The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of deN2O and deNOx Reactors—Review
Abstract
:1. Introduction
2. Technologies for Purifying the Tail Gases from N2O and NOx
- Variant II—selective catalytic reduction of NOx with ammonia (reactions 12 and 13) and selective catalytic reduction of N2O with hydrocarbons (reaction 14, e.g., methane) in the tail gases stream, most commonly used in the plants with the tail gases temperature <425 °C (guaranteed operating temperature range 340–520 °C) [27,53,54]:
- H—height of the catalyst bed, m
- ΔP—pressure drop of a gas flow through the catalyst bed, Pa
- V—linear gas flow velocity, m/s
- x—hydraulic diameter of the shaped catalyst body, m
- ε—bed porosity,
- ρf—density of the gas flowing through the catalyst bed, kg/m3,
- μ—gas viscosity, Pa·s.
- Vi—volume of the shaped catalyst body, m3
- Si—surface of the shaped catalyst body, m2.
3. Various Aspects of Designing Reactors for Purifying the Tail Gases from N2O and NOx
- The required reactor size (dependent on a gas flow rate and catalyst activity);
- The gas temperature at the reactor inlet, required for the effective catalyst operation (eventually, selection of a heat exchanger for the tail gases preheating before they enter the catalytic reactor);
- The allowable gas flow resistances across the catalyst bed;
- Permissible content of nitrogen oxides downstream of the deNOx/deN2O reactor;
- Method of the tail gases purifying from the nitrogen oxides (selective or non-selective catalytic reduction of N2O, direct catalytic N2O decomposition);
- The arrangement (sequence) of the catalyst beds in reactors for simultaneous purifying of the tail gases from N2O and NOx and a method of reducing agent introduction upstream of the second catalyst bed;
- The available free space in the technological line of HNO3 plant, in which the reactor could be installed (downstream of the absorption column).
- -
- Reducing agent injection (this zone can be located upstream of the reactor or inside it);
- -
- Reactor inlet with possible internal elements improving gas flow distribution over the catalyst bed or tail gases mixing with reducing agent;
- -
- Reaction zone with the catalyst bed;
- -
- Reactor outlet.
3.1. Axial Flow Reactor
3.2. Radial Flow Reactor
- It is necessary to ensure uniform gas distribution over the catalyst bed, along the entire length of the catalytic basket to achieve higher nitrogen oxide conversion (uneven gas distribution results in uneven exploitation of the catalyst bed and basket, which may lead to corrosion);
- Settling of the catalyst bed during operation may result in an empty space formation in the upper part of the bed and gas bypassing. In total, 5–20% excess of the catalyst bed should be used in relation to the amount required to achieve the assumed nitrogen oxides conversion, compensating for bed settlement. This amount depends on the mechanical properties of the catalyst and the number of plant shutdowns and start-ups;
- In a radial reactor, a sufficient space between the reactor and the basket side wall should be ensured to distribute the gas stream over the catalyst bed evenly;
- When two or more catalyst beds are used, the deNOx catalyst bed should be placed in the outer annular basket (inward flow of the gas stream). In a radial flow reactor with a co-axial arrangement of the annular catalytic baskets along the reactor diameter, it is not possible to introduce a reducing agent upstream of the second catalyst bed.
Radial Flow Reactor with Triple-Bed Catalytic System
3.3. Radial Flow Reactor with Deflectors
3.4. Radial–Axial Flow Reactors
3.5. Lateral Flow Reactor
3.6. Parallel-Flow Reactor with Deflectors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Symbols | |
| μ | viscosity of the gas flowing through the catalyst bed [Pa·s] |
| ε | bed voidage [m3] |
| density of the gas flowing through the catalyst bed [kg/m3] | |
| density of the shaped catalyst body [kg/m3] | |
| mw | mass of the shaped catalyst body [kg] |
| x | spherical equivalent particle diameter [m] |
| A | Blake–Kozeny–Carman coefficient |
| B | Plummer coefficient |
| H | height of the catalyst bed [m] |
| ∆P | pressure drop across the catalyst bed [Pa] |
| Si | surface of the catalyst body [m2] |
| V | linear gas flow velocity [m/s] |
| Vi | volume of the shaped catalyst body [m3] |
| Vtot | total volume of the catalyst bed [m3] |
References
- IPCC. The IPCC Finalized the Synthesis Report for the Sixth Assessment Report. In Proceedings of the Panel’s 58th Session, Interlaken, Switzerland, 13–19 March 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 24 April 2023).
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Yao, Y. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Prather, M.J.; Hsu, J.; DeLuca, N.M.; Jackman, C.H.; Oman, L.D.; Douglass, A.R.; Funke, B. Measuring and modeling the lifetime of nitrous oxide including its variability. J. Geophys. Res. Atmos. 2015, 120, 5693–5705. [Google Scholar] [CrossRef] [PubMed]
- Wiser, A. Investigation of the Industrial NH3 Oxidation by CFD Simulations Including Detailed Surface Kinetics. Technische Universität: Darmstadt, Germany, 2020. [Google Scholar] [CrossRef]
- Groves, M.; Sasonow, A. Uhde EnviNOx® Technology for NOx and N2O abatement—A contribution to reducing emissions from nitric acid plants. In Proceedings of the Fifth International Symposium on Non-CO2 Greenhouse Gases (NCGG-5), Wageningen, The Netherlands, 30 June–3 July 2009; Available online: https://ucpcdn.thyssenkrupp.com/_legacy/UCPthyssenkruppBAIS/assets.files/download_1/nitrates/uhde_publications_pdf_en_15000012.pdf (accessed on 24 April 2023).
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. Chapter 2. Environ. Sci. 2007, 39, 129–234. Available online: http://www.ipcc.ch/pdf/assessment-report/ar4/wg1/ar4-wg1-chapter2.pdf (accessed on 24 April 2023).
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Woodrow, P. Nitric oxide: Some nursing implications. Intensive Crit. Care Nurs. 1997, 13, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wark, K.; Warner, C.F. Air Pollution: Its Origin and Control, 3rd ed.; U.S. Department of Energy: Washington, DC, USA, 1997; ISBN 0-67399416-3. [Google Scholar]
- USP Technologies. Nitrogen Oxides (NOx) Abatement with Hydrogen Peroxide, Compiled in Part from FMC Technical Data, Pollution Control Release No. 119; Technical Bulletin of USP Technologies; USP Technologies: Atlanta, GA, USA, 2015; Available online: https://www.h2o2.com/industrial/applications.aspx?pid=101 (accessed on 24 April 2023).
- Juzsakova, T.; Al-Jammal, N.; Cretescu, I.; Sebestyén, V.; Le Phuoc, C.; Domokos, E.; Stan, C.D. Case studies for clean technology development in the chemical industry using zeolite based catalysts. Minerals 2018, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Council Decision (EU) 2017/1757 of 17 July 2017 on the Acceptance on Behalf of the European Union of an Amendment to the 1999 Protocol to the 1979 Convention on Long-Range Transboundary Air Pollution to Abate Acidification, Eutrophication and Ground-Level Ozone, Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017D1757&from=ET (accessed on 24 April 2023).
- Reference Document on Best Available Techniques for the Manufacture of Large Inorganic Chemicals—Ammonia, Acids and Fertilisers. 2007. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/lvic_aaf.pdf (accessed on 31 December 2022).
- Emisja Gazów Cieplarnianych. Wybrane Zagadnienia Dotyczące Emisji CO2 w Polsce. 2020, OT-683, Warszawa. Available online: https://www.senat.gov.pl/gfx/senat/pl/senatopracowania/192/plik/ot-683.pdf (accessed on 24 April 2023).
- Bańkowska, K. Global Climate Agreement and Rural Development. Wieś Rol. 2016, 170, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Raport z Rynku CO2. Available online: https://www.kobize.pl/pl/article/aktualnosci-2023/id/2257/raport-z-rynku-co2-styczen-2023 (accessed on 24 April 2023).
- Pérez-Ramırez, J.; Kapteijn, F.; Schöffel, K.; Moulijn, J.A. Formation and control of N2O in nitric acid production: Where do we stand today? Appl. Catal. B Environ. 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Schwefer, M.; Seifert, R.; Fuchs, J.; Ruthardt, K.; Groves, M. Thyssenkrupp Industrial Solutions AG. Method for Removing N2O and NOx from the Nitric Acid Production Process, and an Installation Suitable for Same. U.S. Patent US20170334722, 23 November 2017. Available online: https://scholar.google.com/scholar_lookup?title=Method+for+Removing+N2O+and+NOx+From+the+Nitric+Acid+Production+Process,+and+an+Installation+Suitable+for+Same&author=Schwefer,+M.&author=Seifert,+R.&author=Fuchs,+J.&author=Ruthardt,+K.&author=Groves,+M.&author=Thyssenkrupp+Industrial+Solutions+AG&publication_year=2017 (accessed on 31 December 2022).
- Hevia, M.A.; Pérez-Ramírez, J. Assessment of the low-temperature EnviNOx® variant for catalytic N2O abatement over steam-activated FeZSM-5. Appl. Catal. B Environ. 2008, 77, 248–254. [Google Scholar] [CrossRef]
- Slyusarenko, A. Clariant emission control catalysts. In Proceedings of the SCIF Congress, International Online Symposium, Virtual event, 23–24 June 2022. [Google Scholar]
- Konsolakis, M. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: Catalytic performance, mechanistic considerations, and surface chemistry aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Isupova, L.A.; Ivanova, Y.A. Removal of nitrous oxide in nitric acid production. Kinet. Catal. 2019, 60, 744–760. [Google Scholar] [CrossRef]
- Inger, M.; Wilk, M.; Saramok, M.; Grzybek, G.; Grodzka, A.; Stelmachowski, P.; Sojka, Z. Cobalt spinel catalyst for N2O abatement in the pilot plant operation–long-term activity and stability in tail gases. Ind. Eng. Chem. Res. 2014, 53, 10335–10342. [Google Scholar] [CrossRef]
- Schwefer, M. Catalyst for Decomposing N2O, Its Use and Method for the Production Thereof. U.S. Patent 6890499, 10 May 2005. Available online: https://scholar.google.com/scholar?hl=pl&as_sdt=0%2C5&q=Catalyst+for+decomposing+N2O%2C+its+use+and+method+for+the+production+thereof+&btnG= (accessed on 10 June 2023).
- Schwefer, M.; Siefert, R.; Fuchs, J.; Ruthardt, K.; Groves, M. Method for removing N2O and NOx from the nitric acid production process and an installation suitable for same, Clariant emission control catalysts. In Proceedings of the SCIF Congress, International Online Symposium, Virtual event, 23–24 June 2022. [Google Scholar]
- Perbandt, C.; Bacher, V.; Groves, M.; Schwefer, M.; Siefert, R.; Turek, T. Kinetics and Reactor Design for N2O Decomposition in the EnviNOx® Process. Chem. Ing. Tech. 2013, 85, 705–709. [Google Scholar] [CrossRef]
- Groves, M.C.E.; Sasonow, A. UhdeEnviNOx® technology for NOX and N2O abatement: A contribution to reducing emissions from nitric acid plants. J. Integr. Environ. Sci. 2010, 7 (Suppl. S1), 211–222. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Yang, K.H. The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts. Catalysts 2018, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Gramiccioni, G.A.; Tran, P.; Patchett, J.A.; Gegan, T.A. Catalytic Article Comprising a Coprecipitate of Vanadia, Tungsta, and Titania. U.S. Patent WO2017216690A1, 21 December 2017. Available online: https://patents.google.com/patent/WO2017216690A1/en?oq=WO+2017%2f216690+A1 (accessed on 10 June 2023).
- Spiegel, A.; Odematt, P. Emissions Reduction from Nitric Acid Plants; Nitrogen + Syngas: London, UK, 2012. [Google Scholar]
- Andersen, P.; Doura, K.; Johnson Matthey PLC. Method and Exhaust System for Treating NOx in Exhaust Gas from Stationary Emission Sources. U.S. Patent US20170341022, 30 November 2017. Available online: https://patents.google.com/patent/US20170341022A1/en?oq=US20170341022 (accessed on 10 June 2023).
- Schwefer, M.; Maurer, R.; Turek, T.; Kogel, M.; Uhde Gmbh. Method for the Removal of NOx and N2O from the Residual Gas in Nitric Acid Production. U.S. Patent PL205073B1, 31 March 2010. Available online: https://patents.google.com/patent/PL205073B1/en?oq=PL205073B1 (accessed on 10 June 2023).
- Kułażyński, M. Selective catalytic reduction NO by ammonia over ceramic and active carbon based catalysts. Heat Anal. Thermodyn. Eff. 2011, 351. [Google Scholar] [CrossRef] [Green Version]
- Maurer, S.; Wycisk, M.; Petry, J.; Deuerlein, S.; Zhang, W.; Shi, C.; Takashi, H.; BASF SE. Iron- and Copper-Containing Zeolite Beta from Organotemplate-Free Synthesis and Use Thereof in the Selective Catalytic Reduction of NOx. U.S. Patent US2013/0202524A1, 8 August 2013. Available online: https://patents.google.com/patent/US20130202524A1/en?oq=US2013%2f0202524A1 (accessed on 10 June 2023).
- Schwefer, M.; Siefert, R.; Pinnow, S.; Thyssenkrupp Industrial Solutions, AG. Device and Method for Eliminating NOx and N2O. U.S. Patent US20140363359A1, 11 December 2014. Available online: https://patents.google.com/patent/US20140363359A1/en?oq=US20140363359A1 (accessed on 10 June 2023).
- Chen, J.M.; Tran, P.H.; Durilla, M.; Mack, S.S.; BASF Catalysts LLC. Selective Catalytic Reduction of N2O. U.S. Patent US7438878B2, 21 November 2008. Available online: https://patents.google.com/patent/US7438878B2/en (accessed on 10 June 2023).
- Cheng, X.; Bi, X.T. A review of recent advances in selective catalytic NOx reduction reactor technologies. Particuology 2014, 16, 1–18. [Google Scholar] [CrossRef]
- Busca, G. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Hegedus, L.; Beeckman, J.W.; Pan, W.H.; Solar, J.P.; Grace & Co.-Conn. Catalysts for Selective Catalytic Reduction deNOx Technology. U.S. Patent US4929586, 29 May 1990. Available online: https://patents.google.com/patent/US4929586A/en (accessed on 10 June 2023).
- Li, W.; Liu, F.; Lian, Z.; Xie, L.; Shi, X. Tungsten Oxide Surface Modified Fe2O3 Catalyst as well as Preparation Method and Application Thereof. U.S. Patent CN103170346A, 26 June 2013. Available online: https://patents.google.com/patent/CN103170346A/en?oq=CN103170346A (accessed on 10 June 2023).
- Zhao, F.; Zhang, J.; Chen, L.; Galeano, C.; Huber, S.; Huennekes, E. Selective Catalytic Reduction (SCR) Catalyst Comprising a Composite Oxide Containing V and SB, Preparation Process Thereof, and Use Thereof for Nitrogen Oxides Removal. U.S. Patent WO2017101449A1, 22 June 2017. Available online: https://patents.google.com/patent/WO2017101449A1/RED_FLAGS_Oct.2007_.pdf (accessed on 10 June 2023).
- Elsener, M.; Kröcher, O.; Cristina, M.; Paul Scherrer Institute. Catalyst for a deNOx-Application and a Process for Selective Catalytic Reduction of Nitrogen Oxides. U.S. Patent EP2368628A1, 28 September 2011. Available online: https://patents.google.com/patent/EP2368628A1/de?oq=EP+2368628+ (accessed on 10 June 2023).
- Schwefer, M.; Groves, M.; Perbandt, C.; Siefert, R.; Thyssenkrupp Industrial Solutions, AG. Process and Apparatus for Eliminating NOx and N2O. U.S. Patent US20130149225A1, 13 June 2013. Available online: https://patents.google.com/patent/US20130149225A1/en?oq=US20130149225A1 (accessed on 10 June 2023).
- Bull, I.; Müller, U.; BASF, SE. Copper Containing ZSM-34, Off and/or Eri Zeolitic Material for Selective Reduction of NOx. U.S. Patent WO2012007874, 19 January 2012. Available online: https://patents.google.com/patent/WO2012007874A1/en?oq=WO2012007874 (accessed on 10 June 2023).
- Perbandt, C.; Tyssenkrupp Ind Solutions AG. Reducing the Emission of Nitrogen Oxide When Starting up Systems for Producing Nitric Acid. U.S. Patent WO2015185506, 10 December 2015. Available online: https://patents.google.com/patent/WO2015185506A1/en (accessed on 10 June 2023).
- García, N.M.; Marín, M.M.; Canós, A.C.; Thøgersen, J.R.; Vennestrøm, P.N.R.; HALDOR TOPSØE A/S. Hydrothermally Stable Iron Containing AEI Zeolite SCR Catalyst. U.S. Patent WO2017134001A1, 10 August 2017. Available online: https://patents.google.com/patent/WO2017134001A1/en (accessed on 10 June 2023).
- Derrien, J.Y.; Seigneurin, L.; Rhone Poulenc Industries SA. Catalyst for Removing NOx from Gas Streams. U.S. Patent US4314913, 9 February 1982. Available online: https://patents.google.com/patent/US4314913A/en?oq=US4314913 (accessed on 10 June 2023).
- Usberti, N.; Jablonska, M.; Blasi, M.D.; Forzatti, P.; Lietti, L.; Beretta, A. Design of a “high-efficiency” NH3-SCR reactor for stationary applications. A kinetic study of NH3 oxidation and NH3-SCR over V-based catalysts. Appl. Catal. B Environ. 2015, 179, 185–195. [Google Scholar] [CrossRef]
- Schwefer, M.; Seifert, R.; Fuchs, J.; Ruthardt, K.; Groves, M.; Thyssenkrupp Uhde Gmbh. Method for Removing N2O and NOx from the Nitric Acid Production Process, and an Installation Suitable for Same. U.S. Patent WO2012113516A1, 30 August 2012. Available online: https://patents.google.com/patent/WO2012113516A1/en?oq=WO2012113516A1 (accessed on 31 December 2022).
- Schwefer, M.; Maurer, R.; Turek, T.; Kogel, M.; ThyssenKrupp Industrial Solutions, AG. Method for the Removal of NOx and N2O from the Residual Gas in Nitric Acid Production. U.S. Patent US20030143142A1, 31 July 2003. Available online: https://patents.google.com/patent/US20030143142A1/en?oq=US20030143142A1 (accessed on 10 June 2023).
- Andersen, P.J.; Casci, J.L.; Chen, H.Y.; Fedeyko, J.M.; Johnson Matthey PLC. Disordered Molecular Sieve Supports for the Selective Catalytic Reduction of NOx. U.S. Patent US20120184429, 19 July 2012. Available online: https://patents.google.com/patent/US20120184429A1/en?oq=US+20120184429 (accessed on 10 June 2023).
- Ariga, K.; Aoyama, H.; Ito., Y.; Tosoh Corp. Chabazite-Type Zeolite and Method for Producing Same, Copper Loaded Low-Silica Zeolite and NOx Reductive Removal Catalyst Containing the Zeolite, and Method of NOx Reductive Removal Using This Catalyst. U.S. Patent US 20130280160, 24 October 2013. Available online: https://patents.google.com/patent/US20130280160A1/en (accessed on 10 June 2023).
- EnviNOx®—Reduce NOx Emissions to a Minimum. Available online: https://www.thyssenkrupp-uhde.com/en/products-and-technologies/fertilizer-technologies/nitrate-plants/nox-reduction-with-envinox (accessed on 24 April 2023).
- Groves, M.; Frank, C. EnviNOx®: Process for N2O and NOx Abatementin Nitric Acid Plants. In Proceedings of the Nitrogen + Syngas Conference 2009, Rome, Italy, 22–25 February 2009; Available online: https://scholar.google.com/scholar?hl=pl&as_sdt=0%2C5&q=M.Groves%2C+C.+Frank%2C+Nitrogen%2BSyngas+177-178+%282009%29+EnviNOx%3A+process+for+N2O+and+NOx+abatement+In+Nitric+Acid+Plants&btnG= (accessed on 10 June 2023).
- Kliev, S. EnviNOx Technology for N2O and NOx Reduction. In Proceedings of the SCIF Congress, International Online Symposium, Virtual event, 23–24 June 2021. [Google Scholar]
- Granger, J.F.; Casale, S.A. Method and Apparatus for Removing NOx and N2O from a Gas. U.S. Patent US11325069B2, 10 May 2022. Available online: https://patents.google.com/patent/US11325069B2/en (accessed on 10 June 2023).
- Paramadayalan, T.; Pant, A. Selective catalytic reduction converter design: The effect of ammonia nonuniformity at inlet. Korean J. Chem. Eng. 2013, 30, 2170–2177. [Google Scholar] [CrossRef]
- Nahavandi, M. Selective Catalytic Reduction (SCR) of NO by ammonia over V2O5/TiO2 catalyst in a catalytic filter medium and honeycomb reactor: A kinetic modeling study. Braz. J. Chem. Eng. 2015, 32, 875–893. [Google Scholar] [CrossRef] [Green Version]
- Jodłowski, P.J.; Kryca, J.; Rogulska, A.; Gil, B.; Iwaniszyn, M.; Łojewska, J.; Kołodziej, A. Advantages of a wire gauze structured reactor with a zeolite (Cu-USY) catalyst for NH3-SCR of NOx. Chem. Eng. J. 2013, 214, 319–326. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Tang, X.; Chen, C.; Yi, H. Stainless steel catalyst for air pollution control: Structure, properties, and activity. Environ. Sci. Pollut. Res. 2022, 29, 55367–55399. Available online: https://link.springer.com/content/pdf/10.1007/s11356-022-21079-z.pdf?pdf=button (accessed on 10 June 2023). [CrossRef] [PubMed]
- Ward, A.M.; Chemical Industries PLC. Catalytic Reactor. U.S. Patent WO2001023080A1, 5 April 2001. Available online: https://patents.google.com/patent/WO2001023080A1/en (accessed on 10 June 2023).
- Tarozzo, M.; Filippi, E.; Rizzi, E.; Casale SA. Wall System for Catalytic Beds of Synthesis Reactors and Relative Manufacturing Process. U.S. Patent EP2014356A1, 14 January 2009. Available online: https://patents.google.com/patent/EP2014356A1/en (accessed on 10 June 2023).
- Chen, C.T.; Tan, W.L. Mathematical modeling, optimal design and control of an SCR reactor for NOx removal. J. Taiwan Inst. Chem. Eng. 2012, 43, 409–419. [Google Scholar] [CrossRef]
- Mayerhofer, M.; Govaerts, J.; Parmentier, N.; Jeanmart, H.; Helsen, L. Experimental investigation of pressure drop in packed beds of irregular shaped wood particles. Powder Technol. 2011, 205, 30–35. [Google Scholar] [CrossRef]
- Stolo, A.D.; Polcari, D.S.; Tomida, M. Flow Analysis in a Radial Flow Fixed Bed Reactor. 2014. Available online: https://web.wpi.edu/Pubs/E-project/Available/E-project-121614-125138/unrestricted/Flow_Analysis_MQP.pdf (accessed on 31 December 2022).
- Gry, P. Program to reduce NOx emissions of HNO3 plants with selective catalytic reduction. In Proceedings of the International Conference Industrial Atmospheric Pollution. NOx and N2O Emission Control: Panel of Available Techniques, Paris, France, 21–22 March 2001; Available online: https://scholar.google.com/scholar?hl=pl&as_sdt=0%2C5&q=Program+to+reduce+NOx+emissions+of+HNO3+plants+with+selective+catalytic+reduction&btnG= (accessed on 10 June 2023).
- Braun, H.; Hoeren, A.; Schneiders, T.; Vortmeyer, K.; Pfost, H. Measurement of the mixing quality in premix combustors. Energy Convers. Manag. 1998, 39, 1991–1999. [Google Scholar] [CrossRef]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Peerhossaini, H. Static mixers: Mechanisms, applications, and characterization methods—A review. Chem. Eng. Res. Des. 2014, 92, 205–228. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Wang, J.; Yuan, J. Application of CFD in the optimal design of a SCR–DeNOx system for a 300 MW coal-fired power plant. Comput. Chem. Eng. 2013, 49, 50–60. [Google Scholar] [CrossRef]
- Liu, H.; Guo, T.; Yang, Y.; Lu, G. Optimization and numerical simulation of the flow characteristics in SCR system. Energy Procedia 2012, 17, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Ogidiama, O.V.; Shamim, T. Investigation of dual Layered SCR systems for NOx control. Energy Procedia 2015, 75, 2345–2350. [Google Scholar] [CrossRef] [Green Version]
- Healy, E.C.; Maxwell, J.D.; Hinton, W.S. Innovative Clean Coal Technology (ICCT): Demonstration of Selective Catalytic Reduction (SCR) Technology for the Control of Nitrogen Oxide (NOx) Emission from High-Sulfur, Coal-Fired Boilers-Economic Evaluation of Commercial-Scale SCR Applications for Utility Boilers. No. DOE/PC/89652--T20. Southern Co. Services. 1996. Available online: https://scholar.google.com/scholar?hl=pl&as_sdt=0%2C5&scioq=doi%3A10.1205%2F026387603322302968+&q=EC+Healy%2C+JD+Maxwell%2C+WS+Hinton+-+1996++PDF+Innovative+clean+coal+technology+%28ICCT%29%3A+demonstration+of+selective+catalytic+reduction+%28SCR%29+technology+for+the+control+of+nitrogen+oxide+%28NOx%29+emission+from+high-sulfur%2C+coal-fired+boilers+-+economic+evaluation+of+commercial-scale+SCR+applications+&btnG= (accessed on 10 June 2023).
- Implementation of NOx Emission Reduction in Nitric Acid Plant at NFL, Nangal, PC168/E-1/P-II/Sec.1.0. Available online: https://nationalfertilizers.com/NFL/admin_tender/upload/20181006101050_8_Annex%201.2_SOP%20(%20Revised%20).pdf (accessed on 24 April 2023).
- Lei, Z.; Wen, C.; Chen, B. Optimization of internals for selective catalytic reduction (SCR) for NO removal. Environ. Sci. Technol. 2011, 45, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Structural Design Guideline for Selective Catalytic Reduction Reactor Structures, Publication ICAC-SCR-2. 2002. Available online: https://cdn.ymaws.com/www.icac.com/resource/resmgr/SCR-2.pdf (accessed on 24 April 2023).
- Static Mixer. Available online: https://www.jls-europe.de/products/static-mixer/static-mixer.html?gclid=EAIaIQobChMIooW34ZrR_QIVUgCiAx3e8QRyEAAYASAAEgIsCvD_BwE (accessed on 24 April 2023).
- Towler, G.; Sinnott, R. Design of reactors and mixers. In Chemical Engineering Design; Elsevier: Amsterdam, The Netherlands, 2013; pp. 631–751. [Google Scholar] [CrossRef]
- Thakur, R.K.; Vial, C.; Nigam, K.D.P.; Nauman, E.B.; Djelveh, G. Static mixers in the process industries—A review. Chem. Eng. Res. Des. 2003, 81, 787–826. [Google Scholar] [CrossRef]
- Ghanem, A.; Lemenand, T.; Della Valle, D.; Habchi, C.; Peerhossaini, H. Vortically enhanced heat transfer and mixing: State of the art and recent results. In Heat Transfer Summer Conference; American Society of Mechanical Engineers: New York, NY, USA, 2012; Volume 44786, pp. 21–30. [Google Scholar] [CrossRef]
- Minne, P.; Honecker, H. Reactor for the Catalytic Treatment of a Gas Stream. U.S. Patent WO2021170756A1, 2 September 2021. Available online: https://patents.google.com/patent/WO2021170756A1/en?oq=Pascal+MINNE%2c+Horst+HONECKER+Reactor+for+the+catalytic+treatment+of+a+gas+stream%2c+Thyssenkrupp+Industrial+Solutions+Ag%2c+Thyssenkrupp+Ag++2021+pat+WO2021170756A1 (accessed on 31 December 2022).
- Blanco, J.; Avila, P.; Suárez, S.; Yates, M.; Martin, J.A.; Marzo, L.; Knapp, C. CuO/NiO monolithic catalysts for NOx removal from nitric acid plant flue gas. Chem. Eng. J. 2004, 97, 1–9. [Google Scholar] [CrossRef]
- BASF Catalyst O4-85 Honeycomb Catalysts for DeNOx by SCR. Available online: https://catalysts.basf.com/files/literature-library-a4/BASF_O4-85_Datasheet_Rev.-2019-05_A4.pdf (accessed on 31 December 2022).
- Zardi, U. System and Device to Make Catalytic Basket Walls for Heterogeneous Synthesis Reactors. U.S. Patent US4883646, 28 November 1989. Available online: https://patents.google.com/patent/US4883646A/en (accessed on 10 June 2023).
- Ranade, V.V. 13 Fixed bed and other types of reactors. In Computational Flow Modeling for Chemical Reactor Engineering; Elsevier: Amsterdam, The Netherlands, 2002; pp. 403–423. [Google Scholar] [CrossRef]
- Stabel, U.; Birnbaum, E.; Rizk, F.; Schlichthärle, G. Reactor for Heterogeneous Catalytic Reactions and Method Using Said Reactor. U.S. Patent WO2003002245A1, 9 January 2003. Available online: https://patents.google.com/patent/WO2003002245A1/de?oq=WO+2003002245+ (accessed on 10 June 2023).
- Celik, C.E.; Ackley, M.W.; Praxair Technology Inc. Radial Bed Vessels Having Uniform Flow Distribution. U.S. Patent US8313561, 20 November 2012. Available online: https://patents.google.com/patent/US8313561B2/en (accessed on 10 June 2023).
- Bolz, C.R.; Graziani, K.R.; Harandi, M.N.; Ozawa, Y.; Sorensen, C.M., Jr.; Mobil Oil Corporation. Multiple Catalyst Bed Radial Flow Reactor. U.S. Patent WO1999020384A1, 29 April 1999. Available online: https://patents.google.com/patent/WO1999020384A1/en?oq=WO1999020384A1+ (accessed on 10 June 2023).
- Rizzi, E.; Casale SA. Axial-Radial Flow Catalytic Chemical Reactor with Two Layers of Catalyst. U.S. Patent US10596538, 24 March 2020. Available online: https://patents.google.com/patent/US10596538B2/en?oq=US10596538 (accessed on 10 June 2023).
- Reactor and Pressure Vessel Internals. Available online: https://johnsonscreens.com/products/reactor-and-pressure-vessel-internals/ (accessed on 24 April 2023).
- Filippi, E.; Pizzolitto, C. The past and the future of catalysis and technology in industry: A perspective from Casale SA point of view. Catal. Today 2022, 387, 9–11. [Google Scholar] [CrossRef]
- Platvoet, E.M.J.; Hopkins, S.M. Radial Flow Gas Phase Reactor and Method for Reducing the Nitrogen Oxide Content of a Gas. U.S. Patent US6663839B2, 16 December 2003. Available online: https://patents.google.com/patent/US6663839B2/en?oq=US6663839B2 (accessed on 10 June 2023).
- Schwefer, M.; Groves, M.; Seifert, R.; Maurer, R.; ThyssenKrupp Industrial Solutions, AG. Method and Device for Reducing the NOx and N2O of Gases. U.S. Patent EP1515791B1, 3 August 2011. Available online: https://patents.google.com/patent/EP1515791B1/en?oq=EP+1515791+B1 (accessed on 10 June 2023).
- Travis, A.S. Luigi Casale’s enterprise: Pioneer of global catalytic high-pressure industrial chemistry. Catal. Today 2022, 387, 4–8. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Structured Catalysts and Reactors; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Maaskant, O.L. The Shell Denox System for nitric acid plant. In Proceedings of the Nitrogen 2005 International Conference and Exhibition, Bucharest, Romania, 7 February–2 March 2005. [Google Scholar]
- Van der Grift, C.J.G.; Woldhuis, A.F.; Maaskant, O.L. The shell DENOX system for low temperature NOx removal. Catal. Today 1996, 27, 23–27. [Google Scholar] [CrossRef]
- Vandewalle, L.A.; De, S.; Van Geem, K.M.; Marin, G.B.; Bos, R. Reactor Engineering Aspects of the Lateral Flow Reactor. Ind. Eng. Chem. Res. 2020, 59, 11157–11169. [Google Scholar] [CrossRef]
- Platvoet, E.M.J. Parallel Flow Gas Phase Reactor and Method for Reducing the Nitrogen Oxide Content of a Gas. U.S. Patent US6821490, 23 November 2004. Available online: https://patents.google.com/patent/US6821490B2/en (accessed on 10 June 2023).


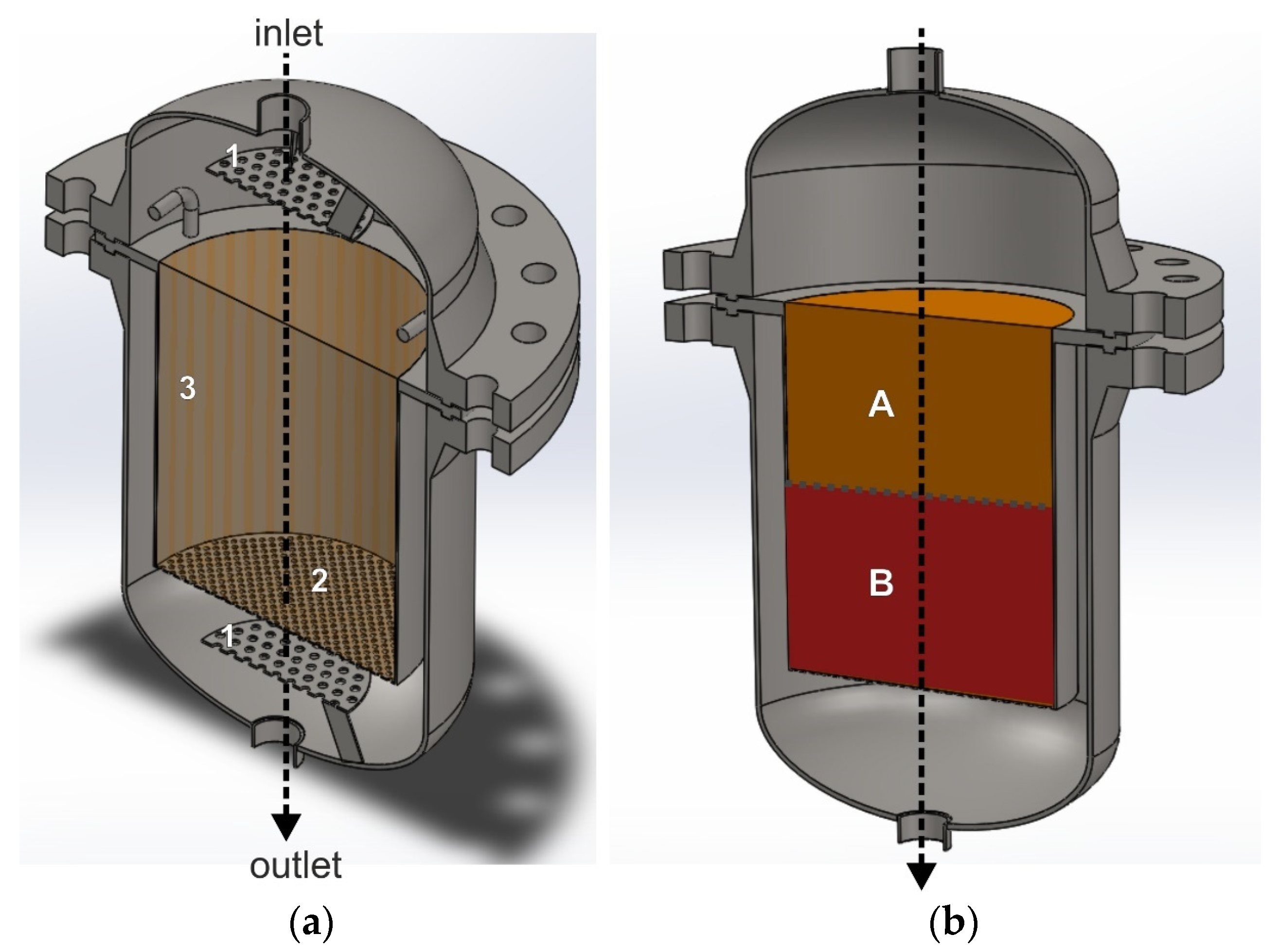

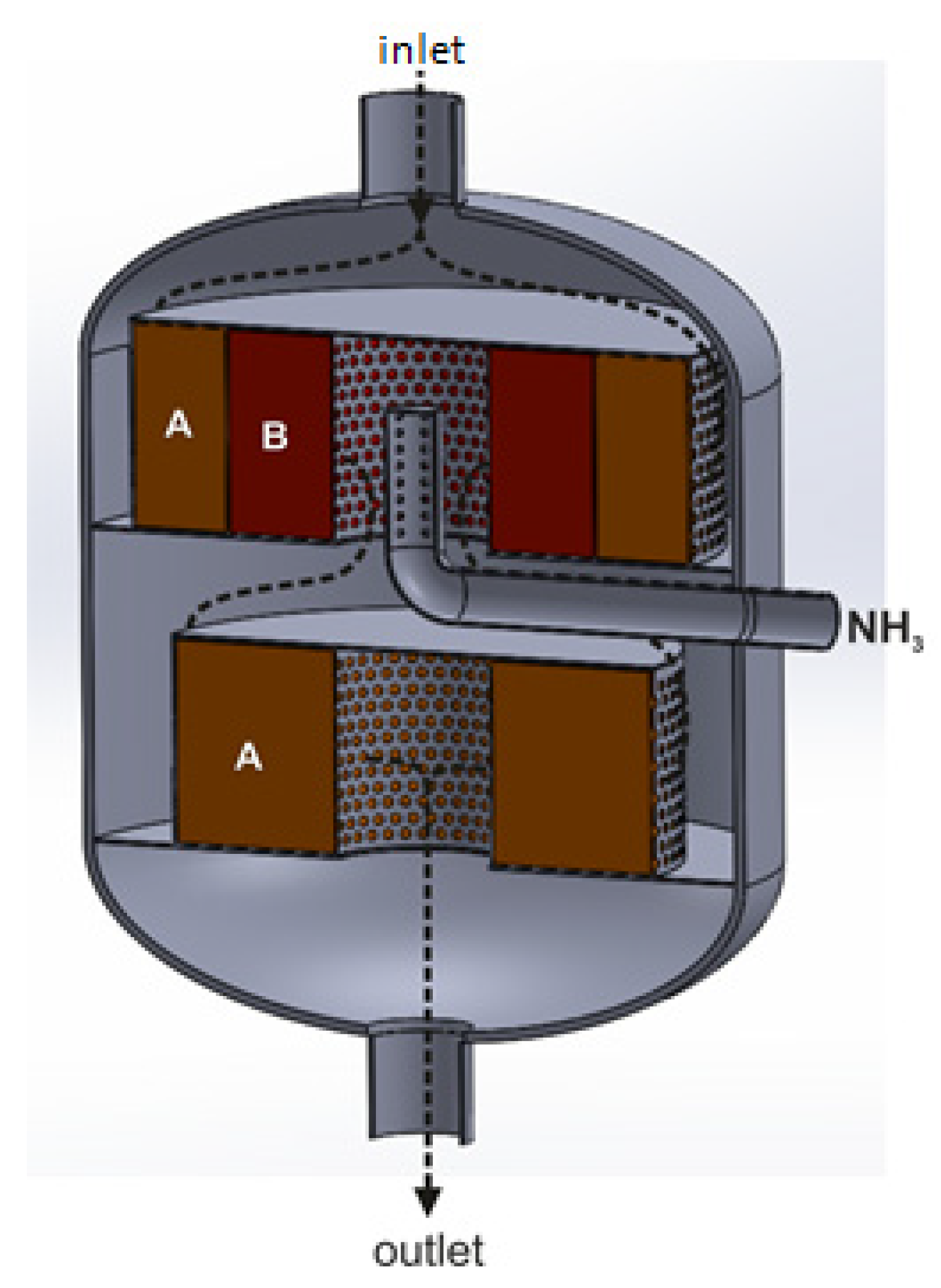






| The Direction of a Gas Flow through the Catalyst Bed | Advantages | Disadvantages |
|---|---|---|
| axial |
|
|
| radial |
|
|
| lateral |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capała, P.; Ruszak, M.; Rudawska, A.; Inger, M.; Wilk, M. The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of deN2O and deNOx Reactors—Review. Appl. Sci. 2023, 13, 7492. https://doi.org/10.3390/app13137492
Capała P, Ruszak M, Rudawska A, Inger M, Wilk M. The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of deN2O and deNOx Reactors—Review. Applied Sciences. 2023; 13(13):7492. https://doi.org/10.3390/app13137492
Chicago/Turabian StyleCapała, Paweł, Monika Ruszak, Anna Rudawska, Marek Inger, and Marcin Wilk. 2023. "The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of deN2O and deNOx Reactors—Review" Applied Sciences 13, no. 13: 7492. https://doi.org/10.3390/app13137492
APA StyleCapała, P., Ruszak, M., Rudawska, A., Inger, M., & Wilk, M. (2023). The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of deN2O and deNOx Reactors—Review. Applied Sciences, 13(13), 7492. https://doi.org/10.3390/app13137492







