Comparison of Electrochemically Deposited Bi and Sn Catalysts onto Gas Diffusion Electrodes for the Electrochemical CO2 Reduction Reaction to Formate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Bi PP Electrodes Based on In-House GDLs
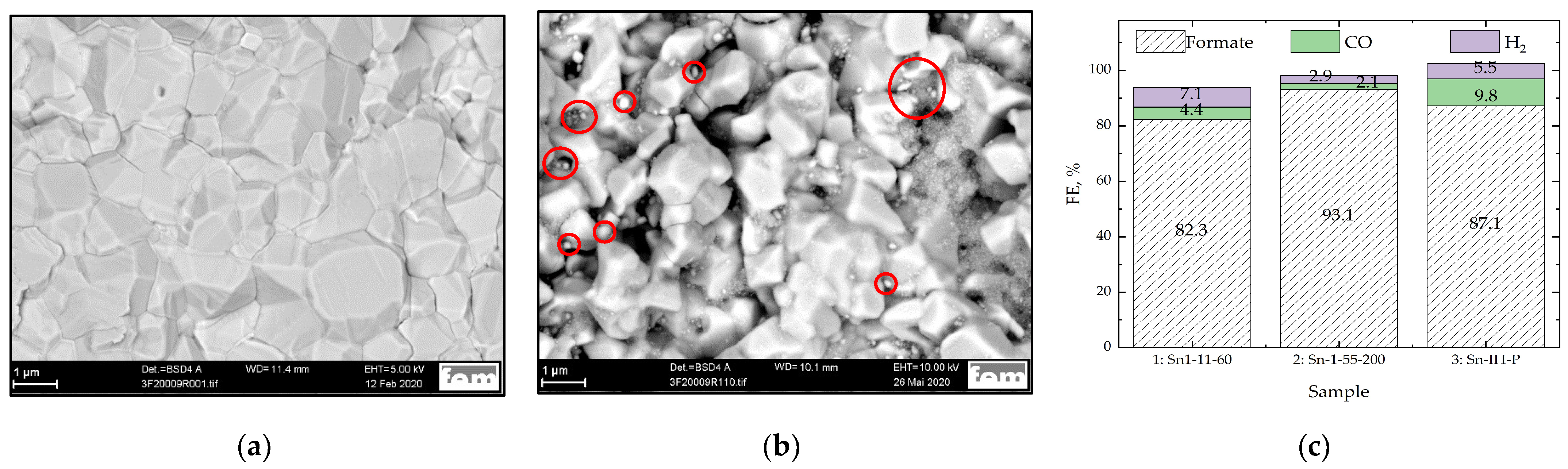
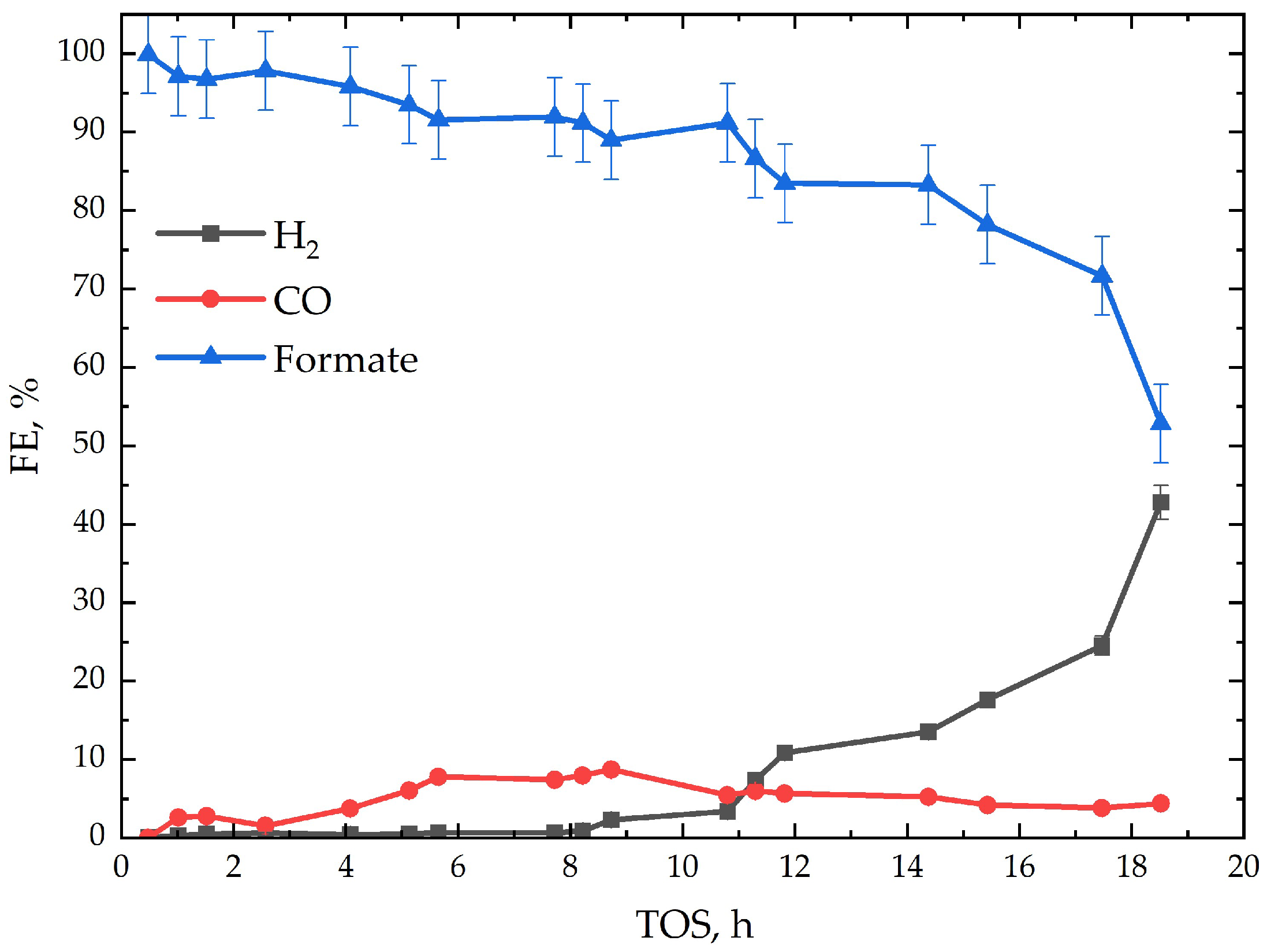
3.2. Bi PP Electrodes Based on 29 BC GDLs
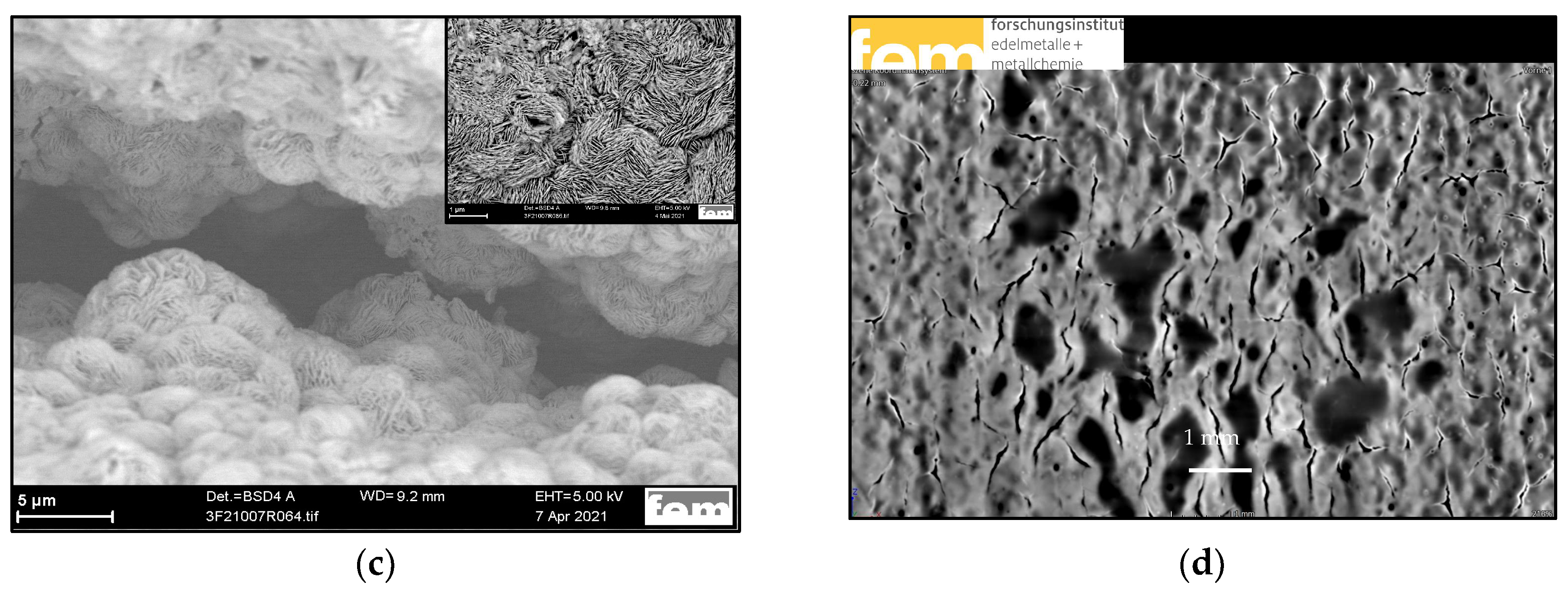
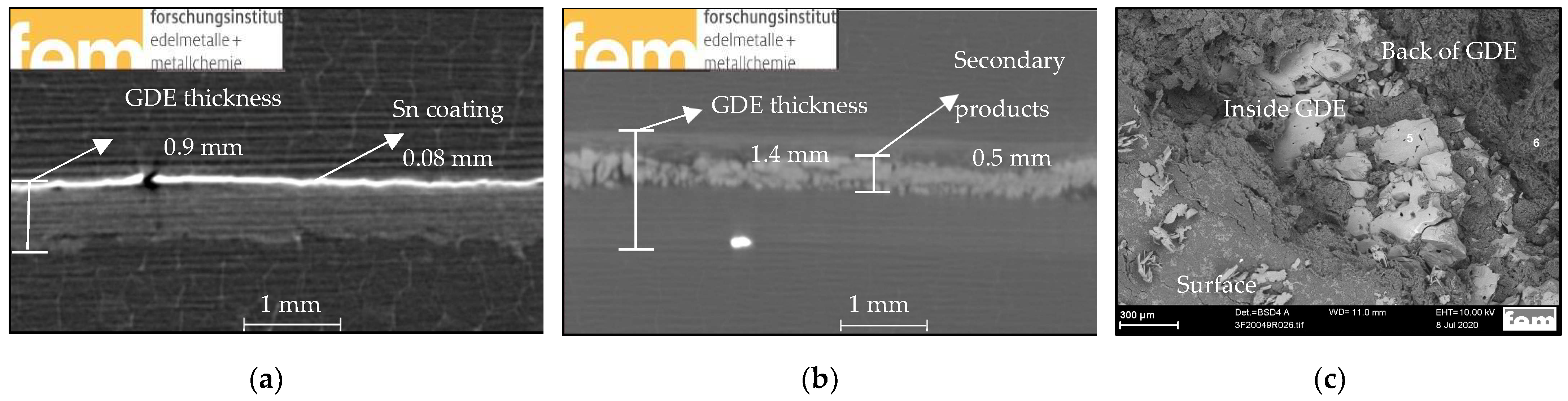
3.3. Sn PP Electrodes Based on In-House GDLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Commercial 29 BC GDL |
| IH | In-house prepared GDL |
| PP | Pulse plating |
| ED | Electrodeposition |
| P | Precipitation |
| NS | Nanostructure |
| NP | Nanoparticles |
| For GDEs: | |
| Bi-BC-PP | Bi deposited on a commercial GDL via pulse plating |
| Bi-IH-PP | Bi deposited on an in-house fabricated GDL via pulse plating |
| Bi-IH-P | Bi deposited on an in-house fabricated GDL via precipitation |
| Sn-BC-PP | Sn deposited on a commercial GDL via pulse plating |
| Sn-IH-PP | Sn deposited on an in-house fabricated GDL via pulse plating |
| Sn-IH-P | Sn deposited on an in-house fabricated GDL via precipitation |
References
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes? Science 2019, 364, eaav3506. [Google Scholar] [CrossRef] [Green Version]
- Abanades, J.C.; Rubin, E.S.; Mazzotti, M.; Herzog, H.J. On the Climate Change Mitigation Potential of CO2 Conversion to Fuels. Energy Environ. Sci. 2017, 10, 2491–2499. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Fernández-Caso, K.; Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Electroreduction of CO2: Advances in the Continuous Production of Formic Acid and Formate. ACS Energy Lett. 2023, 8, 1992–2024. [Google Scholar] [CrossRef]
- Aldaco, R.; Butnar, I.; Margallo, M.; Laso, J.; Rumayor, M.; Dominguez-Ramos, A.; Irabien, A.; Dodds, P.E. Bringing Value to the Chemical Industry from Capture, Storage and Use of CO2: A Dynamic LCA of Formic Acid Production. Sci. Total Environ. 2019, 663, 738–753. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Vo, D.V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Jhong, H.R.M.; Ma, S.; Kenis, P.J. Electrochemical Conversion of CO2 to Useful Chemicals: Current Status, Remaining Challenges, and Future Opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational Catalyst and Electrolyte Design for CO2 Electroreduction towards Multicarbon Products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Perez, P.; Irabien, A. A Techno-Economic Evaluation Approach to the Electrochemical Reduction of CO2 for Formic Acid Manufacture. J. CO2 Util. 2019, 34, 490–499. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Formic Acid Manufacture: Carbon Dioxide Utilization Alternatives. Appl. Sci. 2018, 8, 914. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Sridhar, N. The Electrochemical Reduction of Carbon Dioxide to Formate/Formic Acid: Engineering and Economic Feasibility. ChemSusChem 2011, 4, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Choi, I.; Ahn, S.H.; Hwang, S.J.; Yoo, S.J.; Han, J.; Kim, J.; Park, H.; Jang, J.H.; Kim, S.-K. Analysis on the Effect of Operating Conditions on Electrochemical Conversion of Carbon Dioxide to Formic Acid. Int. J. Hydrogen Energy 2014, 39, 16506–16512. [Google Scholar] [CrossRef]
- Yang, Z.; Oropeza, F.E.; Zhang, K.H.L. P-Block Metal-Based (Sn, In, Bi, Pb) Electrocatalysts for Selective Reduction of CO2 to Formate. APL Mater. 2020, 8, 060901. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.; Li, Y.; Li, Y. Ultrathin Bismuth Nanosheets from in Situ Topotactic Transformation for Selective Electrocatalytic CO2 Reduction to Formate. Nat. Commun. 2018, 9, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, P.; Xu, W.; Qiu, Y.; Zhang, T.; Li, X.; Zhang, H. Ultrathin Bismuth Nanosheets as a Highly Efficient CO2 Reduction Electrocatalyst. ChemSusChem 2018, 11, 848–853. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, J.; Dai, C.; Dong, W.; Tao, C. Bismuth Nano-Flowers as a Highly Selective Catalyst for Electrochemical Reduction of CO2 to Formate. J. Electrochem. Soc. 2018, 165, H594–H600. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Sun, B.; Liu, J.; Xia, Y.; Li, W.; Jiang, C.; He, D.; Xiao, X. In-Situ Structural Evolution of Bi2O3 Nanoparticle Catalysts for CO2 Electroreduction. Int. J. Extrem. Manuf. 2022, 4, 035002. [Google Scholar] [CrossRef]
- Zelocualtecatl Montiel, I.; Dutta, A.; Kiran, K.; Rieder, A.; Iarchuk, A.; Vesztergom, S.; Mirolo, M.; Martens, I.; Drnec, J.; Broekmann, P. CO2 Conversion at High Current Densities: Stabilization of Bi(III) Containing Electrocatalysts under CO2 Gas Flow Conditions; American Chemical Society: Washington, DC, USA, 2022. [Google Scholar]
- Xia, D.; Yu, H.; Xie, H.; Huang, P.; Menzel, R.; Titirici, M.M.; Chai, G. Recent Progress of Bi-Based Electrocatalysts for Electrocatalytic CO2 Reduction. Nanoscale 2022, 14, 7957–7973. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Dimeglio, J.L.; Rosenthal, J. Efficient Reduction of CO2 to CO with High Current Density Using in Situ or Ex Situ Prepared Bi-Based Materials. J. Am. Chem. Soc. 2014, 136, 8361–8367. [Google Scholar] [CrossRef] [Green Version]
- Burdyny, T.; Smith, W.A. CO2 Reduction on Gas-Diffusion Electrodes and Why Catalytic Performance Must Be Assessed at Commercially-Relevant Conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Dinh, C.T. Gas Diffusion Electrode Design for Electrochemical Carbon Dioxide Reduction. Chem. Soc. Rev. 2020, 49, 7488–7504. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Kas, R.; Smith, W.A.; Burdyny, T. Role of the Carbon-Based Gas Diffusion Layer on Flooding in a Gas Diffusion Electrode Cell for Electrochemical CO2 Reduction. ACS Energy Lett. 2021, 6, 33–40. [Google Scholar] [CrossRef]
- Junge Puring, K.; Siegmund, D.; Timm, J.; Möllenbruck, F.; Schemme, S.; Marschall, R.; Apfel, U.P. Electrochemical CO2 Reduction: Tailoring Catalyst Layers in Gas Diffusion Electrodes. Adv. Sustain. Syst. 2021, 5, 2000088. [Google Scholar] [CrossRef]
- De Gregorio, G.L.; Burdyny, T.; Loiudice, A.; Iyengar, P.; Smith, W.A.; Buonsanti, R. Facet-Dependent Selectivity of Cu Catalysts in Electrochemical CO2 Reduction at Commercially Viable Current Densities. ACS Catal. 2020, 10, 4854–4862. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Brown, S.M.; Leonard, M.; Brushett, F.R. Electroreduction of Carbon Dioxide to Formate at High Current Densities Using Tin and Tin Oxide Gas Diffusion Electrodes. J. Appl. Electrochem. 2019, 49, 917–928. [Google Scholar] [CrossRef]
- Li, M.; Idros, M.N.; Wu, Y.; Garg, S.; Gao, S.; Lin, R.; Rabiee, H.; Li, Z.; Ge, L.; Rufford, T.E.; et al. Unveiling the Effects of Dimensionality of Tin Oxide-Derived Catalysts on CO2 Reduction by Using Gas-Diffusion Electrodes. React. Chem. Eng. 2021, 6, 345–352. [Google Scholar] [CrossRef]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullón, J.; Sáez, A.; Montiel, V.; Irabien, A. Sn Nanoparticles on Gas Diffusion Electrodes: Synthesis, Characterization and Use for Continuous CO2 Electroreduction to Formate. J. CO2 Util. 2017, 18, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Löwe, A.; Rieg, C.; Hierlemann, T.; Salas, N.; Kopljar, D.; Wagner, N.; Klemm, E. Influence of Temperature on the Performance of Gas Diffusion Electrodes in the CO2 Reduction Reaction. ChemElectroChem 2019, 6, 4497–4506. [Google Scholar] [CrossRef] [Green Version]
- Manolova, M.; Freudenberger, R.; Hildebrand, J.; Klemm, E.; Bienen, F.; Kopljar, D.; Wagner, N. Sn Electrodeposition on Gas Diffusion Electrodes for the Electrochemical CO2 Reduction. In Proceedings of the 14th European SOFC & SOE Forum 2020, Lucerne, Switzerland, 20–23 October 2020. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Bi, J.; Zhu, Q.; Han, B. Design and Preparation of Electrocatalysts by Electrodeposition for CO2 Reduction. Chem. —Eur. J. 2022, 28, e202200242. [Google Scholar] [CrossRef]
- Puippe, J.-C.; Leaman, F.; American Electroplaters and Surface Finishers Society. Theory and Practice of Pulse Plating; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1986; ISBN 978-0-936569-02-4. [Google Scholar]
- Shah, S.S.A.; Sufyan Javed, M.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.-J. Metal Oxides for the Electrocatalytic Reduction of Carbon Dioxide: Mechanism of Active Sites, Composites, Interface and Defect Engineering Strategies. Coord. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Bienen, F.; Löwe, A.; Hildebrand, J.; Hertle, S.; Schonvogel, D.; Kopljar, D.; Wagner, N.; Klemm, E.; Andreas Friedrich, K. Degradation Study on Tin- and Bismuth-Based Gas-Diffusion Electrodes during Electrochemical CO2 Reduction in Highly Alkaline Media. J. Energy Chem. 2021, 62, 367–376. [Google Scholar] [CrossRef]
- Bienen, F.; Hildebrand, J.; Kopljar, D.; Wagner, N.; Klemm, E.; Friedrich, K.A. Importance of Time-Dependent Wetting Behavior of Gas-Diffusion Electrodes for Reactivity Determination. Chem. Ing. Tech. 2021, 93, 1015–1019. [Google Scholar] [CrossRef]
- Löwe, A.; Schmidt, M.; Bienen, F.; Kopljar, D.; Wagner, N.; Klemm, E. Optimizing Reaction Conditions and Gas Diffusion Electrodes Applied in the CO2 Reduction Reaction to Formate to Reach Current Densities up to 1.8 A Cm–2. ACS Sustain. Chem. Eng. 2021, 9, 4213–4223. [Google Scholar] [CrossRef]
- Kopljar, D.; Wagner, N.; Klemm, E. Transferring Electrochemical CO2 Reduction from Semi-Batch into Continuous Operation Mode Using Gas Diffusion Electrodes. Chem. Eng. Technol. 2016, 39, 2042–2050. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Quintanilla, S.; Irabien, A. Conversion of Carbon Dioxide into Formate Using a Continuous Electrochemical Reduction Process in a Lead Cathode. Chem. Eng. J. 2012, 207–208, 278–284. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Del Castillo, A.; Irabien, A. Continuous Electrochemical Reduction of Carbon Dioxide into Formate Using a Tin Cathode: Comparison with Lead Cathode. Chem. Eng. Res. Des. 2014, 92, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. CO2 Electroreduction to Formate: Continuous Single-Pass Operation in a Filter-Press Reactor at High Current Densities Using Bi Gas Diffusion Electrodes. J. CO2 Util. 2019, 34, 12–19. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Zhao, P.; Xue, X.; Chen, R.; Yang, S.; Ma, J.; Liu, J.; et al. Liquid-Phase Exfoliated Ultrathin Bi Nanosheets: Uncovering the Origins of Enhanced Electrocatalytic CO2 Reduction on Two-Dimensional Metal Nanostructure. Nano Energy 2018, 53, 808–816. [Google Scholar] [CrossRef]
- Lu, P.; Gao, D.; He, H.; Wang, Q.; Liu, Z.; Dipazir, S.; Yuan, M.; Zu, W.; Zhang, G. Facile Synthesis of a Bismuth Nanostructure with Enhanced Selectivity for Electrochemical Conversion of CO2 to Formate. Nanoscale 2019, 11, 7805–7812. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Yang, J.; Wang, L. Electrodeposited Bi Dendrites/2D Black Phosphorus Nanosheets Composite Used for Boosting Formic Acid Production from CO2 Electroreduction. J. CO2 Util. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 Electro-Reduction to Formate via Oxygen Bidentate Intermediate Stabilized by High-Index Planes of Bi Dendrite Catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Liu, X.; Chen, J.; Tian, P.; Dai, S.; Zhu, M.; Han, Y.-F. Probing the Role of Surface Hydroxyls for Bi, Sn and In Catalysts during CO2 Reduction. Appl. Catal. B Environ. 2021, 298, 120581. [Google Scholar] [CrossRef]
- Li, H.; Yue, X.; Qiu, Y.; Xiao, Z.; Yu, X.; Xue, C.; Xiang, J. Selective Electroreduction of CO2 to Formate over the Co-Electrodeposited Cu/Sn Bimetallic Catalyst. Mater. Today Energy 2021, 21, 100797. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, J.; Bei, J.; Zhang, R.; Wang, L.; Xu, Q.; Wang, W. Electrodeposition of Nano-Sized Bismuth on Copper Foil as Electrocatalyst for Reduction of CO2 to Formate. Appl. Surf. Sci. 2017, 393, 191–196. [Google Scholar] [CrossRef]
- Pan, J.; Li, P.; Jiang, X.; Shen, Y.; Wang, M. Electrochemical CO2 Reduction on Few-Atomic-Layer Bismuth Nanosheets. Mater. Today Phys. 2023, 35, 101096. [Google Scholar] [CrossRef]
- Li, L.; Cai, F.; Qi, F.; Ma, D.-K. Cu Nanowire Bridged Bi Nanosheet Arrays for Efficient Electrochemical CO2 Reduction toward Formate. J. Alloy. Compd. 2020, 841, 155789. [Google Scholar] [CrossRef]
- Dutta, A.; Zelocualtecatl Montiel, I.; Kiran, K.; Rieder, A.; Grozovski, V.; Gut, L.; Broekmann, P. A Tandem (Bi2O3 → Bimet) Catalyst for Highly Efficient Ec -CO2 Conversion into Formate: Operando Raman Spectroscopic Evidence for a Reaction Pathway Change. ACS Catal. 2021, 11, 4988–5003. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Smart, S.; Zhu, Z.; Yuan, Z. Shape-Tuned Electrodeposition of Bismuth-Based Nanosheets on Flow-through Hollow Fiber Gas Diffusion Electrode for High-Efficiency CO2 Reduction to Formate. Appl. Catal. B Environ. 2021, 286, 119945. [Google Scholar] [CrossRef]
- Rabiee, H.; Zhang, X.; Ge, L.; Hu, S.; Li, M.; Smart, S.; Zhu, Z.; Yuan, Z. Tuning the Product Selectivity of the Cu Hollow Fiber Gas Diffusion Electrode for Efficient CO2 Reduction to Formate by Controlled Surface Sn Electrodeposition. ACS Appl. Mater. Interfaces 2020, 12, 21670–21681. [Google Scholar] [CrossRef]
- Liu, S.; Hu, B.; Zhao, J.; Jiang, W.; Feng, D.; Zhang, C.; Yao, W. Enhanced Electrocatalytic CO2 Reduction of Bismuth Nanosheets with Introducing Surface Bismuth Subcarbonate. Coatings 2022, 12, 233. [Google Scholar] [CrossRef]
- Liang, X.-D.; Tian, N.; Hu, S.-N.; Zhou, Z.-Y.; Sun, S.-G. Recent Advances of Bismuth-Based Electrocatalysts for CO2 Reduction: Strategies, Mechanism and Applications. Mater. Rep. Energy 2023, 3, 100191. [Google Scholar] [CrossRef]
- Setterfield-Price, B.M.; Dryfe, R.A.W. The Influence of Electrolyte Identity upon the Electro-Reduction of CO2. J. Electroanal. Chem. 2014, 730, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Thorson, M.R.; Siil, K.I.; Kenis, P.J.A. Effect of Cations on the Electrochemical Conversion of CO2 to CO. J. Electrochem. Soc. 2013, 160, F69–F74. [Google Scholar] [CrossRef] [Green Version]
- König, M.; Vaes, J.; Klemm, E.; Pant, D. Solvents and Supporting Electrolytes in the Electrocatalytic Reduction of CO2. iScience 2019, 19, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. Gas–Liquid–Solid Reaction System for CO2 Electroreduction to Formate without Using Supporting Electrolyte. AIChE J. 2020, 66, e16299. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhao, J.; Zhang, X.; Li, M.; Hu, S.; Smart, S.; Rufford, T.E.; Zhu, Z.; Wang, H.; et al. Regulating the Reaction Zone of Electrochemical CO2 Reduction on Gas-Diffusion Electrodes by Distinctive Hydrophilic-Hydrophobic Catalyst Layers. Appl. Catal. B Environ. 2022, 310, 121362. [Google Scholar] [CrossRef]
- Leonard, M.E.; Orella, M.J.; Aiello, N.; Román-Leshkov, Y.; Forner-Cuenca, A.; Brushett, F.R. Flooded by Success: On the Role of Electrode Wettability in CO2 Electrolyzers That Generate Liquid Products. J. Electrochem. Soc. 2020, 167, 124521. [Google Scholar] [CrossRef]
- Nwabara, U.O.; Cofell, E.R.; Verma, S.; Negro, E.; Kenis, P.J.A. Durable Cathodes and Electrolyzers for the Efficient Aqueous Electrochemical Reduction of CO2. ChemSusChem 2020, 13, 855–875. [Google Scholar] [CrossRef]
- Nwabara, U.O.; Hernandez, A.D.; Henckel, D.A.; Chen, X.; Cofell, E.R.; de-Heer, M.P.; Verma, S.; Gewirth, A.A.; Kenis, P.J.A. Binder-Focused Approaches to Improve the Stability of Cathodes for CO2 Electroreduction. ACS Appl. Energy Mater. 2021, 4, 5175–5186. [Google Scholar] [CrossRef]
- Kim, B.; Hillman, F.; Ariyoshi, M.; Fujikawa, S.; Kenis, P.J.A. Effects of Composition of the Micro Porous Layer and the Substrate on Performance in the Electrochemical Reduction of CO2 to CO. J. Power Sources 2016, 312, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, L.M.; Koopman, C.I.; Forner-Cuenca, A.; Vermaas, D.A. When Flooding Is Not Catastrophic─Woven Gas Diffusion Electrodes Enable Stable CO2 Electrolysis. ACS Appl. Energy Mater. 2022, 5, 15125–15135. [Google Scholar] [CrossRef] [PubMed]
- Merino-Garcia, I.; Alvarez-Guerra, E.; Albo, J.; Irabien, A. Electrochemical Membrane Reactors for the Utilisation of Carbon Dioxide. Chem. Eng. J. 2016, 305, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Oßkopp, M.; Löwe, A.; Lobo, C.M.S.; Baranyai, S.; Khoza, T.; Auinger, M.; Klemm, E. Producing Formic Acid at Low PH Values by Electrochemical CO2 Reduction. J. CO2 Util. 2022, 56, 101823. [Google Scholar] [CrossRef]
- Van Daele, K.; De Mot, B.; Pupo, M.; Daems, N.; Pant, D.; Kortlever, R.; Breugelmans, T. Sn-Based Electrocatalyst Stability: A Crucial Piece to the Puzzle for the Electrochemical CO2 Reduction toward Formic Acid. ACS Energy Lett. 2021, 6, 4317–4327. [Google Scholar] [CrossRef]
- Alfath, M.; Lee, C.W. Recent Advances in the Catalyst Design and Mass Transport Control for the Electrochemical Reduction of Carbon Dioxide to Formate. Catalysts 2020, 10, 859. [Google Scholar] [CrossRef]
- Endrődi, B.; Bencsik, G.; Darvas, F.; Jones, R.; Rajeshwar, K.; Janáky, C. Continuous-Flow Electroreduction of Carbon Dioxide. Prog. Energy Combust. Sci. 2017, 62, 133–154. [Google Scholar] [CrossRef]
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and Challenges in Electrochemical CO2 Reduction Processes: An Engineering and Design Perspective Looking beyond New Catalyst Materials. J. Mater. Chem. A 2020, 8, 1511–1544. [Google Scholar] [CrossRef]
- Perry, S.C.; Leung, P.; Wang, L.; Ponce de León, C. Developments on Carbon Dioxide Reduction: Their Promise, Achievements, and Challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- De Mot, B.; Hereijgers, J.; Duarte, M.; Breugelmans, T. Influence of Flow and Pressure Distribution inside a Gas Diffusion Electrode on the Performance of a Flow-by CO2 Electrolyzer. Chem. Eng. J. 2019, 378, 122224. [Google Scholar] [CrossRef]
- Chen, Z.; Rodriguez, A.G.; Nunez, P.; van Houtven, D.; Pant, D.; Vaes, J. Experimental Investigation of Anion Exchange Membrane Water Electrolysis for a Tubular Microbial Electrosynthesis Cell Design. Catal. Commun. 2022, 170, 106502. [Google Scholar] [CrossRef]
- Yang, H.; Kaczur, J.J.; Sajjad, S.D.; Masel, R.I. Performance and Long-Term Stability of CO2 conversion to Formic Acid Using a Three-Compartment Electrolyzer Design. J. CO2 Util. 2020, 42, 101349. [Google Scholar] [CrossRef]
- Schweiss, R.; Meiser, C.; Damjanovic, T.; Galbiati, I.; Haak, N. SIGRACET® Gas Diffusion Layers for PEM Fuel Cells, Electrolyzers and Batteries (White Paper); SGL Group: Wiesbaden, Germany, 2016. [Google Scholar]
- Li, M.; Idros, M.N.; Wu, Y.; Burdyny, T.; Garg, S.; Zhao, X.S.; Wang, G.; Rufford, T.E. The Role of Electrode Wettability in Electrochemical Reduction of Carbon Dioxide. J. Mater. Chem. A 2021, 9, 19369–19409. [Google Scholar] [CrossRef]
- Bertin, E.; Garbarino, S.; Roy, C.; Kazemi, S.; Guay, D. Selective Electroreduction of CO2 to Formate on Bi and Oxide-Derived Bi Films. J. CO2 Util. 2017, 19, 276–283. [Google Scholar] [CrossRef]
- Pander, J.E.; Baruch, M.F.; Bocarsly, A.B. Probing the Mechanism of Aqueous CO2 Reduction on Post-Transition-Metal Electrodes Using ATR-IR Spectroelectrochemistry. ACS Catal. 2016, 6, 7824–7833. [Google Scholar] [CrossRef]
- Egetenmeyer, A.; Radev, I.; Durneata, D.; Baumgärtner, M.; Peinecke, V.; Natter, H.; Hempelmann, R. Pulse Electrodeposited Cathode Catalyst Layers for PEM Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 13649–13660. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria; National Association of Corrosion Engineers: Houston, TX, USA, 1966. [Google Scholar]
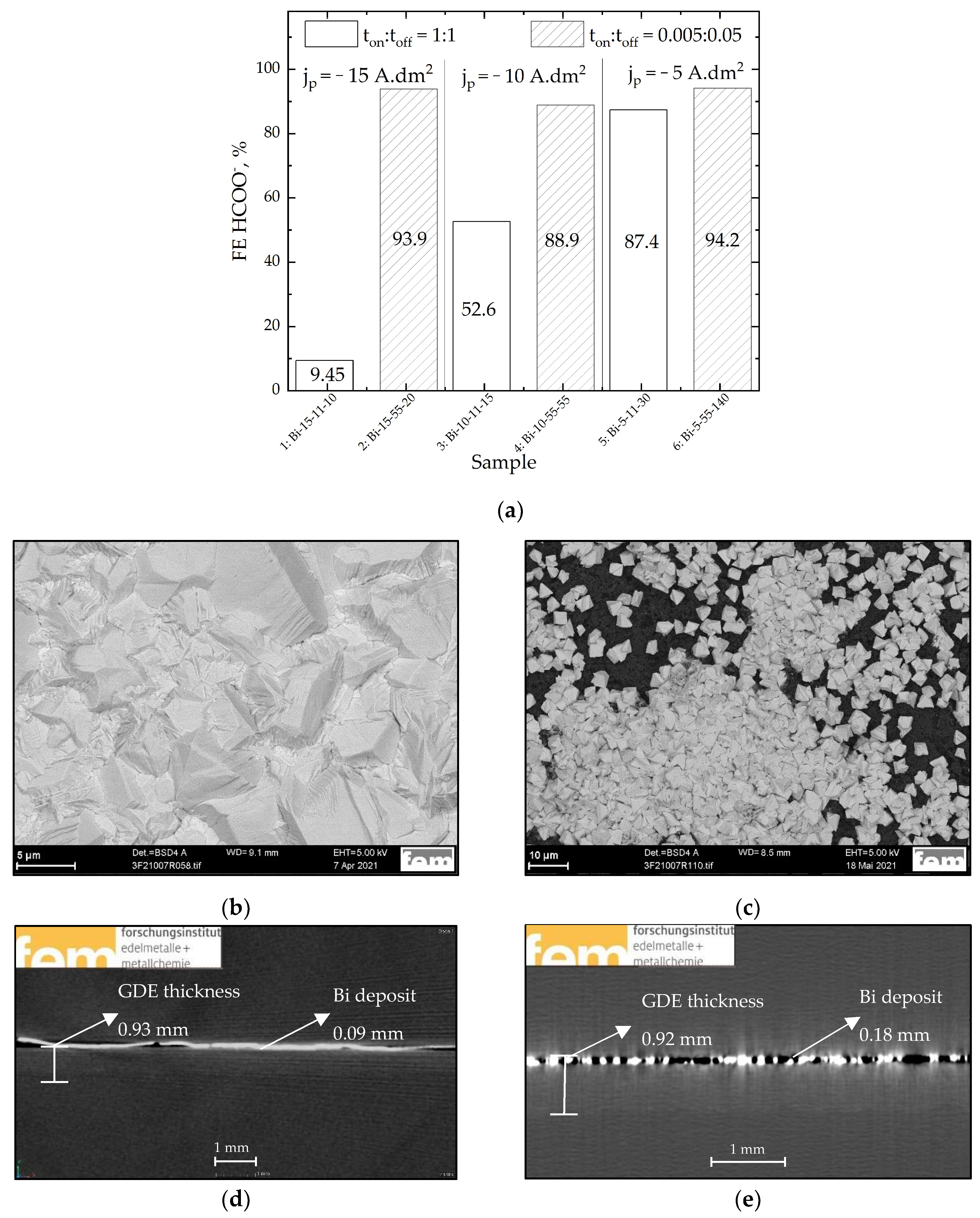
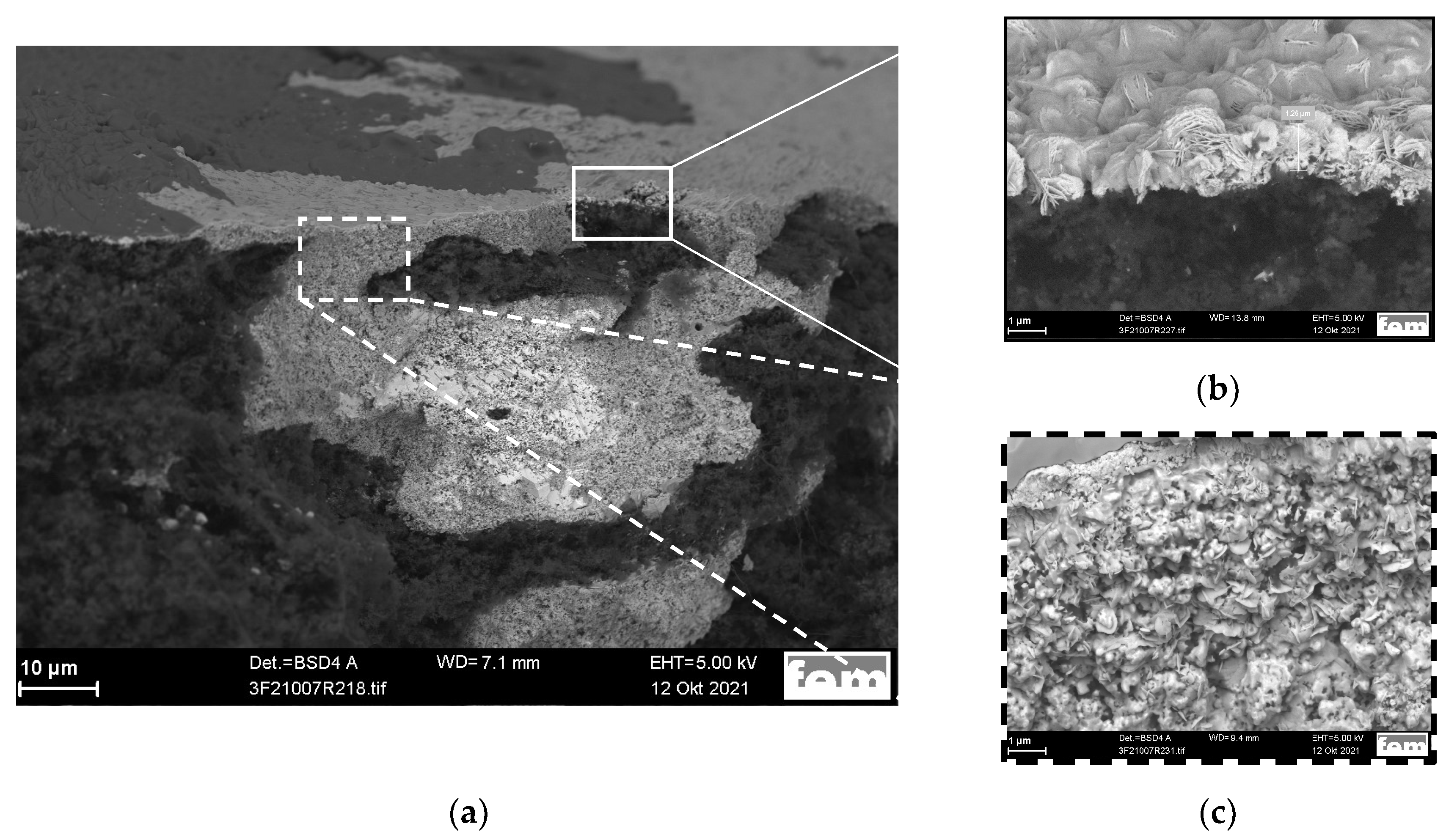
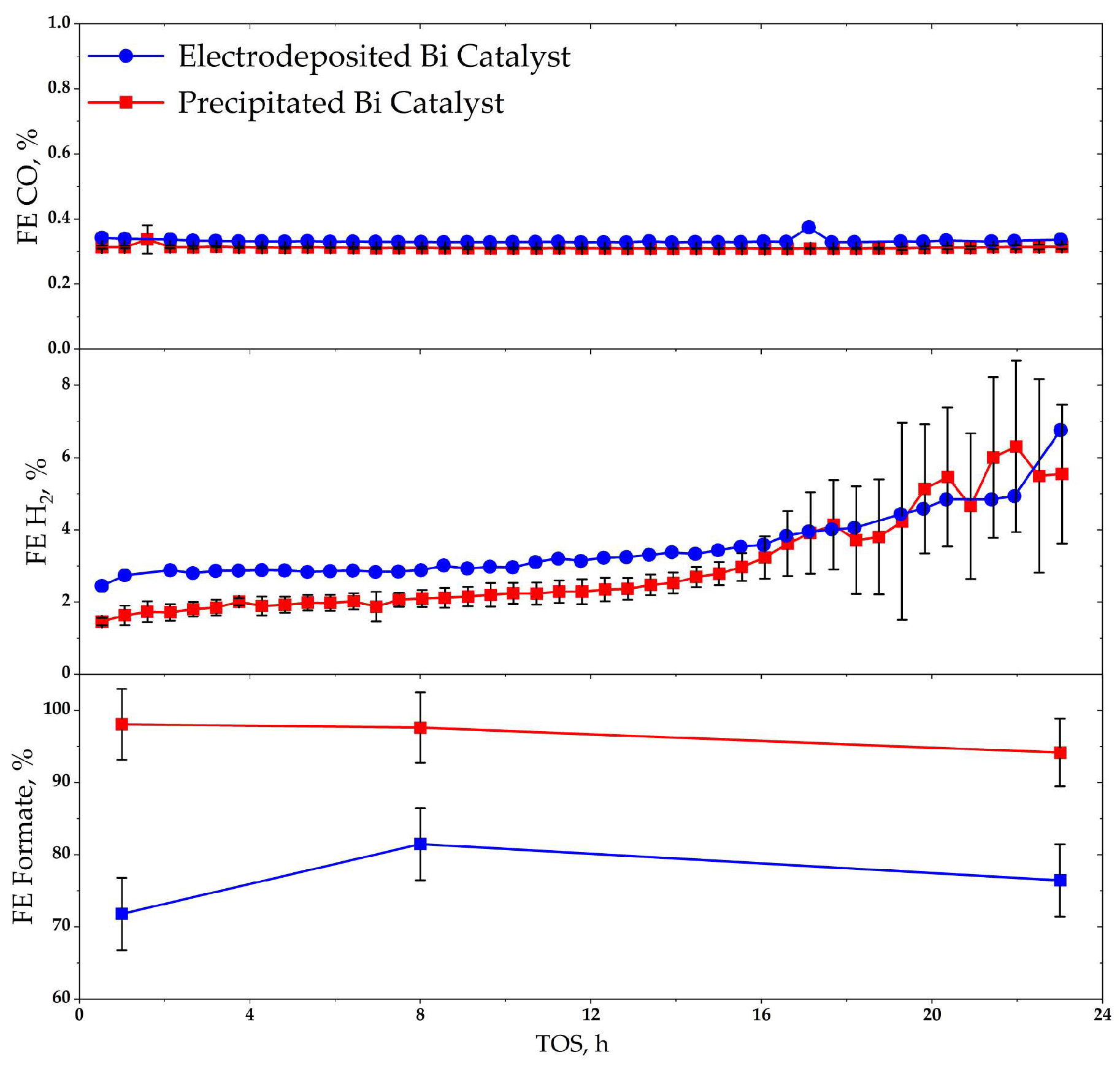
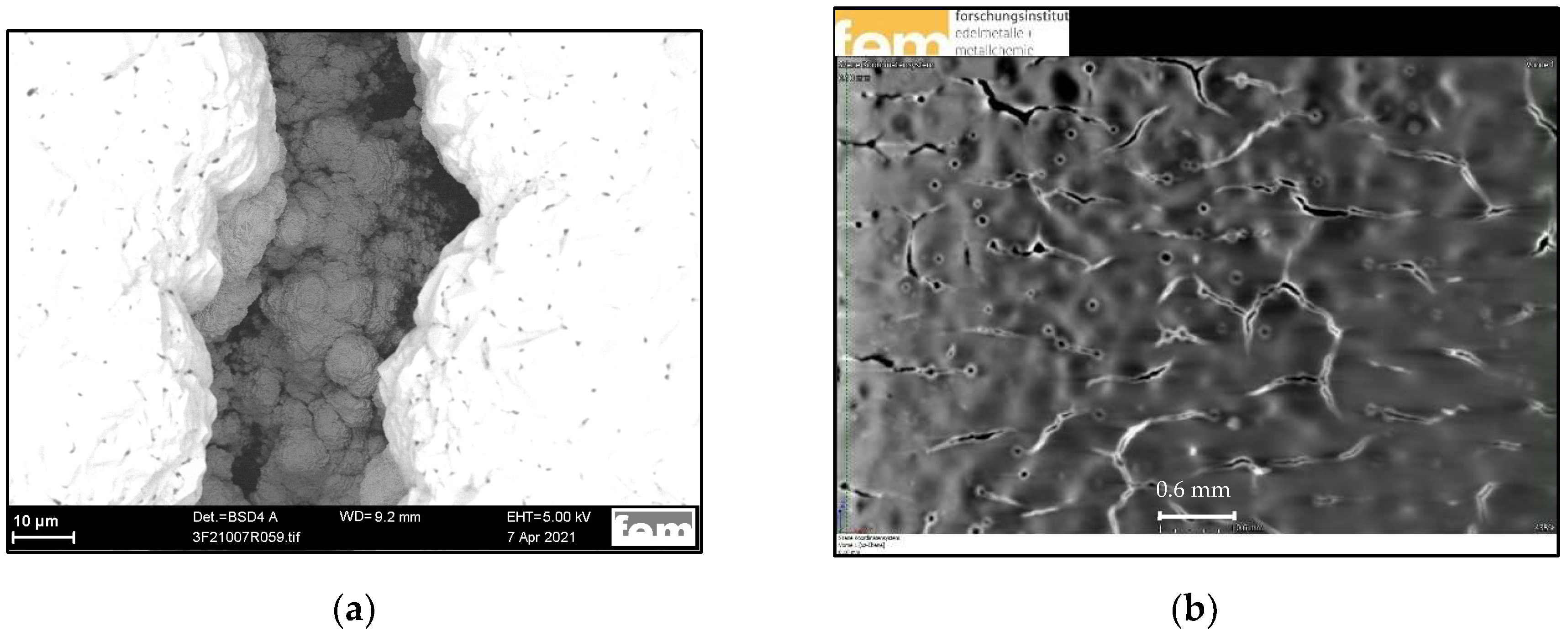
| Bi | Sn | −jav, mA·cm−2 | −jp, mA·cm−2 | ton:toff, s:s | θ, % | f, Hz | t, min |
|---|---|---|---|---|---|---|---|
| Bi-1-11-60 | Sn-1-11-60 | 0.5 | 1 | 1:1 | 50 | 0.5 | 60 |
| Bi-5-11-30 | Sn-5-11-30 | 2.5 | 5 | 1:1 | 50 | 0.5 | 30 |
| Bi-10-11-15 | Sn-10-11-15 | 5 | 10 | 1:1 | 50 | 0.5 | 15 |
| Bi-15-11-10 | Sn-15-11-10 | 7.5 | 15 | 1:1 | 50 | 0.5 | 10 |
| Bi-1-55-200 | Sn-1-55-200 | 0.09 | 1 | 0.005:0.05 | 9 | 18.18 | 200 |
| Bi-5-55-140 | Sn-5-55-140 | 0.45 | 5 | 0.005:0.05 | 9 | 18.18 | 140 |
| Bi-10-55-55 | Sn-10-55-55 | 0.91 | 10 | 0.005:0.05 | 9 | 18.18 | 55 |
| Bi-15-55-20 | Sn-15-55-20 | 1.36 | 15 | 0.005:0.05 | 9 | 18.18 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolova, M.; Hildebrand, J.; Hertle, S.; Sörgel, Ş.; Kassner, H.; Klemm, E. Comparison of Electrochemically Deposited Bi and Sn Catalysts onto Gas Diffusion Electrodes for the Electrochemical CO2 Reduction Reaction to Formate. Appl. Sci. 2023, 13, 7471. https://doi.org/10.3390/app13137471
Manolova M, Hildebrand J, Hertle S, Sörgel Ş, Kassner H, Klemm E. Comparison of Electrochemically Deposited Bi and Sn Catalysts onto Gas Diffusion Electrodes for the Electrochemical CO2 Reduction Reaction to Formate. Applied Sciences. 2023; 13(13):7471. https://doi.org/10.3390/app13137471
Chicago/Turabian StyleManolova, Mila, Joachim Hildebrand, Sebastian Hertle, Şeniz Sörgel, Holger Kassner, and Elias Klemm. 2023. "Comparison of Electrochemically Deposited Bi and Sn Catalysts onto Gas Diffusion Electrodes for the Electrochemical CO2 Reduction Reaction to Formate" Applied Sciences 13, no. 13: 7471. https://doi.org/10.3390/app13137471
APA StyleManolova, M., Hildebrand, J., Hertle, S., Sörgel, Ş., Kassner, H., & Klemm, E. (2023). Comparison of Electrochemically Deposited Bi and Sn Catalysts onto Gas Diffusion Electrodes for the Electrochemical CO2 Reduction Reaction to Formate. Applied Sciences, 13(13), 7471. https://doi.org/10.3390/app13137471






