Abstract
High levels of lipids and cholesterol, particularly LDL, in blood are considered the most common risk factors for cardiovascular diseases that threaten human life. Recently, interest has increased in the use of medicinal plants to treat various diseases due to their lack of side effects. The current study aims to investigate the effect of Ajwa date (AD) and germinated barley (GB) on the lipid profile in rats fed a high-fat diet (HFD). Thirty rats were distributed into five groups (six per group) as follows: the negative control group, the positive control group fed a HFD, and the other three groups that were fed a HFD supplemented with a mixture of AD and GB in equal ratios with different proportions of 20, 30, and 40% in the diet. There was an increase in moisture, protein, phenols, and vitamin C content and a decrease in the content of ash, carbohydrates, fats, and beta-glucans in GB. Blood total cholesterol levels decreased significantly (83.53, 70.12, and 73.55 mg/dL) in the groups fed the AD and GB mixtures in different percentages (20, 30, and 40%). Likewise, the AD and GB mixtures recorded a significant decrease in the level of triglycerides in the treated groups, with no significant effect on the high-density lipoprotein and low-density lipoprotein. There was a significant improvement in the level of alanine aminotransferase, while the level of aspartate aminotransferase was not affected by the treatment. In summary, AD and GB mixtures can modulate the lipid profile alterations caused by HFD through their phytochemical constituents, particularly beta-glucans.
1. Introduction
Dyslipidemia is one of the major risk factors of cardiovascular disease (CVD) and chronic non-communicable diseases (NCDs) and is characterized by an increase in one or more abnormal serum lipid concentrations of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), or high-density lipoprotein cholesterol (HDL-C), which contribute to raising morbidity and mortality rates globally. This affects both health and economic status and raises worldwide healthcare costs [1,2]. Dyslipidemia is divided into primary and secondary types. Primary dyslipidemia is genetic and secondary dyslipidemia is acquired, as it is caused by obesity or diabetes [3]. Dyslipidemia can damage blood vessels by triggering oxidative stress, inflammation, and immune dysregulation [4,5]. The use of herbalism and bioactive compounds from plants has increased worldwide because of their beneficial impacts on cardiovascular diseases, dyslipidemia, and diabetes. Moreover, the side effects associated with medicinal plants are fewer than those associated with chemical medications [6]. Therefore, many people prefer to improve their lipid profile through dietary interventions or at least by integrating dietary changes with medication treatment. The use of plant-based medicines is not related to their positive effects on cholesterol, but to the fact that dietary supplements are perceived (erroneously) to be safer for consumers. The breakthrough in the use of dietary supplements for the prevention of diseases related to high cholesterol levels is related to the discovery of fermented red rice. Specifically, rice upon fermentation by Monascus purpureus allows for the production of monacolin, which is structurally equivalent to lovastatin. However, equally to other statins, monacolin K displayed anaphylaxis, hepatotoxicity, central nervous system complaints, rhabdomyolysis effects, and a high possibility of developing diabetes mellitus. This is the motivation because the search for new therapeutic plants for the control of cholesterol levels is important today, as is finding a new anti-cholesterolemic agent that can prevent high levels of cholesterol with mechanisms different from states [7].
Barley (Hordeum vulgare L.) is an ancient plant of the Poaceae family and an essential cereal crop that is increasing worldwide due to high demand, covering about 9.4% of the world cereal production area [8]. Due to its nutritional value and the high amounts of biologically active compounds that are present in barley, there is a growing interest in its use as a component in food products, among all cereals. Barley grains contain the highest amount of β-glucans and other bioactive compounds with beneficial effects, such as phenolic compounds, vitamin C, tocols, and sterols [9,10]. The phytochemicals present in barley exhibit a high anti-oxidative ability, immunostimulant potentials, and anti-inflammatory effects. In addition, it has been proven previously that they can suppress LDL cholesterol while raising HDL cholesterol levels [11]. The germination approach improves the nutritional value and increases the bioactive components in grains [12].
Additionally, germination softens the granular structure, improves the digestibility of grains and seeds, and reduces anti-nutritional components [12,13]. Furthermore, [12] reported that germinated barley (GB) improves the lipid profile in rats with steatohepatitis by feeding them a high-fat diet. However, limited reports were completed regarding the potential use of germinated barley in reducing lipid release after feeding rats with HFD.
Ajwa date (AD, Phoenix dactylifera L.) is one of the most abundant and popular dates and is cultivated in Al Madinah, western Saudi Arabia. Ajwa date has high nutritional and great medicinal value [14]. Fresh dates contain high amounts of antioxidants, carotenoids, anthocyanins, phenolics, and free and bound phenolic compounds among fruit varieties. Moreover, it contains very high levels of phenolics due to its exposure to high temperatures and a hot climate [15]. According to traditional and alternative medicine, AD dates provide several health benefits, including anticholesteremic, anti-inflammatory, antioxidant, antidiabetic, hepatoprotective, and anticancer effects [16]. A previous study has shown the therapeutic potential of AD in rats treated with isoproterenol to induce myocardial infarction. This study reported that the oral administration of Ajwa extract improved blood lipid profile, inflammation, and cardio-protectiveness, alongside having strong antioxidants [17].
Based on the therapeutic effects of AD and GB, we hypothesized that the mixture of GB and AD would enhance the lipid profile and liver enzymes after feeding rats with HFD. Thus, the present study was designed firstly to determine the germination’s effect on barley’s phytochemical contents. Secondly, this study aimed to investigate the effect of the Ajwa date (AD) and germinated barley (GB) on the lipid profile in rats fed a high-fat diet (HFD).
2. Materials and Methods
2.1. Ajwa Date Preparation
The first-grade Ajwa dates (AD) were purchased from the local market in Al-Baday’ Governorate, Al-Qassim region, Saudi Arabia. The Ajwa dates were washed with sterilized water and then dried; the cores were removed and then chopped by an electric chopper and stored at 4 °C until extraction and their use in the experiment.
2.2. Preparation for Germinated Barley
The barley was purchased from the local market in Al-Bada’i Governorate, Al-Qassim region, Saudi Arabia. After removing the broken grains, dust, and impurities, the grains were washed and soaked in sterilized water for 4 h, and then distributed on wet gauze in plastic baskets at 18 ± 2 °C for 3–4 days until germination. After that, the germinated plants were air-dried for 48 h and then milled. The dried germinated barely (GB) was milled by an electric mill until a powder was obtained and passed through sieves (sifters) with holes of 2 mm. Then, it was kept at 4 °C until the extraction, analysis, and use in the experiment.
2.3. Estimation of the Chemical Composition of Barley and GB
The chemical composition of barley before and after germination was estimated according to the methods mentioned in the America Association of Cereal Chemists [18], where the moisture content, ash percentage, and total protein percentage were estimated using the Keldahl method (the conversion factor for barley is 5.83) [19].
The fat percentage was estimated using Folch’s method [19]. Calculating the percentage of carbohydrates using the difference method [18] was completed according to the following equation:
The content of beta-glucan fibers in barley was estimated before and after germination using chemical reagents from Megazyme International Co., (Wicklow, Ireland). Vitamin C was also determined for barley powder, as was germinated barley, according to the method of [20] using 2,6-dichlorophenol-indophenol dye.
2.4. Preparation of AD and GB for Antioxidant Activities
A total of 1 g each of the Ajwa date, normal barley, and germinated barley was extracted by 10 mL of the solvent (aqueous ethanol 50%). The extracts were mixed with a magnetic stirrer for an hour (1000 revolutions per minute) and then left in the dark for a full day. After that, the extracts were centrifuged for 10 min at a low temperature, and the liquid was separated for the determination of the total phenols and antioxidants according to the method [21,22].
Determination of 2,2-Diphenylpicrylhydrazyl in AD and GB
According to the methods of [23,24], the DPPH (2,2-diphenylpicrylhydrazyl) radical scavenging activity and TPC (total phenolic content) were determined in the Ajwa date and germinated barely. The outcomes regarding TPC were presented as milligrams of GAE (gallic acid equivalents)/g of dry weight, while the outcomes regarding DPPH were expressed as a percentage.
2.5. Animals and Experimental Design
Thirty Wistar Albino rats were purchased from the Experimental Animals Unit at the College of Pharmacy, King Saud University and their weight ranged from 150 to 200 g. They were placed in clean plastic cages with a controlled temperature (25 ± 2 °C), a humidity of about 50 ± 10%, and a light and dark cycle of 12 h. Rats were allowed free access to water and fed a normal diet (ND) or a high-fat diet (HFD, 20% fats) that was prepared by the Experimental Animals Unit at the College of Pharmacy, King Saud University in Riyadh, Saudi Arabia), according to the method [25].
After the rats were allowed to adapt to the laboratory environment for seven to ten days, the experiment was conducted according to the instructions of the College of Agriculture and Veterinary Medicine at Qassim University for experimental animals. The rats were divided into five groups (n = 6 rats in each group), as follows: (1) the first group (NC; negative control): rats were fed a standard diet throughout the experimental period. (2) The second group (PC; positive control): rats were fed a HFD throughout the twelve weeks of the experimental period. (3), (4), and (5) The third (ABM20), fourth (ABM30), and fifth groups (ABM40): rats were fed a HFD in addition to a mixture of AD and GB in a ratio of (1:1) in different proportions (20, 30, and 40 gm/100 gm of the diet weight) daily for 12 weeks, respectively. The HFD contained 60% of fat by energy, as reported by [26,27]. Ethical approval was granted by the Committee of Research Ethics (Institutional Review Board, IRB) of Qassim University, Saudi Arabia (approval no. 21-05-08).
At the end of the experiment, the animals fasted for 12 h before sample collection (blood and tissues). The food consumption and conversion ratio for each animal and its weight were recorded during the experimental period (12 weeks). Moreover, the initial and final body weights were recorded, and body weight gain was calculated. At the end of the experiment, blood was pinched and centrifuged directly (3000 rpm, 10 min), and the collected serum was stored at −20 °C until biochemical analysis. Hepatic tissues were discarded and weighted for each group. An electronic balance estimated all previous traits. The liver index was calculated using the following formula: liver index percentage = liver weight/body weight × 100.
2.6. Lipid Profile Assessment
Serum total cholesterol (TC) levels and triglyceride (TG) were measured using assays kits BC1985 and BC0625 acquired from Solarbio Company (Beijing, China) according to the methods of [28,29], following the manufacturer’s protocols. The method of [30] was followed to assess the serum HDL-C (high-density lipoprotein cholesterol) levels, while the other lipid parameters, such as LDL-C (high-density lipoprotein cholesterol) and very LDL-C, were mathematically considered following the technique depicted by [31].
2.7. Liver Functions Tests
The concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were measured using an automatic biochemistry analyzer (Hitachi Ltd., Tokyo, Japan) according to the methods of [32] and [33], respectively, at a wavelength of 540 nm.
2.8. Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences SPSS 22nd edition. The arithmetic mean standard error and one-way analysis of variance (ANOVA) were found, followed by Tukey’s test. Significant differences were found between the results at the level of significance (p ≤ 0.05) according to the method. Only the chemical composition of barley before and after germination was performed using the t-test to express the data as mean ± standard error.
3. Results
3.1. Chemical Composition of GB
The chemical composition of the barley before and after germination was analyzed to measure the most abundant compounds in germinated barley. As seen in Table 1, the moisture percentage, protein, and vitamin C content were higher in the germinated barley than in the bare seed. Moreover, the results showed a decrease in the ash percentage from 1.59 to 1.26% after germination. Likewise, there was a decrease in the carbohydrate content from 78.47 to 75.59%, and the fat content of GB compared to normal barley decreased from 3.25 to 2.03%.

Table 1.
Chemical composition of barley before and after germination.
3.2. Antioxidant Activities of Ajwa, Barley, and GB (TPC and DPPH)
As demonstrated in Table 2, the TPC content of the alcoholic extract (ethanol 50%) of normal barley and GB was (2.617 ± 0.28 and 3.335 ± 0.06 mg GAE.g), respectively. This means that GB recorded a significant increase in TPC compared to normal barley. In addition, the DPPH showed a slight decrease in GB compared to normal barley. Regarding the Ajwa date, it exerted the highest DPPH and TPC (85.47 ± 1.50% and 3.496 ± 0.02 mg GAE.g), respectively, compared to barley and GB.

Table 2.
The antioxidant assays (TPC and DPPH) in Ajwa date, barley, and germinated barley.
3.3. Body Weight Changing
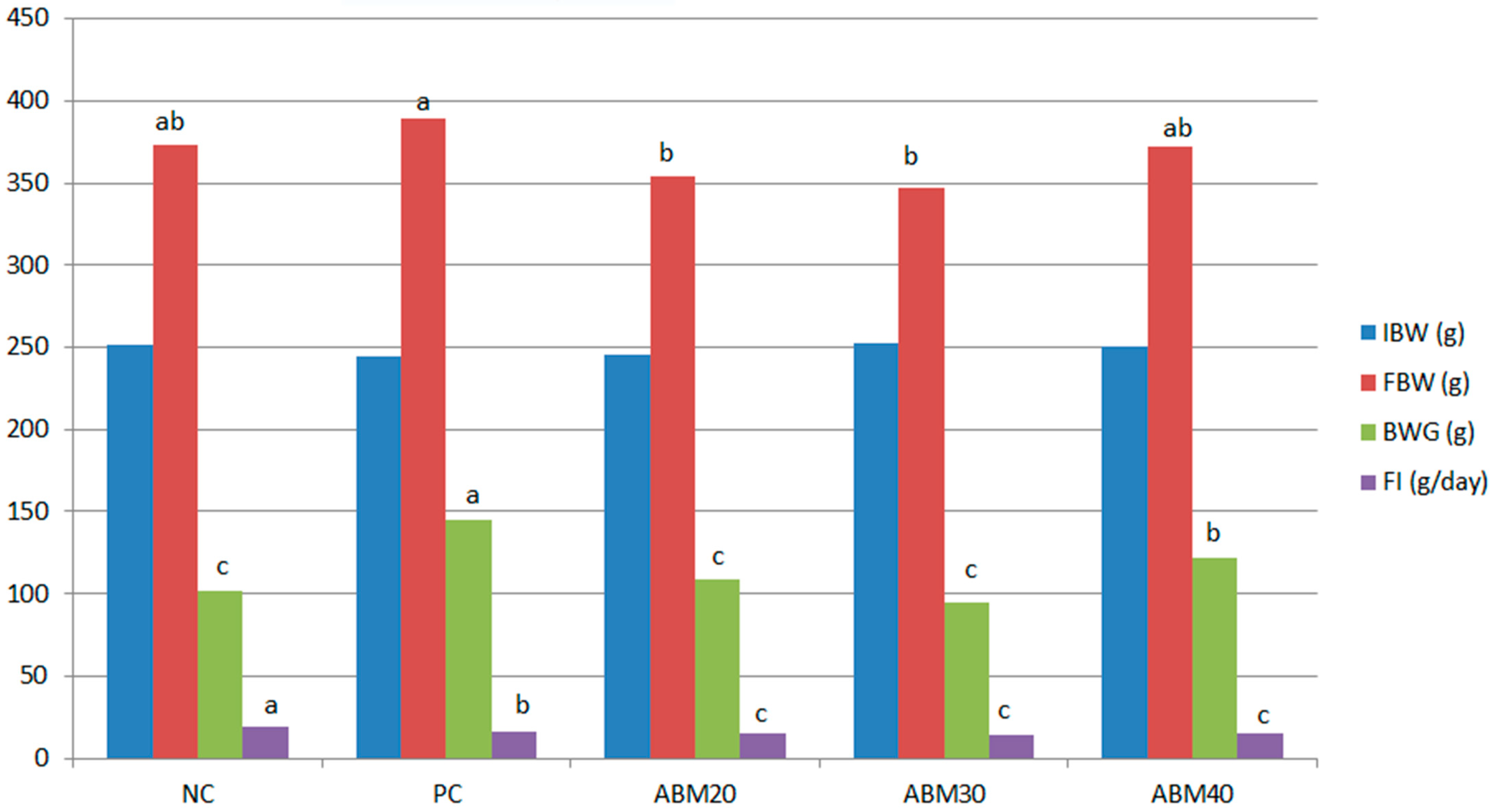
The effects of the date mixture and germinated barley in different doses of 20, 30, and 40% at a ratio of (1:1) on the weight of rats fed high-fat diets (Table 3, and Figure 1) are clear in the table, which shows statistical differences among the treatments. Both ABM20 and ABM30 significantly reduced the final body weights compared to the PC group, while no significant difference was detected among PC, NC, and ABM40. For the body weight gains, rats fed a HFD with ABM20 and ABM30, as well as rats in the control group, had the lowest values, while the highest values of body weight gains were detected in the PC group. Rats fed with ABM had an improvement in feed conversion rates compared to the NC and PC groups.

Table 3.
The effect of a mixture of Ajwa date and germinated barley on body weight changes in rats fed a high-fat diet.
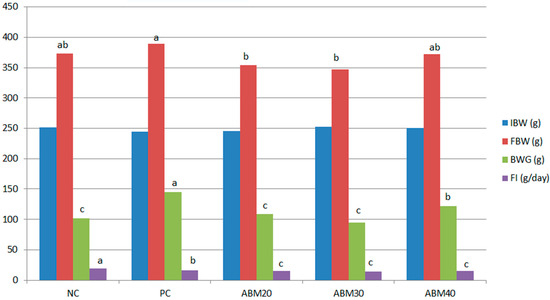
Figure 1.
The effect of a mixture of Ajwa date and germinated barley on body weight changes (IBW; initial body weight, FBW; final body weight, BWG; body weight gain, and FI, food intake) in rats fed a high-fat diet. NC = Negative control, PC = Positive control, ABM20 = Ajwa barley mix 20%, ABM30 = Ajwa barley mix 30%, ABM40 = Ajwa barley mix 40%. %. a–c Values don’t have the same letter are different (p ≤ 0.05); values are expressed as mean ± standard error.
3.4. Liver Indices
Table 4 shows the liver changes in rats fed ND, HFD, or HFD supplemented with a mixture of Ajwa dates and germinated barley in different proportions.

Table 4.
The effect of a mixture of Ajwa date and germinated barley on hepatic indices in rats fed a high-fat diet.
Rats fed a HFD showed greater liver weights than other groups (p ≤ 0.05), while rats in groups ABM20 and ABM30 had increased liver weights relative to other groups. Rats fed a HFD treated with ABM20 showed a non-significant effect when compared to the NC group (p ≥ 0.05). The ABM40 group exhibited intermediate values. This indicates that ABM may enhance liver metabolism, thus reducing liver weights and indices.
3.5. Blood Lipid Profile
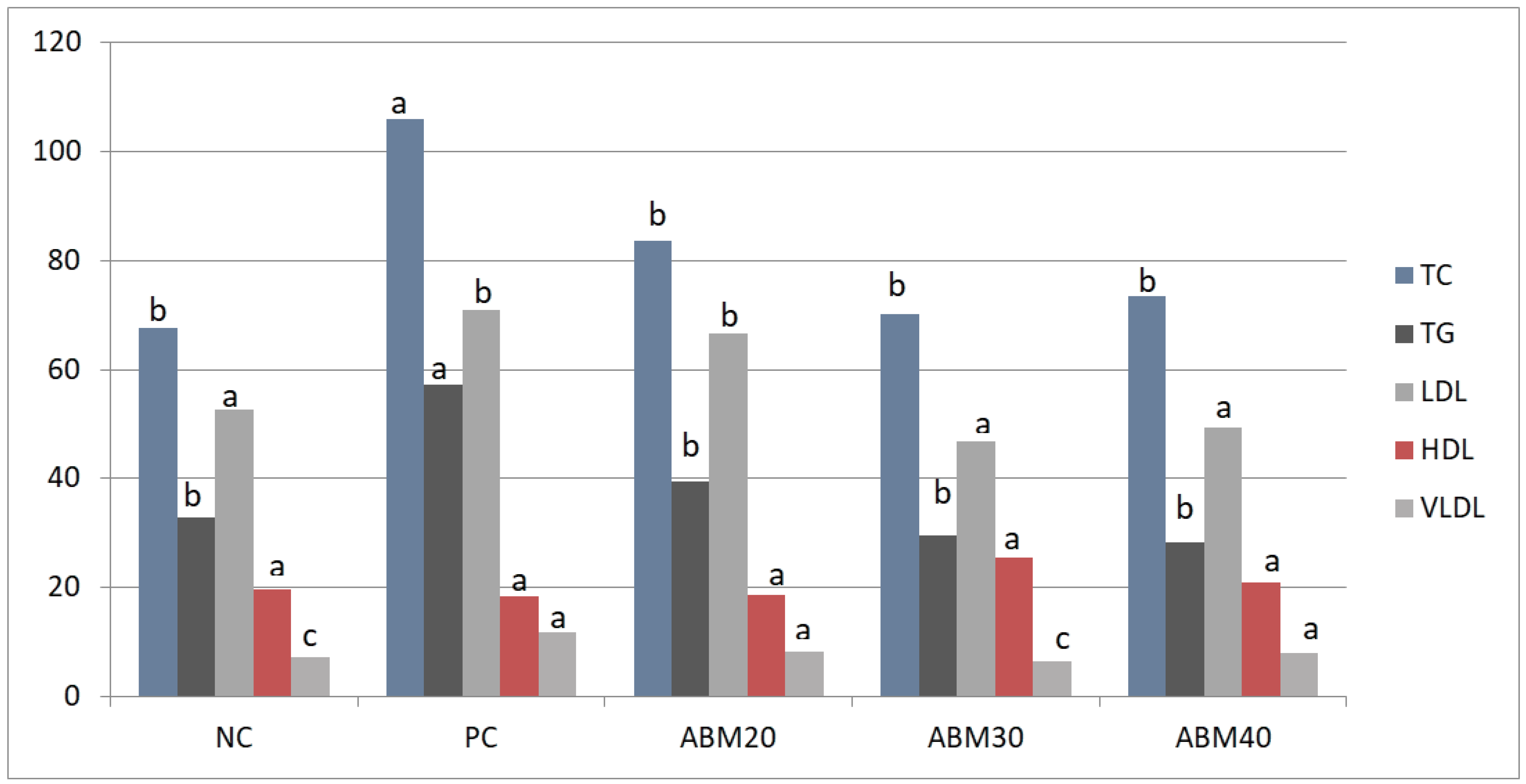
After gaining a better insight into the lipid changes in rats fed a HFD treated with different levels of ABM, it is clear that there is a statistically significant effect in all blood lipid profiles, as depicted in Table 5 and Figure 2. All ABM groups showed significantly reduced TC and TG compared to the PC group, while non-significant differences were detected between the treated groups and the NC group. LDL values were significantly reduced in rats fed a HFD treated with 30 and 40% of ABM, as compared to the ABM20 and PC groups. HDL did not cause significant changes in all experimental groups. However, LDL tended to be lower in the ABM30 and ABM40 groups than the positive group PC. The values of VLDL decreased significantly in ABM20 and ABM40 compared to other groups. The highest values of VLDL were shown in PC, with significant differences to other groups, while both groups ABM20 and ABM40 showed intermediate values.

Table 5.
Effect of Ajwa date mixture and germinated barley on blood lipid profile in rats fed a high-fat diet.
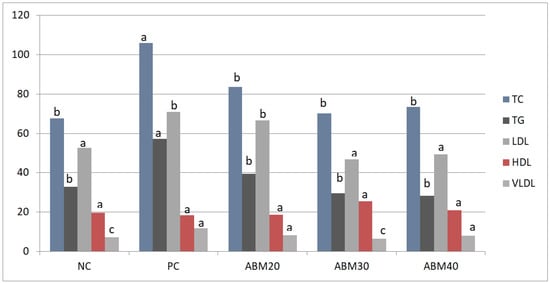
Figure 2.
Effect of Ajwa date mixture and germinated barley on blood lipid profile in rats fed a high-fat diet. TC, total cholesterol; TG, total glycerides; LDL, low-density lipoprotein; HDL, high-density lipoproteins; VLDL, very-low-density lipoproteins. NC = Negative control, PC = Positive control, ABM20 = Ajwa barley mix 20%, ABM30 = Ajwa barley mix 30%, ABM40 = Ajwa barley mix 40%. a–c Values don’t have the same letter are different (p ≤ 0.05); values are expressed as mean ± SE.
3.6. Liver Function
In the current data, ALT and AST values were significantly increased (p ≤ 0.05) in rats fed a HFD and treated with different ABM (Table 6). Rats fed a HFD treated with ABM had significantly lower values of ALT than the PC group. ABM-treated groups significantly reduced the ALT levels. All ABM groups had greater AST values than the PC group, while the lowest values of AST were detected in the NC group.

Table 6.
The effect of Ajwa date and germinated barley on liver function in rats fed a high-fat diet.
4. Discussion
Recently, with the increase in ultra-processed foods, diets are enriched with different types of fats, resulting in hyperlipidemia (HYL), obesity, and cardiovascular diseases. Significant increases in TC, TG, VLDL, and LDL and decreases in HDL are the main categories for hyperlipidemia. Consequently, ameliorating HYL is substantial for avoiding and remedying cerebrovascular and cardiovascular syndromes and dropping social stress. In the current study, the authors described the potential of AD and GB in reducing the lipid profile due to their antihyperlipidemic and antioxidant effects. The mixture of AD and GB can effectively reduce lipid profile (TC, TG, VLDL, and LDL) in rats and increase HDL fed with HFD, as demonstrated in the present study.
The chemical composition of the barley before and after germination was analyzed to measure the effectiveness of germination on the barley with a high percentage of protein, vitamin C, and lower contents of fats for GB. This diversity of components between the seed and germinated barley may lead to the production of some amino acids during protein biosynthesis [34]. Moreover, decreased percentages of fat in the germinated barley may lead to the breakdown of lipids into simpler compounds during the germination process [35], and improve the beta oxidation of fats to provide more energy for cellular activity [36]. A decrease in the beta-glucan fiber content of barley after germination was also observed in these results. These results agree with the results of [9], which showed that germination causes an increase in the activity of beta-glucan enzymes, which are the primary enzymes responsible for breaking down the endosperm cell walls during germination, and thus reduce the beta-glucan content, and this decrease is inversely proportional to the increase in germination time.
The content of phenolic substances in barley increased with a statistically significant increase after germination compared to normal barley. This result is consistent with the results obtained by [37,38]. One of the main advantages of germination is that the antioxidant properties of grain can be enhanced by increasing the content of phenols, and this increase depends on the type of grain grown and the germination conditions. It was found from previous studies that using a mixture of organic solvent and water at a rate ranging between 50 and 70% allows for the extraction of most of the phenolic components from plant samples [39,40]. It also showed that the total polyphenol content of Ajwa dates ranges from 245 to 455.8 mg/100 g.
The amount of extracted phenols is greatly affected by the extraction solvent used, its concentration, and the degree of maturity of the dates, as the phenols content decreases significantly with the dates’ ripening progression. This is consistent with the researcher’s conclusion. Epidemiological studies have shown that the consumption of foods high in phenols is associated with reduced cardiovascular disease, inflammation, and cancer-related mortality [41]. Moreover, the mixture of AD and GB (ABM) decreased the liver indices and enzymes, especially ALT, compared to the rats induced with the hyperlipidemia group (PC). We supposed that this improvement in lipid profile is a response to rats fed a HFD, which could be attributed to the potent antioxidant ability of AD and GB, as shown in Table 2. Moreover, based on the chemical composition of GB, higher contents of carbohydrates and vitamin C are higher in GB than in barley seed (Table 1).
Geminated seeds or sprout seeds present more active phytochemicals or carotenoids than seeds, demonstrating many health-promoting abilities, including reducing TC, anti-obesity, anti-oxidants, and inflammatory abilities [42,43]. Moreover, this research indicated that the mixture of GB and AD was related to reducing lipid accumulation in rats fed a HFD, as reported previously by [44,45] in hepatocytes and adipocytes. The anti-hyperlipidemic ability of barley sprouts might be associated with their high contents of saponarin, which considerably suppressed TC and TG gathering, and was accredited by decreases in TAG synthesis-related transcriptomics. Flavonoids and polyphenols in germinated barley and AD comprise several hydroxyl groups (-OH) which exert their antioxidant abilities [46] by binding to oxidative markers, as mentioned in our data. Moreover, many previous studies have shown the therapeutic roles of geminated barely as an anti-obesity functional food [47,48]. Moreover, the mixture of barley sprout and Indian gooseberry significantly reduced the serum lipid profile and weights of rats fed a HFD [47]. Several studies have indicated that AD has antihyperlipidemic action due to its anti-obesity qualities [39].
In human studies, AD seed effectively reduced TC, LDL, and TG by 19.4, 22.5, and 25.78% in the treated group compared to the placebo group [49]. This machinery of the anti-obesity qualities of GB can promote lipolysis and inhibit lipid synthesis due to its contents of saponarin and ellagic acid [47].
Liver enzymes are responsible for the normal function of hepatocytes, and the increase in ALT and AST might be associated with liver injury [50]. In the current study, rats fed a HFD displayed increased ALT and AST levels, indicating liver injury. This research indicates that feeding the rats a high-fat diet (HFD) during the experiment period led to possible damage or inflammation in the liver cells, which caused the aminotransferase enzymes (ALT, AST) to rise above the standard levels, which was evident in the results of the groups (ABM 30 and 40%).
A mixture of AD and GB at a ratio of 20, 30, and 40% restored the ALT enzyme to a level near that of the NC group. Unexpectedly, there was an increase in AST values recorded in ABM at a ratio of 20, 30, and 40%. This elevation of the AST enzyme may be due to the low beta-glucan content recorded in GB, since beta-glucan supplementation reduced liver damage and oxidative stress, which is represented by the significant lowering of AST [51]. This later study supposed that the increasing of AST might be related to the liver damages in rats. Thus, further research would be conducted to illustrate this topic and support the increasing levels of AST. The authors did not examine any histological features for a more in-depth discovering of the hepatocytes status.
Nevertheless, ALT is a more specific indicator of liver inflammation than AST [52], as AST may also be elevated in diseases affecting other organs, such as the heart or muscles [53].
Treating rats induced with hepatic steatosis with GB prevented the weakening of hepatocyte function and diminished inflammation [11]. β-glucans are recognized for their glucoregulatory actions, promoting hepatic health from the damages caused by HFD [54]. Moreover, [55] reported that GB has a hepatoprotective agent against heavy metals in rats. Epidemiological studies have shown that the consumption of foods high in phenols is associated with reduced cardiovascular disease, inflammation, and cancer-related mortality [12,42]. The TG was lower in all rats co-treated with AD and GB. Similar to our results, AD significantly reduced TC and glucose, indicating a protective agent against obesity in animal studies [56,57]. The dietary fiber of β-glucans can increase the abundance of probiotics, which can accelerate the secretion of unconjugated bile acids and thus help to regulate the level of TC in the blood short-chain fatty acids (SCFAs) resulting from the fermentation of food fibers inhibiting the activity of enzymes that synthesize TC within the body, thus reducing blood TC levels [24].
The synergistic effects of AD and GB in reducing the lipid profile and exhibiting anti-hyperlipidemic effects may be due to their effect on TC and TG, involving avoiding their absorption by blocking the intestinal barriers related to enzymes that are responsible for the transportation of both molecules through the intestinal cavity [25,27]. Moreover, many phenolic compounds have different pharmacological properties on dyslipidemia or oxidative and inflammatory stress, as well as on risk factors for cardiovascular diseases. Based on the presented data, we suggested that the mixture of AD and GB may produce a higher effective strategy in promoting lipolysis and inhibiting lipogenesis.
5. Conclusions
The present results confirmed that a mixture of AD and GB (especially ABM 30 or ABM 40% mixed with Ajwa date and germinated barley) has an anti-hyperlipidemic ability, which was evidenced by the reduced level of TG, TC, LDL, and VLDL in rat models. Moreover, a mixture of AD and GB can effectively reduce the liver index and body weight of rats fed a HFD. AD and GB are potentially therapeutically beneficial for tackling hyperlipidemia due to their phytochemical constituents and vitamin C content. This strategy may produce a more effective method for promoting lipolysis and inhibiting lipogenesis. Furthermore, clinical research is needed in this context to recommend AD and GB mixtures for hyperlipidemic patients.
Author Contributions
Conceptualization, R.A.; methodology, R.A.; validation, formal analysis, investigation, resources, data curation, R.A.; writing—original draft preparation, R.M.A.; writing—review and editing, R.M.A.; supervision, R.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, for its financial support for this research under the number (CAVM-2022-1-1-J-27088) during the academic year 1444 AH/2022 AD.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and was approved by the Institutional Review Board of Health Affairs. This study was approved by the Committee of Research Ethics, Deanship of Scientific Research, Qassim University (21-05-08).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, for its financial support for this research under the number (CAVM-2022-1-1-J-27088). The authors also thank Qassim University for its technical support. The authors appreciate Essam M. Hamad for providing help during the experimental research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Johani, J.J.; Alzahrani, G.S.; Aljehani, S.M. Risk factors of dyslipidemia among Saudi population, 2017. Egypt. J. Hosp. Med. 2018, 71, 2262–2265. [Google Scholar]
- Hadi, A.; Ghaedi, E.; Khalesi, S.; Pourmasoumi, M.; Arab, A. Effects of synbiotic consumption on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Nutr. 2020, 59, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, W.; Luo, L.; Xu, C. Dyslipidemia: Causes, symptoms and treatment. Int. J. Trend Sci. Res. Dev. 2021, 5, 1013–1016. [Google Scholar]
- Noh, J.-W.; Yang, H.-K.; Jun, M.-S.; Lee, B.-C. Puerarin attenuates obesity-induced inflammation and dyslipidemia by regulating macrophages and TNF-alpha in obese mice. Biomedicines 2022, 10, 175. [Google Scholar] [CrossRef]
- Boarescu, P.-M.; Boarescu, I.; Pop, R.M.; Roşian, Ş.H.; Bocșan, I.C.; Rus, V.; Mada, R.O.; Popa, I.D.; Neagu, N.; Bulboacă, A.E. Evaluation of Oxidative Stress Biomarkers, Pro-Inflammatory Cytokines, and Histological Changes in Experimental Hypertension, Dyslipidemia, and Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 1438. [Google Scholar] [CrossRef]
- Khorshidi, M.; Zarezadeh, M.; Moradi Moghaddam, O.; Emami, M.R.; Kord-Varkaneh, H.; Mousavi, S.M.; Alizadeh, S.; Heshmati, J.; Olang, B.; Aryaeian, N. Effect of evening primrose oil supplementation on lipid profile: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 2628–2638. [Google Scholar] [CrossRef]
- Mannino, G.; Iovino, P.; Lauria, A.; Genova, T.; Asteggiano, A.; Notarbartolo, M.; Porcu, A.; Serio, G.; Chinigò, G.; Occhipinti, A.; et al. Bioactive Triterpenes of Protium heptaphyllum Gum Resin Extract Display Cholesterol-Lowering Potential. Int. J. Mol. Sci. 2021, 22, 2664. [Google Scholar] [CrossRef]
- Sorour, M.; Ramadan, B.; El Sayed, M.A.-H.; Ahmed, W.K. Effect of Soaking and Malting Process on Chemical Compostion, Bioactive Compounds and Antioxidant Activity of some Egytian Barley Varities. J. Food Dairy Sci. 2020, 11, 313–319. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Ahmad, N.; Imran, A.; Niaz, B.; Afzaal, M.; Imran, M.; Tufail, T.; Javed, A. Functional and health-endorsing properties of wheat and barley cell wall’s non-starch polysaccharides. Int. J. Food Prop. 2018, 21, 1463–1480. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; García, M.d.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Al-Shali, R.A.; Ramadan, W.S. Germinated barley downregulates hepatic stearoyl-CoA desaturase-1 enzyme gene expression in a hepatic steatohepatitis rat model. Anat. Sci. Int. 2020, 95, 489–497. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Lu, H.; Shu, Q.; Zhang, Y.; Chen, Q. New perspectives on physiological, biochemical and bioactive components during germination of edible seeds: A review. Trends Food Sci. Technol. 2022, 123, 187–197. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Abdu, S.B. Ameliorative influence of ajwa dates on ochratoxin A-induced testis toxicity. J. Microsc. Ultrastruct. 2018, 6, 134. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, R.; Khan, M.A.; Upadhyay, S.; Husain, I.; Srivastava, A.N. Cytostatic and anti-tumor potential of Ajwa date pulp against human hepatocellular carcinoma HepG2 cells. Sci. Rep. 2019, 9, 245. [Google Scholar] [CrossRef]
- Al-Yahya, M.; Raish, M.; AlSaid, M.S.; Ahmad, A.; Mothana, R.A.; Al-Sohaibani, M.; Al-Dosari, M.S.; Parvez, M.K.; Rafatullah, S. ‘Ajwa’dates (Phoenix dactylifera L.) extract ameliorates isoproterenol-induced cardiomyopathy through downregulation of oxidative, inflammatory and apoptotic molecules in rodent model. Phytomedicine 2016, 23, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the American Association of Cereal Chemists; Amer Assn of Cereal Chemists: Constanta, Romania, 2000; Volume 1. [Google Scholar]
- Jones, B.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentage of Protein; US Department of Agriculture: Washington, DC, USA, 1931.
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Ruck, J.A. Chemical Methods for Analysis of Fruits and Vegetables; no. 1154; Research Station Summerland, Research Branch Canada, Department of Agriculture: Summerland, BC, Canada; Department Agriculture Canada: Summerland, BC, Canada, 1961.
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.-G.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharmacol. 2018, 70, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Abdelghffar, E.A.; Obaid, W.A.; Mohammedsaleh, Z.M.; Ouchari, W.; Eldahshan, O.A.; Sobeh, M. Ajwa dates (Phoenix dactylifera L.) attenuate cisplatin-induced nephrotoxicity in rats via augmenting Nrf2, modulating NADPH oxidase-4 and mitigating inflammatory/apoptotic mediators. Biomed. Pharmacother. 2022, 156, 113836. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, Y.; Wang, D.; Zhao, W.; Hu, X.; Chen, F.; Zhao, X. Protective Effects of Dietary Resveratrol against Chronic Low-Grade Inflammation Mediated through the Gut Microbiota in High-Fat Diet Mice. Nutrients 2022, 14, 1994. [Google Scholar] [CrossRef]
- Li, L.; Zhai, S.; Wang, R.; Kong, F.; Yang, A.; Wang, C.; Yu, H.; Li, Y.; Wang, D. Anti-Obesity Effect of Auricularia delicate Involves Intestinal-Microbiota-Mediated Oxidative Stress Regulation in High-Fat-Diet-Fed Mice. Nutrients 2023, 15, 872. [Google Scholar] [CrossRef]
- Abell, L.L.; Levy, B.B.; Brodie, B.B.; Kendall, F.E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J. Biol. Chem. 1952, 195, 357–366. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef]
- Assmann, G.; Schriewer, H.; Schmitz, G.; Hägele, E. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin. Chem. 1983, 29, 2026–2030. [Google Scholar] [CrossRef]
- Tremblay, A.J.; Morrissette, H.; Gagné, J.-M.; Bergeron, J.; Gagné, C.; Couture, P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with β-quantification in a large population. Clin. Biochem. 2004, 37, 785–790. [Google Scholar] [CrossRef]
- DeRosa, G.; Swick, R.W. Metabolic implications of the distribution of the alanine aminotransferase isoenzymes. J. Biol. Chem. 1975, 250, 7961–7967. [Google Scholar] [CrossRef]
- Murray, R.; Kaplan, A. Aspartate aminotransferase. In Clinical Chemistry. Theory, Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; CV Mosby Company: Maryland Heights, MO, USA, 1984; pp. 1105–1108. [Google Scholar]
- Afify, A.E.-M.; Abbas, M.S.; Abd El-Lattefi, B.M.; Ali, A.M. Chemical, rheological and physical properties of germinated wheat and naked barley. Int. J. Chemtech Res. 2016, 9, 521–531. [Google Scholar]
- Youssef, M.K.E.; El-Fishawy, F.; Ramadan, E.; Abd El-Rahman, A.M. Nutritional assessment of barley, talbina and their germinated products. Front. Sci. 2013, 3, 56–65. [Google Scholar]
- Idowu, A.T.; Olatunde, O.O.; Adekoya, A.E.; Idowu, S. Germination: An alternative source to promote phytonutrients in edible seeds. Food Qual. Saf. 2020, 4, 129–133. [Google Scholar] [CrossRef]
- Leitao, C.; Marchioni, E.; Bergaentzlé, M.; Zhao, M.; Didierjean, L.; Miesch, L.; Holder, E.; Miesch, M.; Ennahar, S. Fate of polyphenols and antioxidant activity of barley throughout malting and brewing. J. Cereal Sci. 2012, 55, 318–322. [Google Scholar] [CrossRef]
- Lotfy, T.M.R.; Agamy, N.; Younes, N. Dietry Impact of Normal and Germinated Barely Grains Products on body weight and Lipid Profile of Diabetic Rats. Alex. Sci. Exch. 2021, 42, 121–132. [Google Scholar] [CrossRef]
- Alqarni, M.M.M.; Osman, M.A.; Al-Tamimi, D.S.; Gassem, M.A.; Al-Khalifa, A.S.; Al-Juhaimi, F.; Mohamed Ahmed, I.A. Antioxidant and antihyperlipidemic effects of Ajwa date (Phoenix dactylifera L.) extracts in rats fed a cholesterol-rich diet. J. Food Biochem. 2019, 43, e12933. [Google Scholar] [CrossRef]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Tarzi, B.G.; Gharachorloo, M.; Baharinia, M.; Mortazavi, A. The effect of Germination on phenolic content and antioxidant activity of chickpea. Iran J. Pharm. Res. 2012, 11, 1137–1143. [Google Scholar]
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A potential cereal for producing healthy and functional foods. Food Qual. Saf. 2022, 6, fyac012. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S.; Wang, L.; Liu, X.; Ma, Y.; Tong, L.; Zhang, Y.; Wang, F. Effect of an Environment Friendly Heat and Relative Humidity Approach on γ-Aminobutyric Acid Accumulation in Different Highland Barley Cultivars. Foods 2022, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jeong, E.; Jo, S.M.; Park, J.; Kim, J.Y. Comparative Study of the Effects of Light Controlled Germination Conditions on Saponarin Content in Barley Sprouts and Lipid Accumulation Suppression in HepG2 Hepatocyte and 3T3-L1 Adipocyte Cells Using Barley Sprout Extracts. Molecules 2020, 25, 5349. [Google Scholar] [CrossRef]
- Barley Sprout Water Extract and Saponarin Mitigate Triacylglycerol Accumulation in 3T3-L1 Adipocytes. J. Med. Food 2022, 25, 79–88. [CrossRef] [PubMed]
- Islam, M.Z.; Yu, D.S.; Lee, Y.T. The effect of heat processing on chemical composition and antioxidative activity of tea made from barley sprouts and wheat sprouts. J. Food Sci. 2019, 84, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, J.-L.; Park, M.-R.; Lee, J.W.; Kim, O.-K.; Lee, J. Indian gooseberry and barley sprout mixture prevents obesity by regulating adipogenesis, lipogenesis, and lipolysis in C57BL/6J mice with high-fat diet-induced obesity. J. Funct. Foods 2022, 90, 104951. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kawk, H.-W.; Kim, S.-H.; Lee, H.-J.; Seo, J.-W.; Kim, J.-T.; Jang, S.-H.; Kim, M.-J.; Kim, Y.-M. Anti-obesity effect of hot water extract of barley sprout through the inhibition of adipocyte differentiation and growth. Metabolites 2021, 11, 610. [Google Scholar] [CrossRef]
- Jubayer, F.; Kayshar, S.; Rahaman, M. Effects of Ajwa date seed powder on serum lipids in humans: A randomized, double-blind, placebo-controlled clinical trial. J. Herb. Med. 2020, 24, 100409. [Google Scholar] [CrossRef]
- Lala, V.; Goyal, A.; Minter, D.A. Liver function tests. In StatPearls [internet]; StatPearls Publishing: Tampa, FL, USA, 2020. [Google Scholar]
- Erkol, H.; Kahramansoy, N.; Kordon, O.; Büyükaşık, O.; Serin, E.; Ulaş, N. Effects of beta-glucan on hepatic damage caused by obstructive jaundice. Ulus Travma Acil Cerrahi Derg. 2011, 17, 303–307. [Google Scholar] [CrossRef]
- Hall, P.; Cash, J. What is the real function of the liver ‘function tests? Ulst. Med. J. 2012, 81, 30. [Google Scholar]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Cmaj 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Hübner, F.; O’Neil, T.; Cashman, K.D.; Arendt, E.K. The influence of germination conditions on beta-glucan, dietary fibre and phytate during the germination of oats and barley. Eur. Food Res. Technol. 2010, 231, 27–35. [Google Scholar] [CrossRef]
- Eldamaty, H.S.E.; Elbasiouny, H.; Elmoslemany, A.M.; Abd El-Maoula, L.M.; El-Desoky, O.I.; Rehan, M.; Abd El Moneim, D.; Zedan, A. Protective Effect of Wheat and Barley Grass Against the Acute Toxicological Effects of the Concurrent Administration of Excessive Heavy Metals in Drinking Water on the Rats Liver and Brain. Appl. Sci. 2021, 11, 5059. [Google Scholar] [CrossRef]
- Ahmed, A.; Arshad, M.U.; Saeed, F.; Tufail, T. Exploring the role of date pit based drinks against hyperglycemia and hypercholesterolemia. Prog. Nutr. 2019, 21, 307–320. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).