Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification
Abstract
1. Introduction
2. Mature Technologies for Water and Wastewater Remediation
2.1. Chlorination
2.1.1. Applications of Chlorination
2.1.2. Chlorination Process
2.1.3. Mechanism of Chlorination
2.1.4. Advantages and Disadvantages of Chlorination
2.2. Ozonation
2.2.1. Applications of Ozonation
2.2.2. Ozonation Process
2.2.3. Advantages and Disadvantages of Ozonation
2.3. Ultraviolet Radiation
2.3.1. Applications of UV Radiation
2.3.2. UV Radiation Process
2.3.3. Advantages and Disadvantages of UV Radiation
3. More Recent Technology: Non-Thermal Plasma
3.1. Non-Thermal Plasma Overview
3.2. Applications of Plasma Purification
3.3. Plasma Purification Process
3.4. Advantages and Disadvantages of Plasma Purification
4. Tertiary Water and Wastewater Pollutants
4.1. Contaminants of Emerging Concern
4.2. Pathogens in Drinking Water
4.3. Inorganic Contaminants in Drinking Water
5. Comparison between Mature Methods and More Recent Non-Thermal Plasma Technology
5.1. Materials and Methods
5.1.1. Plasma Applications in Water Purification
5.1.2. Planning-Energy and Costs
5.1.3. Calculations
Operating Cost Calculations
Capital Cost Calculations
Energy Yield Calculations
| Non-Thermal Plasma Reactor/Discharge Type | Pollutant Description | Operation Conditions | Degradation Performance | Ref. | |
|---|---|---|---|---|---|
| Type | Category/Class | ||||
| Plasma catalysis/dielectric barrier discharge (DBD) | Phenol | Organic | Applied voltage: 16 kV Frequency: 50 Hz Discharge current: 0.56 mA Gas flow rate: 3.2 mL/s | Contact time: 50 s Removal efficiency: 99% | [81] |
| Gas–liquid DBD reactor | Carbamazepine | Organic: EDC | Applied power: 0.7 W Air flow rate: 1 L/min Initial concentration (Co): 20 mg/L Liquid flow rate: 6 mL/min | Contact time: 3 min Removal efficiency: 100% Energy density: 25 kJ/L | [82] |
| DBD | Oestrone | Organic: EDC | Applied voltage: 80 kV Frequency: 50 Hz Initial concentration (Co): 2 mg/L | Contact time: 15 min Removal efficiency: >80% Energy yield: 777–737 × 10−6 g/kWh | [71] |
| DBD | PFOS | Organic: EDC | Applied voltage: 130 kV Frequency: 17 Hz Applied Power: 322 W Peak Current: 40A Initial concentration (Co): 1 ppb | Contact time: 60 min Removal efficiency: >85% | [83] |
| DBD | E. coli | Pathogen | Applied voltage: 4.16 kV Frequency: 27.6 Hz Discharge current: 13.01 A Initial concentration (Co): 1 × 108 cfu mL−1 | Contact time: 60 min Removal efficiency: >99% Energy (duty): 0.24 J/s | [84] |
| Cold atmospheric plasma jet | Cryptosporidium | Pathogen | Applied power: 549 W Frequency: 47 kHz | Contact time: 3 min Removal efficiency: 2.03 log inactivation | [85] |
| Radio-frequency (RF) atmospheric pressure plasma jet (APPJ) | Norovirus (feline calicivirus (FCV)) | Pathogen | Applied power: 2.5 W Frequency: 13.36 MHz Ar gas flow rate: 1.5 standard litres per min (SLM) | Contact time: 15 s Removal efficiency: 6.0 log inactivation | [86] |
| DBD photocatalyst | Chloroform | Disinfection byproduct | Applied high voltage (AC): 20 kV Frequency: 52–30,000 Hz Chloroform vapours (in air) flow rate: 0.3 L/min Initial concentration (Co): 85 ppm | Contact time: 2 s Removal efficiency: 70% | [43] |
| Corona discharge | 1 Bromate | Disinfection byproduct | Peak voltage: 20 kV Current: 13.8 A Peak power: 203 kW Frequency: 30 Hz Initial concentration (Co): 30 µM | Contact time: 60 min Removal/reduction efficiency: 95% Energy: 0.16 J | [87] |
| Micro discharge plasma jet (MDPJ)/DBD | E. coli | Pathogen | Applied voltage: 1.01–1.66 kV Frequency (Transformer): 60 Hz Air/Nitrogen gas flow rates: 2–4 L/min Initial concentration (No): 2.4 × 107 CFU/mL | Contact time: 40 min Removal efficiency: 99.9% | [88] |
| Non-thermal plasma (NTP)/spark discharge plasma | Enterococcus faecalis (E. faecalis) and E. coli | Pathogens | Applied voltage: 10 kV Frequency: 30 Hz Initial concentration (No): 1 × 108 CFU/mL | Contact time: 12 min (E. faecalis) and 15 min (E. coli) Removal efficiency: 8-log CFU reduction (E. faecalis and E. coli) | [89] |
| Type | Contaminant | Chlorination Technology | Ozonation Technology | ||||
|---|---|---|---|---|---|---|---|
| CT (mg/L × min) | Conc * × Time # | % Removal | CT (mg/L × min) | Conc * × Time # | % Removal | ||
| Organic | Phenol | 5570 [90] | 5570 × 1 | 99 | 1200 [91] | 80 × 15 | >99 |
| Organic: EDC | Carbamazepine | 1065 [92] | 17.75 × 60 | 40 | 2.2 [93] | 0.44 × 5 | >99.9 |
| Organic: EDC | Oestrone | IDA 1 | IDA 1 | IDA 1 | 0.06 [94] | 4.4 × 0.014 | 99.8 |
| Organic: EDC | PFOS | 1440 [95] | 4 × 360 | 83 | 840 [96] | 3.5 × 240 | 43 |
| Inorganic | Manganese | 1.161 [97,98] | 2.322 × 0.5 | 99 | 0.792 [99] | 1.584 × 0.5 | 99 |
| Inorganic | Iron | 0.496 [97,98] | 0.992 × 0.5 | 99 | 0.344 [99] | 0.688 × 0.5 | 99 |
| Pathogen | E. coli | 0.25 [100] | 0.25 × 1 | 99.99 | 0.05 [101] | IDA 1 | 99.99 |
| Pathogen | Cryptosporidium | 7200 [102] | 80 × 90 | 99 | 6.2 [103] | IDA 1 | >99 |
| Pathogen | Norovirus | 2 [104] | IDA 1 | 99.9 | 1.3 [105] | IDA 1 | 99.99 |
| Disinfection byproduct | Chloroform | + 2 | + 2 | ||||
| Disinfection byproduct | Bromate | + 2 | + 2 | ||||
| Type | Contaminant | Non-Thermal Plasma Technology | |||
|---|---|---|---|---|---|
| Plasma Type | PT (W × Min) | Power * × Time # | % Removal | ||
| Organic | Phenol | DBD 1 | 2430 [81] | 9 × 0.83 | 98 |
| Organic: EDC | Carbamazepine | DBD 1 | 36 [82] | 12 × 3 | 99.99 |
| Organic: EDC | Oestrone | DBD 1 | 60,000 [71] | 4000 × 15 | 83.6 |
| Organic: EDC | PFOS | DBD 1 | 19,320 [83] | 322 × 60 | 93.5 |
| Inorganic | Manganese | IDA 2 | |||
| Inorganic | Iron | IDA 2 | |||
| Pathogen | E. coli | DBD 1 | 1080 [84] | 54 × 20 | 99.9 |
| Pathogen | Cryptosporidium | AC 3 Gliding Arc | 1647 [85] | 549 × 3 | >99 |
| Pathogen | Norovirus | DBD1 | 24 [106] | 12 × 2 | 99.99 |
| Disinfection by-product | Chloroform | DBD1 | 18 [43,71] | 600 × 0.03 | 80 |
| Disinfection by-product | Bromate | + 4 | |||
6. Results
6.1. Typical Operating Conditions for Non-Thermal Plasma-Based Contaminant Degradation
6.2. Efficiency Tables
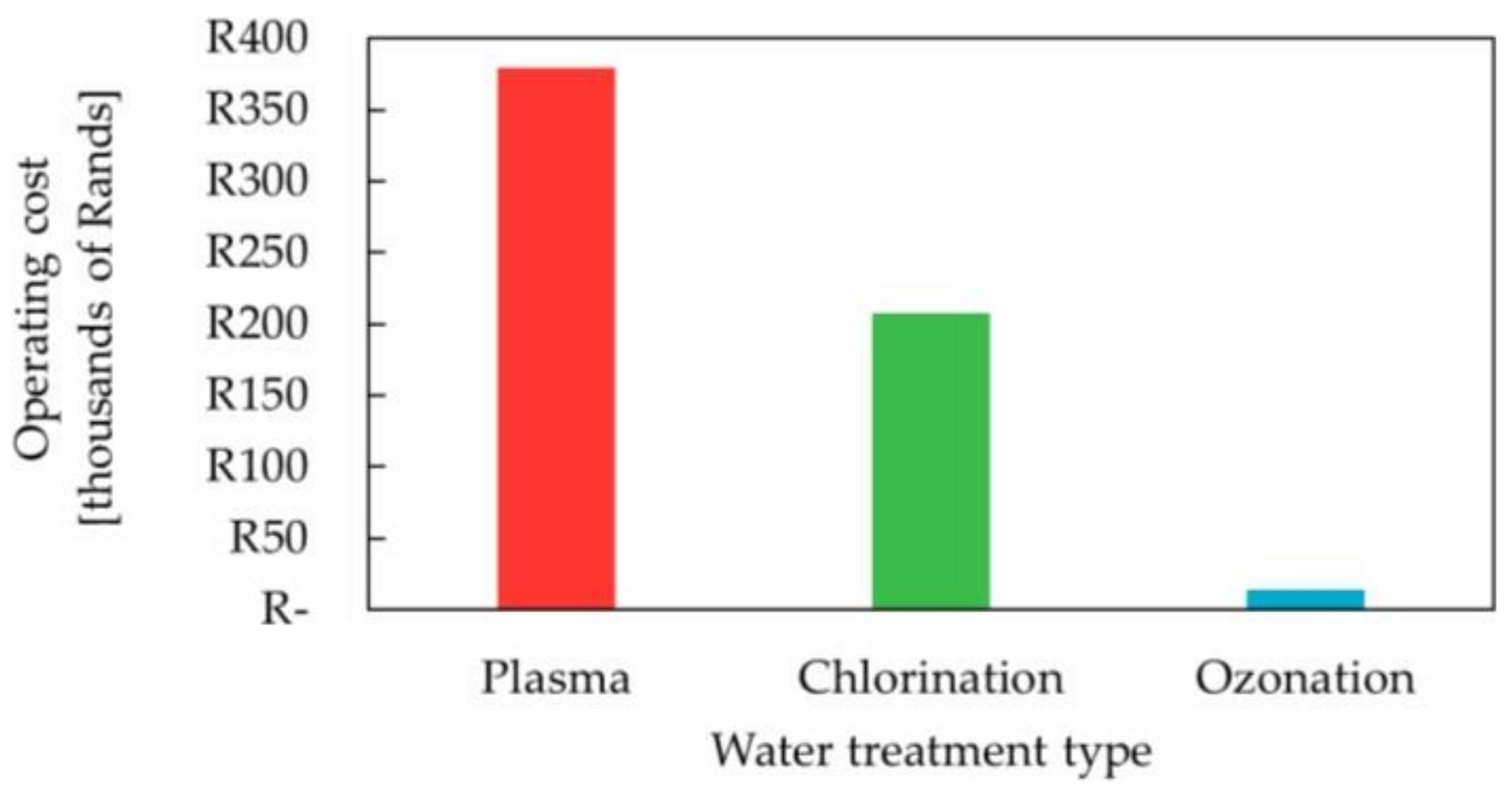
6.3. Operating Cost
6.4. Capital Cost
6.5. Energy Yield
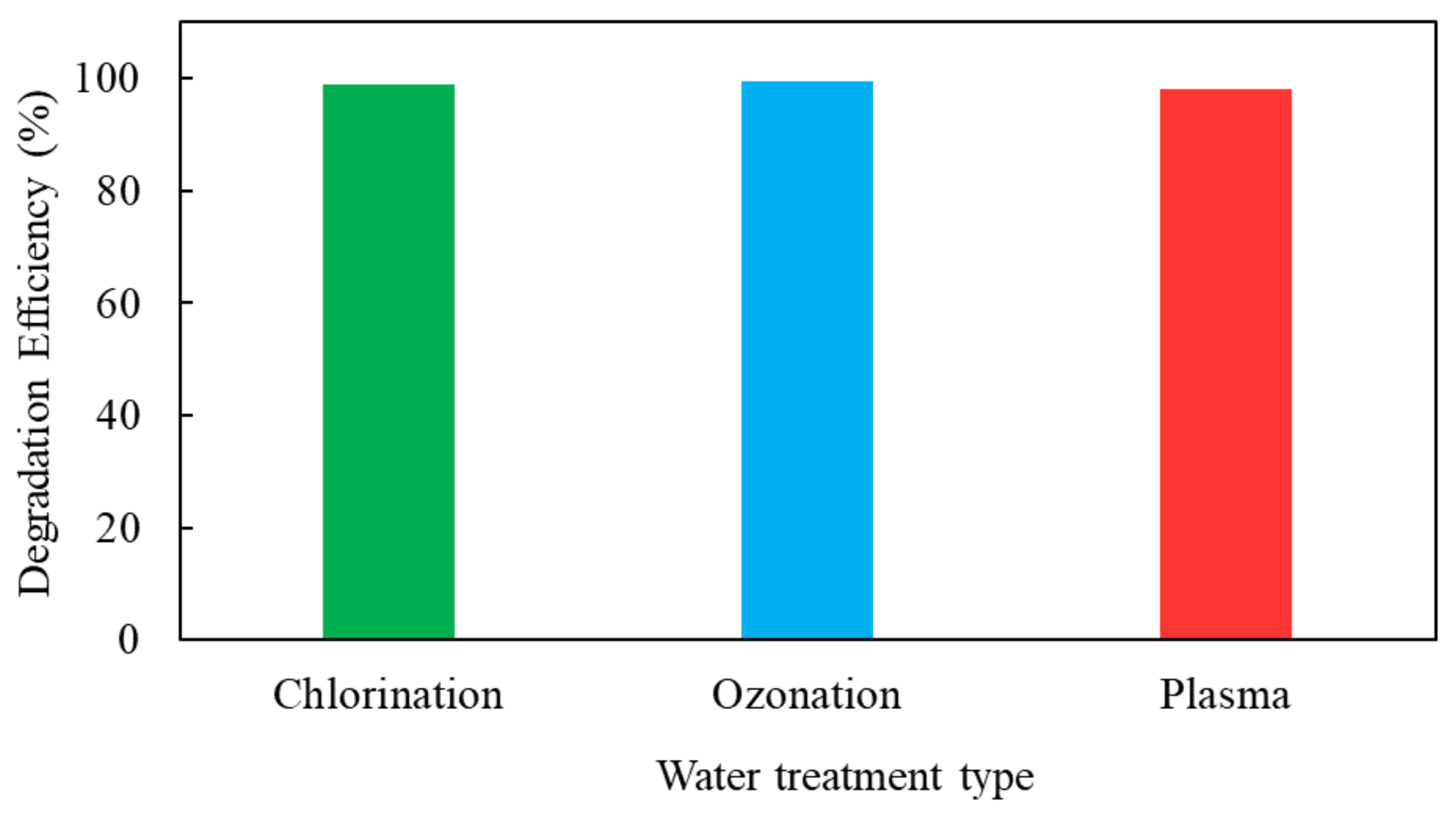
6.6. Degradation Efficiency
| Contaminant Description | Non-Thermal Plasma Technology | Chlorination Technology | Ozonation Technology | |||||
|---|---|---|---|---|---|---|---|---|
| Plasma Type | PT (W × min) | % Removal | CT (mg/L × min) | % Removal | CT (mg/L × min) | % Removal | ||
| Organic | Phenol | DBD 1 | 2430 [81] | 98 | 5570 [90] | 99 | 1200 [91] | >99 |
| Organic: EDC | Carbamazepine | DBD 1 | 36 [82] | 99.99 | 1065 [92] | 40 | 2.2 [93] | >99.9 |
| Organic: EDC | Oestrone | DBD 1 | 60,000 [71] | 83.6 | IDA 1 | IDA 1 | 0.06 [94] | 99.8 |
| Organic: EDC | PFOS | DBD 1 | 19,320 [83] | 93.5 | 1440 [95] | 83 | 840 [96] | 43 |
| Inorganic | Manganese | IDA 2 | 1.161 [97,98] | 99 | 0.792 [99] | 99 | ||
| Inorganic | Iron | IDA 2 | 0.496 [97,98] | 99 | 0.344 [99] | 99 | ||
| Pathogen | E. coli | DBD 1 | 1080 [84] | 99.9 | 0.25 [100] | 99.99 | 0.05 [101] | 99.99 |
| Pathogen | Cryptosporidium | AC 3 Gliding Arc | 1647 [85] | >99 | 7200 [102] | 99 | 6.2 [103] | >99 |
| Pathogen | Norovirus | DBD 1 | 24 [106] | 99.99 | 2 [104] | 99.9 | 1.3 [105] | 99.99 |
| Disinfection byproduct | Chloroform | DBD 1 | 18 [43,71] | 80 | + 2 | + 2 | ||
| Disinfection byproduct | Bromate | + 4 | + 2 | + 2 | ||||
7. Discussion
7.1. Efficiency Table Analysis
7.2. Operating Cost Analysis
7.3. Capital Cost Analysis
7.4. Energy Yield Analysis
7.5. Chemical Demand Analysis
7.6. Degradation Efficiency
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brusseau, M.; Pepper, I.; Gerb a, C. Environmental and Pollution Science, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 393–418. [Google Scholar]
- Ameta, R.; Ameta, S.C. Advanced Oxidation Processes for Waste Water Treatment, 1st ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–12. [Google Scholar]
- Gago-Ferrero, P.; Gros, M.; Ahrens, L.; Wiberg, K. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharmaceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Sorengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass loads, source apportionment, and risk estimation of organic micropollutants from hospital and municipal wastewater in recipient catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gadupudi, C.K.; Rice, L.; Xiao, L.; Kantamaneni, K. Endocrine disrupting compounds removal methods from wastewater in the United Kingdom: A review. Sci 2021, 3, 11. [Google Scholar] [CrossRef]
- van Zijl, M.C. Estrogenic Activity, Target Endocrine Disrupting Chemical Levels and Potential Health Risks of Bottled Water and Water from Selected Distribution Points in Pretoria and Cape Town. PhD Thesis, University of Pretoria, Pretoria, South Africa, 2016. [Google Scholar]
- Amor, C.; Marchão, L.; Lucas, M.S.; Peres, J.A. Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water 2019, 11, 205. [Google Scholar] [CrossRef]
- Flowrox Plasma Oxidizer. Available online: https://flowrox.com/article/plasma-water-treatment/ (accessed on 5 July 2021).
- Chlorine and Drinking Water. Available online: https://chlorine.americanchemistry.com/Chlo-rine/DrinkingWaterFAQ/ (accessed on 5 July 2021).
- Water Purification Methods-and the South African Position. Available online: https://www.instrumentation.co.za/article.aspx?pklarticleid=2906 (accessed on 5 July 2021).
- National Research Council (US) Safe Drinking Water Committee. Drinking Water and Health; National Academies Press (US): Washington, DC, USA, 1980; Volume 2. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234590/ (accessed on 3 April 2023).
- Chlorination. Available online: https://sswm.info/sswm-solutions-bop-markets/affordable-wash-services-and-products/ (accessed on 5 July 2021).
- MSR SE200TM Community Chlorine Maker. Available online: https://www.msrgear.com/ie/products/global-health/se200-community-chlorine-maker/10275.html (accessed on 5 July 2021).
- Safe Drinking Water Foundation. What is Chlorination Fact Sheet. Available online: https://www.safewater.org/fact-sheets-1/2017/1/23/what-is-chlorination (accessed on 3 April 2023).
- Lenntech Disinfection By-products Types. Available online: https://www.lenntech.com/processes/disinfection/byproducts/disinfection-byproducts-types.htm (accessed on 5 July 2021).
- Jameson, P.B.; Hung, Y.-T.; Kuo, C.Y.; Bosela, P.A. Cryptosporidium outbreak (water treatment failure): North Battleford, Saskatchewan, spring 2001. J. Perform. Constr. Facil. 2008, 22, 342–347. [Google Scholar] [CrossRef]
- Bright Blue CCS Ozone Systems for Prepared Water Disinfection. Available online: https://www.wassertec.co.za/ozone-water-products/bright-blue-ccs/ (accessed on 5 July 2021).
- How Is Aqueous Ozone Used in Olympic Swimming Pools? Available online: https://purozo.co.uk/aqueous-ozone-in-olympic-swimming-pools/ (accessed on 5 July 2021).
- Eco-logical Technology: Medical Ozone Use in Hospitals, Clinics and Nursing Homes. Available online: https://www.eco3.co.za/medical_ozone.htm (accessed on 5 July 2021).
- United States Environmental Protection Agency. Wastewater Technology Fact Sheet: Ozone Disinfection; Office of Water: Washington, DC, USA, 1999.
- Van Der Walt, M.; Krüger, M.; Van Der Walt, C. The South African Oxidation and Disinfection Manual; Technical Report; Water Research Commission: Pretoria, South Africa, 2009; ISBN 9781770058637. [Google Scholar]
- Ozone Applications: Drinking Water. Available online: https://www.lenntech.com/library/ozone/drinking/ozone-applications-drinking-water.htm (accessed on 5 July 2021).
- Ozonation in Water Treatment. Available online: https://www.knowyourh2o.com/indoor-4/ozonation-in-water-treatment (accessed on 3 April 2023).
- Gould, J.P.; Weber, W.J., Jr. Oxidation of phenols by ozone. J. Water Pollut. Control Fed. 1976, 48, 47–60. [Google Scholar]
- Iron and Manganese Removal with Ozone. Available online: https://ozonesolutions.com/blog/iron-and-manganese-removal-with-ozone/ (accessed on 3 April 2023).
- de Oliveira Pereira, R.; de Alda, M.L.; Joglar, J.; Daniel, L.A.; Barceló, D. Identification of new ozonation disinfection byproducts of 17β-estradiol and estrone in water. Chemosphere 2011, 84, 1535–1541. [Google Scholar] [CrossRef]
- Richardson, S.D. Disinfection by-products and other emerging contaminants in drinking water. Trends Analyt. Chem. 2003, 22, 666–685. [Google Scholar] [CrossRef]
- Environmental Technology Initiative. What Is Ozone Disinfection? Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Ozone Generators That Are Sold as Air Cleaners. Available online: https://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners (accessed on 5 July 2021).
- UV Disinfection Drinking Water Treatment. Available online: https://www.knowyourh2o.com/indoor-4/ultraviolet-disinfection (accessed on 3 April 2023).
- Rhode Island Department of Health; University of Rhode Island Cooperative Extension Water Quality Program. Ultraviolet Radiation Treatment of Drinking Water Supplies; Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Lin, J. Conventional Water Treatment Processes for Removing Pharmaceutical and Endocrine Disrupting Compounds. Master’s Thesis, Graduate School of the University of Massachusetts, Massachusetts, MA, USA, 2011. [Google Scholar]
- American Air and Water: UV Water Disinfection. Available online: https://www.americanairandwater.com/uv-water-applications.htm (accessed on 5 July 2021).
- Pros and Cons of UV filtration. Available online: https://haguewaterofmd.com/pros-and-cons-of-uv-fil-tration/ (accessed on 5 July 2021).
- Dave, H.; Ledwani, L.; Nema, S.K. The Impact and Prospects of Green Chemistry for Textile Technology, 1st ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 199–249. [Google Scholar] [CrossRef]
- Zeghioud, H.; Nguyen-Tri, P.; Khezami, L.; Amrane, A.; Assadi, A.A. Review on discharge Plasma for water treatment: Mechanism, reactor geometries, active species and combined processes. J. Water Process Eng. 2020, 38, 101664. [Google Scholar] [CrossRef]
- Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. Water decontamination using non-thermal plasma: Concepts, applications, and prospects. J. Environ. Chem. Eng. 2020, 8, 104377. [Google Scholar] [CrossRef]
- Malik, M.A. Water purification by plasmas: Which reactors are most energy efficient? Plasma Chem. Plasma Process. 2010, 30, 21–31. [Google Scholar] [CrossRef]
- Yusuf, A.; Amusa, H.K.; Eniola, J.O.; Giwa, A.; Pikuda, O.; Dindi, A.; Bilad, M.R. Hazardous and emerging contaminants removal from water by plasma-based treatment: A review of recent advances. Chem. Eng. J. Adv. 2023, 14, 100443. [Google Scholar] [CrossRef]
- Tak, G.; Gallagher, M.; Gangoli, S.; Gutsol, A.; Fridman, A. Use of non-thermal atmospheric pressure plasma for air cleaning and sterilization. In IEEE Conference Record-Abstracts, Proceedings of IEEE International Conference on Plasma Science, Monterey, CA, USA, 20–23 June 2005; IEEE: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Fridman, N.; Lahav, O. Formation and minimization of bromate ions within non-thermal-plasma advanced oxidation. Desalination 2011, 280, 273–280. [Google Scholar] [CrossRef]
- Aljundi, I.H. Bromate formation during ozonation of drinking water: A response surface methodology study. Desalination 2011, 277, 24–28. [Google Scholar] [CrossRef]
- Shahna, F.G.; Ebrahimi, H.; Jaleh, B.; Bahrami, A. Decomposition of gas-phase chloroform using nano-photocatalyst downstream the novel non-thermal plasma reactor: By-products elimination. Int. J. Environ. Sci Technol. 2015, 12, 3489–3498. [Google Scholar] [CrossRef]
- Sciex: Contaminants of Emerging Concern. Available online: https://sciex.com/applications/environmental-testing/contaminants-of-emerging-concern (accessed on 5 July 2021).
- Wee, S.Y.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Occurrence of multiclass endocrine disrupting com- pounds in a drinking water supply system and associated risks. Sci. Rep. 2010, 10, 17755. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Momba, M.; Kibambe, G.; Thobela, K.; Kgositau, T.; Mahlangu, P. The Removal of Endocrine Disrupting Compounds by Wastewater Treatment Plants; Technical Report to the Water Research Commission; Tshwane University of Technology: Tshwane, South Africa, 2017. [Google Scholar]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine disruptors in water and their effects on the reproductive system. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety. Global Assessment of the State-of-the-Science of Endocrine Disruptors; Technical Report; World Health Organisation: Geneva, Switzerland, 2002. [Google Scholar]
- Björvang, R.D.; Damdimopoulou, P. Persistent environmental endocrine disrupting chemicals in ovarian follicular fluid and in vitro fertilization treatment outcome in women. Ups. J. Med. Sci. 2020, 125, 85–94. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Wise, A.; O’Brien, K.; Woodruff, T. Are oral contraceptives a significant contributor to the estrogenicity of drinking water? Environ. Sci. Technol. 2011, 45, 51–60. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Gwanzura, E. Degradation Studies of Carbamazepine and Escherichia coli in Wastewater Using a Non-Thermal Electrical Discharge Reactor. Master’s Thesis, University of KwaZulu-Natal, KwaZulu-Natal, South Africa, 2020. [Google Scholar]
- Corsini, E.; Luebke, R.W.; Germolec, D.R.; DeWitt, J.C. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014, 230, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Farré, M.; Eljarrat, E.; Diaz, S.; Barceló, D. Liquid Chromatography, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 389–410. [Google Scholar]
- Environmental and Occupational Health Surveillance Program. Perfluorinated Chemicals (PFCs) in Drinking Water; Technical Report; New Jersey Department of Health: Trenton, NJ, USA, 2016.
- Coliform Bacteria in Drinking Water Supplies. Available online: https://www.health.ny.gov/environmental/water/drinking/coliform_bacteria.htm (accessed on 5 July 2021).
- Coliform Bacteria? Examples, Characteristics, Fecal/Total Count Tests. Available online: https://www.microscopemaster.com/coliform.html (accessed on 5 July 2021).
- Indicators of Drinking Water Quality. Available online: https://waterandhealth.org/safe-drinking-water/indicators-drinking-water-quality (accessed on 5 July 2021).
- E. coli Infection from Food or Water. Available online: https://www.healthlinkbc.ca/health-top-ics/hw133795 (accessed on 5 July 2021).
- World Health Organisation. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organisation: Geneva, Switzerland, 2017; pp. 30–630. ISBN 978-92-4-154995-0. [Google Scholar]
- Centers for Disease Control and Prevention: Water-Related Diseases and Contaminants in Public Water Systems. Available online: https://www.cdc.gov/healthywater/drinking/public/water_diseases.html (accessed on 5 July 2021).
- National Health Service Norovirus: Vomiting Bug. Available online: https://www.nhs.uk/conditions/norovirus/ (accessed on 5 July 2021).
- South Dakota Department of Agriculture and Natural Resources: Inorganic Contaminants. Available online: https://denr.sd.gov/des/dw/IOCs.aspx (accessed on 5 July 2021).
- American Water Works Association: Contaminants of Concern. Available online: https://www.awwa.org/Resources-Tools/Resource-Topics/Inorganic-Contaminants (accessed on 5 July 2021).
- Penn State Extension: Iron and Manganese in Private Water Systems. Available online: https://extension.psu.edu/iron-and-manganese-in-private-water-systems (accessed on 5 July 2021).
- Bruce, I.D.; Sharon, O.S.; Wayne, E.W. Drinking Water: Iron and Manganese. NebGuide Series. Natural Resources/Water Management. 2021, G1714, pp. 1–6. Available online: https://extensionpubs.unl.edu/publication/9000016364707/drinking-water/ (accessed on 5 July 2021).
- Manganese: Get Informed. Available online: https://www.knowyourh2o.com/indoor-6/manganese (accessed on 3 April 2023).
- Rana, A.G.; Tasbihi, M.; Schwarze, M.; Minceva, M. Efficient advanced oxidation process (AOP) for photocatalytic contaminant degradation using exfoliated metal-free graphitic carbon nitride and visible light-emitting diodes. Catalysts 2021, 11, 662. [Google Scholar] [CrossRef]
- Office of Water. Disinfection Profiling and Benchmarking Technical Guidance Manual; Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 2020.
- Sarangapani, C.; Danaher, M.; Tiwari, B.; Lu, P.; Bourke, P. Efficacy and mechanistic insights into endocrine disruptor degradation using atmospheric air plasma. Chem. Eng. J. 2017, 326, 700–714. [Google Scholar] [CrossRef]
- How Temperature Impacts Onsite Wastewater Treatment. Available online: https://www.onsiteinstaller.com/online_exclusives/2017/05/how_temperature_impacts_onsite_wastewater_treatment (accessed on 5 July 2021).
- Division of Toxicology and Environmental Medicine, Applied Toxicology Branch. Toxicological Profile For phenol; Technical Report; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2008.
- Trading Economics: South Africa Prime Lending Rate. Available online: http://www.tradingeconomics.com/south-africa/bank-lending-rate (accessed on 28 September 2021).
- Tribe, M.A.; Alpine, R.L.W. Scale economies and the “0.6 Rule”. Eng. Cost. Prod. Econ. 1986, 10, 271–278. [Google Scholar] [CrossRef]
- Barillas, L. Design of a prototype of water purification by plasma technology as the foundation for an industrial wastewater plant. J. Phys. Conf. Ser. 2015, 591, 012057. [Google Scholar] [CrossRef]
- STATSSA: South Africa Consumer Price Index (CPI) (1960–2021). Available online: https://tradingeconomics.com/south-africa/consumer-price-index-cpi (accessed on 28 September 2022).
- Eskom. Eskom Tariffs & Charges Booklet 2020/2021; Technical Report; Eskom: Sunninghill, South Africa, 2020. [Google Scholar]
- Eskom. Tariffs & Charges 2009/10; Technical Report; Eskom: Sunninghill, South Africa, 2009. [Google Scholar]
- Loiteh Ozone Generators. Available online: www.loiteh.ee/eng/ozone-generators (accessed on 28 September 2021).
- Bubnov, A.G.; Burova, E.Y.; Grinevich, V.I.; Rybkin, V.V.; Kim, J.K.; Choi, H.S. Plasma-catalytic de- composition of phenols in atmospheric pressure dielectric barrier discharge. Plasma Chem. Plasma Process. 2006, 26, 19–30. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, S.; Iya-Sou, D.; Cavadias, S.; Ognier, S. Carbamazepine removal from water by dielectric barrier discharge: Comparison of ex situ and in situ discharge on water. Chem. Eng. Process. Process Intensif. 2012, 56, 10–18. [Google Scholar] [CrossRef]
- Palma, D.; Papagiannaki, D.; Lai, M.; Binetti, R.; Sleiman, M.; Minella, M.; Richard, C. PFAS degradation in ultrapure and groundwater using non-thermal plasma. Molecules 2021, 26, 924. [Google Scholar] [CrossRef]
- Akter, M.; Yadav, D.K.; Ki, S.H.; Choi, E.H.; Han, I. Inactivation of infectious bacteria using nonthermal biocompatible plasma cabinet sterilizer. Int. J. Mol. Sci. 2020, 21, 8321. [Google Scholar] [CrossRef]
- Craighead, S.; Hertrich, S.; Boyd, G.; Sites, J.; Niemira, B.A.; Kniel, K.E. Cold atmospheric plasma jet inactivates Cryptosporidium parvum oocysts on cilantro. J. Food Prot. 2020, 83, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Williams, P.; Gangal, U.; Youssef, M.M.; El-Sohaimy, S.A.; Bruggeman, P.J.; Goyal, S.M. Virucidal Effect of Cold Atmospheric Gaseous Plasma on Feline Calicivirus, a Surrogate for Human Norovirus. Appl. Environ. Microbiol. 2015, 81, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Lakhian, V.; Dickson-Anderson, S.E. Reduction of bromate and chlorate contaminants in water using aqueous phase corona discharge. Chemosphere 2020, 255, 126864. [Google Scholar] [CrossRef]
- Ma, S.; Kim, K.; Huh, J.; Hong, Y. Characteristics of microdischarge plasma jet in water and its application to water purification by bacterial inactivation. Sep. Purif. Technol. 2017, 188, 147–154. [Google Scholar] [CrossRef]
- Rashmei, Z.; Bornasi, H.; Ghoranneviss, M. Evaluation of treatment and disinfection of water using cold atmospheric plasma. J. Water Health 2016, 14, 609–616. [Google Scholar] [CrossRef]
- Cleary, E.J. Phenol Wastes Treatment by Chemical Oxidation, 1st ed.; Ohio River Valley Water Sanitation Commission: Cincinnati, OH, USA, 1951; pp. 17–34. [Google Scholar]
- Mooketsi, O.I. Evaluation of Ozone for the Removal of Phenolic Compounds in Wastewater from the Merisol Plant (Sasolburg). Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2008. [Google Scholar]
- Zhou, S.; Xia, Y.; Yao, T.; Shi, Z.; Zhu, S. Degradation of carbamazepine by UV/chlorine advanced oxidation process and formation of disinfection by-products. Environ. Sci. Pollut. Res. 2016, 23, 16448–16455. [Google Scholar] [CrossRef] [PubMed]
- Kraakström, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Ozonation of carbamazepine and its main transformation products: Product determination and reaction mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 23258–23269. [Google Scholar] [CrossRef] [PubMed]
- Pešoutová, R.; Stříteský, L.; Hlavínek, P. A pilot scale comparison of advanced oxidation processes for estrogenic hormone removal from municipal wastewater effluent. Water Sci. Technol. 2014, 70, 70–75. [Google Scholar] [CrossRef]
- Belkouteb, N.; Franke, V.; McCleaf, P.; Köhler, S.J.; Ahrens, L. Removal of per- and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant—Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Res. 2020, 182, 115913. [Google Scholar] [CrossRef]
- Yu-Chen Lin, A.; Sri Chandana, P.; Chang, C.-Y.; Hong, P.A.; Hsueh, H.F. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. J. Hazard. Mater. 2012, 243, 272–277. [Google Scholar] [CrossRef]
- Clean Water Store: How much Chlorine to Inject to Treat Iron & Manganese before Iron Filters? Available online: https://www.cleanwaterstore.com/blog/much-chlorine-inject-treat-iron-manganese-iron-filters/ (accessed on 5 July 2021).
- Khadse, G.K.; Patni, P.M.; Labhasetwar, P.K. Removal of iron and manganese from drinking water supply. Sustain. Water Resour. Manag. 2015, 1, 157–165. [Google Scholar] [CrossRef]
- Gates, D.; Krasner, S. Guidance Manual: Alternative Disinfectants and Oxidants; Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 1999.
- Bowman, G.; The Wisconsin State Lab of Hygiene; Mealy, R. The Role of Chlorine in Dealing with Water Contamination; Technical Report; The Wisconsin Department of Natural Resources: Wisconsin, WI, USA, 2004.
- Huizenga, J. How much Ozone Do I Need to Destroy Bacteria and Viruses? Available online: https://www.oxidationtech.com/blog/how-much-ozone-do-i-need-to-destroy-bacteria-and-viruses/ (accessed on 5 July 2021).
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Guidance Manual for Compliance with the Filtration and Disinfection Required for Public Water Systems Using Surface Water Sources; Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 1991.
- Kanna, C.R. Inactivation of Viruses in Water by Chlorination Using Bacteriophages as Model Organisms. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015. [Google Scholar]
- Concept of Ozone Disinfection. Available online: https://spartanwatertreatment.com/ozone-disinfection-ct/ (accessed on 5 July 2021).
- Mohamed, H.; Nayak, G.; Rendine, N.; Wigdahl, B.; Krebs, F.C.; Bruggeman, P.J.; Miller, V. Nonthermal plasma as a novel strategy for treating or preventing viral infection and associated disease. Front. Phys. 2021, 9, 683118. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Cao, H.; Yang, C.; Guo, Z.; Shi, Y.; Li, W.; Zhao, H.; Sun, J.; Xie, Y. Degradation of phenolic compounds by dielectric barrier plasma: Process optimization and influence of phenol substituents. Chem. Eng. J. 2020, 385, 123732. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Hassan, M.; Ma, Z.; Dong, L.; Xie, Y.; He, Y. Efficient degradation of Bisphenol A by dielectric barrier discharge non-thermal plasma: Performance, degradation pathways and mechanistic consideration. Chemosphere 2022, 286, 131627. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jo, J.-O.; Whitehead, J.C. Degradation of an azo dye Orange II using a gas phase dielectric barrier discharge reactor submerged in water. Chem. Eng. J. 2008, 142, 56–64. [Google Scholar] [CrossRef]
- Vasikaran, E.M.; Murugesan, P.; Moses, J.A.; Anandharamakrishnan, C. Performance of non-thermal plasma reactor for removal of organic and inorganic chemical residues in aqueous media. J. Electrost. 2022, 115, 103671. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naicker, K.-I.; Kaweesa, P.; Daramola, M.O.; Iwarere, S.A. Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification. Appl. Sci. 2023, 13, 6243. https://doi.org/10.3390/app13106243
Naicker K-I, Kaweesa P, Daramola MO, Iwarere SA. Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification. Applied Sciences. 2023; 13(10):6243. https://doi.org/10.3390/app13106243
Chicago/Turabian StyleNaicker, Kaamil-Inaam, Paul Kaweesa, Michael O. Daramola, and Samuel A. Iwarere. 2023. "Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification" Applied Sciences 13, no. 10: 6243. https://doi.org/10.3390/app13106243
APA StyleNaicker, K.-I., Kaweesa, P., Daramola, M. O., & Iwarere, S. A. (2023). Non-Thermal Plasma Review: Assessment and Improvement of Feasibility as a Retrofitted Technology in Tertiary Wastewater Purification. Applied Sciences, 13(10), 6243. https://doi.org/10.3390/app13106243









