Construction and Identification of a Food-Grade Recombinant Cyberlindnera jadinii Strain Expressing Lignin Peroxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and Strains
2.2. Primer Design
2.3. Construction of a Homologous Integrated Expression Vector
2.3.1. Cloning of the lip Gene
2.3.2. Cloning of C. jadinii GAP Promoter Sequence, GAP Terminator Sequence, Cycloheximide Resistance Gene (CYH) and 18S rDNA Fragment
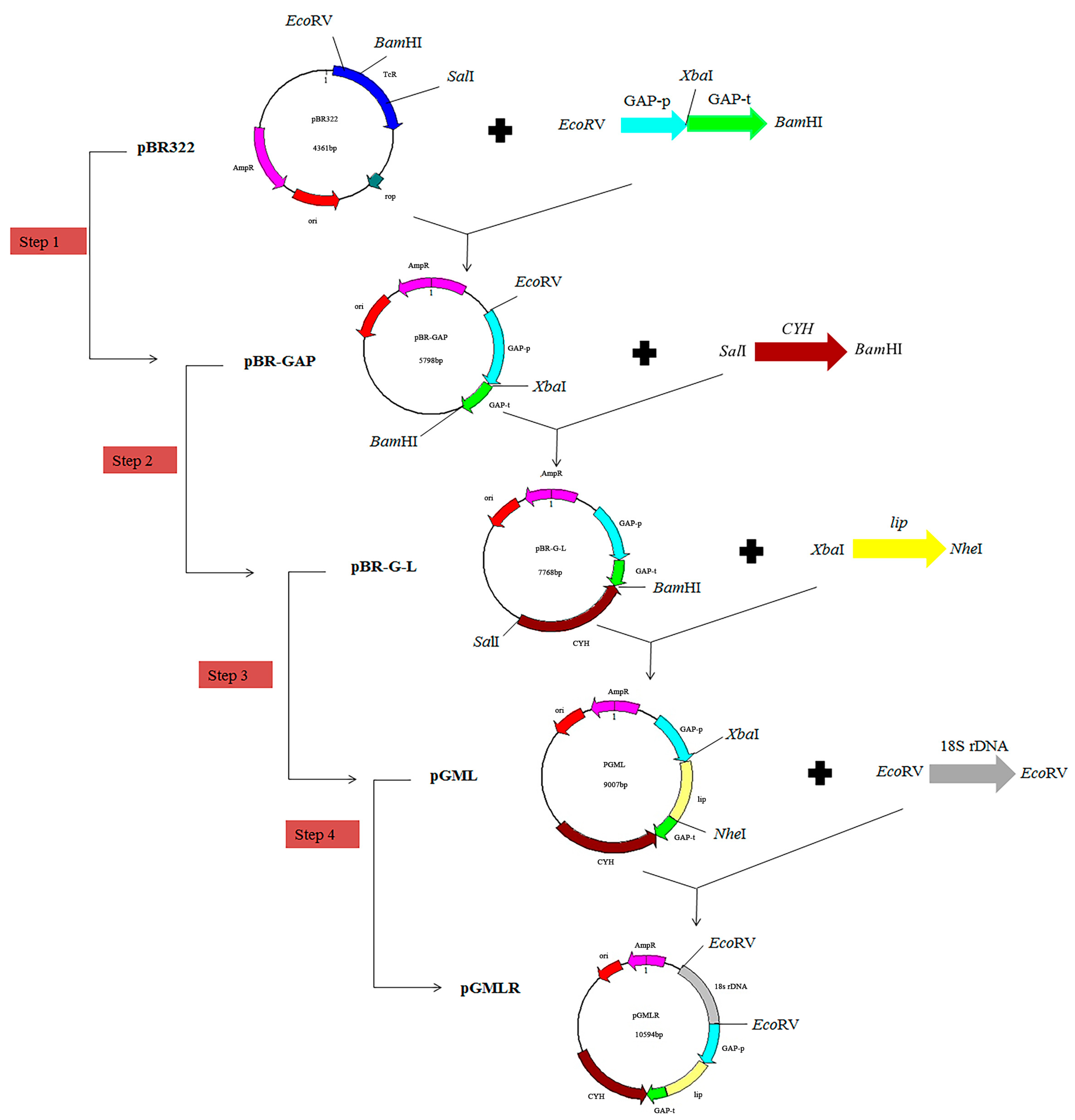
2.3.3. Construction of the pGMLR Vector
2.4. Construction of Recombinant Food-Grade C. jadinii Expressing lip
2.5. Identification and Genetic Stability Study of Transformants
2.6. Detection of Lignin Peroxidase Expression and Enzyme Activity
2.7. Phenotype Characterization Using the Biolog MicroStation™ System
3. Results
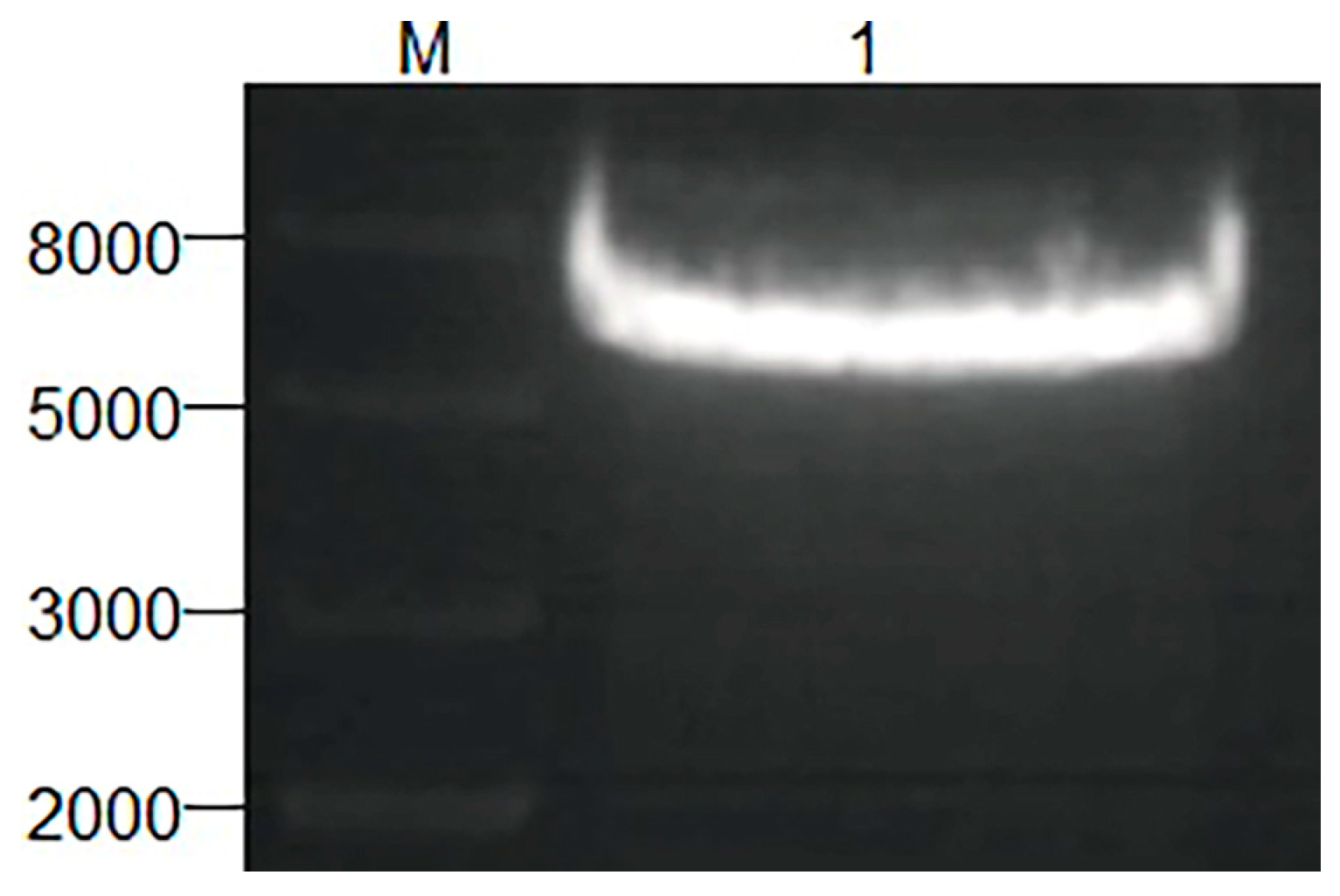
3.1. Construction of the Homologous Integration Expression Vector pGMLR
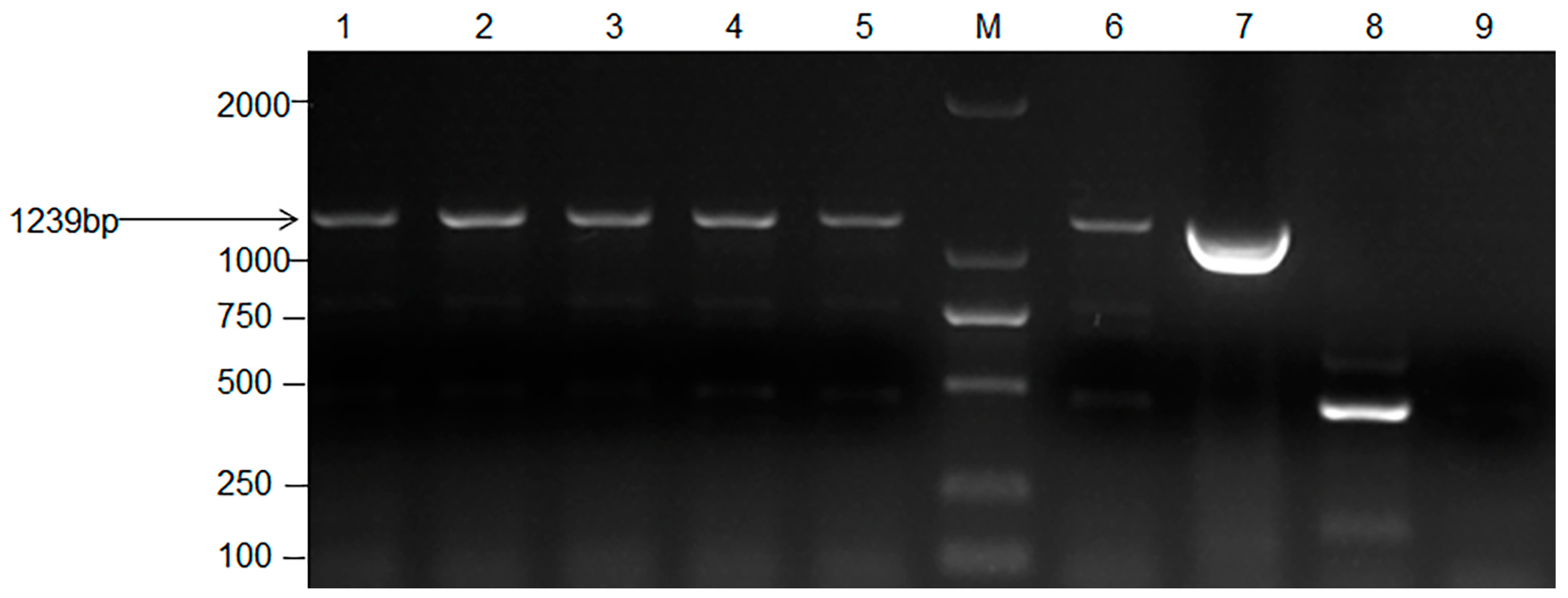
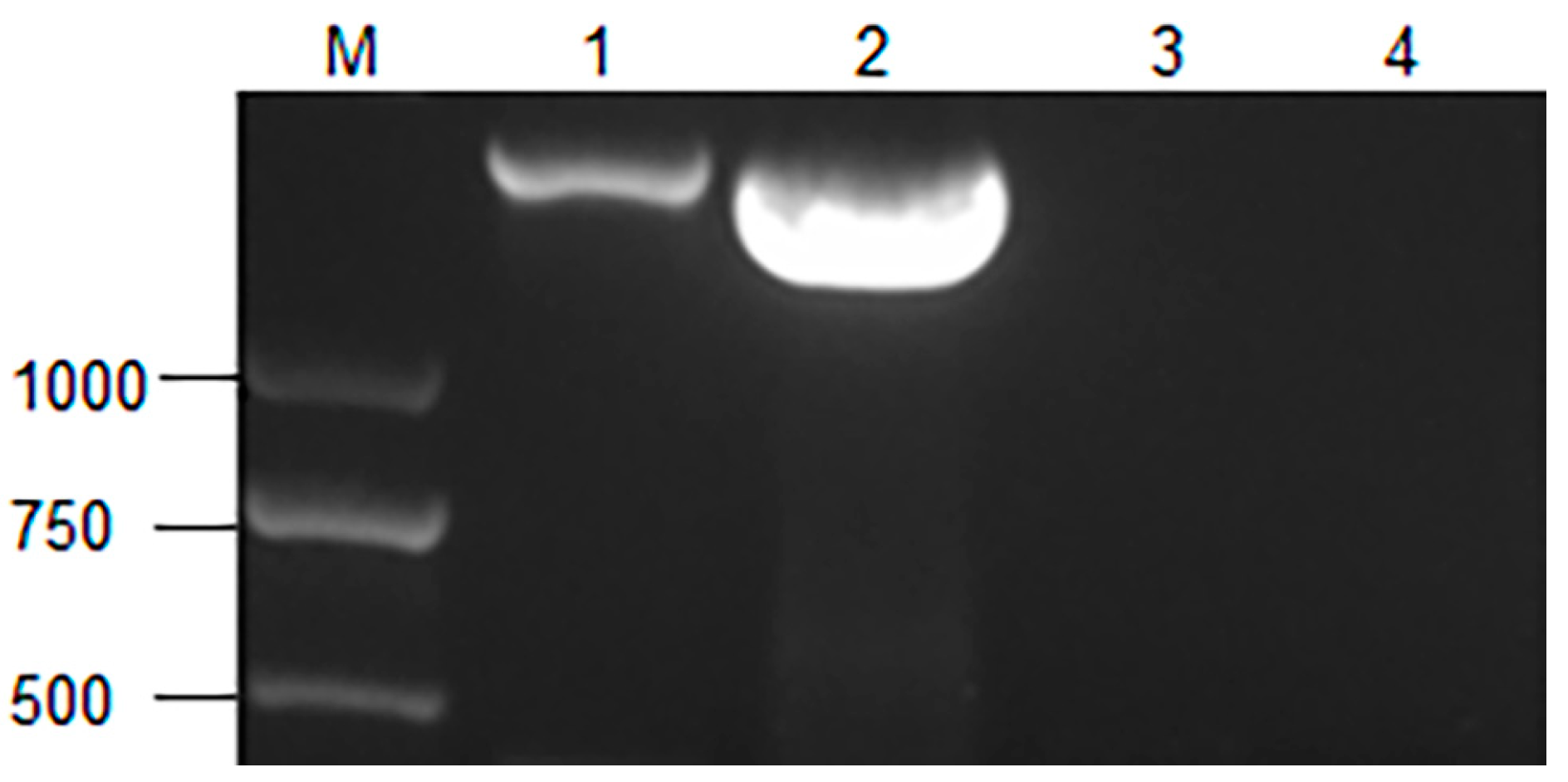
3.2. Identification and Genetic Stability Study of Transformants
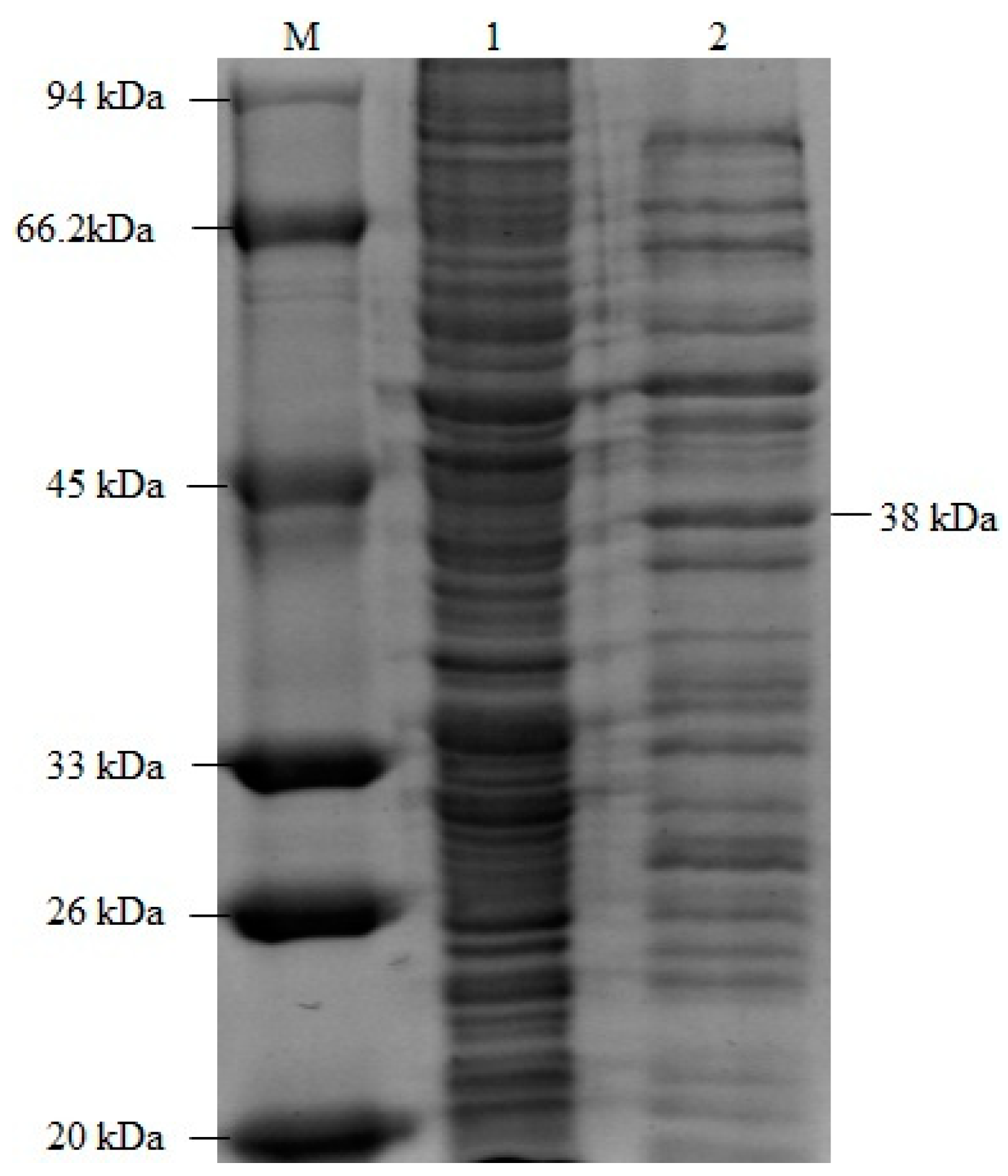
3.3. Expression of Recombinant Lignin Peroxidase Protein
3.4. Measuring the Enzyme Activity of Recombinant Lignin Peroxidase
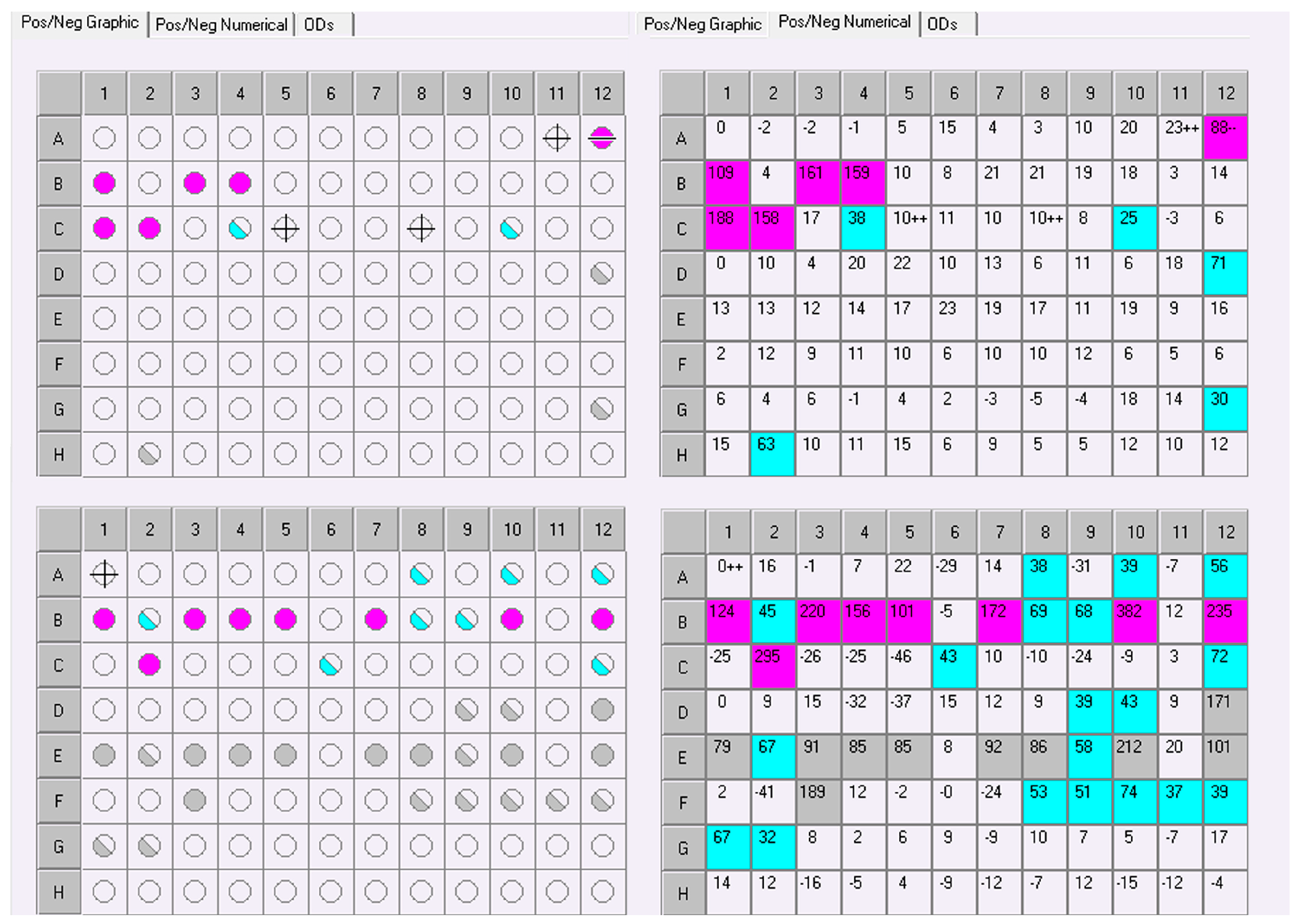
3.5. Phenotype Profiling with the Biolog MicroStation™ System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. chrysosporium | Phanerodontia chrysosporium |
| LiP | Lignin peroxidase enzyme |
| MnP | Mn-dependent peroxidase enzyme |
| C. jadinii | Cyberlindnera jadinii |
| lip | lignin peroxidase gene |
| FDA | Food and Drug Authority USA |
| CYH | Cycloheximide |
| GAPD | Glyceraldehyde-3-phosphate dehydrogenase |
| GAP-p | Glyceraldehyde-3-phosphate dehydrogenase promoter |
| GAP-t | Glyceraldehyde-3-phosphate dehydrogenase terminator |
| PDA | Potato Dextrose Agar |
| YPD | Yeast Potato Dextrose |
| SOE | Splicing Overlap Extension |
| VA | Veratryl Alcohol |
References
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; de Polizeli, M.L.; Silva, D.P.D.; Ruzene, D.S.; Vicente, A.A.; Jorge, J.A.; Terenzi, H.F.; Teixeira, J.A. Production of xylanolytic enzymes by Aspergillus terricola in stirred tank and airlift tower loop bioreactors. J. Ind. Microbiol. Biotechnol. 2011, 38, 1979–1984. [Google Scholar] [CrossRef]
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilization of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Yang, S.G.; Li, J.H.; Meng, Z.; Zheng, Z. Review on anaerobic biodegradation of lignocellulose. Trans. CSAE 2006, 22, 120–124. [Google Scholar]
- Zhang, L.X.; Tu, Y.; Li, Y.L.; Wang, H.M.; Diao, Q.Y. Effects of Different Microbes and Their Combinations on Rumen Degradation Rate of Corn Stalk. Chin. J. Anim. Nutr. 2014, 26, 2433–2444. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.H. Research Progress on Degradation of Agricultural Waste Straw by White Rot Fungi. J. Liaoning Inst. Sci. Technol. 2020, 22, 17–19. [Google Scholar]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Correa, R.C.G.; Peralta, R.A.; Moreira, R.; Polizeli, M.; Souza, C.; Peralta, R.M. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar]
- Wang, F.Q.; Xie, H.; Chen, W.; Du, F.G.; Song, A.D. Biological pretreatment of corn stover with ligninolytic enzyme for high efficient enzymatic hydrolysis. Bioresour. Technol. 2013, 144, 572–578. [Google Scholar] [CrossRef]
- Singh, D.; Chen, S.L. The white-rot fungus Phanerochaete chrysosporium: Conditions for the production of lignin-degrading enzymes. Appl. Microbiol. Biotechnol. 2010, 81, 399–417. [Google Scholar]
- Belinky, P.A.; Flikshtein, N.; Lechenko, S.; Gepstein, S.; Dosoretz, C.G. Reactive Oxygen Species and Induction of Lignin Peroxidase in Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2003, 69, 6500–6506. [Google Scholar] [CrossRef]
- Glenn, J.K.; Morgan, M.A.; Mayfield, M.B.; Kuwahara, M.; Gold, M.H. An Extracellular H2O2—Requir Enzyme Preparation Involved in Ligni Biodegradation by the White Rot Basidiomycete Phaenerochaete chrysosporium. Biochem. Biophy Res. Commun. 1983, 114, 1077–1083. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-Degrading Enzyme from the Hymenomycete Phaenerochaete Chrysosporium Burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Sadaqat, B.; Khatoon, N.; Malik, A.Y.; Jamal, A.; Farooq, U.; Ali, M.I.; He, H.; Liu, F.J.; Guo, H.G.; Urynowicz, M.; et al. Enzymatic decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Sci. Rep. 2020, 10, 20240. [Google Scholar] [CrossRef]
- Thanh Mai Pham, L.; Eom, M.H.; Kim, Y.H. Inactivating effect of phenolic unit structures on the biodegradation of lignin by lignin peroxidase from Phanerochaete chrysosporium. Enzym. Microb. Technol. 2014, 61–62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S. Role of various bacterial enzymes in complete depolymerization of lignin: A review. Biocatal. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Colonia, B.S.O.; Woiciechowski, A.L.; Malanski, R.; Letti, L.A.J.; Soccol, C.R. Pulp improvement of oil palm empty fruit bunches associated to solid-state biopulping and biobleaching with xylanase and lignin peroxidase cocktail produced by Aspergillus sp. LPB-5. Bioresour. Technol. 2019, 285, 121361. [Google Scholar] [CrossRef]
- Liu, H.J.; Li, L.M. Research progress of lignin peroxidase. China Feed 2010, 2, 20–26. [Google Scholar]
- Wang, W.; Wen, X. Expression of lignin peroxidase H2 from Phanerochaete chrysosporium by multi-copy recombinant Pichia strain. J. Environ. Sci. 2009, 21, 218–222. [Google Scholar] [CrossRef]
- Liu, J.L.; Liu, T.J.; Yu, Y.; Li, T.M.; Yi, H. Expression of lignin degrading enzyme system in Pichia pastoris and the activity of degrading lignin. Jiangsu Agric. Sci. 2015, 43, 58–62. [Google Scholar]
- Xiao, J.L.; Zhang, S.T.; Sun, X.Z.; Chen, G. Construction of an engineered Saccharomyces cerevisiae for lignin peroxidase production. Acta Microbiol. Sin. 2020, 60, 951–962. [Google Scholar]
- Hong, Y.R.; Chen, Y.L.; Farh, L.; Yang, W.J.; Liao, C.H.; Shuan, D. Recombinant Candida utilis for the production of biotin. Appl. Microbiol. Biotechnol. 2005, 71, 211–221. [Google Scholar] [CrossRef]
- Kondo, K.; Saito, T.; Kajiwara, S.; Takagi, M.; Misawa, N. A transformation system for the yeast Candida utilis; use of a modified endogenous ribosomal protein gene as a drug-resistant marker and ribosomal DNA as an integration target for vector DNA. J. Bacteriol. 1995, 177, 7171–7177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Luo, J.X.; Zhang, T.Y.; Chen, S.C.; Guan, W.J. Constitutive expression of human angiostatin in Pichia pastoris using the GAP promoter. Aata Genet. Sin. 2004, 31, 552–557. [Google Scholar]
- Yang, H.L.; Wang, W.; Bao, H.F.; Cui, C.S.; Hu, S. Construction of integrative vector of Candida utilis. Acta Microbiol. Sin. 2009, 49, 316–323. [Google Scholar]
- Liu, X.Y.; Shen, Y.; Guo, T.A.; Bao, X.M. Construction of a ribosomal DNA multi-copy integration vector and application in the industrial Saccharomyces cerevisiae strain. J. Shandong Univ. 2005, 40, 105–109. [Google Scholar]
- Tuo, D.C.; Shen, W.T.; Yan, P.; Li, X.Y.; Zhou, P. A Novel E. coli-Free Method for the Rapid Construction of Full-Length cDNA Infectious Clone of Papaya leaf distortion mosaic virus (PLDMV) by Yeast Homologous Recombination System. Chin. J. Trop. Crops 2017, 38, 1492–1500. [Google Scholar]
- Horton, R.M.; Cai, Z.; Ho, S.N.; Pease, L.R. Gene splicing by overlap extension: Tailor -made genes using the polymerase chain reaction. Biotechniques 1990, 8, 528–535. [Google Scholar] [CrossRef]
- Fan, H.C.; He, C. Evaluation of Physiological and Metabolic Characteristics of New Xylose-Utilizing Yeasts and the Construction of Genetic Expression System. Master’s Thesis, Jiangnan University, Wuxi, China, 2015. [Google Scholar]
- Wang, H.; Lu, F.; Sun, Y.; Du, L. Heterologous expression of lignin peroxidase of Phanerochaete chrysosporium in Pichia methanolica. Biotechnol. Lett. 2004, 26, 1569–1573. [Google Scholar] [CrossRef]
- Johnson, T.M.; Li, J.K. Heterologous expression and characterisation of an active lignin peroxidase from Phanerchaete chrysosporium using recombinant baculovirus. Arch. Biochem.Biophys. 1991, 291, 371–378. [Google Scholar] [CrossRef]
- Doyle, W.A.; Smith, A.T. Expression of lignin peroxidase H8 in Escherichia coli: Folding and activation of the recombinant enzyme with Ca2+ and haem. Biochem. J. 1996, 315, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.J.; Readin, N.S.; Aust, S.D. Expression of the lignin peroxidase H2 gene from Phanerochaete chrysosporium in Escherichia coli. Biochem. Biophys. Res. Commun. 1998, 249, 146–150. [Google Scholar] [CrossRef]
- Aifa, M.S.; Sayadi, S.; Gargouri, A. Heterologous expression of lignin peroxiedase of Phanerochaete chrysosporium in Aspergillus niger. Biotechnol. Lett. 1999, 21, 849–853. [Google Scholar] [CrossRef]
- Gaowa; Qiburi; Sachula; Su, S.F.; Wu, Q.H.; Huhe; Yu, P. Study on some biological characteristics of recombinant Candida utilis expressing lignin-degrading enzyme. China Feed 2022, 1, 53–59. [Google Scholar]


| Primers | Primers Sequence (5′ to 3′) | Size (bp) | Source | |
|---|---|---|---|---|
| 1 | GAP-p1 | GGATATCTTACAGCGAGCACTCAA EcoRV | 975 | C. jadinii |
| GAP-p2 | GCTCTAGAATGTTGTTTGT XbaI | |||
| 2 | GAP-t1 | CTAGCTAGCTATGACTTTTAT NheI | 462 | C. jadinii |
| GAP-t2 | GGGATCCTTCATTCATCCCTCACTATCG BamHI | |||
| 3 | L41-P4 | CGTCGACAGTAAGTATGAAAAGAGC SalI | 1970 | C. jadinii |
| L41-RM4 | GGGATCCGG GTTTGGTCTATGTTGCT BamHI | |||
| mL41-P5 | AACCAAGCAAGTTTTCCAC | CAA code Gln | C. jadinii | |
| mL41-R5 | GTGGAAAACTTGCTTGGTT | |||
| 4 | 18S rDNA-P3 | CGATATCTGCCAGTAGTCATATGC EcoRV | 1587 | C. jadinii |
| 18S rDNA-R3 | CGATATCTGACTTGCGCTTACTAG EcoRV | |||
| 5 | Lip-R1+1 | GCTCTAGAATGGCCTTCAAGCAGCTCTTC XbaI | 1239 | P. chrysosporium |
| Lip-F1+1 | CTAGCTAGCTTAGGCCTTGTGCGGGG NheI | |||
| 6 | M1-1S | GGGCCCTTCTGGGTCTTGTAATT | pGMLR | |
| M1-1AS | GCATATGACTACTGGCAAGTAAGTATGAAAAGAGCCAAT | |||
| M2-1S | ATTGGCTCTTTTCATACTTACTTGCCAGTAGTCATATGC | |||
| M2-1AS | GCCCTGTATCGTTATTTATTGTCACTACCTCCCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, G.; He, Q.; Wan, T.; Bai, S.; Hu, H.; Yu, P. Construction and Identification of a Food-Grade Recombinant Cyberlindnera jadinii Strain Expressing Lignin Peroxidase. Appl. Sci. 2023, 13, 6277. https://doi.org/10.3390/app13106277
Gong G, He Q, Wan T, Bai S, Hu H, Yu P. Construction and Identification of a Food-Grade Recombinant Cyberlindnera jadinii Strain Expressing Lignin Peroxidase. Applied Sciences. 2023; 13(10):6277. https://doi.org/10.3390/app13106277
Chicago/Turabian StyleGong, Gaowa, Qiburi He, Tingting Wan, Sule Bai, He Hu, and Peng Yu. 2023. "Construction and Identification of a Food-Grade Recombinant Cyberlindnera jadinii Strain Expressing Lignin Peroxidase" Applied Sciences 13, no. 10: 6277. https://doi.org/10.3390/app13106277
APA StyleGong, G., He, Q., Wan, T., Bai, S., Hu, H., & Yu, P. (2023). Construction and Identification of a Food-Grade Recombinant Cyberlindnera jadinii Strain Expressing Lignin Peroxidase. Applied Sciences, 13(10), 6277. https://doi.org/10.3390/app13106277





