO is followed by the functionalization of rGO with hydrophilic groups, leading to finally water-dispersible graphene derivatives. Although functionalized rGO (f-rGO) has lower conductivity compared to FLG, it outcompetes pristine graphene due to the great advantage of its high dispersibility.
Nonetheless, the conductivity of pure graphene ink is low for most applications and not comparable with that of metals, restricting its extensive use. Several efforts have been devoted to increase the electrical conductivity of graphene inks. Among other things, a graphene/carbon nanotube hybrid has been developed that can be easily mixed with hydrophilic polymers and resins to produce highly conductive water-based nanocomposites suitable for gravure printing [
11]. The development of a gravure ink based on a water dispersible and highly conductive
f-rGO starting from GO and using 2,4-diamino benzene sulfonic acid (2,4-DBSA) as a reducing agent and functionalization agent has recently been presented by Belessi et al. [
19].
Another interesting approach to increasing the electrical conductivity of graphene inks is to decorate graphene nanosheets with various metal nanostructures. Graphene nanosheets doped with Ag nanoparticles have been proven to be a promising material due to their potential applications in many fields, including preparing a hybrid ink and thus taking full advantage of these two materials [
7,
20,
21,
22,
23]. Recently, a hybrid nanocomposite derived from the combination of silver nanowires and
f-rGO has been formed for the development of stable gravure printing inks with enhanced conductivity, which is promising for electronic applications [
24].
It is reasonably supposed that Ag nanoparticles that stand between graphene nanosheets decrease the intersheet resistance, while silver nanowires can furthermore form extra pathways for charge movement. However, the mechanism of the effect of the metal structure on the improved conductivity of metal-graphene is still unclear. On the other hand, due to the weak bonding between silver and graphene surfaces, it is not always successful in obtaining homogeneous Ag-graphene hybrid inks. Therefore, it is still a challenge to develop new graphene-based/metal hybrid inks for printing conductive patterns and to explore the structure-effect of metal nanoparticles on the conductivity of graphene-based patterns.
In this article, the in-situ formation and direct deposition of AgNPs on two different substrates—pristine FLG and f-rGO—with several Ag percentages are described. The use of two different forms of graphene—the hydrophobic and mainly aromatic FLG nanosheets and the hydrophilic and less aromatic f-rGO—offers the opportunity to shed light, for the first time, on the role of the carbon substrate in the morphology and the properties of the final hybrid.
The two different types of hybrids that were formed were compared as regards dispersibility in water and electrical conductivity as a function of the Ag percentage. Furthermore, a representative
f-rGO/AgNPs sample was used for the formulation of a gravure water-based conductive ink. The latter was achieved without any addition of rheological modifiers, other common additives, or organic alcohols, improving the sustainability of the printing process. The electrical properties of the printed patterns were tested using two different kinds of paper. Emphasis was given to the formulation of gravure ink because gravure as a printing method has played a part in the growth of the global economy over the last eighty years. Nowadays, in emerging economies such as those of Asia and Africa, gravure is considered the most effective and popular for industrial applications compared to other printing methods [
25]. Additionally, gravure offers the ability to print a variable ink film thickness at high speeds and for high-volume production. Simultaneously, gravure produces high-quality patterns using a variety of substrates and the same set-up that prints conventional printing inks. Finally, its offer to the circular economy is mainly due to the reusability of the printing cylinders and also due to the potential for recovery of metals (Cu or Cr) during plating processes [
25].
2. Materials and Methods
2.1. Materials
Graphite (Sigma-Aldrich, St. Louis, MO, USA, powder <20 μm, synthetic), DMF, NaBH4, AgNO3, potassium chlorate (purum >99.0%), and 2,4-diaminobenzenesulfonic acid (98%) were purchased from Merck KGaA (Darmstadt, Germany). Νitric acid (65%) and sulfuric acid (95–97%) were purchased from Riedel-de Haen (Munich, Germany) and Merck, KGaA, (Darmstadt, Germany), respectively, and were used as provided.
2.2. Method Section
Graphene preparation. A total of 300 mg of graphite was dispersed in 300 mL of DMF and sonicated for 1 h in bath sonicator. The unexfoliated graphite that precipitates after 1 h of standing was separated from the liquid phase. The dispersed material, a few layers of graphene nanosheets (FLG), was isolated from the solvent by filtration using hydrophobic nylon membrane filters (0.45 μm) [
26].
Graphite oxide preparation [
27]. A total of 5 g of graphite was added to a cold mixture of concentrated sulfuric acid (80 mL) and nitric acid (40 mL). Then 40 g of potassium chlorate was slowly added by stirring and continuously cooling. The reaction was stopped 18 h later by pouring the mixture into water, and GO was isolated by centrifugation and washed several times with water until neutral. The product was air-dried.
Functionalization and reduction of graphene oxide (
f-rGO). The functionalized reduced graphene oxide was prepared according to ref. [
19]. A total of 100 mg of GO were dispersed in deionized water under sonication for 30 min (110 W, 40 kHz). A total of 300 mg of 2,4 diaminobenzenesulfonic acid was then added, and the mixture was heated under reflux for 5 h. The product was isolated by centrifugation (9000 rpm) and washed extensively with water, alkaline water, ethanol, and acetone, then air-dried.
Deposition of Ag nanoparticles on f-rGO (f-rGO/AgNPs). A total of 4 mg of f-rGO was dispersed in 20 mL of DMF, followed by the addition of 60 mg of NaBH4. A certain amount of AgNO3 (0.25 Μ) was diluted in 3 mL H2O and added dropwise into the reaction mixture for 30 min. The mixture was then centrifuged (10,000 rpm, 20 min), and the precipitate was washed twice with water. This procedure was used to produce f-rGO/AgNPs nanocomposites with the following theoretical Ag percentages and the added volume of AgNO3 in parenthesis: 15% (28 μL), 25% (52 μL), 30% (68 μL), 40% (108 μL), and 57% (216 μL).
Deposition of Ag nanoparticles on FLG (FLG/AgNPs). A total of 6 mg of FLG nanosheets was dispersed in 20 mL of DMF, followed by the addition of 60 mg of NaBH4. A certain amount of AgNO3 (0.25 Μ) was diluted in 3 mL of H2O and added dropwise into the reaction mixture for 30 min. Then, 200 mg of sodium citrate diluted in 3 mL of water was added dropwise into the reaction mixture. The aggregate that formed was isolated by centrifugation (10,000 rpm, 20 min) and washed by redispersion in 20 mL of water and centrifugation (10,000 rpm, 20 min). Finally, the solid composite was dispersed in 1 mL of water. This procedure was used to produce FLG/AgNPs nanocomposites with the following Ag percentages and the volume of AgNO3 in parenthesis: 15% (40 μL), 20% (56 μL), 30% (96 μL), 45% (183 μL), and 60% (336 μL).
Preparation of the f-rGO/AgNPs ink formulation. An appropriate amount of the sample f-rGO/AgNP2 was mixed with the underproduction system of resins R-UDP (solid content approx. 42%) and water to produce an ink with a ratio of pigment solids/resin solids of 50/50. The mixing of ink components is made using a homogenizer (Witeg Labortechnik GmbH, Wertheim, Germany) and a vortex mixer (LLG-uniTEXER, LabLogistics Group GmbH, Meckenheim, Germany).
2.3. Characterization
Electrical measurements. A Lucas Labs Pro4 Resistivity System (Lucas Signatone Corp., Gilroy, CA, USA) and a Keithley 2400 Source Meter (Keithley Instruments, Cleveland, OH, USA) were used to measure the sheet resistance (Rs) of the samples and the ink via the 4-point probe method after deposition of 400 µg of each sample on coated paper, drying at room temperature, and “polishing” using mild pressure so that films with an average thickness of about 5 µm were formed.
Thermogravimetric analysis (TGA) was carried out with a TA Instrument Q500 Thermogravimetric Analyzer under ambient air with a heating rate of 10 °C min−1 up to 850 °C. UV-Vis spectra were recorded in water dispersion with a Hitachi Digilab, Model U2800-Double-Beam-UV/Vis (Hitachi, Berkshire, UK). Scanning electron microscopy (SEM) was carried out on a Zeiss EVO-MA10 (Carl Zeiss Microscopy GmbH, Jena, Germany). X-ray diffraction (XRD) patterns were conducted using a D-500 Siemens (Munich, Germany) diffractometer (CuKα, λ = 1.54 Å). Transmission electron microscopy (TEM), wherein samples were prepared by casting a droplet of a dilute aqueous suspension of particles onto copper grids coated by a Formvar carbon film. Images were obtained using a JEOL, JEM-2100 instrument operating at 200 kV.
Gravure printing. The G1-5 printability tester (IGT Testing Systems, Almere, The Netherlands) was used for the printing tests using the chromium-plated printing cylinder 402.226 (60, 80, 100, and 140 lines cm−1; screen angle 53; stylus angle 130; and cell volumes of 16, 11, 9, and 7 mL m−2) and the 402.474 special disc for printed electronics having 12 different engravings. The printing force between the printing disc and the substrate was 200 N, and the printing speed was 0.6 m/s. The f-rGO/AgNPs ink was gravure printed onto a commercially high glossy photo paper (265 g m−2, @work) suitable for inkjet and on a standard IGT coated paper (C2846, IGT Testing Systems).
3. Results and Discussion
Two graphene substrates were used in this study: pristine FLG and
f-rGO. FLG nanosheets were produced by LPE of graphite [
25], while
f-rGO was produced by the subsequent functionalization and reduction of GO with 2,4 diaminobenzenesulfonic acid [
19]. The two substrates have a common basic graphene structure; however, they are quite different. FLG, consisting of a few graphene layers (usually 1–5), is highly aromatic and mostly hydrophobic since only a few oxygen groups are formed on the surface during the preparation treatment from graphite. On the other hand,
f-rGO consists mostly of one layer; it is less aromatic due to the defects that have been created in the preparation step of GO and highly hydrophilic due to the functional groups that have been added during the treatment of GO with 2,4 diamino benzene sulfonic acid. Both substrates, pristine FLG and
f-rGO, are well dispersed in DMF.
Ag
+ ions were slowly added to the dispersion of the graphene substrate and the reducing agent—NaBH
4—in DMF. The reduction of Ag
+ ions led to the development of Ag nanoparticles, preferably on graphene surfaces (see
Figure 1). By altering the amount of Ag
+ ions added,
f-rGO/AgNP and FLG/AgNP hybrids with different Ag percentages were produced.
In the next step, the structure and properties of the nanohybrids were studied in detail. Due to the thermal stability of AgNPs, TG analysis was the preferred way to verify the Ag percentage of the as-produced nanohybrids. The thermal profiles of the
f-rGO/AgNPs and FLG/AgNPs were recorded as shown in
Figure 2. Under air, the
f-rGO/AgNPs samples exhibited an overall weight loss that ranged between 43% and 85% up to 700 °C, reflecting the residue of AgNPs after the combustion of the
f-rGO content of the hybrids. The percentage of AgNPs in the hybrid was in good agreement with the starting amount of Ag
+ ions (see the theoretical Ag percentage in the inset of
Figure 2), indicating that the deposition of AgNPs onto
f-rGO surfaces was strongly favored. Similar weight loss and good agreement with the theoretical Ag percentage were also recorded for the FLG/AgNPs between 500 and 600 °C. A 10% weight loss between 200 and 400 °C of the
f-rGO/AgNPs samples was due to the removal of the functional groups of
f-rGO, while in the same temperature region, FLG/AgNPs samples have lost less than 3% of their weight.
The
f-rGO samples have a strong hydrophilic character due to the amino denzenesulfonic groups [
19], and this is reasonably affecting the final hybrids, inducing high dispersibility of the hybrid
f-rGO/AgNPs in water, a fact that further enhances the rheological properties and the stability of the related conductive inks. In
Figure 3, certain amounts of the different Ag percentages of the
f-rGO/AgNPs hybrids were suspended in water, affording stable dispersions. The FLG/AgNPs hybrid samples are based on FLG nanosheets, which have, like pristine graphene, much fewer functional groups—usually few carboxy or hydroxy groups—that formed during the preparation procedure. Therefore, FLG/AgNPs hybrids showed much less stability in water due to the hydrophobic character of FLG nanosheets.
The morphological characteristics of the hybrids were elucidated through the TEM and SEM microscopy images (see
Figure 4). TEM images in
Figure 4a,b and SEM images in
Figure 4c,d correspond to
f-rGO/AgNPs hybrids with the highest and lowest Ag percentages, respectively. Similarly, SEM images in
Figure 4g,h are from FLG/AgNPs hybrids with the lowest and highest Ag percentages, respectively. EDS analysis confirmed the Ag percentage for both hybrids. TEM image in
Figure 4b shows that in the case of the lowest Ag percentage, spherical-shaped AgNPs with an average size of 12 nm have been formed, and most of them are finely dispersed on the
f-rGO surface. A few aggregates were also observed (see yellow circles in
Figure 4b). In the case of the hybrid with the highest Ag percentage, as shown in
Figure 4a, many more and larger aggregates have been formed. In both cases, AgNPs not deposited on the graphene substrate were not observed. SEM images show better the morphology of the hybrids, revealing the phylomorphous character of the graphene derivatives and the decoration of the nanosheets with Ag nanoparticles. The amount of AgNPs and their aggregates was obviously higher in the hybrids with a high percentage of Ag.
The UV-Vis spectrum of FLG/AgNPs showed a characteristic absorption curve with a maximum at 284 nm due to the
π,π* transitions of the pristine graphene nanosheets (see
Figure 5). The same band in the spectrum of
f-rGO/AgNPs was recorded at 266 nm, showing a blue shift that is attributed to the partly oxidized character of
f-rGO. After the AgNPs development onto the graphene surfaces, a characteristic broad absorption appeared due to the plasmon resonance of AgNPs around 400 nm. This band is very broad for both hybrids, and a second maximum appears at 600 nm at the higher Ag percentage in the case of
f-rGO/AgNPs. This type of absorption band indicates the formation of large Ag nanospheres, aggregates or anisotropic nanoparticles like rods or wires. The intensity of the absorption is proportional to the amount of AgNPs deposited, as estimated before.
The XRD patterns of the hybrids also indicate the formation of AgNPs. The analysis of the XRD pattern of the sample
f-rGO/AgNP
5 (57%
w/
w Ag) showed a weak and broad peak located around 23°, which corresponds to an interlayered spacing of 3.3 Å between 002 atomic planes due to the partly graphitic character of the product,
f-rGO/AgNPs (see
Figure 6). The sharp peaks at 38.18°, 44.3°, 64.4°, and 77.4° were assigned to the (111), (200), (220), and (311) planes of face-centered cubic (fcc) Ag crystals (4-0783 JCPDS card). The crystallized size of the Ag nanoparticles was calculated using the Scherrer equation (average value from all peaks) to 11 ± 2.4 nm, in accordance with the size of the nanoparticles as estimated by the TEM images.
To examine the electric conductivity of the
f-rGO/AgNPs and FLG/AgNPs hybrids, circular spots were formed by drop casting on paper and air-dried. To have comparable results, spots of similar size were formed with the same amount of hybrid (400 μg dispersed in 50 μL οf water) under certain conditions. The values of the sheet resistance (R
s) are presented in
Table 1.
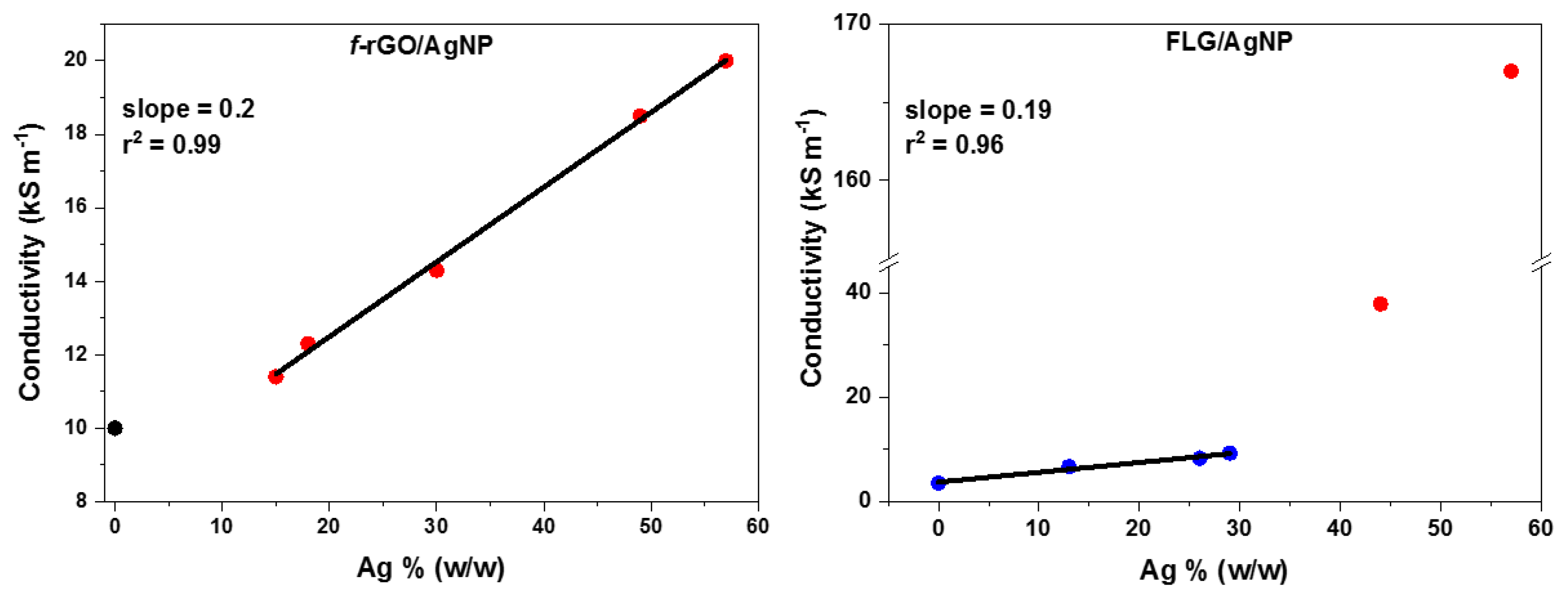
The two hybrids contain AgNPs at several percentages between 13 and 57%. The
f-rGO/AgNPs hybrid showed mildly increased conductivity that ranged between 11 and 20 kS m
−1, starting from 10 kS m
−1 for the pure
f-rGO. Specifically, as depicted in
Figure 7, the electrical conductivity of the
f-rGO/AgNPs hybrid showed a linear increase versus Ag percentage with a slope of 0.2 and a threshold around 10% (
w/
w). Below this percentage, the conductivity seems to be stable, close to 10 kS m
−1, and it represents the conductive network of graphene nanosheets (see
Figure 7).
The FLG/AgNPs hybrid also showed mild conductivity at low Ag percentages, which increased remarkably at higher Ag percentages. There is a very similar linear increase with a slope of 0.19 between 13 and 30% (w/w), and then the conductivity increases radically with a slope close to 10.
This behavior could be explained by assuming the existence of two different pathways for the electrons through the hybrid surface. The first slope in both hybrids, which is close to 0.2 for both hybrids, indicates the formation of pathways with mild conductivity that could be reasonably attributed to the intercalation of AgNPs between the graphene nanosheets and the formation of graphene-Ag-graphene interconnections with lower resistance than graphene-graphene in the pure graphene substrates. The second slope, which is observed only in the FLG/AgNPs hybrid and indicates much more effective electron mobility, could be attributed to the formation of new pathways that were formed only by rows of aggregated AgNPs. The fact that the second slope is not observed in the hybrid f-rGO/AgNPs indicates that AgNPs are much better dispersed in f-rGO than FLG nanosheets. Finally, it is concluded that AgNPs increase the conductivity of both graphene substrates without the assistance of an annealing process.
In the recent literature, graphene-based inks produced from liquid exfoliation of graphite in various solvents or solvent/surfactant systems showed sheet resistance values between 20 and 2 × 10
3 Ω sq
−1 but only after annealing at several temperatures between 100 and 950 °C [
28]. Karagiannidis et al. showed that graphene ink in carboxymethycellulose sodium saltwater solution can reach a sheet resistance of 2 Ω sq
−1 after annealing at 100 °C [
29]. As regards conductive inks based on hybrids of graphene nanosheets and Ag nanoparticles, several reports have shown the contribution of Ag nanoparticles to electrical conductivity. As an example, Deng et al. used a graphene/Ag nanoparticle hybrid as ink for inkjet printing, achieving a low resistivity of 20 Ω sq
−1 after annealing at 400 °C with an Ag percentage of 30%
w/
w [
30]. Marriati et al. recently presented a water-based graphene/AgNPs hybrid ink with sheet resistance of about 9 kΩ sq
−1, which, however, decreased remarkably after six inkjet printing cycles [
31]. They also observed that one hour of annealing at 100 °C can increase the conductivity by about 60%. The combination of a high concentration of Ag nanoparticles with an annealing process in the graphene/Ag hybrid nanostructures promotes a sintering procedure between Ag nanoparticles that leads to the formation of better conductive silver networks and therefore remarkably reduces the resistance of the hybrid. In addition, a high-temperature treatment of graphene hybrid ink-printed patterns often removes organic additives and solvents that are insulators and improves the final conductivity. However, annealing is often a forbidding or unfavorable process, especially with sensitive printing substrates such as paper and polymer films.
Considering that a conductive graphene/AgNPs hybrid is much more cost-effective and contains a lower Ag percentage, it is concluded that hybrid
f-rGO/AgNPs has an advantage. Based on this, the
f-rGO/AgNP
2 sample (containing approximately 18% Ag
w/
w) was further selected as the functional pigment for preparing a conductive ink with a ratio of solid pigment/resin of 50/50. The as-prepared ink was applied with the gravure printing method using two different kinds of coated papers under the same printing and environmental conditions (temperature and relative humidity). Generally, the interaction between ink and substrate affects various parameters such as wetting, penetration, and solvent rate evaporation. In this case, the best printing quality was obtained with the glossy photo paper (lower contact angle than IGT standard coated paper), where more adequate wetting and more uniform spreading of the water-based ink on its top layer were achieved (see
Figure 8). The formation of such a smooth ink film on the glossy substrate denotes that
f-rGO/AgNPs remained dispersible and stable in the ink as water evaporated. Thus, the photo paper was selected for further printing investigation and study in this work (see
Figure 9).
According to example
Figure 8, the correct choice of paper for electronic applications is critical for the emergence of ink characteristics. Substrates with good wetting properties provide reproducible printed patterns, and their high quality is required for direct contact between conductive particles and, by extension, the production of working electronic devices [
32,
33]. As a general rule, if the surface energy of a substrate is higher than the surface tension of an ink, then the contact angle is small and the ink is highly spread. Additionally, the porosity of papers is another important parameter relevant to the uniform deposition of inks on the printing substrates. Recently, Ruhkopf et al. [
33] reported that improved electrical properties were obtained when papers with porous coatings were used as substrates for graphene ink deposition.
Usually, printed graphene or Ag/graphene-based conductive inks require a thermal stage to create conductive paths. In this case, the high dispersion of the composite hybrid
f-rGO/AgNP
2 in the resin matrix allows the formation of conductive pathways without the addition of dispersants, rheology modifiers, or organic alcohols and without any thermal posttreatment. The R
s values of the printed zones in
Figure 9a showed a gradually increased conductivity that followed the thickness of the printed zones. Similarly, multiple printing passes via ink-jet printing [
34] using the
f-rGO ink showed that the resulted printed item can be used for practical applications, e.g., sensors or microheaters [
34,
35,
36].