Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Procurement
2.2. Method
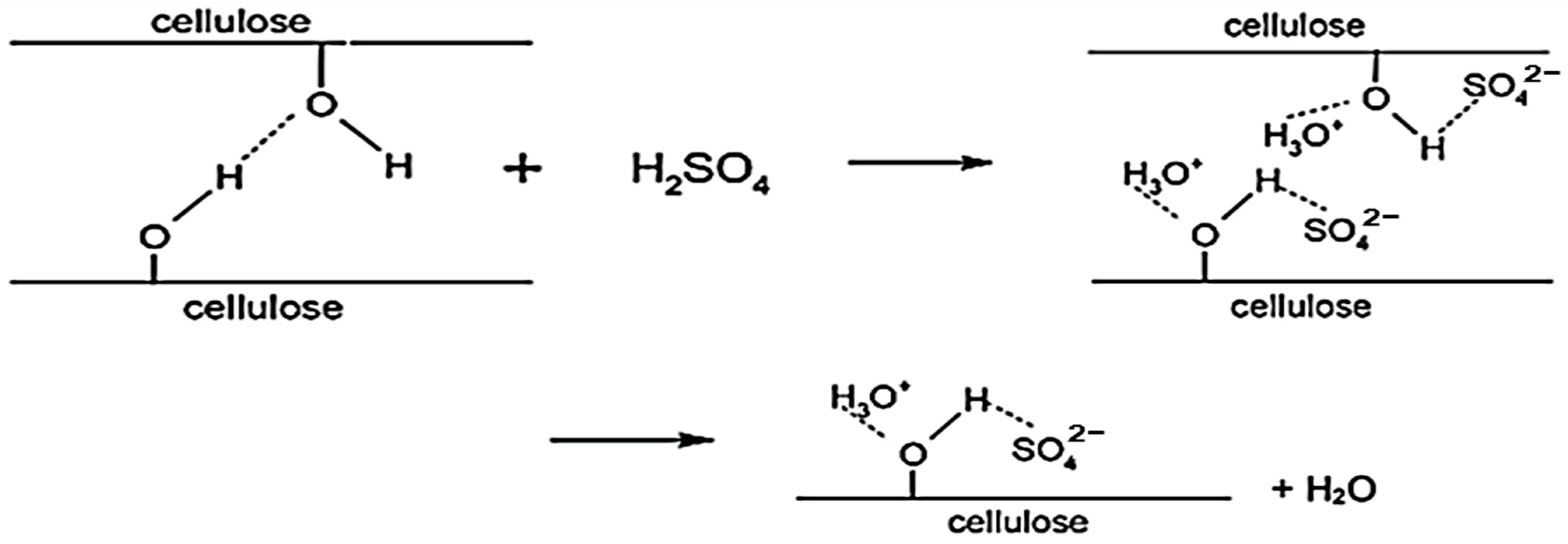
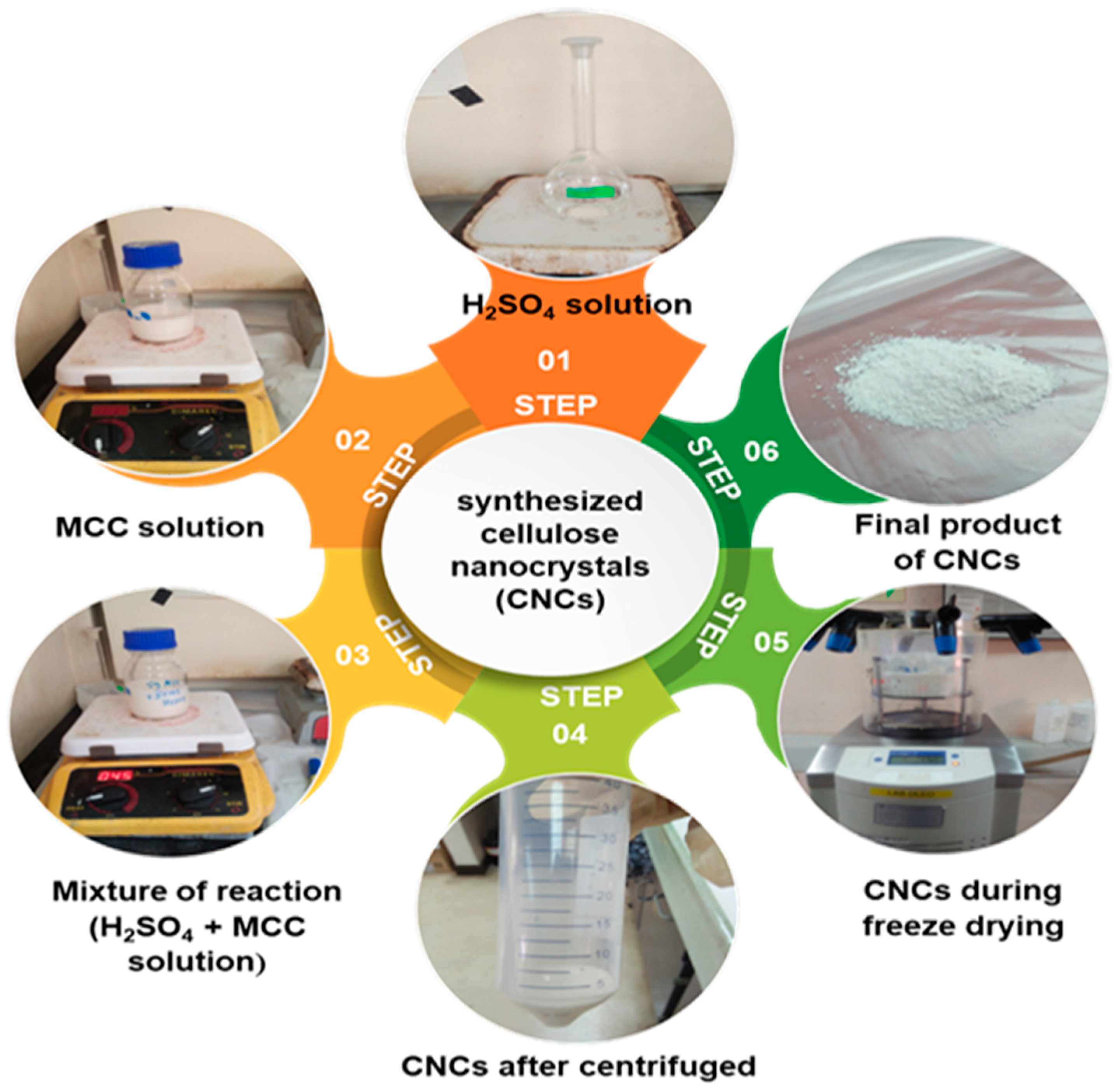
2.2.1. Preparation of Cellulose Nanocrystals (CNCs) via Sulfuric Acid Hydrolysis
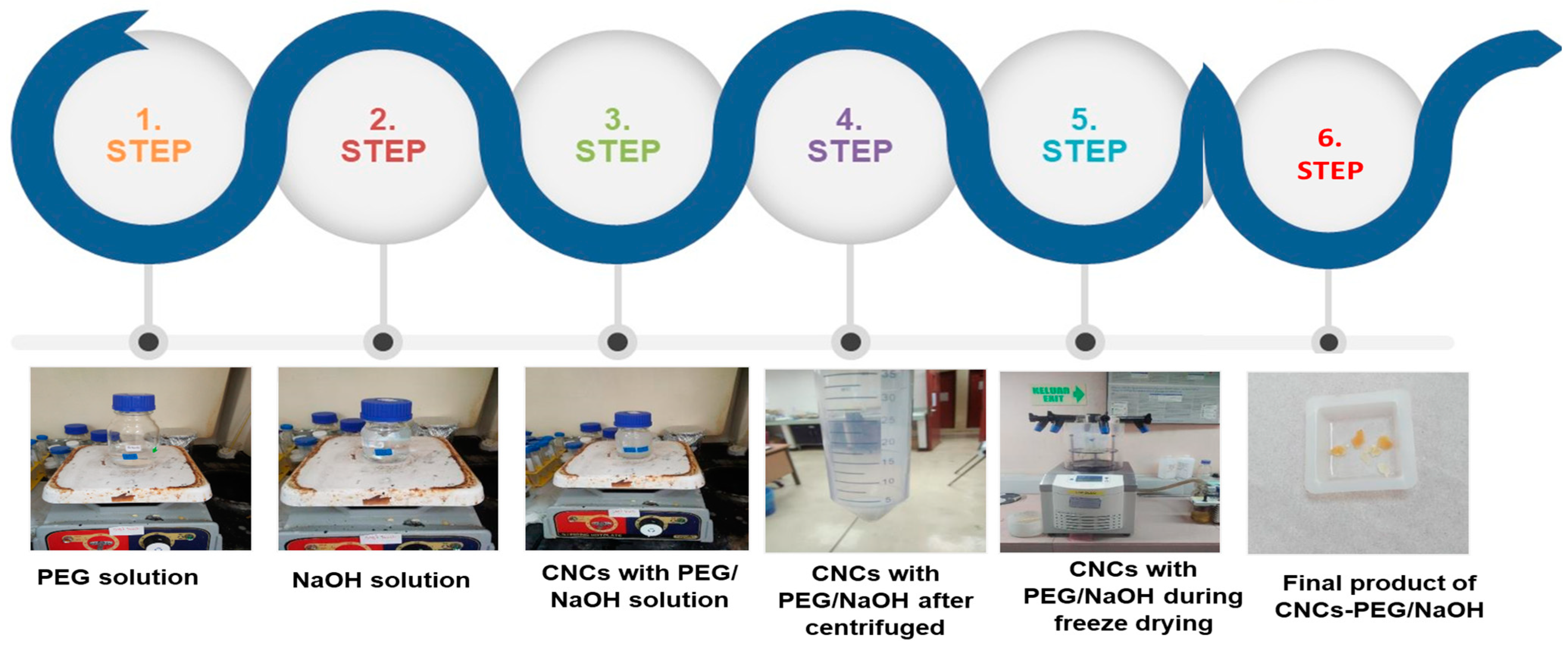
2.2.2. Synthesis of (CNCs-PEG/NaOH)
2.3. CNCs Characterization
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. X-ray Diffraction (XRD) Analysis
2.3.3. Thermogravimetric and Derivative Thermogravimetry (TGA/DTG) Analysis
2.3.4. Zeta Potential Characterization
2.3.5. Dynamic Light Scattering (DLS) Analysis
2.3.6. Transmission Electron Microscopy (TEM) Analysis
2.3.7. Emission Scanning Electron Microscopy (FE-SEM) Imaging
2.3.8. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
2.3.9. In Vitro Magnetic Resonance Imaging (MRI) Studies
2.3.10. Cell Survival Test
2.3.11. Cell Culture Preparation
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Analysis
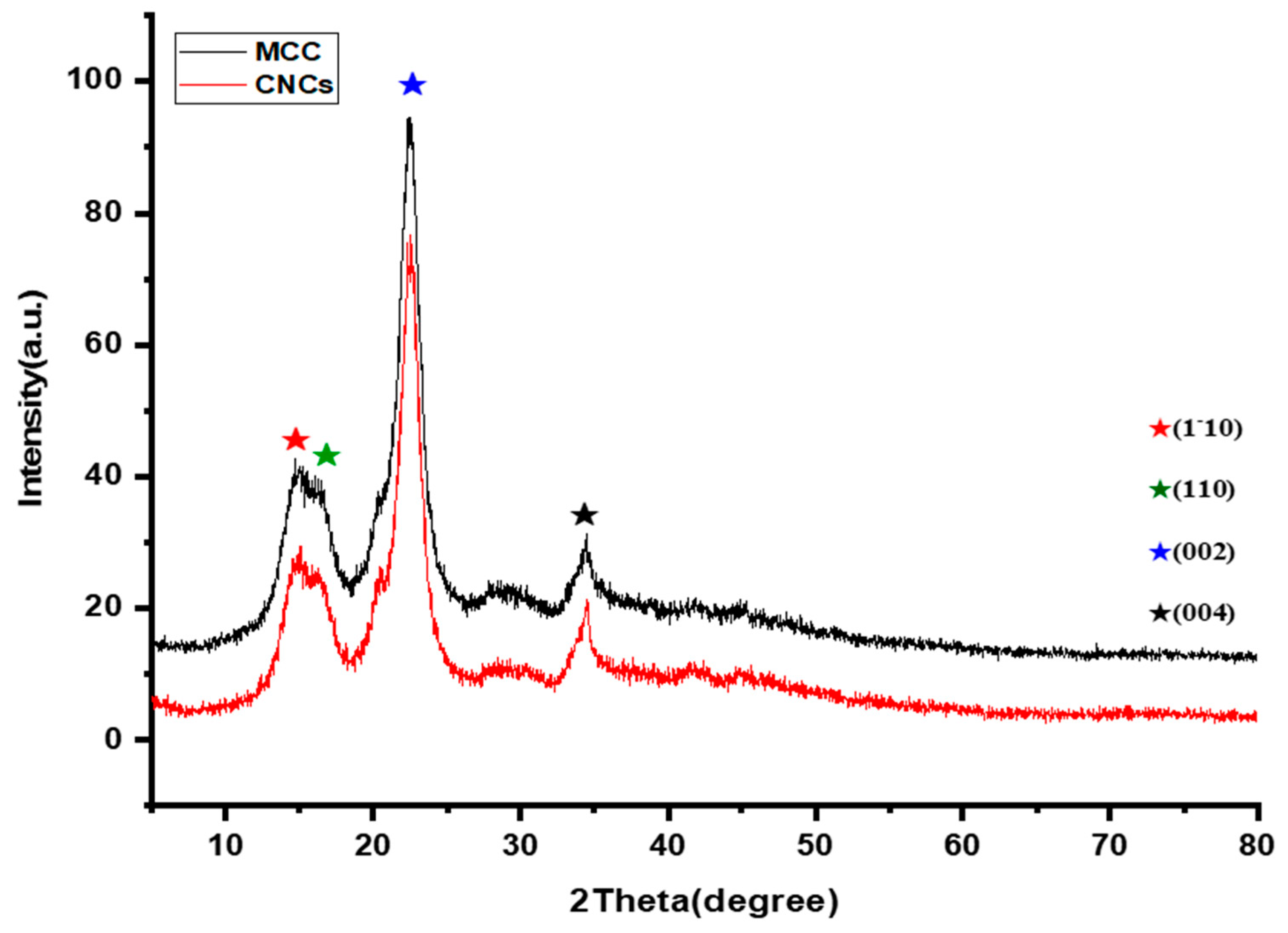
3.2. XRD Analysis
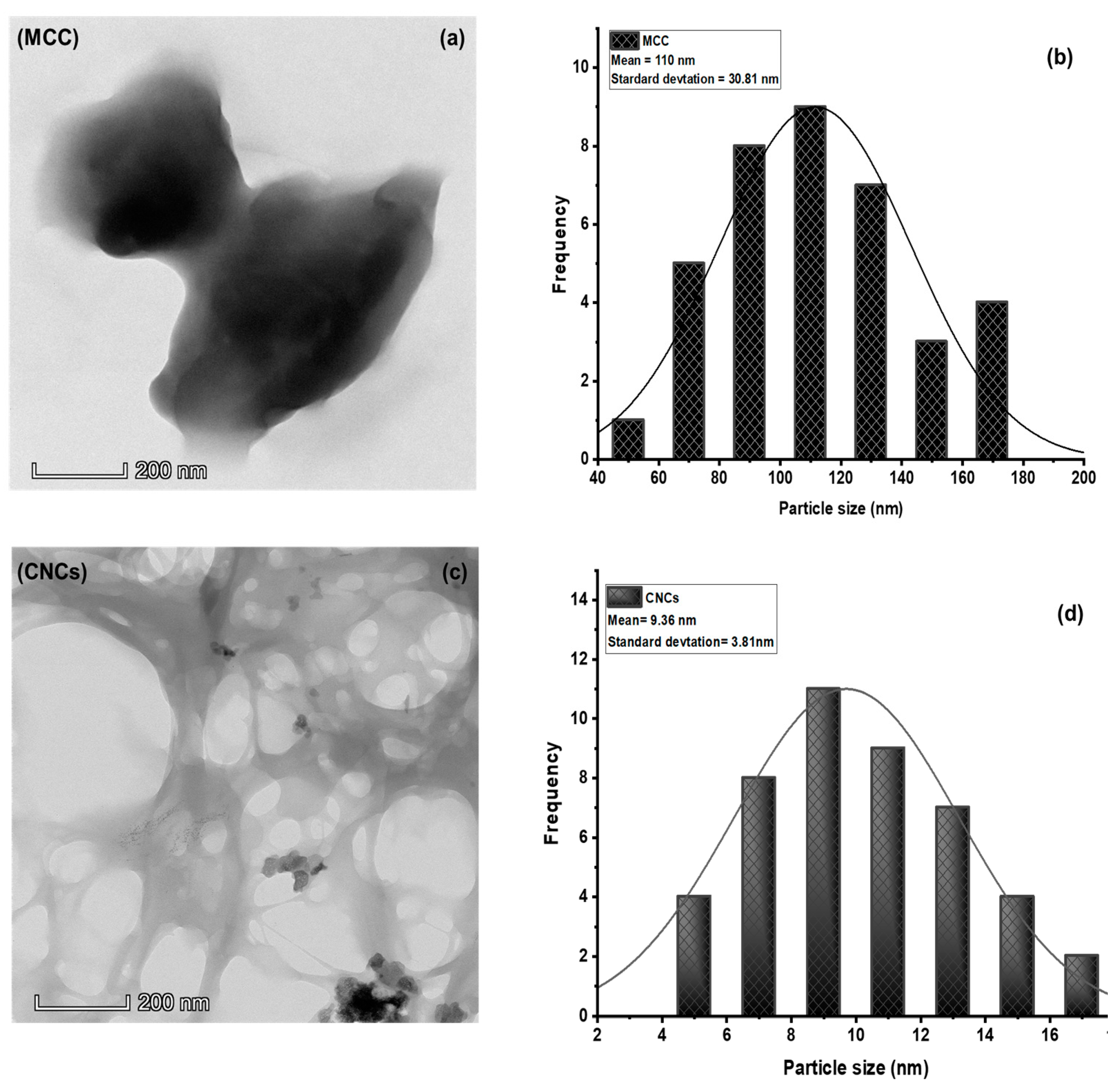
3.3. Transmission Electron Microscopy (TEM) Analysis
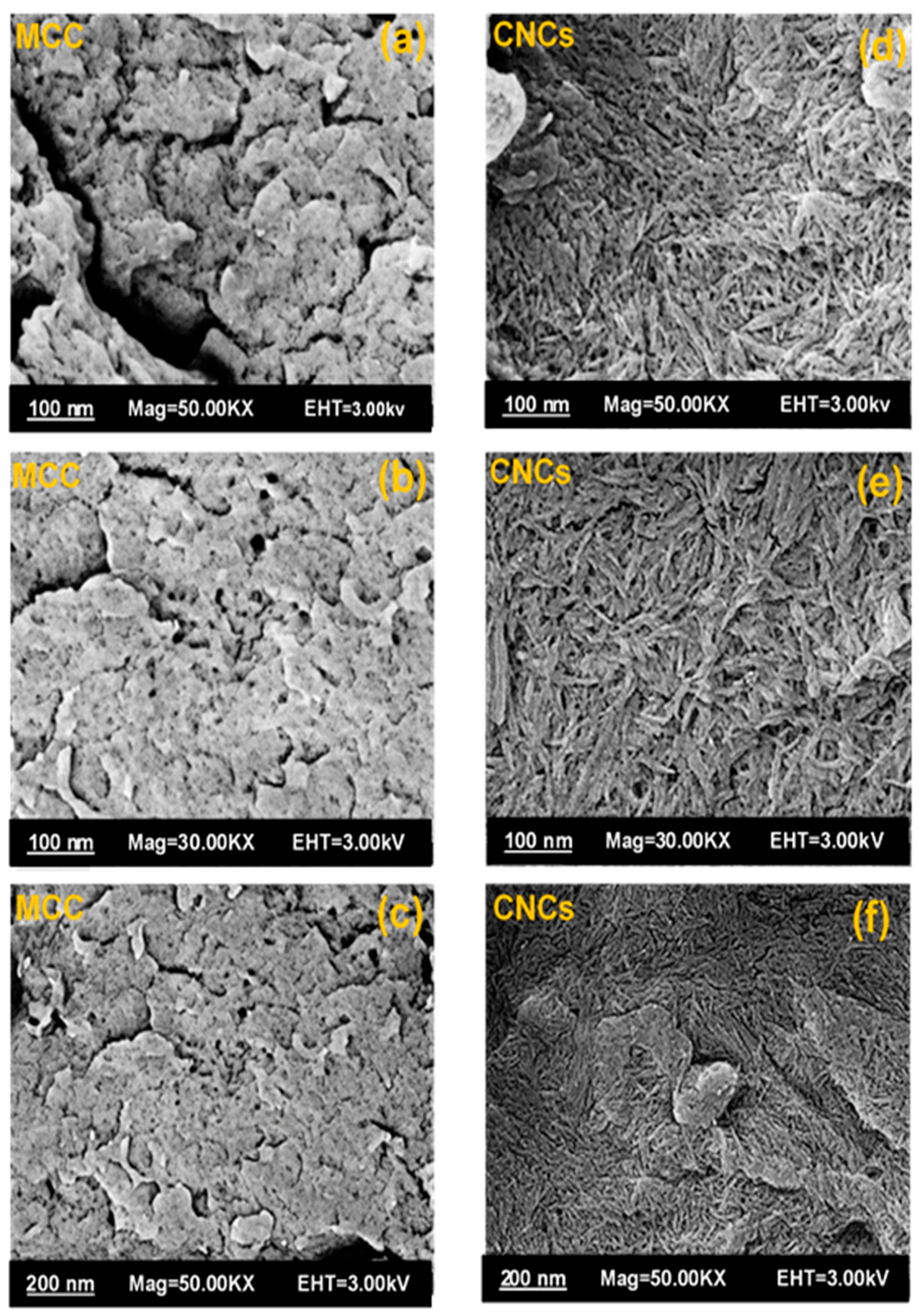
3.4. Field-Emission Scanning Electron Microscopy (FESEM) Analysis
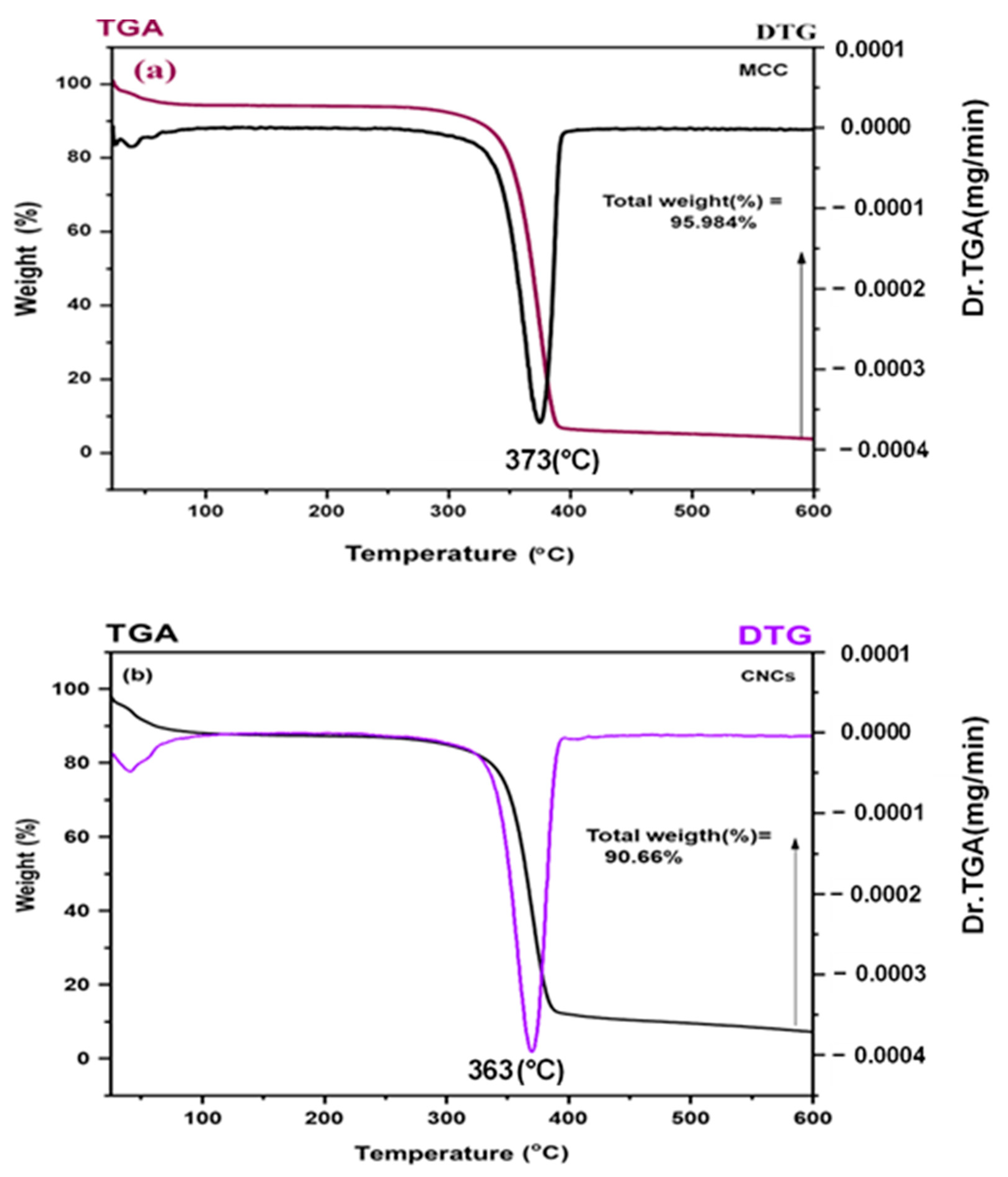
3.5. TGA/DTG Analysis
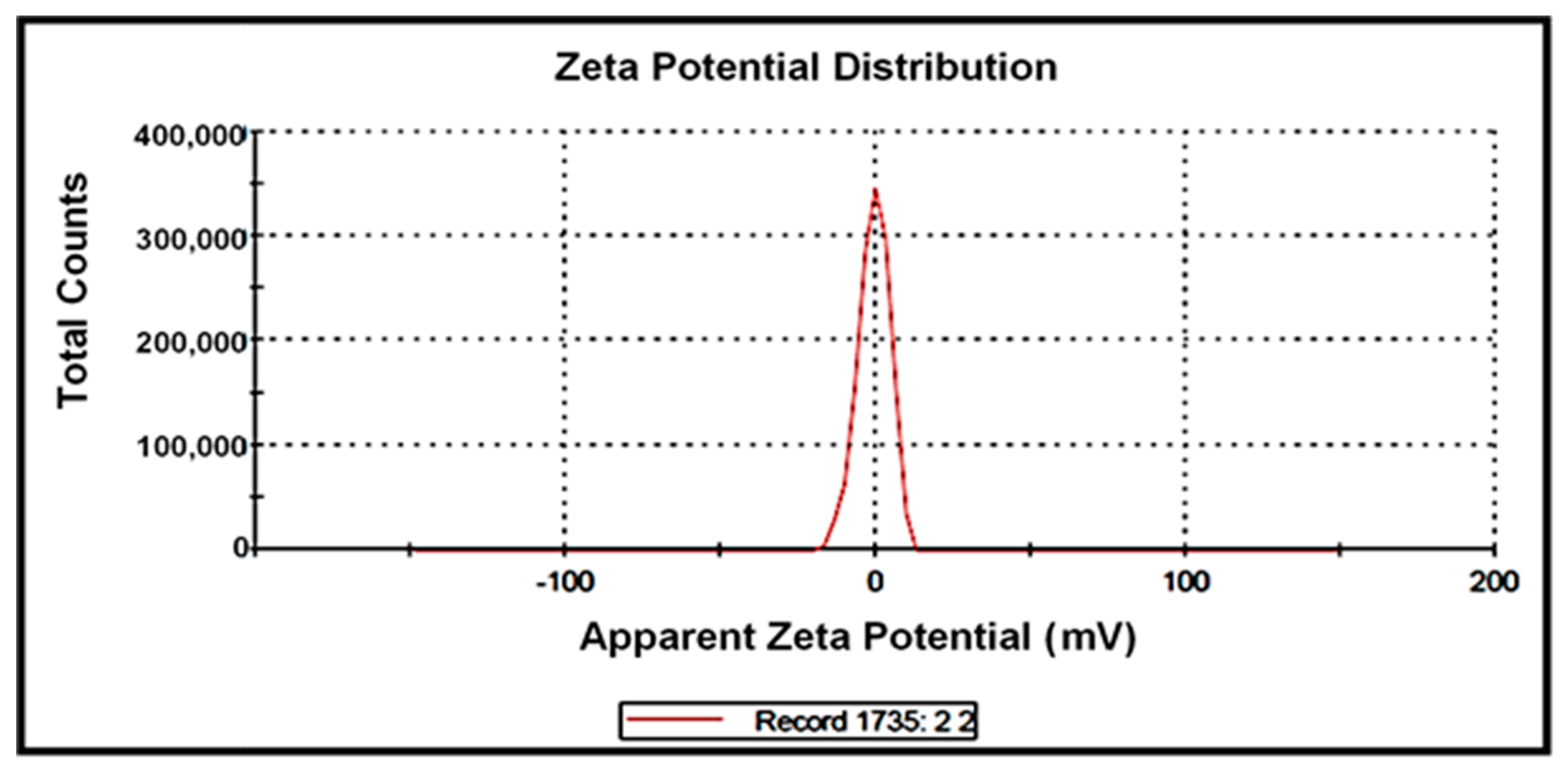
3.6. Zeta (ζ) Potential Characterization
3.7. Nuclear Magnetic Resonance (NMR) Analysis)
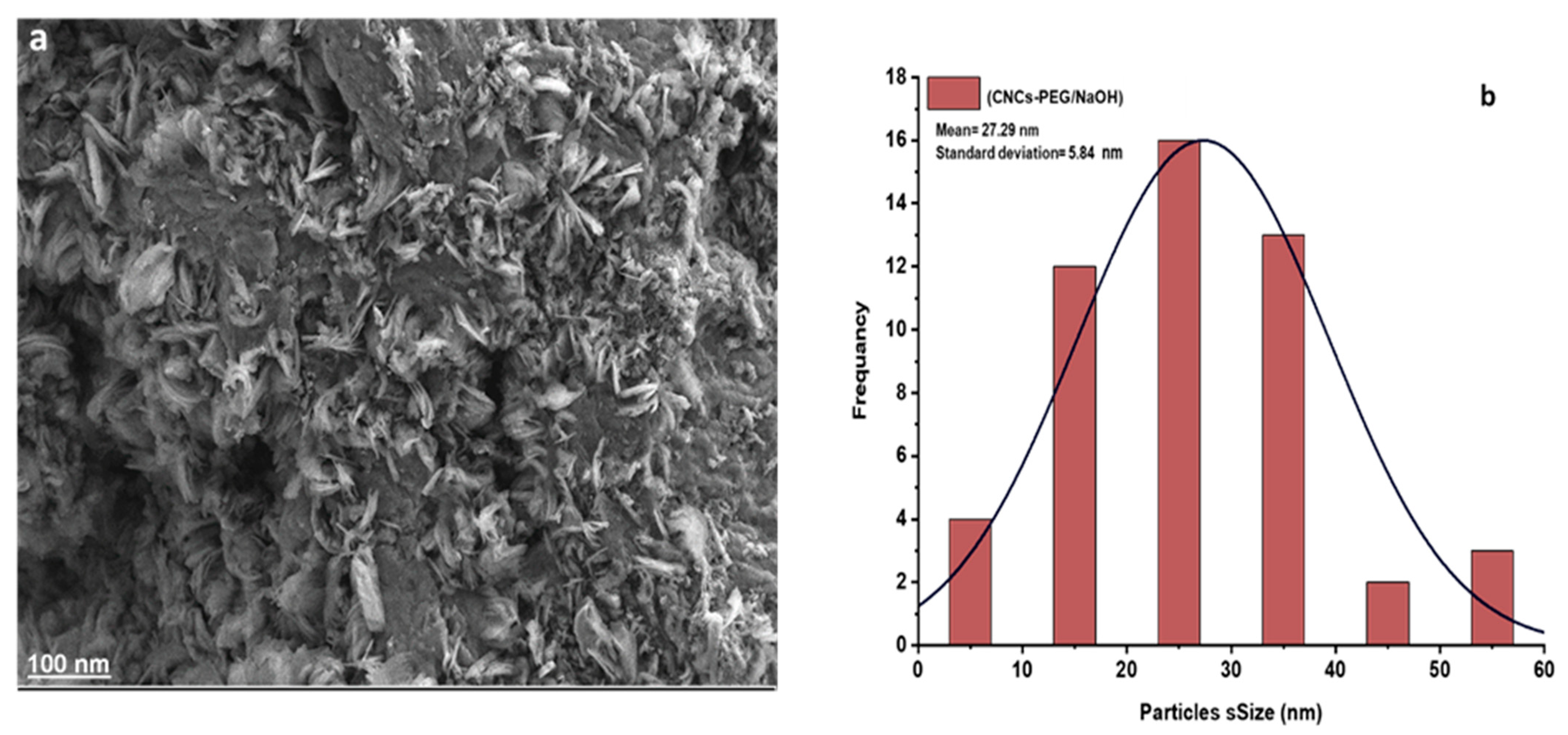
3.8. Analysis Using Field-Emission Scanning Electron Microscopy (FESEM)
3.9. Zeta (ζ) Potential Characterization
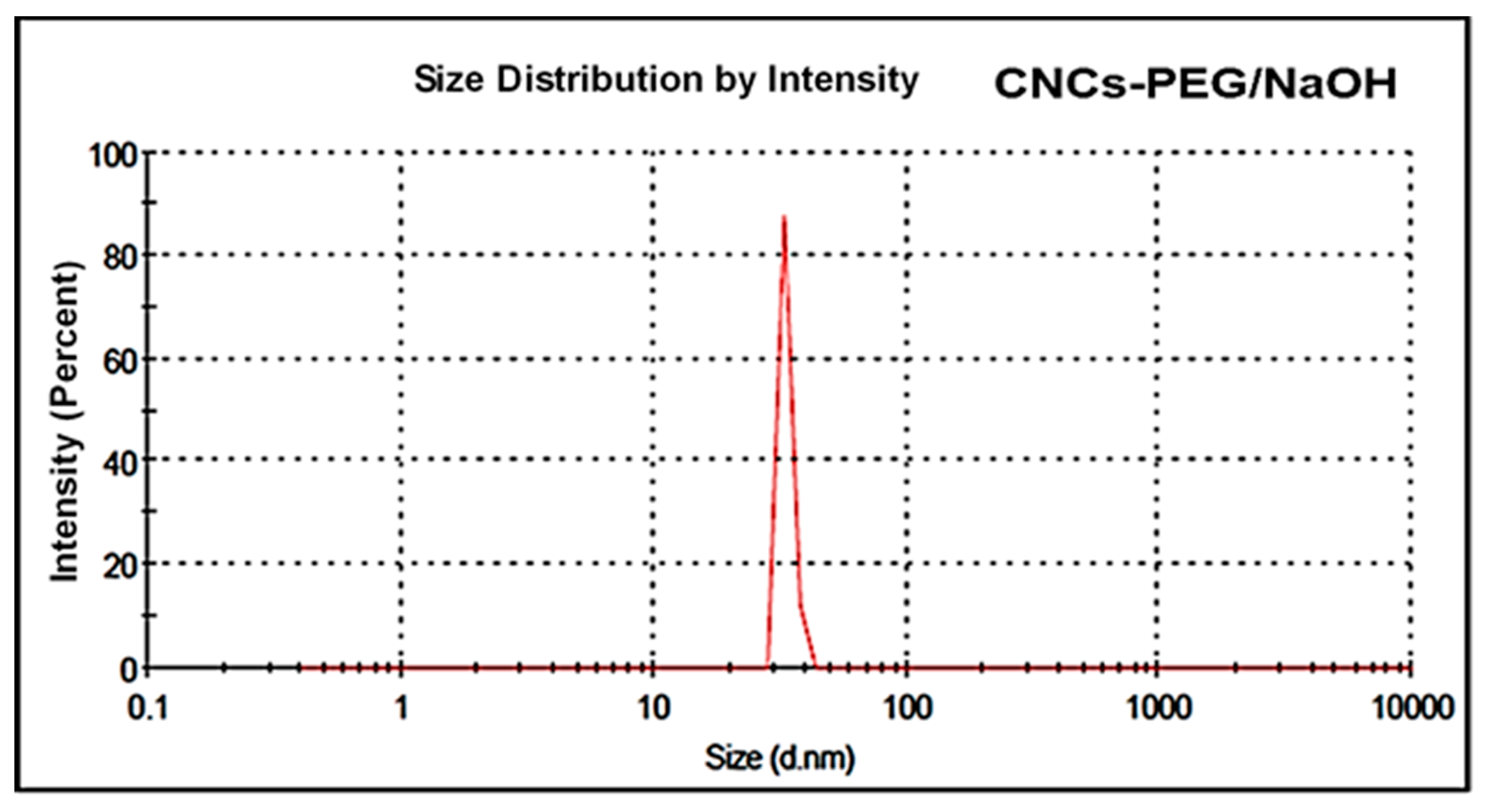
3.10. Dynamic Light Scattering (DLS) Analysis
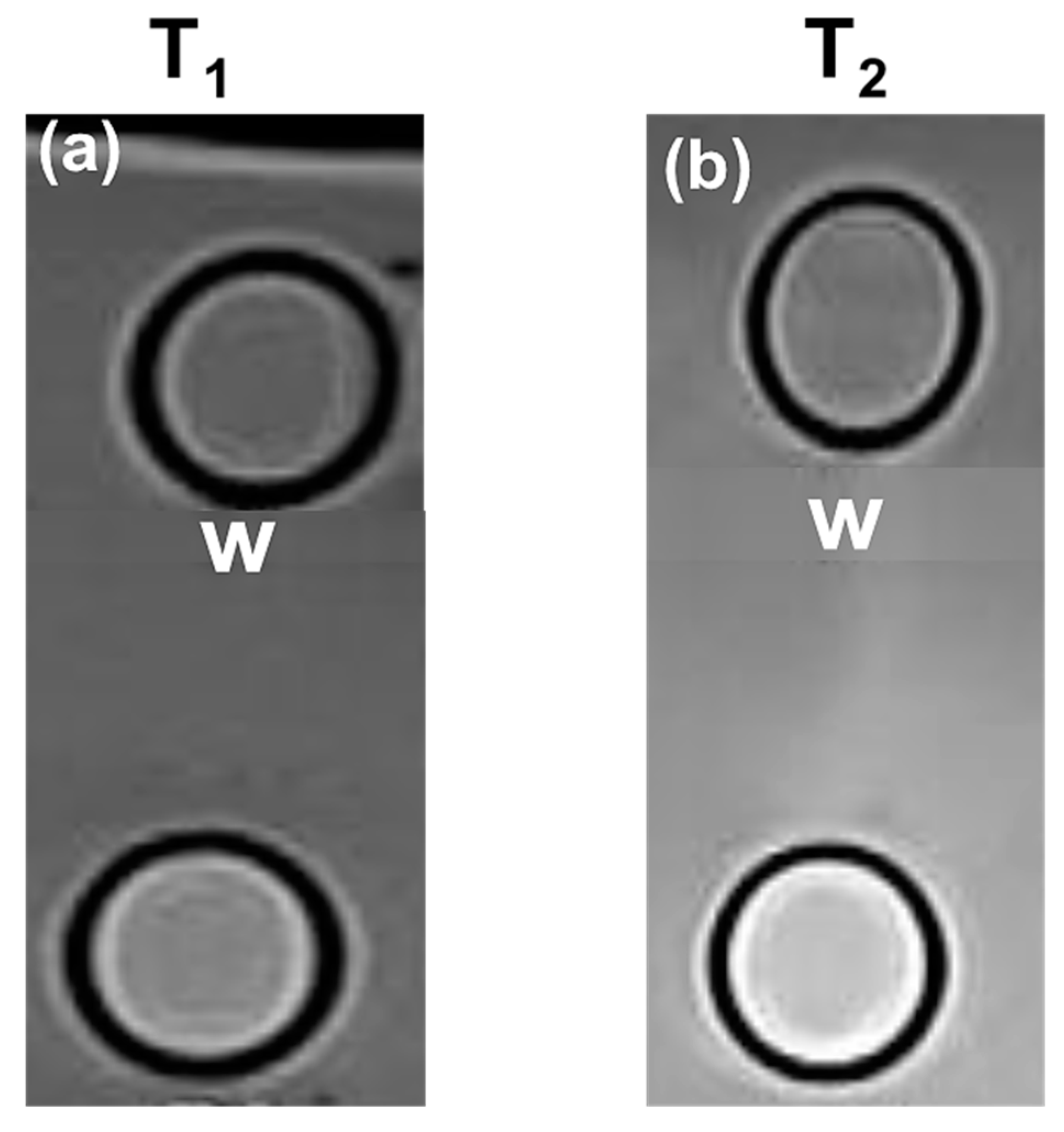
3.11. T1-Weighted and T2-Weighted MR Images
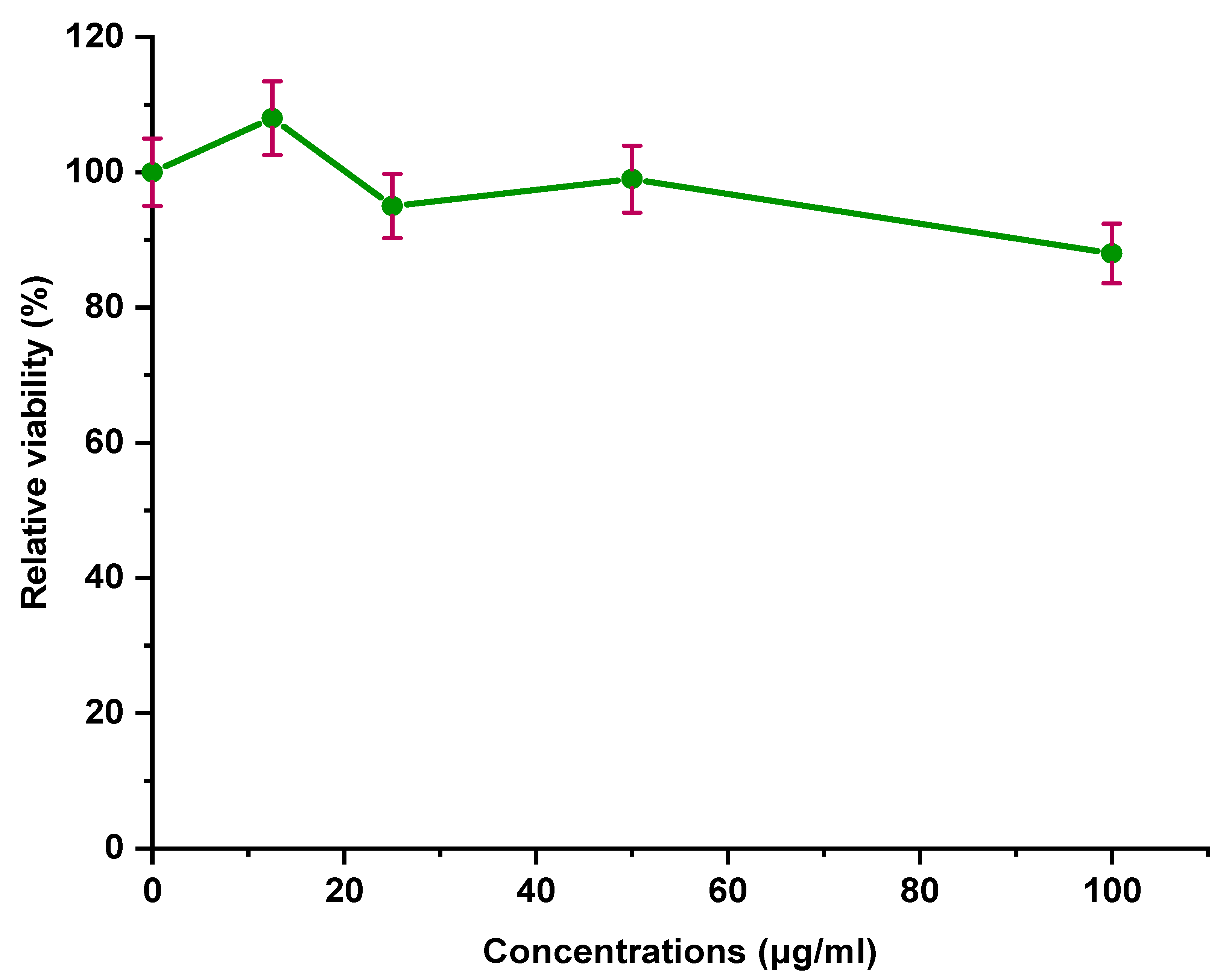
3.12. Descriptive Statistics of MTT Assay
3.13. Effects of Concentration of (CNCs-PEG/NaOH) on Hep G2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Samples | Weight Initial | Weight after Hydrolysis | % Yield |
|---|---|---|---|
| CNCs | 0.5 | 0.445 | 89% |
| CNCs-PEG/NaOH | 12.5 | 11 | 88% |
| Samples | Ia | Ib | Crystallinity Index (%) |
|---|---|---|---|
| MCC | 70 | 90 | 0.78 |
| CNCs | 120 | 139 | 0.86 |
| Samples | Icr | Iam | Crystallinity (I222) (%) |
|---|---|---|---|
| MCC | 93.80 | 23.54 | 74.90 |
| CNCs | 76.66 | 12.39 | 83.84 |
| Viability (%) | Replicate | Positive Control (10 mM) | Negative Control | Responses of (CNCs-PEG/NaOH)/(μg/mL) | |||
| 12.5 | 25 | 50 | 100 | ||||
| n = 1 | 13 | 100 | 106 | 95 | 98 | 85 | |
| n = 2 | 100 | 110 | 97 | 99 | 89 | ||
| n = 3 | 100 | 108 | 95 | 100 | 90 | ||
| Mean | NA | 100 | 108 | 95 | 99 | 88 | |
| Standard Error Mean | NA | 0 | 2 | 2 | 3 | 3 | |
References
- Coseri, S. Cellulose: To depolymerize… or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Nanoparticles of Amorphous Cellulose and Their Properties. Am. J. Nanosci. Nanotechnol. 2013, 1, 41–45. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Fatona, A.; Berry, R.M.; Brook, M.A.; Moran-Mirabal, J.M. Versatile Surface Modification of Cellulose Fibers and Cellulose Nanocrystals through Modular Triazinyl Chemistry. Chem. Mater. 2018, 30, 2424–2435. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Endes, C.; Camarero-Espinosa, S.; Mueller, S.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Clift, M.J.D. A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 2016, 14, 1–14. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, 05486. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Liu, E.; He, L.; Guan, Q.; Zhang, J.; Peng, L. Development of a general kinetic model for organic acid-catalyzed hydrolysis of corn stalk. Cellulose 2021, 28, 6935–6952. [Google Scholar] [CrossRef]
- Chu, Y.; Song, R.; Zhang, L.; Dai, H.; Wu, W. Water-dispersible, biocompatible and fluorescent poly(ethylene glycol)-grafted cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 153, 46–54. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Hu, X.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021, 186, 591–615. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, Q.; Li, T.; Yang, M.; Yao, Q.; Hu, H.-Y. Gadolinium-Labeled Aminoglycoside and Its Potential Application as a Bacteria-Targeting Magnetic Resonance Imaging Contrast Agent. Anal. Chem. 2018, 90, 1934–1940. [Google Scholar] [CrossRef]
- Cao, Y.-B.; Ren, H.-T.; Hu, C.-S.; Meng, Q.-X.; Liu, Q. In-situ formation behavior of NbC-reinforced Fe-based laser cladding coatings. Mater. Lett. 2015, 147, 61–63. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Chong, Y.; Ma, Y.; Zhang, H.; Deng, Z.; Zhang, Z. Graphene oxide based theranostic platform for T1-weighted magnetic resonance imaging and drug delivery. Appl. Mater. Interfaces 2013, 5, 13325–13332. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, H.; Liang, Z.; Feng, J.; Lu, Y.; Huang, L.; Shen, Z. Biodegradable and biocompatible exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging of tumors. J. Nanobiotechnol. 2022, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, N.; Poojar, P.; Ashwini, R.; Qurishi, Y.; Geethanath, S.; Srivastava, C. Ultrafine graphene oxide–CoFe2O4 nanoparticle composite as T1 and T2 contrast agent for magnetic resonance imaging. RSC Adv. 2016, 6, 17423–17429. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Zhao, K.; Tang, J.; Wang, X.-Y.; Li, L.-D.; Chen, N.-X.; Wang, Y.-J.; Shi, S.; Zhang, X.; Malaisamy, S.; et al. Gd-dots with strong ligand–water interaction for ultrasensitive magnetic resonance renography. ACS Nano 2017, 11, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Ittrich, H.; Peldschus, K.; Raabe, N.; Kaul, M.; Adam, G. Superparamagnetic Iron Oxide Nanoparticles in Biomedicine: Applications and Developments in Diagnostics and Therapy. Fortschr. Röntgenstr. 2013, 185, 1149–1166. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.X.; Sun, Q.; Zhao, H.L.; Lei, H.; Lan, M.B.; Cheng, Z.X.; Wang, X.L.; Dou, S.X.; Lu, G.Q. Ultrasmall Manganese Ferrite Nanoparticles as Positive Contrast Agent for Magnetic Resonance Imaging. Adv. Health Mater. 2013, 2, 958–964. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, D.; Bao, J.; Chen, Q.; Liu, G.; Chen, Z.; Gao, J. A Synergistically EnhancedT1-T2Dual-Modal Contrast Agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z.-L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Sfiligoj, M.; Hribernik, S.; Kurečič, M.; Urbanek Krajnc, A.; Kreže, T.; Stana Kleinschek, K. Surface Properties of Non-Conventional Cellulose Fibres; Springer International Publishing: Cham, Switzerland, 2019; pp. 61–71. [Google Scholar] [CrossRef]
- Song, M.-L.; Yu, H.-Y.; Chen, L.-M.; Zhu, J.-Y.; Wang, Y.-Y.; Yao, J.-M.; Zou, Z.; Tam, K.C. Multibranch Strategy To Decorate Carboxyl Groups on Cellulose Nanocrystals To Prepare Adsorbent/Flocculants and Pickering Emulsions. ACS Sustain. Chem. Eng. 2019, 7, 6969–6980. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Vigneshwaran, N. A novel process for synthesis of spherical nanocellulose by controlled hydrolysis of microcrystalline cellulose using anaerobic microbial consortium. Enzym. Microb. Technol. 2013, 52, 20–25. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.A.; Bodîrlău, R. Structural changes evidenced by FTIR spectroscopy in cellulose materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioResources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Fitriani; Aprilia, N.A.S.; Arahman, N. Properties of nanocrystalline cellulose from pineapple crown leaf waste. IOP Conf. Ser. Mater. Sci. Eng. 2020, 796, 012007. [Google Scholar] [CrossRef]
- French, A.D.; Cintron, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493. [Google Scholar] [CrossRef]
- Kamel, S.; Jahangir, K. Optimization of Carboxymethylation of Starch in Organic Solvents. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 511–519. [Google Scholar] [CrossRef]
- Rahbar Shamskar, K.; Heidari, H.; Rashidi, A. Study on Nanocellulose Properties Processed Using Different Methods and Their Aerogels. J. Polym. Environ. 2019, 27, 1418–1428. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Yousefi, T.; Torab-Mostaedi, M.; Ghasemi, M.; Ghadirifar, A. Synthesis of Gd2O3 nanoparticles: Using bulk Gd2O3 powders as precursor. Rare Met. 2015, 34, 540–545. [Google Scholar] [CrossRef]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Van den Berg, O.; Capadona, J.R.; Weder, C. Preparation of Homogeneous Dispersions of Tunicate Cellulose Whiskers in Organic Solvents. Biomacromolecules 2007, 8, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Mariano, M.; Cercená, R.; Soldi, V. Thermal characterization of cellulose nanocrystals isolated from sisal fibers using acid hydrolysis. Ind. Crops Prod. 2016, 94, 454–462. [Google Scholar] [CrossRef]
- Ting, S.S. Comparative properties analysis between microcrystalline cellulose and cellulose nanocrystals extracted from rice straw. Malays. J. Microsc. 2019, 15, 146–154. [Google Scholar]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.U.H.A.; Gul, S.; Arjomandi, J.; Bilal, S. Study on the thermal decomposition kinetics and calculation of activation energy of degradation of poly (o-toluidine) using thermogravimetric analysis. Iran. J. Chem. Chem. Eng. 2018, 37, 193–204. [Google Scholar]
- Park, J.; Lee, E.; Hwang, N.-M.; Kang, M.; Kim, S.C.; Hwang, Y.; Hyeon, T. One-Nanometer-Scale Size-Controlled Synthesis of Monodisperse Magnetic Iron Oxide Nanoparticles. Angew. Chem. 2005, 117, 2932–2937. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.; Cintil, J.; Thomas, S.; John, M.; Narine, S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Faradilla, R.H.F.; Lee, G.; Rawal, A.; Hutomo, T.; Stenzel, M.H.; Arcot, J. Nanocellulose characteristics from the inner and outer layer of banana pseudo-stem prepared by TEMPO-mediated oxidation. Cellulose 2016, 23, 3023–3037. [Google Scholar] [CrossRef]
- Khouri, S. Experimental Characterization and Theoretical Calculations of Responsive Polymeric Systems. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2010. [Google Scholar]
- Mohaiyiddin, M.S.; Lin, O.H.; Owi, W.T.; Chan, C.H.; Chia, C.H.; Zakaria, S.; Akil, H.M. Characterization of nanocellulose recovery from Elaeis guineensis frond for sustainable development. Clean Technol. Environ. Policy 2016, 18, 2503–2512. [Google Scholar] [CrossRef]
- Morais, J.P.S.; de Freitas Rosa, M.; Nascimento, L.D.; Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B. Surface modification of cellulose nanocrystals (CNC) by a cationic surfactant. Ind. Crops Prod. 2015, 65, 45–55. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Liu, C.-F.; Ren, J.-L.; Xu, F.; Liu, J.-J.; Sun, J.-X.; Sun, R.-C. Isolation and Characterization of Cellulose Obtained from Ultrasonic Irradiated Sugarcane Bagasse. J. Agric. Food Chem. 2006, 54, 5742–5748. [Google Scholar] [CrossRef]
- da Silva, R.; Sierakowski, M.R.; Bassani, H.P.; Zawadzki, S.F.; Pirich, C.L.; Ono, L.; de Freitas, R.A. Hydrophilicity improvement of mercerized bacterial cellulose films by polyethylene glycol graft. Int. J. Biol. Macromol. 2016, 86, 599–605. [Google Scholar] [CrossRef]
- Goetz, L.; Foston, M.; Mathew, A.P.; Oksman, K.; Ragauskas, A.J. Poly(methyl vinyl ether-co-maleic acid)−Polyethylene Glycol Nanocomposites Cross-Linked In Situ with Cellulose Nanowhiskers. Biomacromolecules 2010, 11, 2660–2666. [Google Scholar] [CrossRef]
- McCormick, D.A.; Connors, B.W.; Lighthall, J.W.; Prince, D.A.; Rule, M.E.; Vargas-Irwin, C.E.; Donoghue, J.P.; Truccolo, W.; Jacob, V.; Mitani, A.; et al. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 1985, 54, 782–806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhang, D.; Zhao, D.; Liu, N.; Shi, F.; Qin, W. Bright white upconversion emission from Yb3+, Er3+, and Tm3+-codoped Gd2O3 nanotubes. Phys. Chem. Chem. Phys. 2010, 12, 7620–7625. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, N.; Sarlak, N. Fabrication of a dual T1 and T2 contrast agent for magnetic resonance imaging using cellulose nanocrystals/Fe3O4 nanocomposite. Eur. Polym. J. 2019, 118, 128–136. [Google Scholar] [CrossRef]
- Majeed, S.; Shivashankar, S.A. Rapid, microwave-assisted synthesis of Gd2O3 and Eu: Gd2O3 Nanocrystals: Characterization, magnetic, optical and biological studies. J. Mater. Chem. B 2014, 2, 5585–5593. [Google Scholar] [CrossRef]




| Samples | Peak Position, 2θ (o) | Crystal Indices, (hkl) | Interplanar Distance, (dhkl) (Å) | Lattice Parameter, a ≠ = b ≠ c (Å) | Volume of the Unit Cell, (Å3) | ||
|---|---|---|---|---|---|---|---|
| MCC& CNCs | 17.09 | 020 | 5.20 | 8.26 | 10.40 | 7.88 | 677 |
| 22.98 | 200 | 4.3 | |||||
| 34.54 | 004 | 1.97 | |||||
| Samples | Lattice Parameters | Particle Size (nm) | Grain Size Range (nm) | Intensity (cps) | |||
|---|---|---|---|---|---|---|---|
| Crystal Indices (hkl) | Peak Position, 2θ (o) | Peak Width at Half Maximum Intensity (FWHM) (o) | Peak Width at Half Maximum Intensity (FWHM) (radian) | ||||
| MCC | 002 | 22.7 | 1.20 | 0.020933 | 7.06 | 7.06–17.66 | 93.80 |
| 004 | 34.5 | 0.49 | 0.008547 | 17.66 | |||
| CNCs | 002 | 22.7 | 1.52 | 0.026516 | 5.57 | 5.57–11.90 | 77.34 |
| 004 | 34.5 | 0.73 | 0.012734 | 11.90 | |||
| Samples | Peak Position, 2θ (o) | θ (o) | Cos θ | Peak Width at Half Maximum Intensity (FWHM) (o) | Peak Width at Half Maximum Intensity (FWHM) (Radian) | Sin θ | 4Sin θ | βCos θ | Strain, |
|---|---|---|---|---|---|---|---|---|---|
| MCC | 16.4 | 8.20 | 0.9898 | 0.33 | 0.005767 | 0.1426 | 0.5705 | 0.00571 | 1.43 × 10−3 |
| 22.7 | 11.35 | 0.9804 | 1.20 | 0.020933 | 0.1967 | 0.7868 | 0.02052 | 5.13 × 10−3 | |
| 34.5 | 17.25 | 0.9550 | 0.49 | 0.008547 | 0.2965 | 1.1861 | 0.00816 | 2.04 × 10−3 | |
| CNCs | 16.4 | 8.20 | 0.9898 | 0.60 | 0.010467 | 0.1426 | 0.5705 | 0.01036 | 2.5 × 10−3 |
| 22.7 | 11.35 | 0.9804 | 1.52 | 0.026516 | 0.1967 | 0.7868 | 0.02599 | 6.4 × 10−3 | |
| 34.5 | 17.25 | 0.9550 | 0.73 | 0.012734 | 0.2965 | 1.1861 | 0.01216 | 3.04 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whba, F.; Mohamed, F.; Idris, M.I.; Yahya, M.S. Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Appl. Sci. 2023, 13, 6316. https://doi.org/10.3390/app13106316
Whba F, Mohamed F, Idris MI, Yahya MS. Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Applied Sciences. 2023; 13(10):6316. https://doi.org/10.3390/app13106316
Chicago/Turabian StyleWhba, Fathyah, Faizal Mohamed, Mohd Idzat Idris, and Mohd Syukri Yahya. 2023. "Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI" Applied Sciences 13, no. 10: 6316. https://doi.org/10.3390/app13106316
APA StyleWhba, F., Mohamed, F., Idris, M. I., & Yahya, M. S. (2023). Surface Modification of Cellulose Nanocrystals (CNCs) to Form a Biocompatible, Stable, and Hydrophilic Substrate for MRI. Applied Sciences, 13(10), 6316. https://doi.org/10.3390/app13106316






