Heavy Metals in Post-Exploitation Reservoirs—The Bagry Lake Case Study (Poland)
Abstract
1. Introduction
2. Data, Methods and Techniques
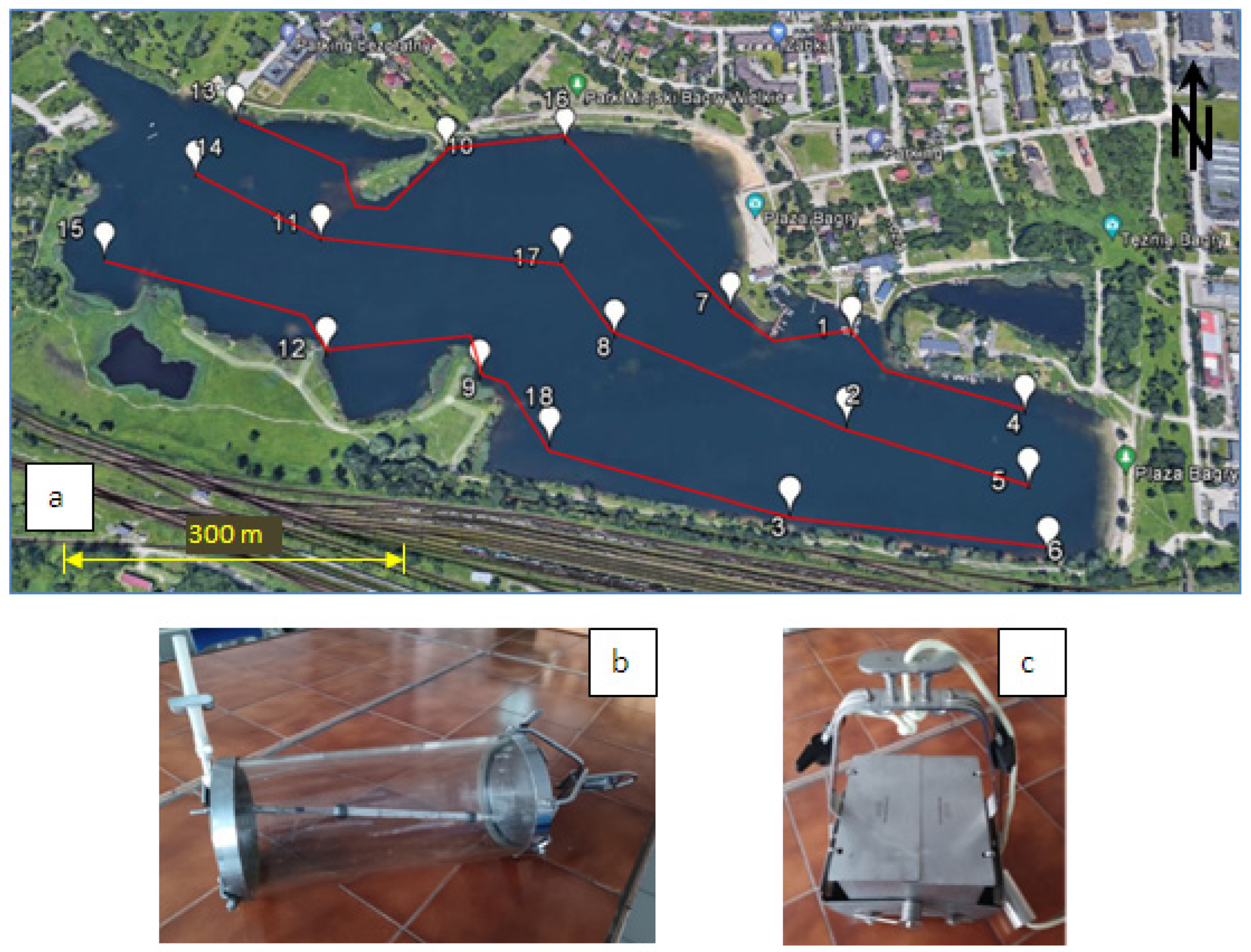
2.1. Lake Description
2.2. Materials and Methods
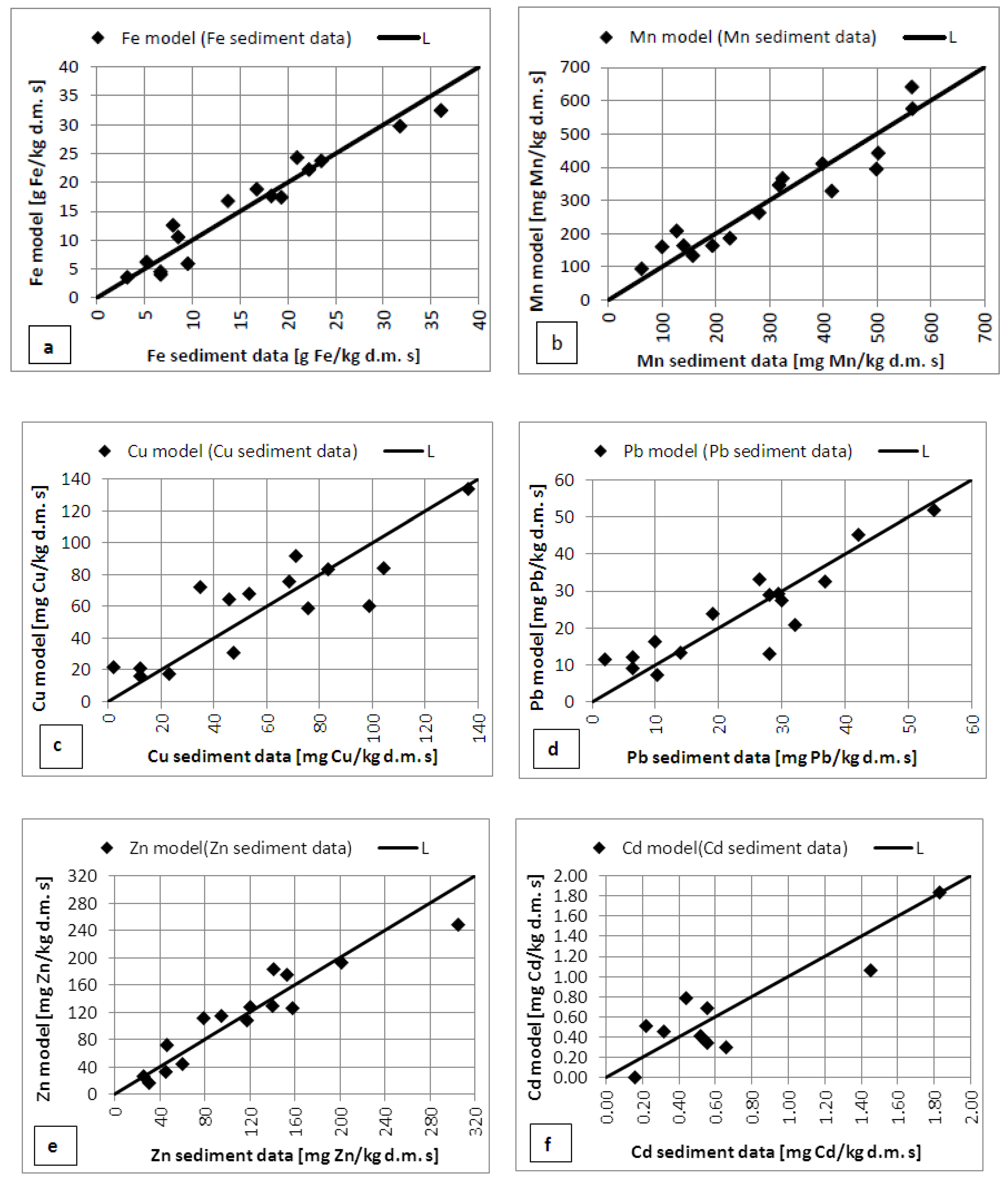
2.3. Model of Relationships between Metals in Sediment
2.4. Method of Determining Mutual Relationships between Metals in Water and Sediments
2.5. Model of Equilibrium between the Metal Concentration in Supernatant and in Sediment
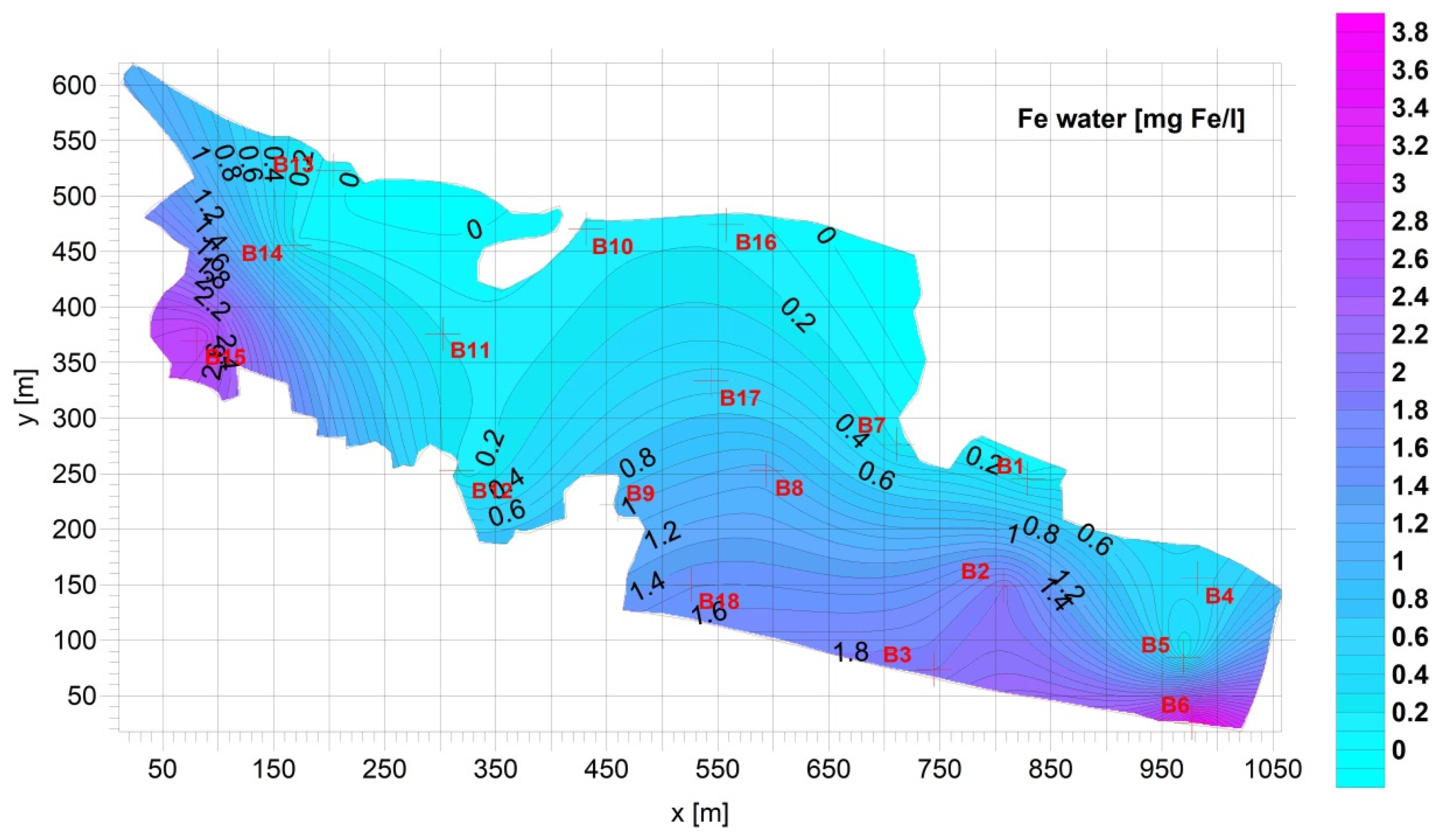
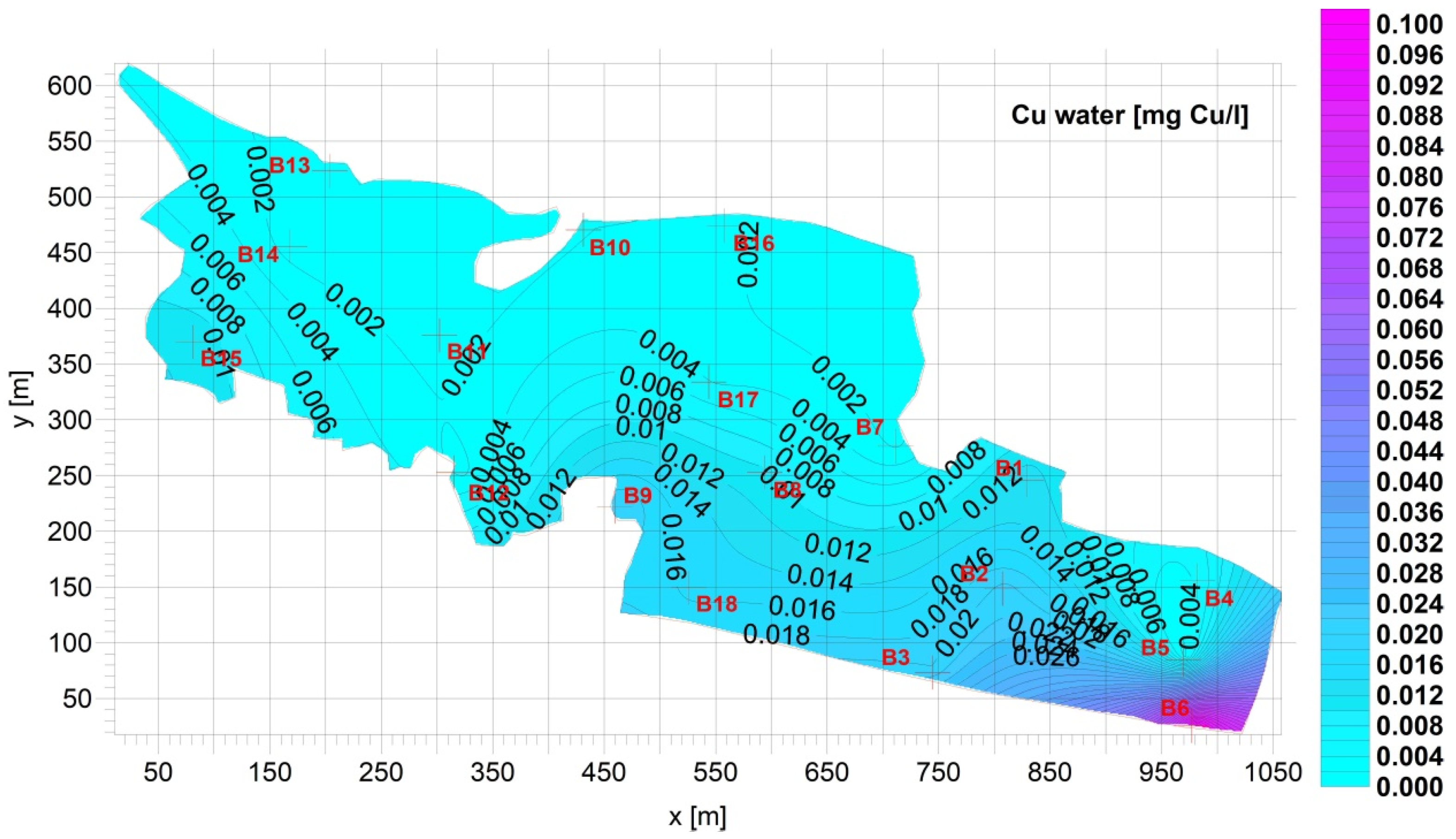
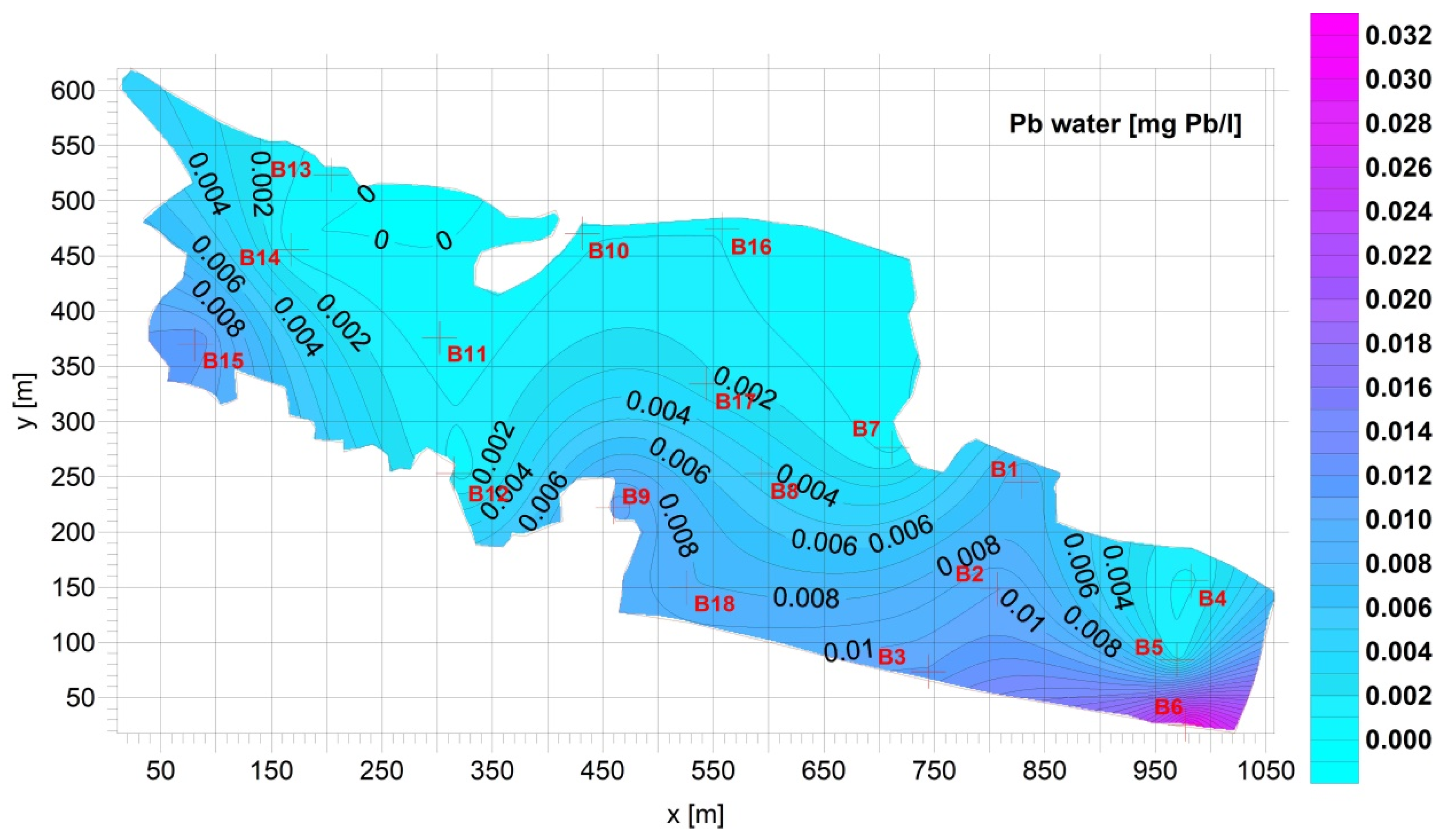
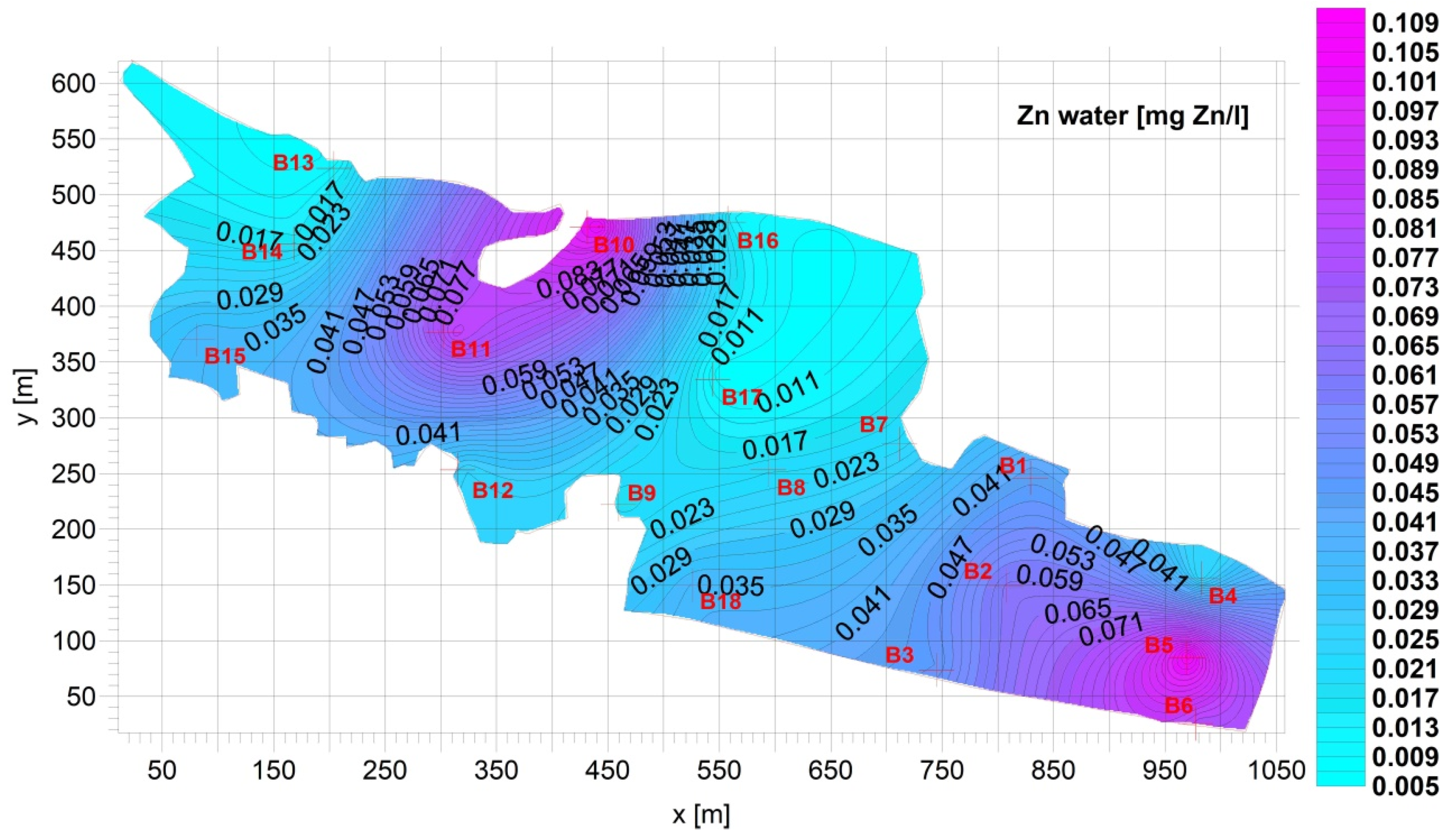
3. Results
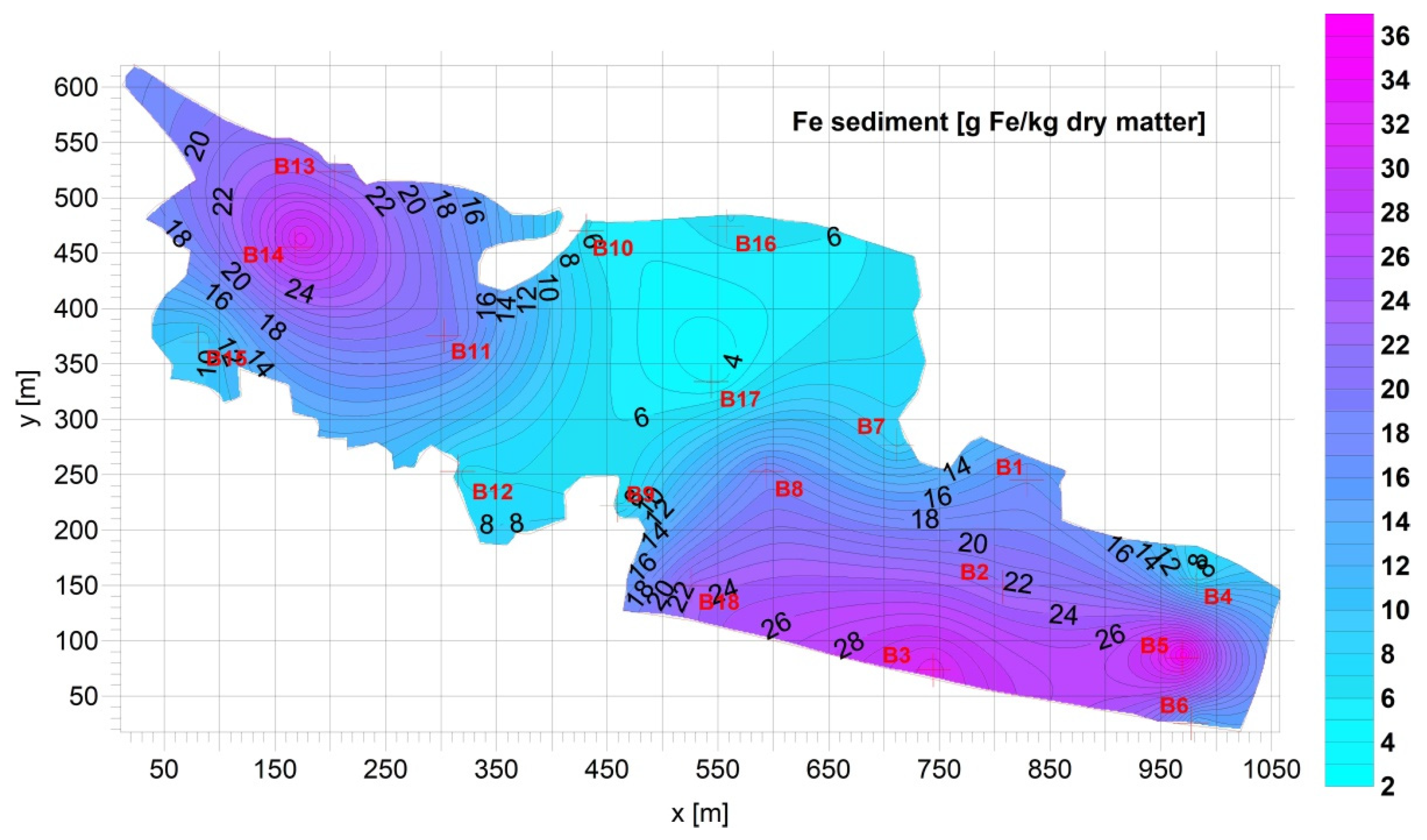
3.1. Sediment
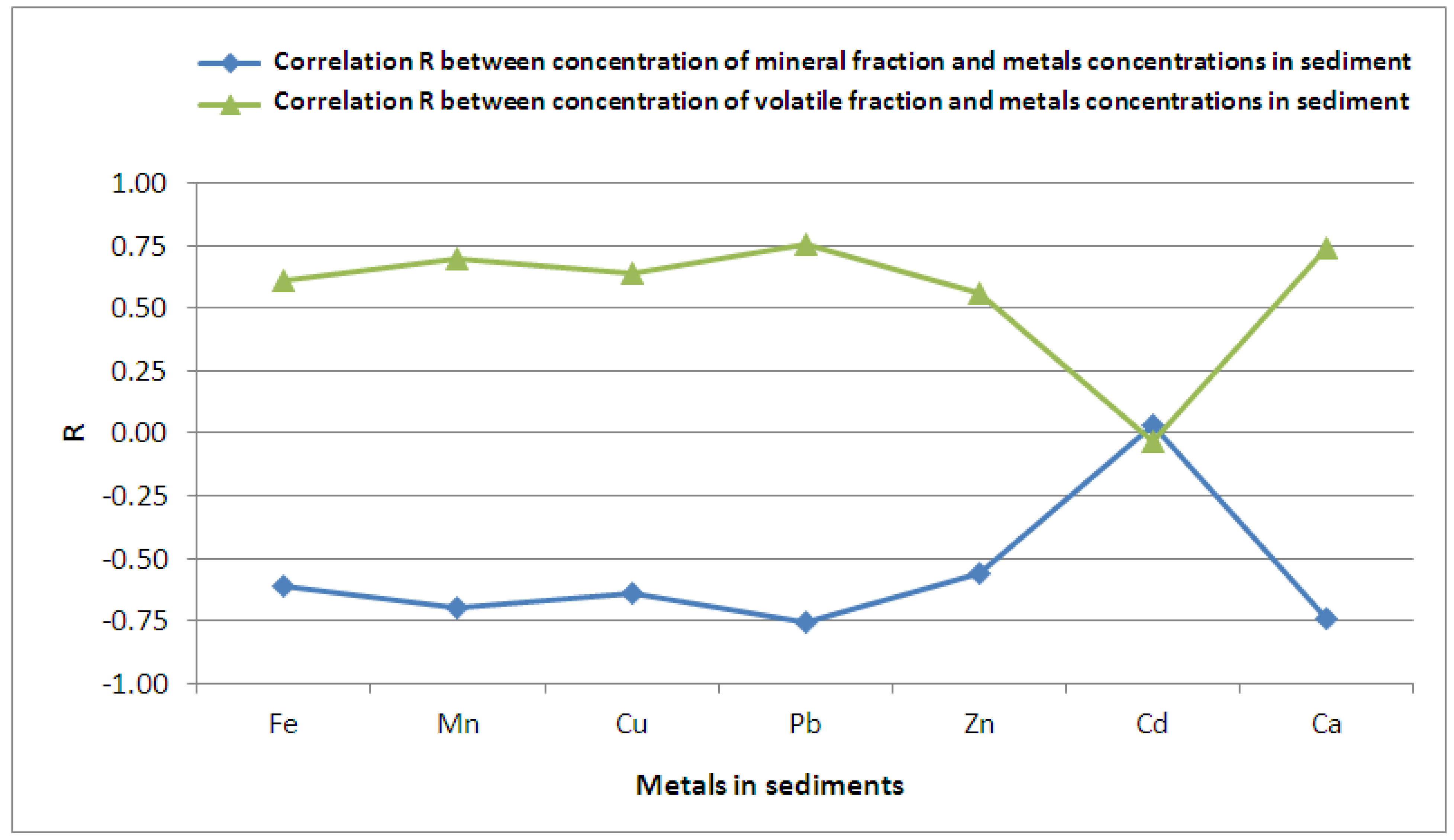
 Figure 3); such correlation is not true in case of Cd (positive correlation). Therefore, a high mineral fraction will be followed by a generally lower concentration of Fe, Mn, Cu, Pb, Zn and Ca in the sediment, except for Cd (
Figure 3); such correlation is not true in case of Cd (positive correlation). Therefore, a high mineral fraction will be followed by a generally lower concentration of Fe, Mn, Cu, Pb, Zn and Ca in the sediment, except for Cd ( Figure 3). Mostly, it means that metals tend to accumulate in the volatile fraction while cadmium, on the other hand, will accumulate in mineral matter.
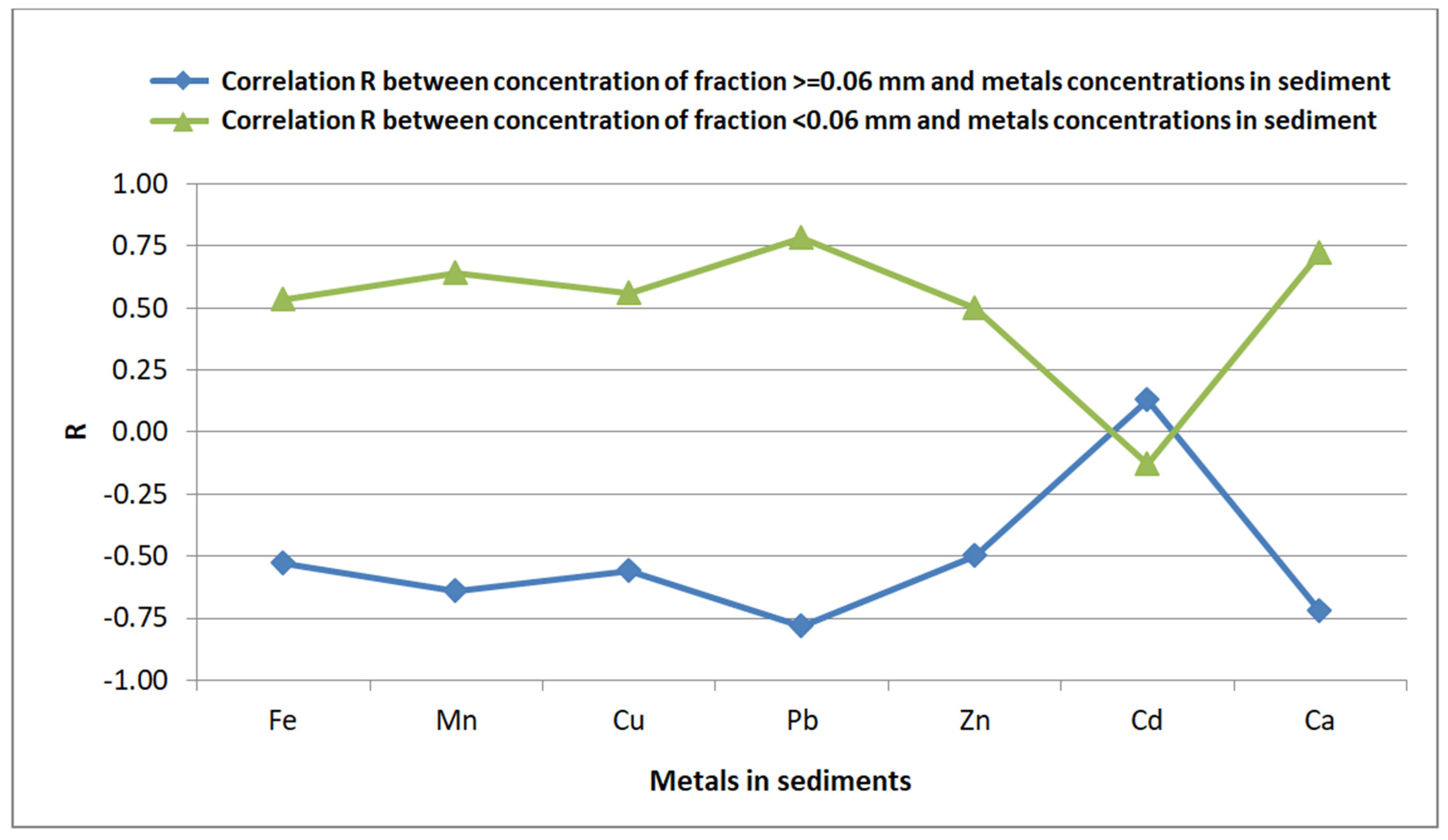
Figure 3). Mostly, it means that metals tend to accumulate in the volatile fraction while cadmium, on the other hand, will accumulate in mineral matter. ) and the metals in the sediment is observed for Fe, Mn, Cu, Pb, Zn and Ca (Figure 4); only for Cd the correlation is negative. Since the fine fraction (<0.06 mm) comprises both mineral and organic matter it means that fine particles predominate in accumulation of metals, except for Cd. An increase in the fine fraction <0.06 mm (
) and the metals in the sediment is observed for Fe, Mn, Cu, Pb, Zn and Ca (Figure 4); only for Cd the correlation is negative. Since the fine fraction (<0.06 mm) comprises both mineral and organic matter it means that fine particles predominate in accumulation of metals, except for Cd. An increase in the fine fraction <0.06 mm ( ) is followed by higher metal concentration in the sediment, again except for Cd. For the fraction ≥0.06 mm (
) is followed by higher metal concentration in the sediment, again except for Cd. For the fraction ≥0.06 mm ( ), the opposite is true: its higher concentration in the sediment results in a lower metal concentration, except for Cd (Figure 4). Therefore, looking at the line (
), the opposite is true: its higher concentration in the sediment results in a lower metal concentration, except for Cd (Figure 4). Therefore, looking at the line ( ) in Figure 3, it can be assumed that metals accumulate in the fine volatile fraction (<0.06 mm,
) in Figure 3, it can be assumed that metals accumulate in the fine volatile fraction (<0.06 mm,  Figure 4) of the sediment but not in the fine mineral fraction (see a blue line
Figure 4) of the sediment but not in the fine mineral fraction (see a blue line  in Figure 3). In the case of Cd, the opposite it true: Cd accumulates mainly in the mineral fraction (
in Figure 3). In the case of Cd, the opposite it true: Cd accumulates mainly in the mineral fraction ( Figure 3) with particles ≥ 0.06 mm (
Figure 3) with particles ≥ 0.06 mm ( Figure 4).
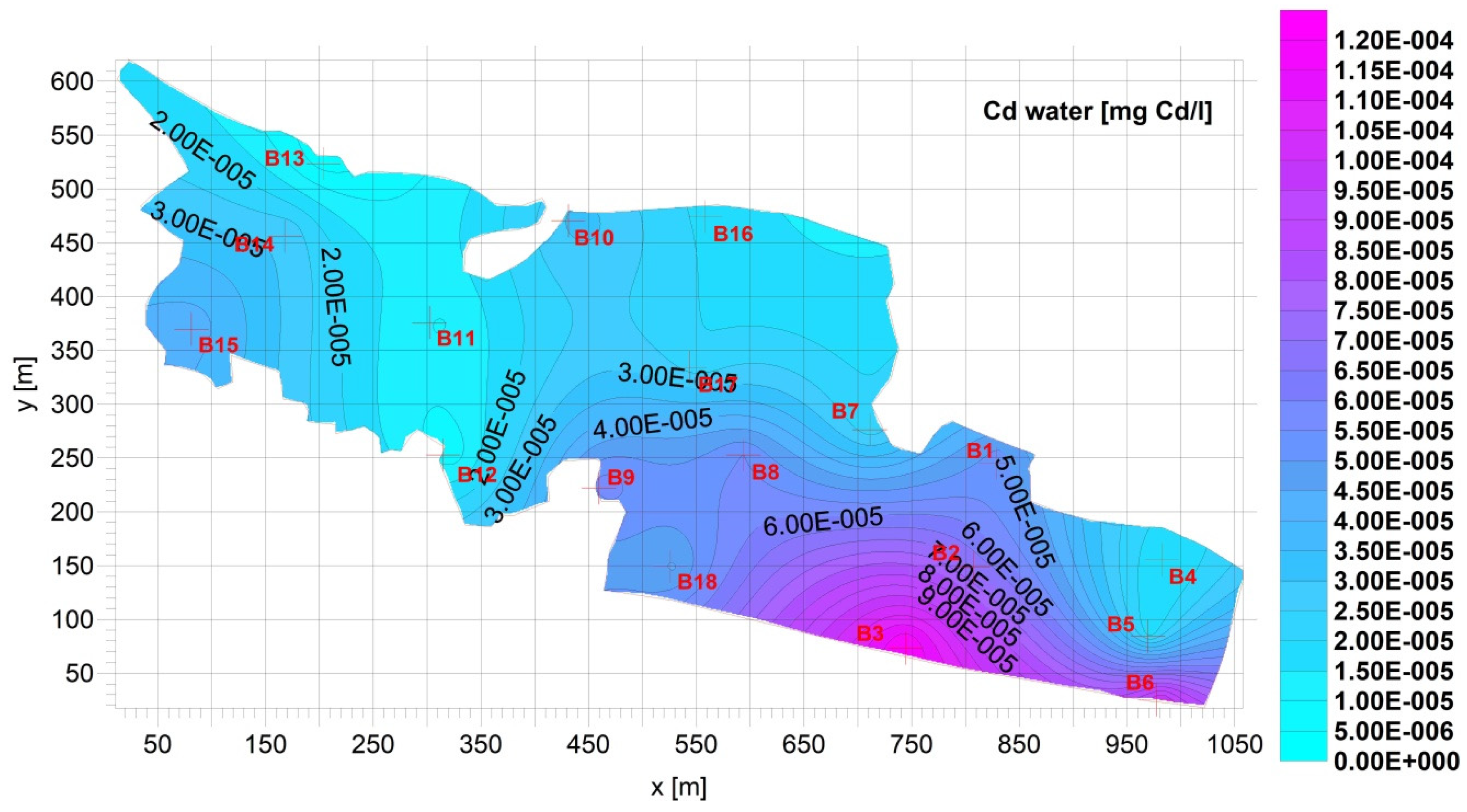
Figure 4).3.2. Supernatant
4. Relationships between Metals in Sediment
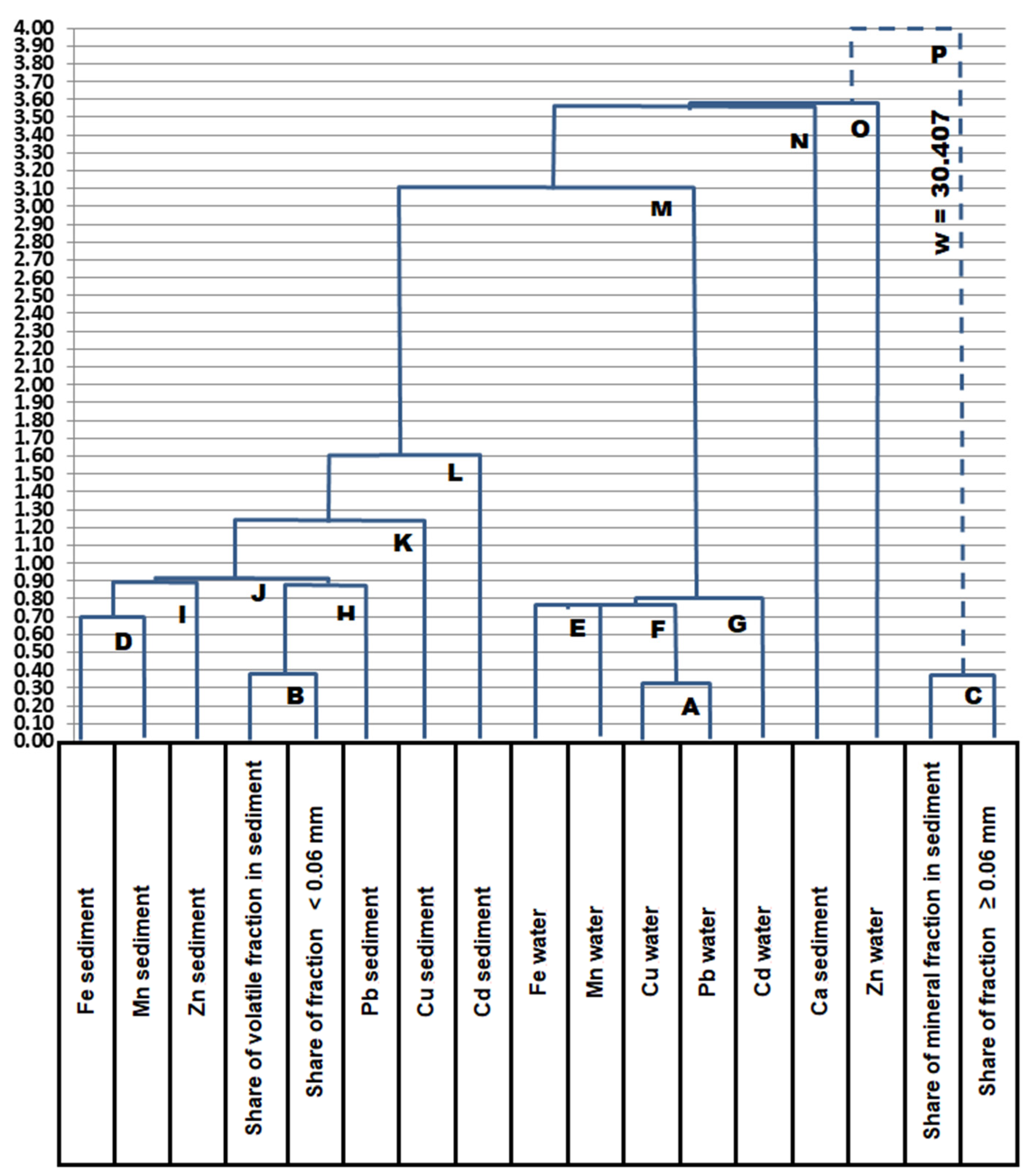
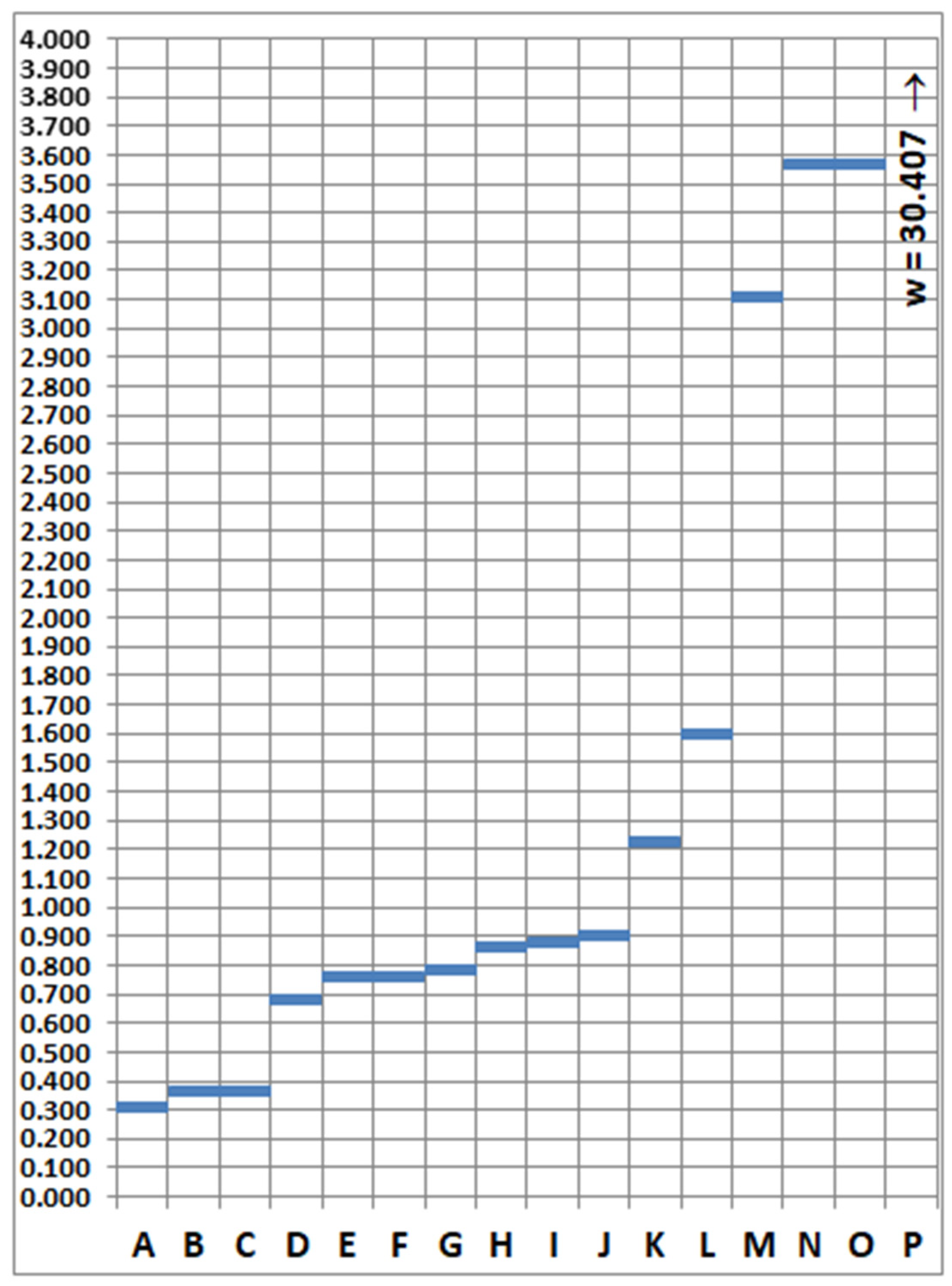
5. Relationships between Metals in Supernatant and Sediments
- Clusters for metals in the sediment;
- Clusters for metals in the supernatant without zinc;
- Cluster for the sediment mineral fraction and the fraction of particles ≥ 0.06 mm.
- Cluster D: Fe sediment, Mn sediment;
- Cluster E: Fe supernatant, Mn supernatant;
- Cluster A: Cu supernatant, Pb supernatant;
- Cluster B: a volatile mass fraction, a mass fraction of particles < 0.06 mm (silty and clay fractions);
- Cluster C: a mineral mass fraction, a mass fraction of particles ≥ 0.06 mm (sand fraction).
6. Equilibrium between the Metal Concentration in Supernatant and in Sediment
- Iron in the supernatant does not affect its concentration in mineral and volatile fractions of the sediment (,). At a substantial iron concentration in the sediments, model (6) did not detect its accumulation from the supernatant (, ), or there was the maximum accumulation not dependent on the metal concentration in the supernatant. Iron is mostly accumulated in the volatile fraction, while its concentration in the mineral fraction is 2.510 g/kg d.m. (64 times lower).
- Manganese in the supernatant does not affect its concentration in mineral and volatile fractions of the sediment (, ). Model (6) did not detect any accumulation from the supernatant to the sediment (,), or the maximum accumulation was not dependent on the metal concentration in the supernatant. Manganese is present mainly in the volatile fraction; its concentration in the mineral fraction is 62.57 g/kg d.m. (47 times lower).
- Copper in the supernatant correlates with copper in mineral and volatile fractions of the sediments. Model (6) showed the possibility of adsorption (general accumulation) from the supernatant to both sediment fractions. Copper is mainly present in the volatile fraction; in the mineral fraction its concentration is 9.583 mg/kg d.m. (62 times lower).
- Lead in the supernatant does not correlate with its concentration in the mineral and volatile fractions of the sediment (, ), or the maximum accumulation is not dependent on the metal concentration in the supernatant. Model (6) showed the possible adsorption (general accumulation) in the sediment volatile fraction. Lead is present mainly in the volatile fraction (302.8 mg/kg d.m. v.).
- Zinc in the supernatant correlates with zinc in the mineral and volatile fractions of the sediment. Model (6) showed the possible adsorption (general accumulation) of zinc in both sediment fractions. Zinc is present mainly in the volatile fraction; in the mineral fraction its concentration is barely 0.1176 mg/kg d.m. m (10,878 times lower).
- Model (6) showed that cadmium can be adsorbed (general accumulation) in both sediment fractions. However, its adsorption (general accumulation) in the mineral fraction is almost negligible. Cadmium is mainly adsorbed (general accumulation) in the volatile fraction. Its concentration in the mineral fraction is close to zero, while in the volatile one it reaches 12.788 mg/kg d.m. v.
| Metal | [% Mew/% Mem] | [% Mew/% Mev] | ||
|---|---|---|---|---|
| Fe | – | – | – | – |
| Mn | – | – | – | – |
| Cu | 0.8057 | 0.1943 | 4.149 | 104.7 |
| Pb | 0 | 1 | – | – |
| Zn | 0.01159 | 0.9884 | 0.3051 | 3.832 |
| Cd | 4.207 × 10−16 | ~1 | 0.2025 | 1.777 |
7. Discussion
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kabata-Pendias, A.; Pendias, H. Biogeochemia pierwiastków śladowych. Biogeochemistry of Trace Elements; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1999; ISBN 83-01-12823-2. [Google Scholar]
- Kownacka, M.; Kownacki, A. Vertical distribution of zoocenoses in the streams of the Tatra, Caucasus and Balkan Mts. Verh. Des Int. Ver. Für Limnol. 1972, 18, 742–750. [Google Scholar] [CrossRef]
- Swansburg, E.O.; Fairchild, W.L.; Fryer, B.J.; Ciborowski, J.J.H. Mouthpart deformities and community compositions of Chironomidae (Diptera) larvae downstream of metal mines in New Brunswick, Canada. Environ. Toxicol. Chem. 2002, 21, 2675–2684. [Google Scholar] [CrossRef] [PubMed]
- Gower, A.M.s.G.; Kent, M.; Foulkes, M.E. Relationship between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-west England. Freshwater Biol. 2006, 32, 199–221. [Google Scholar] [CrossRef]
- Martinez, E.A.; Moore, B.C.; Schaumloffel, J.; Dasgupta, N. Induction of morphological deformities in Chironomus tentans exposed to zinc and lead-spiked sediments. Environ. Toxicol. Chem. 2009, 20, 2475–2481. [Google Scholar] [CrossRef]
- Ross, K.; Cooper, N.; Bidwell, J.R.; Elder, J. Genetic diversity and metal tolerance of two marine species: A comparison between populations from contaminated and reference sites. Mar. Poll. Bull. 2002, 44, 671–679. [Google Scholar] [CrossRef]
- Florea, M.A.; Büsselberg, D. Metals and metal compounds: Occurrence, use, benefits and toxic cellular effects. Biometals 2006, 19, 419–427. [Google Scholar] [CrossRef]
- Michailova, P.; Szarek-Gwiazda, E.; Kownacki, A. Effect of contaminants on the genome of chironomids (Chironomidae, Diptera) live in sediments of Dunajec River and Czorsztyn Reservoir (Southern Poland). Water Air Soil Pollut. 2009, 202, 245–256. [Google Scholar] [CrossRef]
- Michailova, P.; Szarek-Gwiazda, E.; Kownacki, A. Does biodiversity of macroinvertebrates and genome response of Chironomidae larvae (Diptera) reflect heavy metal pollution in a small pond. Environ. Monit. Assess. 2012, 184, 1–14. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Gulan, L.; Stajic, J.M.; Zeremski, T.; Durlević, U.; Valjarević, A. Radionuclides and Metals in the Parks of the City of Belgrade, Serbia: Spatial Distribution and Health Risk Assessment. Forests 2022, 13, 1648. [Google Scholar] [CrossRef]
- Li, J.; Zuo, Q.; Feng, F.; Jia, H. Occurrence and Ecological Risk Assessment of Heavy Metals from Wuliangsuhai Lake, Yellow River Basin, China. Water 2022, 14, 1264. [Google Scholar] [CrossRef]
- Demina, L.; Gablina, I.; Budko, D.; Dara, O.; Solomatina, A.; Gorkova, N.; Smirnova, T. Geochemical Fractions of Heavy Metals in Bottom Sediments of the Pobeda Hydrothermal Cluster in the Mid-Atlantic Ridge (17°07′–17°08′ N). Minerals 2021, 11, 591. [Google Scholar] [CrossRef]
- Pertsemli, E.; Voutsa, D. Distribution of heavy metals in Lakes Doirani and Kerkini, Northern Greece. J. Hazard. Mater. 2007, 148, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef]
- Ying, W.; Yang, F.; Bick, A.; Oron, G.; Herzberg, M. Extracellular Polymeric Substances (EPS) in a Hybrid Growth Membrane Bioreactor (HG-MBR): Viscoelastic and Adherence Characteristics. Environ. Sci. Technol. 2010, 44, 8636–8643. [Google Scholar] [CrossRef]
- Doney, S.; Tw, L.; Ma, S.; Gn, G.; Ij, P.; Rc, P.; Fm, M. Faculty Opinions recommendation of Biochemistry: A cadmium enzyme from a marine diatom. Nature 2005, 435, 42. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Priority List of Hazardous Substances. 2002. Available online: https://www.atsdr.cdc.gov/SPL/ (accessed on 10 November 2022).
- Rozporządzenie Ministra Gospodarki Morskiej i Żeglugi Śródlądowej z dnia 12 lipca 2019, Regulation of the Minister of Maritime Economy and Inland Navigation of July 12, 2019, Poland. Dz. U. 2019, poz. 1311. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 4 May 2023).
- Szarek-Gwiazda, E. Czynniki kształtujące stężenia metali ciężkich w rzece Rabie i niektórych zbiornikach zaporowych, Factors influencing the concentration of heavy metals in the Raba River and some dam reservoirs. Stud. Nat. 2013, 60, 1–146, (In Kraków). [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M.; Stahl, D. Cluster Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Tian, J.; Guo, K.; Sun, Y.; Lin, R.; Chen, T.; Zhang, B.; Liu, Y.; Yang, T. Solvent-Free Synthesis of Magnetic Sewage Sludge-Derived Biochar for Heavy Metal Removal from Wastewater. Int. J. Environ. Res. Public Health 2023, 20, 155. [Google Scholar] [CrossRef]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Sangiumsak, N.; Punrattanasin, P. Adsorption Behavior of Heavy Metals on Various Soils. Pol. J. Environ. Stud. 2014, 23, 853–865. [Google Scholar]
- EPA-SAB-EPEC-90–006; Report of the Sediment Criteria Subcommittee of the Ecological Processes and Effects Committee—Evaluation of the Equilibrium Partitioning (EqP) Approach for Assessing Sediment Quality. U.S. Environmental Protection Agency: Washington, DC, USA, 1990.
- Burgess, R.M.; Kane Driscoll, S.B.; Ozretich, R.J.; Mount, D.R.; Reiley, M.C. Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Procedures for the Determination of the Freely Dissolved Interstitial Water Concentrations of Nonionic Organics; EPA/600/R-02/012; United States Environmental Protection Agency, Office of Research and Development, National Health and Environmental Effects Research Laboratory, Atlantic Ecology Division: Washington, DC, USA, 2012. [Google Scholar]
- Kasztelewicz, Z. Poprawianie Krajobrazu: Rekultywacja Terenów Pogórniczych w Polskich Kopalniach Odkrywkowych. Cz. III; Surowce i Maszyny Budowlane, Landscaping. Reclamation of Post-Mining Areas in Polish Opencast Mines; Raw Materials and Construction Machinery: Kraków, Poland, 2011; Volume 3, pp. 91–93. [Google Scholar]
- Pietrzyk-Sokulska, E. Zbiorniki Wodne w Województwie Małopolskim Jako Istotny Element Jakości Środowiska. Cz. 2, Charakterystyka wybranych antropogenicznych zbiorników wodnych województwa małopolskiego, (Water reservoirs in the Małopolskie voivodship as an important element of environmental quality. Characteristics of selected anthropogenic water reservoirs in the Małopolskie voivodeship). Zesz. Nauk. Inst. Gospod. Surowcami Miner. I Energią Pol. Akad. Nauk. 2011, 2, 37–65. [Google Scholar]
- Czaplicka, A.; Kubala, M.; Pokrzywa, K. Historia powstania Zalewu Bagry oraz jakość jego wód w aspekcie wykorzystania rekreacyjnego, w: Prokocim dawniej i dziś, (The History of the Creation of the Bagry Lake and the Quality of Its Waters in Terms of Recreational Use, in: Prokocim in the Past and Today), Kraków. Tow. Przyj. Prokocimia 2018, 205–222. ISBN 978-83-920955-7-6. Available online: http://prokocim2017.wis.pk.edu.pl/Prokocim_650.pdf (accessed on 4 May 2023).
- Standard Methods for the Examination of Water and Wastewater, American Waterworks Association, 24th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2022; ISBN 13978–0875532998.
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the protection and management of aquatic sediment quality in Ontario. Ont. Minist. Environ. Tor. 1992. Available online: https://atrium.lib.uoguelph.ca/xmlui/handle/10214/15797 (accessed on 4 May 2023).
- Gawałkiewicz, R. Bagry Lake—Volume 1. A bibliographic query of literature and archive data in explaining the evolution of the water. Folia Quat. 2017, 16, 115–126. [Google Scholar] [CrossRef]
- Available online: https://www.hornkrakow.pl/klub/info-o-bagrach/ (accessed on 24 December 2022).
- Hermanowicz, W. Chemia Sanitarna, (Sanitary Chemistry); Wydawnictwo “Arkady”: Warszawa, Poland, 1984. [Google Scholar]
- Rozporządzenie Ministra Infrastruktury z dnia 25 czerwca 2021 r. w sprawie klasyfikacji stanu ekologicznego, potencjału ekologicznego i stanu chemicznego oraz sposobu klasyfikacji stanu jednolitych części wód powierzchniowych, a także środowiskowych norm jakości dla substancji priorytetowych, (Regulation of the Minister of Infrastructure of June 25, 2021 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classifying the Status of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances, Poland). Dz. U. z 2021, poz. 1475. Available online: sap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 4 May 2023).
- Dojlido, J. Chemia Wody, (Water Chemistry); Wydawnictwo “Arkady”: Warszawa, Poland, 1987. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystem, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; Volume 35, ISBN 9780127447605. [Google Scholar]
- Głowacka, M. [red.], Metaloznawstwo, (Metallography); Wydawnictwo Politechniki Gdańskiej: Gdańsk, Poland, 1996. [Google Scholar]
- Zhang, C.; Zhang, R. Matrix proteins in the outer shells of molluscs. Mar. Biotechnol. 2006, 8, 572–586. [Google Scholar] [CrossRef]
- Turekian, K.; Wadepohl, K. Distribution of the Elements in Some Major Units of the Earth’s. Crust. Geol. Soc. Am. Bul. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Gruca-Rokosz, R.; Bartoszek, L.; Tomaszek, J.A. Heavy metals in the bottom sediments of the Solina Reservoir. Environ. Prot. Eng. 2004, 30, 45–50. [Google Scholar]
- Reczyńska-Dutka, M. Ecology of some waters in the forest—Agricultural basin of the River Brynica near the Upper Silesian Industrial Region. Atmospheric heavy metal pollution of the bottom sediments of the reservoir at Kozłowa Góra. Acta Hydrobiol. 1985, 27, 465–476. [Google Scholar]
- Pasternak, K. Zawartość miedzi, cynku i manganu w wodzie zbiornika zaporowego w Goczałkowicach oraz kilku innych zbiornikach, (The content of copper, zinc and manganese in the water of the dam reservoir in Goczałkowice and several other reservoirs). Acta Hydrobiol. 1971, 13, 159–177. [Google Scholar]
- Reczyńska-Dutka, M. Ecology of some waters in the forest-agricultural basin of thr River Brynica near the Upper Silesian Industrial Region. Chemical composition of the water. Heavy metals. Acta Hydrobiol. 1985, 27, 451–464. [Google Scholar]
- Ryborz-Masłowska, S.; Moraczewska-Majkut, K.; Krajewska, J. Metale ciężkie w wodach i osadach dennych zbiornika w Kozłowej Górze na Górnym Śląsku, (Heavy metals in waters and bottom sediments of the reservoir in Kozłowa Góra in Upper Silesia). Arch. Ochr. Sr. 2000, 26, 127–140. [Google Scholar]

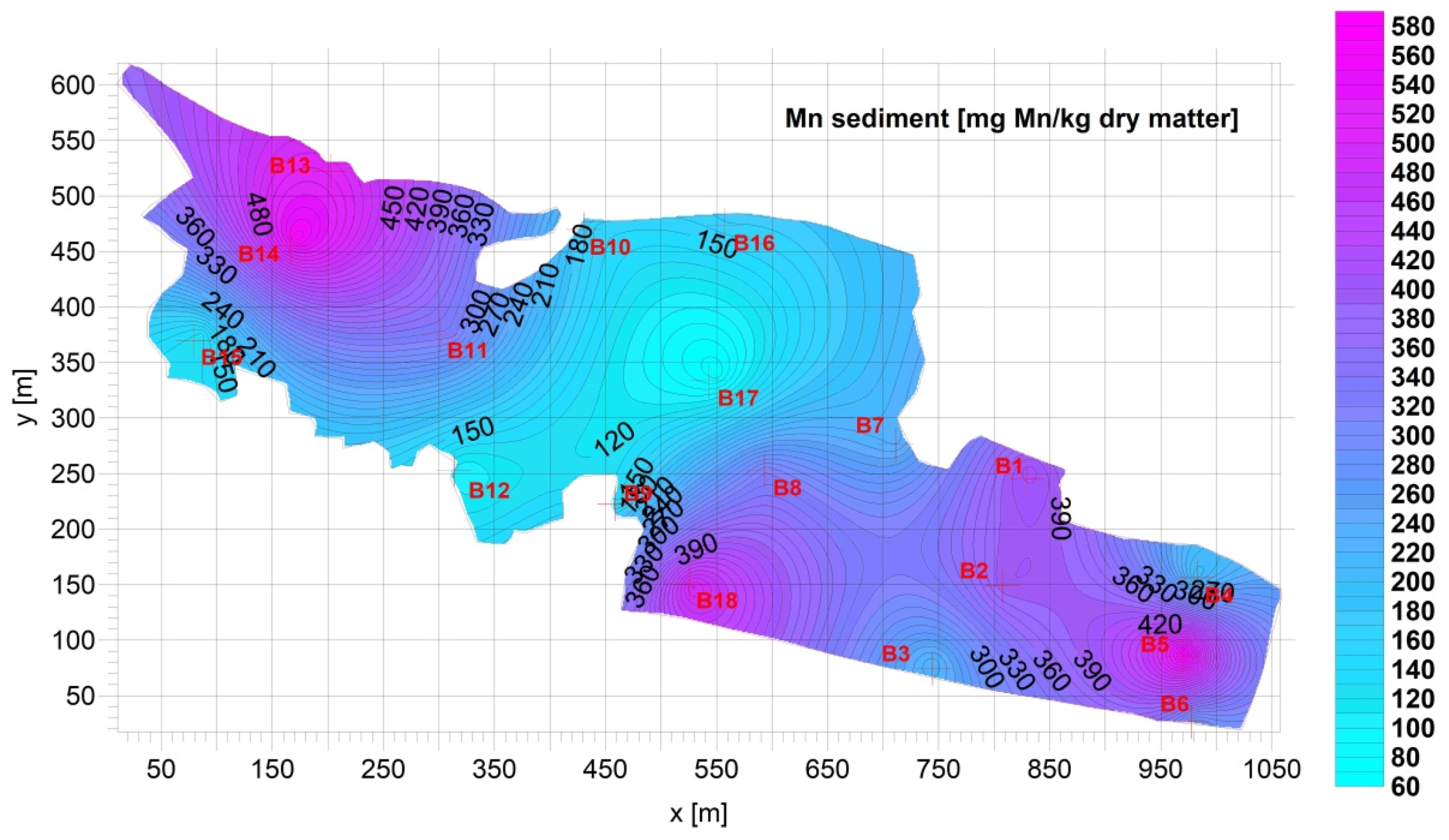
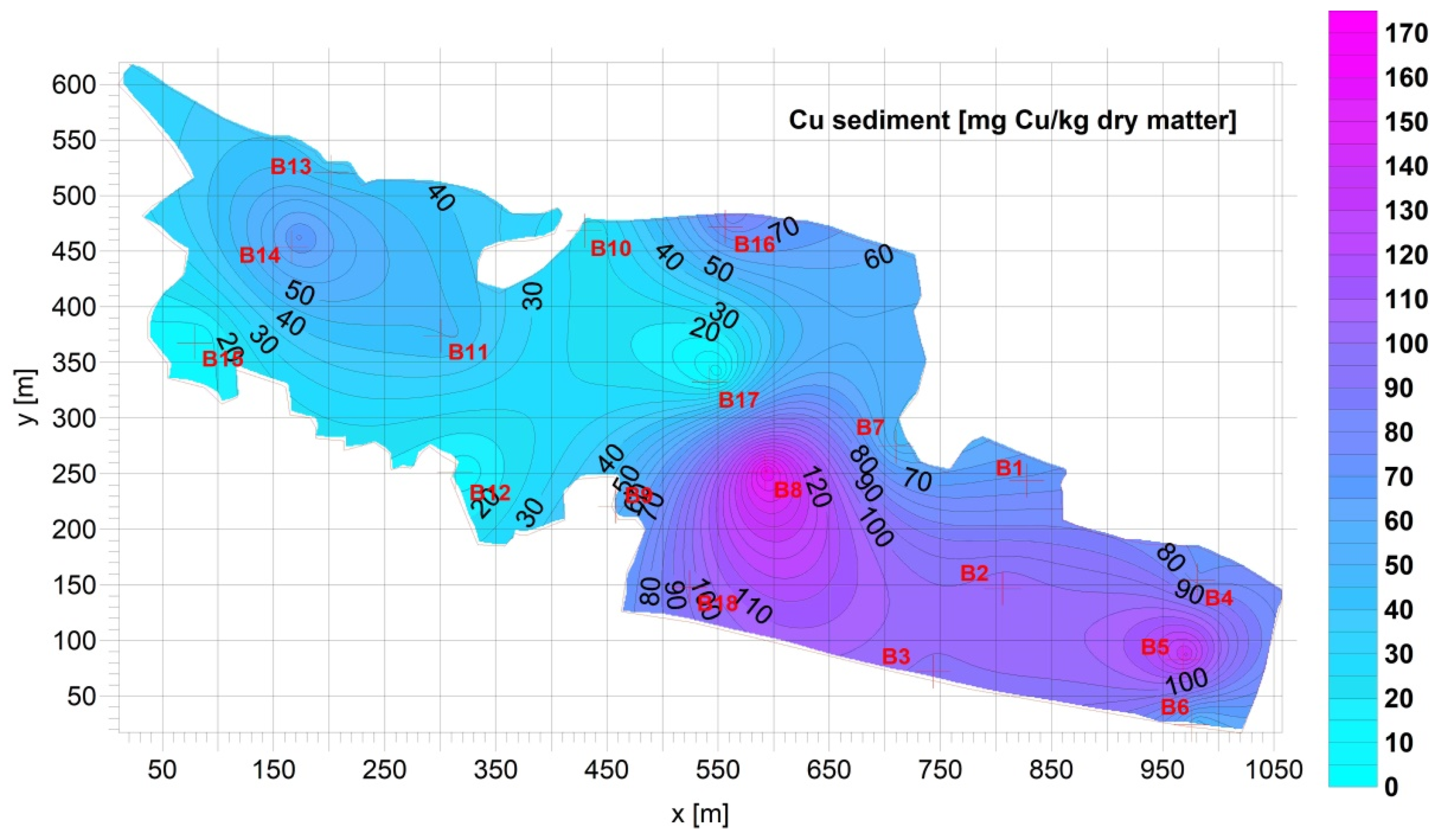

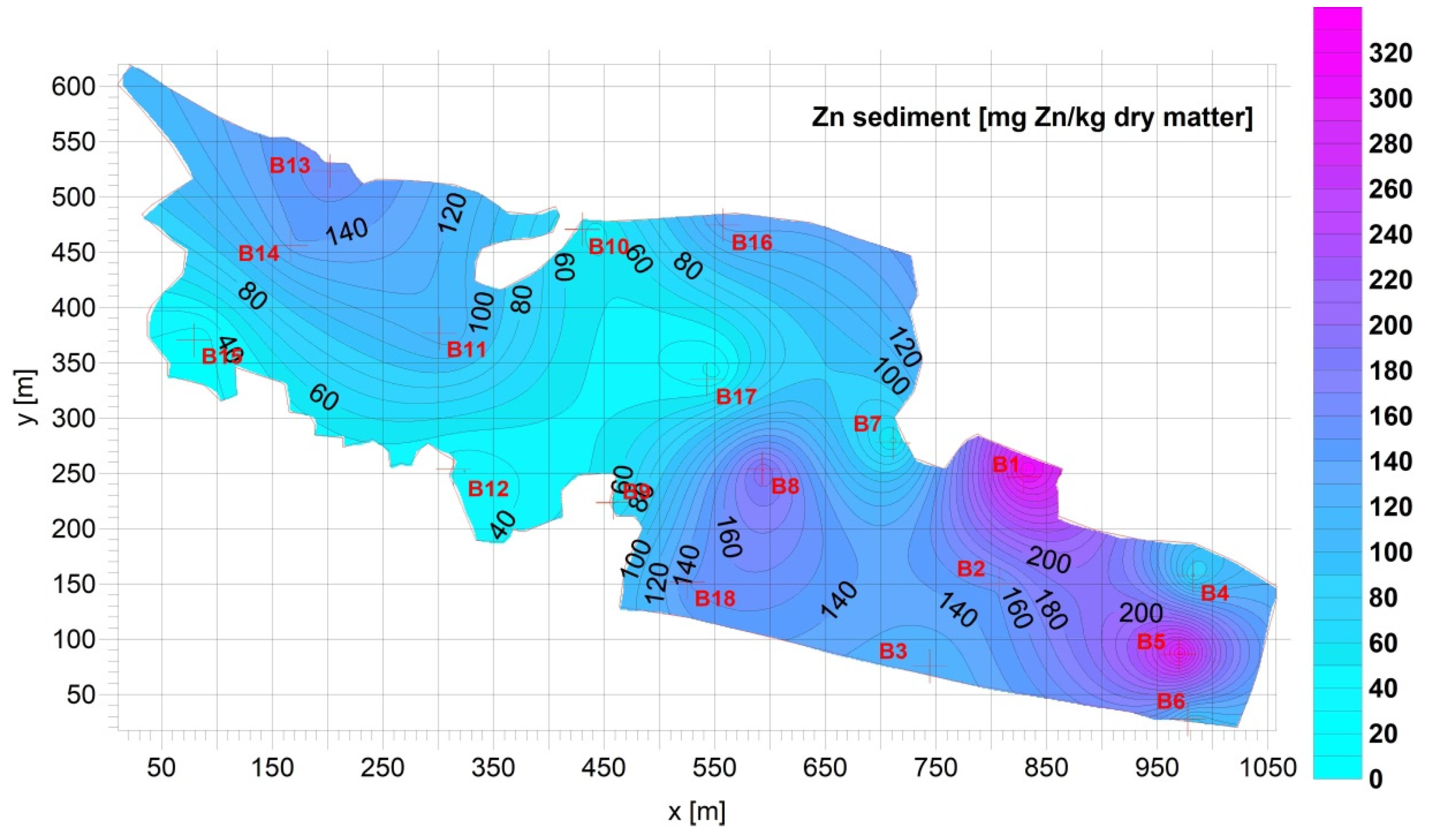
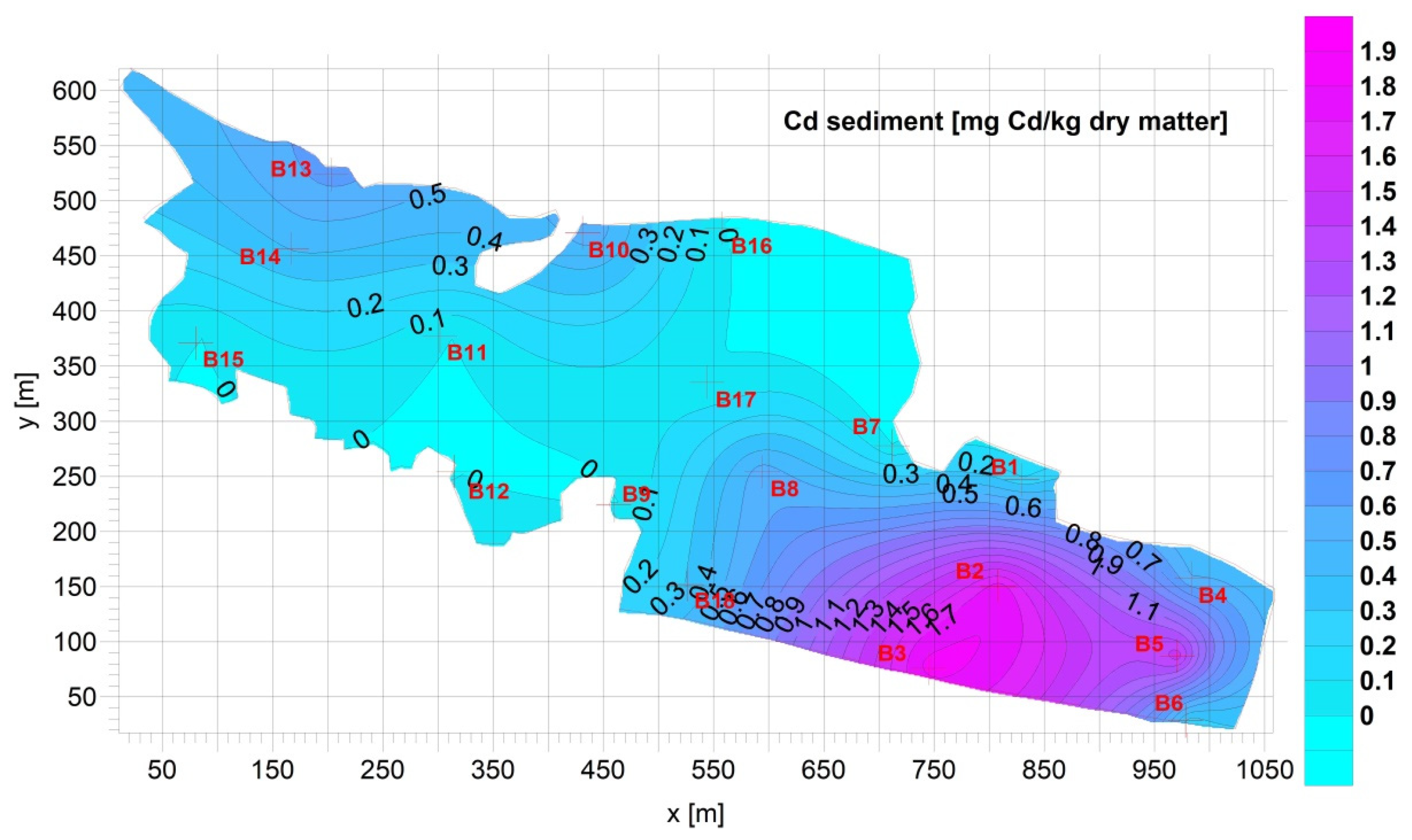


| Fe [g Fe/kg d.m.] | Mn [mg Mn/kg d.m.] | Cu [mg Cu/kg d.m.] | Pb [mg Pb/kg d.m.] | Zn [mg Zn/kg d.m.] | Cd [mg Cd/kg d.m.] | Ca [g Ca/kg d.m.] | |
|---|---|---|---|---|---|---|---|
| Average= | 16.04 | 292.7 | 65.53 | 24.33 | 122.9 | 0.7709 | 64.45 |
| Standard deviation= | 9.803 | 159.4 | 42.06 | 13.65 | 84.48 | 0.5845 | 32.27 |
| Min= | 3.162 | 61.60 | 2.113 | 1.962 | 25.35 | 0.1592 | 28.70 |
| Max= | 36.03 | 566.4 | 161.0 | 54.11 | 321.0 | 1.831 | 144.8 |
| Fe [mg Fe/L] | Mn [mg Mn/L] | Cu [mg Cu/L] | Pb [mg Pb/L] | Zn [mg Zn/L] | Cd [mg Cd/L] | |
|---|---|---|---|---|---|---|
| Average | 0.8928 | 0.09733 | 0.01261 | 0.005663 | 0.04165 | 4.172 × 10−5 |
| Standard deviation= | 1.044 | 0.07402 | 0.02157 | 0.007396 | 0.03117 | 3.060 × 10−5 |
| Min= | 0.05300 | 0.03000 | 0.0005274 | 3.330 × 10−5 | 0.007400 | 7.200 × 10−6 |
| Max= | 3.690 | 0.3010 | 0.09659 | 0.03081 | 0.1065 | 0.0001200 |
| Constant | Multiplier for Concentration [g Fe/kg d.m.] Others [mg …../kg d.m.] | Significance F | R | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe Sediment | Mn Sediment | Cu Sediment | Pb Sediment | Zn Sediment | Cd Sediment | ||||
| Fe sediment | −1.185 | 0.03727 | 0.2328 | 2.653 × 10−8 | 0.9652 | ||||
| Mn sediment | 47.02 | 17.85 | −0.9139 | 4.524 × 10−7 | 0.9457 | ||||
| Cu sediment | 0.7386 | 1.718 | 0.1315 | 3.394 × 10−4 | 0.8411 | ||||
| Pb sediment | 3.087 | 1.551 | −0.01271 | 3.030 × 10−5 | 0.8934 | ||||
| Zn sediment | −15.68 | 0.1369 | 0.7184 | 1.658 | 2.088 × 10−6 | 0.9415 | |||
| Cd sediment | 0.4990 | 0.06407 | −0.003081 | 5.309 × 10−3 | 0.8810 | ||||
| Clusters (Definitions): | Distances between Clusters w | Distances between Clusters [%] | Cluster |
|---|---|---|---|
| Cu supernatant + Pb supernatant = A | 0.312 | 1.025 | A |
| Volatile fraction + mass fraction < 0.06 mm = B | 0.362 | 1.189 | B |
| Mineral fraction + mass fraction ≥ 0.06 mm = C | 0.362 | 1.189 | C |
| Fe sediments + Mn sediments = D | 0.680 | 2.235 | D |
| Fe supernatant + Mn supernatant = E | 0.757 | 2.489 | E |
| E + A = F | 0.758 | 2.492 | F |
| F + Cd supernatant =G | 0.787 | 2.588 | G |
| Pb sediment +B = H | 0.866 | 2.846 | H |
| D + Zn sediment = I | 0.880 | 2.895 | I |
| I + H = J | 0.900 | 2.960 | J |
| J + Cu sediment = K | 1.230 | 4.044 | K |
| K + Cd sediment = L | 1.599 | 5.259 | L |
| G + L = M | 3.109 | 10.224 | M |
| M + Ca sediment = N | 3.566 | 11.728 | N |
| N + Zn supernatant = O | 3.570 | 11.742 | O |
| O + C = P | 30.407 | 100.000 | P |
| Fe Supernatant | Mn Supernatant | Cu Supernatant | Pb Supernatant | Zn Supernatant | Cd Supernatant | Fe Sediment | Mn Sediment | Cu Sediment | Pb Sediment | Zn Sediment | Cd Sediment | Ca Sediment | Mineral Fraction in Sample | Volatile Fraction in Sample | Mass Fraction ≥ 0.06 mm | Mass Fraction < 0.06 mm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe Supernatant | 1.0000 | ||||||||||||||||
| Mn Supernatant | 0.7974 | 1.0000 | |||||||||||||||
| Cu Supernatant | 0.7970 | 0.7740 | 1.0000 | ||||||||||||||
| Pb Supernatant | 0.8981 | 0.8113 | 0.9547 | 1.0000 | |||||||||||||
| Zn Supernatant | 0.1762 | 0.1989 | 0.2697 | 0.2315 | 1.0000 | ||||||||||||
| Cd Supernatant | 0.7249 | 0.6087 | 0.6919 | 0.7859 | 0.1357 | 1.0000 | |||||||||||
| Fe Sediment | 0.0452 | 0.0953 | 0.0010 | 0.0211 | 0.3009 | 0.3062 | 1.0000 | ||||||||||
| Mn Sediment | −0.1310 | 0.0345 | −0.0262 | −0.0639 | 0.2139 | 0.0490 | 0.8271 | 1.0000 | |||||||||
| Cu Sediment | 0.0531 | 0.1059 | 0.0428 | 0.0370 | 0.1587 | 0.2807 | 0.6309 | 0.5394 | 1.0000 | ||||||||
| Pb Sediment | −0.0049 | 0.0378 | 0.0835 | −0.0154 | 0.2552 | 0.0461 | 0.7433 | 0.7399 | 0.6881 | 1.0000 | |||||||
| Zn Sediment | −0.2014 | −0.0579 | −0.0410 | −0.0420 | 0.2600 | 0.1051 | 0.6578 | 0.7506 | 0.6905 | 0.6557 | 1.0000 | ||||||
| Cd Sediment | 0.1206 | −0.1909 | −0.1712 | −0.0807 | 0.2827 | 0.3133 | 0.5302 | −0.0162 | 0.3951 | 0.3597 | 0.0660 | 1.0000 | |||||
| Ca Sediment | −0.0909 | 0.2224 | −0.0007 | −0.0359 | −0.2303 | −0.1693 | 0.0552 | 0.2700 | 0.1681 | 0.2637 | 0.2115 | −0.4603 | 1.0000 | ||||
| Mineral fraction in sample | −0.0354 | −0.2514 | −0.0679 | −0.0362 | −0.0839 | −0.0503 | −0.6104 | −0.7007 | −0.6412 | 0.5692 | −0.5630 | 0.0329 | −0.7407 | 1.0000 | |||
| Volatile fraction in sample | 0.0354 | 0.2514 | 0.0679 | 0.0362 | 0.0839 | 0.0503 | 0.6104 | 0.7007 | 0.6412 | −0.5692 | 0.5630 | −0.0329 | 0.7407 | −1.0000 | 1.0000 | ||
| Mass fraction ≥ 0.06 mm | 0.0632 | −0.1464 | −0.0272 | 0.0411 | 0.0515 | 0.0237 | −0.5320 | −0.6423 | −0.5601 | −0.7837 | −0.4968 | 0.1275 | −0.7225 | 0.9404 | −0.9404 | 1.0000 | |
| Mass fraction < 0.06 mm | −0.0632 | 0.1464 | 0.0272 | −0.0411 | −0.0515 | −0.0237 | 0.5320 | 0.6423 | 0.5601 | 0.7837 | 0.4968 | −0.1275 | 0.7225 | −0.9404 | 0.9007 | −1.0000 | 1.0000 |
| Metal | R | Average Value | |||||
|---|---|---|---|---|---|---|---|
[mg/kg d.m. m] | [mg/kg d.m. v] | ||||||
| Fe | 2.510 | 0.000 | 1.611 × 102 | 0.000 | 0.783 | 2.510 g/kg d.m. m | 161.1 g/kg d.m. v |
| Mn | 62.57 | 0.000 | 2.931 × 103 | 0.000 | 0.799 | 62.57 | 2930.9 |
| Cu | 32.77 | 0.2410 | 6.290 × 102 | 9.552 × 10−3 | 0.796 | 9.583 | 597.7 |
| Pb | 0.000 | 0.000 | 3.028 × 102 | 0.000 | 0.878 | 0.000 | 302.8 |
| Zn | 8.956 × 102 | 3.277 | 3.092 × 103 | 0.2610 | 0.884 | 0.1176 | 1279.3 |
| Cd | 1.144 × 104 | 4.938 | 3.509 × 103 | 0.5627 | 0.847 | 9.052 × 10−17 | 12.788 |
| Reservoir | Fe [g/kg] | Mn [mg/kg] | Cu [mg/kg] | Pb [mg/kg] | Zn [mg/kg] | Cd [mg/kg] |
|---|---|---|---|---|---|---|
| Bagry (currently analysed) | 3.16–36.03 | 61.60–566.4 | 2.11–161.0 | 1.96–54.11 | 25.35–321.0 | ≤1.831 |
| Dobczycki [20] | 10.3–43.8 | 156–2355 | 9.3–44.2 | 11.5–40.7 | 16.5–264.4 | 0.33–2.4 |
| Czorsztyński [20] | 14.1–39.9 | 21.5–336 | 14.8–34.1 | 12–23.2 | 84.7–296.1 | 0.7–2.1 |
| Rożnowski [20] | 20.6–38.1 | 619–1381 | 27.5–38.4 | 16.9–26.3 | 78.5–189 | 1.4–2.6 |
| Solina [42] | nd | nd | 21.69–37.57 | 10.73–33.71 | nd | 0.12–1.4 |
| Kozłowa Góra [43] | nd | 109–784 | 6.8–112.5 | 105–1373 | 290–1875 | 2.8–22.7 |
| Reservoir | Fe [mg/L] | Mn [mg/L] | Cu [mg/L] | Pb [mg/L] | Zn [mg/L] | Cd [mg/L] |
|---|---|---|---|---|---|---|
| Bagry (currently analysed) | 0.053–3.690 | 0.030–0.301 | 0.000527–0.0966 | 3.33 × 10-5–0.0308 | 0.00740–0.1065 | 7.2 × 10−6–0.000120 |
| Dobczycki [20] | 0.003–0.970 | 0.0015–1.880 | 0.0007–0.0218 | 0.0001–0.0068 | 0.0010–0.0862 | 1 × 10−5–0.00062 |
| Czorsztyński [20] | 0.0031–0.186 | 0.0033–0.050 | 0.0005–0.0079 | 0.0002–0.0030 | 0.0847–0.2961 | 2 × 10−5–0.0002 |
| Rożnowski [20] | 0.020–0.247 | 0.0083–0.1736 | 0.0013–0.0106 | 0.0001–0.0027 | 0.0040–0.0318 | 1 × 10−5–0.00024 |
| Solina [44] | 0.020–0.030 | 0.0432–0.0441 | 0.00569–0.00626 | nd | 0.023–0.02325 | nd |
| Kozłowa Góra (Mn i Cu—[45], Zn, Pb, Cd—[46]) | nd | 0.0199–0.5936 | 0.0017–0.0167 | 0.002–0.074 | 0.012–0.190 | 0.0002–0.0080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielski, A.; Czaplicka, A. Heavy Metals in Post-Exploitation Reservoirs—The Bagry Lake Case Study (Poland). Appl. Sci. 2023, 13, 5884. https://doi.org/10.3390/app13105884
Bielski A, Czaplicka A. Heavy Metals in Post-Exploitation Reservoirs—The Bagry Lake Case Study (Poland). Applied Sciences. 2023; 13(10):5884. https://doi.org/10.3390/app13105884
Chicago/Turabian StyleBielski, Andrzej, and Anna Czaplicka. 2023. "Heavy Metals in Post-Exploitation Reservoirs—The Bagry Lake Case Study (Poland)" Applied Sciences 13, no. 10: 5884. https://doi.org/10.3390/app13105884
APA StyleBielski, A., & Czaplicka, A. (2023). Heavy Metals in Post-Exploitation Reservoirs—The Bagry Lake Case Study (Poland). Applied Sciences, 13(10), 5884. https://doi.org/10.3390/app13105884





