Optimization of Soybean Protein Extraction Using By-Products from NaCl Electrolysis as an Application of the Industrial Symbiosis Concept
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Industrial Co- and By-Products
2.2. Approximate Characterization of Soybean Flour
2.3. Alkaline Solutions By-Product Characterization
2.4. Protein Extraction
2.5. Experimental Design and Statistical Analysis
2.6. Gel Electrophoresis
2.7. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.8. Functional Properties
3. Results and Discussion
3.1. Characterization of the Industrial Co- and By-Products
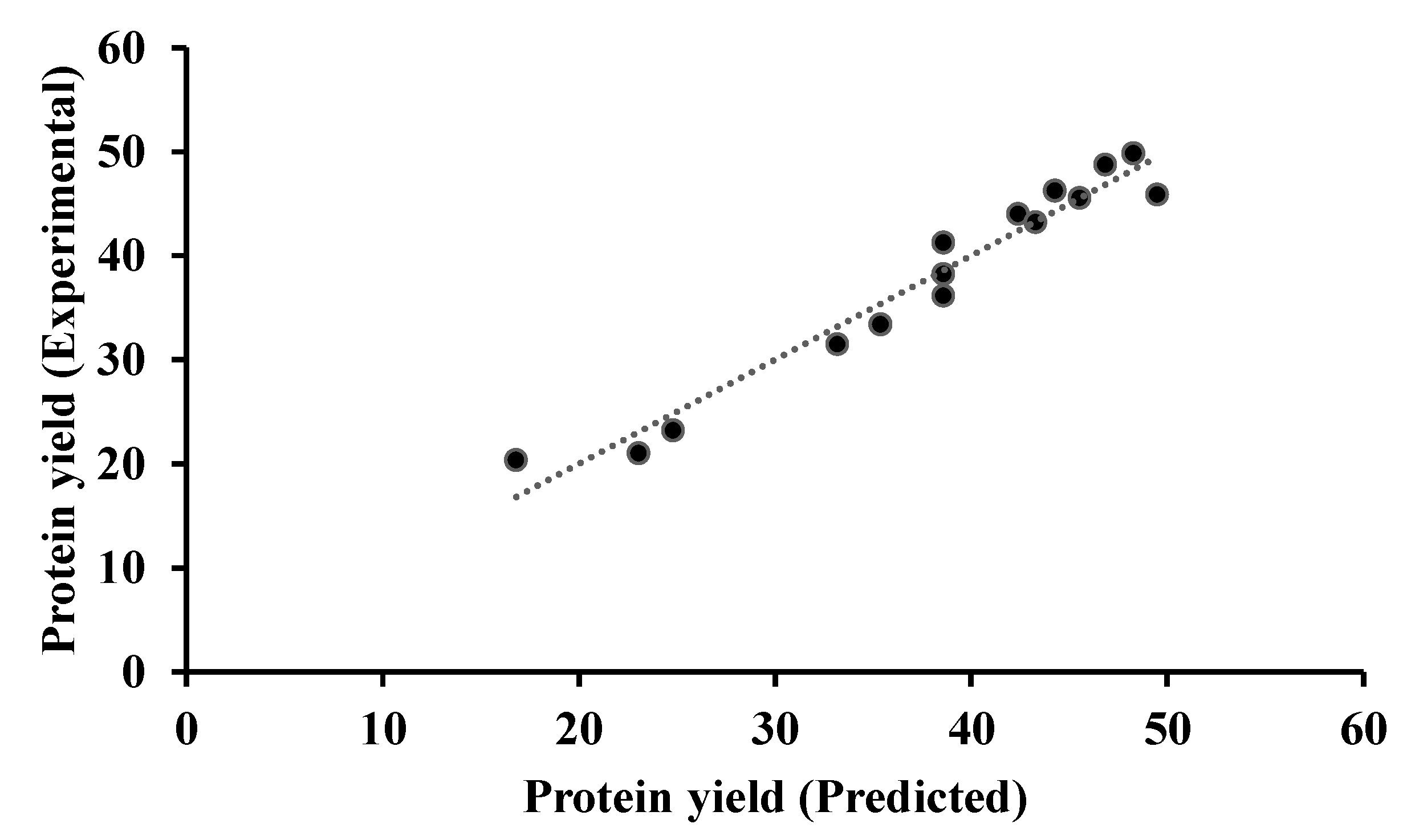
3.2. Modeling
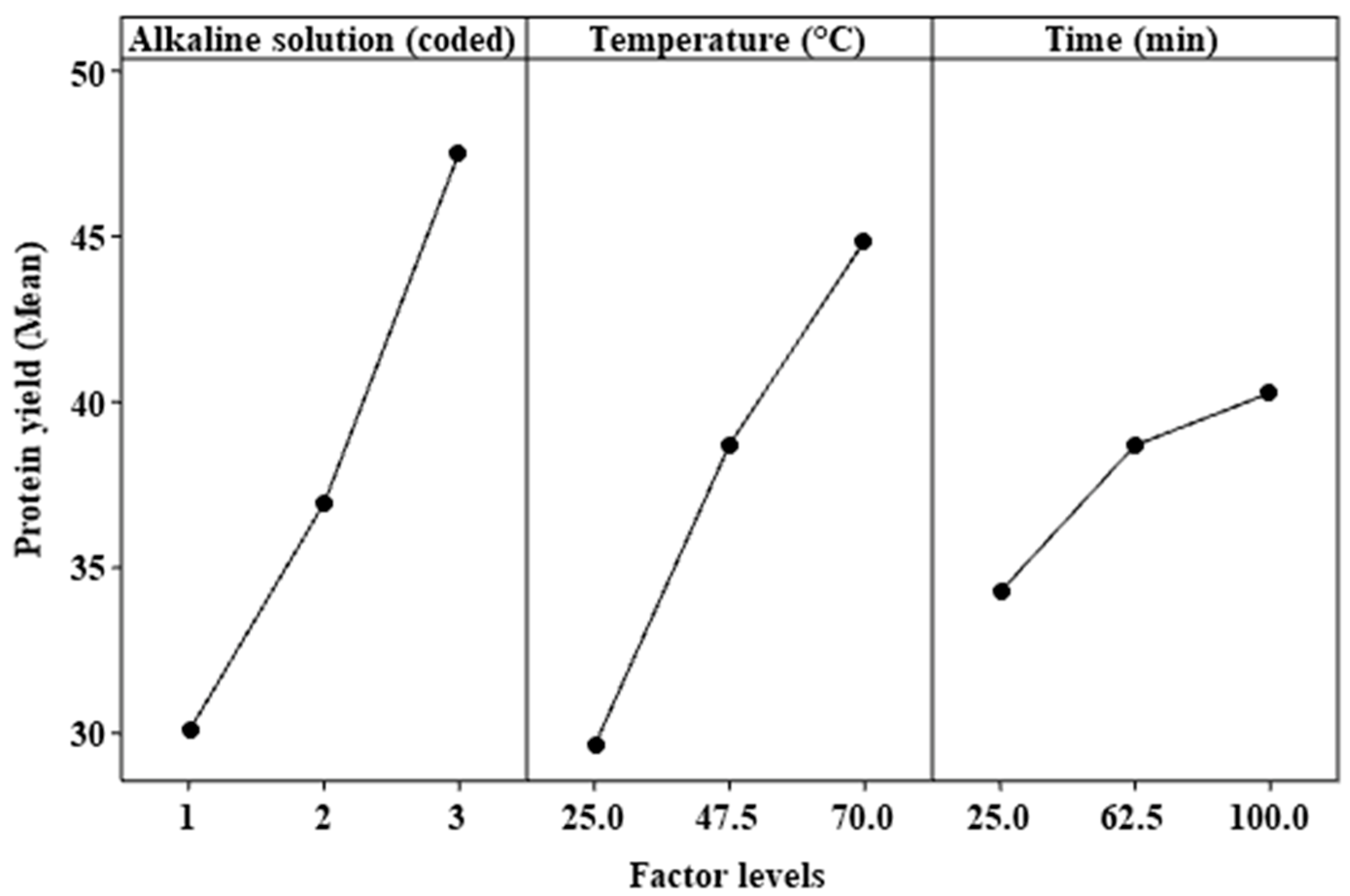
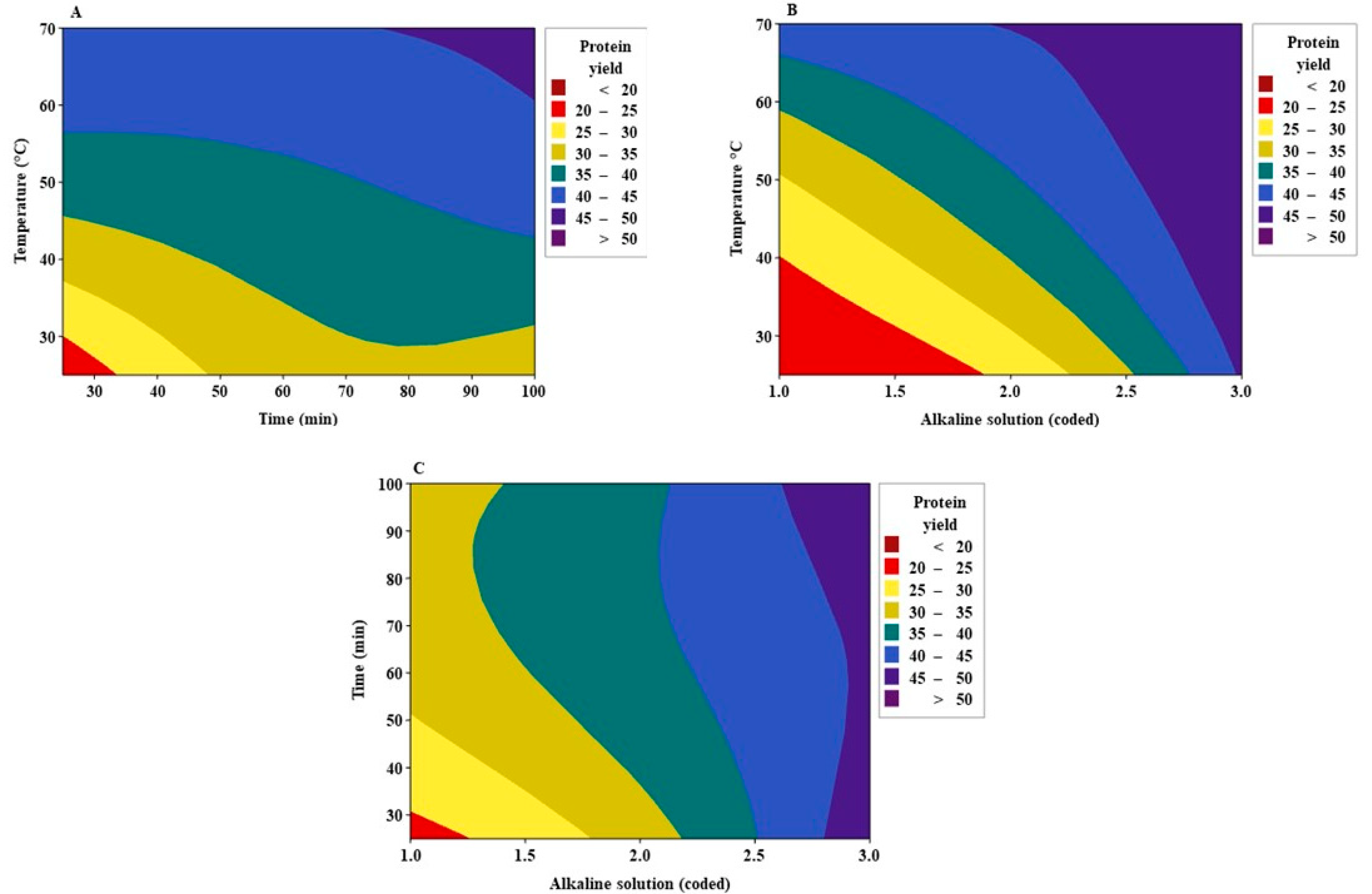
3.3. Effect of Factors and Interactions
3.4. Optimization
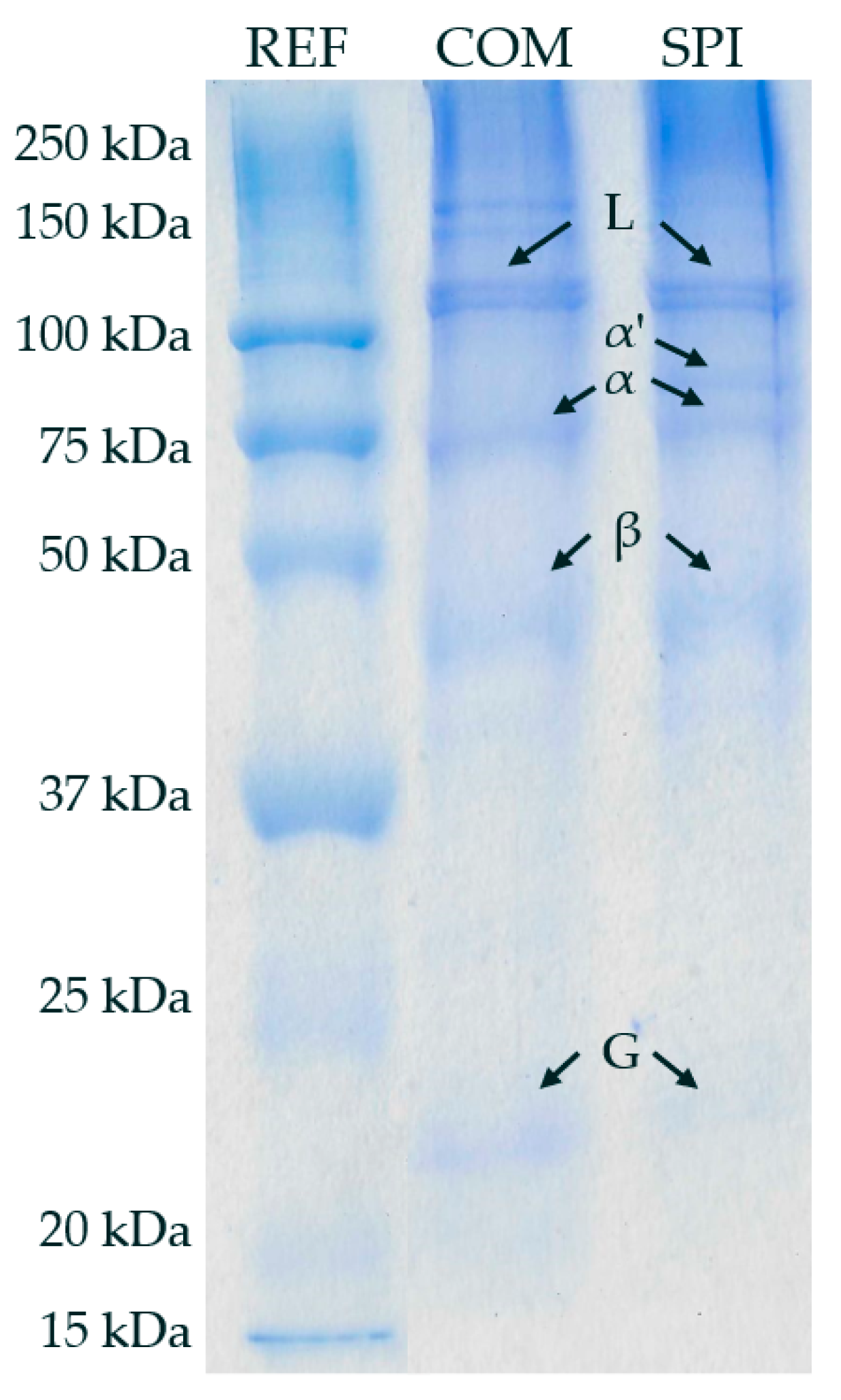
3.5. Molecular Weight (MW) of SPI
3.6. Structural Analysis by ATR-FTIR
3.7. Urease Activity (UA) and Functional Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, B.; Yeo, Z.; Kohls, P.; Herrmann, C. Industrial symbiosis: Exploring big-data approach for waste stream discovery. Procedia CIRP 2017, 61, 353–358. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; pp. 1–156. ISBN 9781315764788. [Google Scholar]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef] [Green Version]
- North Carolina Soybean Producers Association. Uses of Soybeans. Available online: https://ncsoy.org/media-resources/uses-of-soybeans/ (accessed on 6 June 2021).
- Lazo-Vélez, M.A.; Chuck-Hernandez, C.; Serna-Saldívar, S.O. Evaluation of the functionality of five different soybean proteins in yeast-leavened pan breads. J. Cereal Sci. 2015, 64, 63–69. [Google Scholar] [CrossRef]
- Farzana, T.; Mohajan, S.; Saha, T.; Hossain, N.; Haque, Z. Formulation and nutritional evaluation of a healthy vegetable soup powder supplemented with soy flour, mushroom, and moringa leaf. Food Sci. Nutr. 2017, 5, 911–920. [Google Scholar] [CrossRef]
- Elisia, H.; De Barros, A.; Vinicius, C.; Natarelli, L.; Maria, I.; Tavares, D.C.; Valério, E.; Vilas, D.B.; Franco, M. Nutritional clustering of cookies developed with cocoa shell, soy, and green banana flours using exploratory methods. Food Bioproc. Technol. 2020, 13, 1566–1578. [Google Scholar]
- Riska, N. Formulating baked-roll cake by using a composite of wheat flour, soy flour, and vegetable flour (Carrot, Purple Yam, and Red Bean) as a healthy snack for children aged 5–6 years at kindergarten in Tangerang. Int. J. Multidiscip. Curr. Res. 2017, 5, 1087–1093. [Google Scholar]
- Azarbad, H.R.; Tehrani, M.M.; Rashidi, H. Optimization of gluten-free bread formulation using sorghum, rice, and millet flour by D-optimal mixture design approach. J. Agric. Sci. Technol. 2019, 21, 101–115. [Google Scholar]
- Farzana, T.; Mohajan, S.; Hossain, N.; Ahmed, M.M. Formulation of a protein and fibre enriched soy-mushroom health drink powder compared to locally available health drink powders. Mal. J. Nutr. 2017, 23, 129–138. [Google Scholar]
- Cho, H.; Jung, Y.; Auh, J.; Lee, D. Effects of jet-milled defatted soy flour on the physicochemical and sensorial properties of hamburger patties. Food Sci. Anim. Resour. 2017, 37, 840–846. [Google Scholar] [CrossRef]
- Berk, Z. Isolated soybean protein (ISP). In Technology of Production of Edible Flours and Protein Products from Soybeans; FAO Agricultural Services Bulletin No. 97: Rome, Italy, 1992. [Google Scholar]
- Wongkanya, R.; Chuysinuan, P.; Pengsuk, C.; Techasakul, S.; Lirdprapamongkol, K.; Svasti, J.; Nooeaid, P. Electrospinning of alginate / soy protein isolated nano fibers and their release characteristics for biomedical applications. J. Sci. Adv. Mater. Devices 2017, 2, 309–316. [Google Scholar] [CrossRef]
- Aydogdu, A.; Yildiz, E.; Ayhan, Z.; Aydogdu, Y.; Sumnu, G.; Sahin, S. Nanostructured poly (lactic acid)/soy protein/HPMC films by electrospinning for potential applications in food industry. Eur. Polym. J. 2019, 112, 477–486. [Google Scholar] [CrossRef]
- González-Estrada, R.R.; Chalier, P.; Ragazzo-Sánchez, J.A. Antimicrobial soy protein based coatings: Application to Persian lime (Citrus latifolia Tanaka) for protection and preservation. Postharvest Biol. Technol. 2017, 132, 138–144. [Google Scholar] [CrossRef]
- Tansaz, S.; Liverani, L.; Vester, L.; Boccaccini, A.R. Soy protein meets bioactive glass: Electrospun composite fibers for tissue engineering applications. Mater. Lett. 2017, 199, 143–146. [Google Scholar] [CrossRef]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy protein: Impacts, production, and applications. In Sustainable Protein Sources Advances for a Healthier Tomorrow, 1st ed.; Nadathur, S., Wanasundara, J.P.D., Scanlin, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 23–45. ISBN 9780128027783. [Google Scholar]
- Gentile, L. Protein-polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Frossard, P. Kinetics of formation and functional properties of conjugates prepared by dry-state incubation of -lactoglobulin/ acacia gum electrostatic complexes. J. Agric. Food Chem. 2005, 53, 9089–9099. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.R.; Lopes-da-Silva, J.A. Critical evaluation of the functionality of soy protein isolates obtained from different raw materials. Eur. Food Res. Technol. 2019, 245, 199–212. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. A comparative study between the electro-activation technique and conventional extraction method on the extractability, composition and physicochemical properties of canola protein concentrates and isolates. Food Biosci. 2015, 11, 56–71. [Google Scholar] [CrossRef]
- Gerliani, N.; Hammami, R.; Aïder, M. Extraction of protein and carbohydrates from soybean meal using acidic and alkaline solutions produced by electro-activation. Food Sci. Nutr. 2020, 8, 1125–1138. [Google Scholar] [CrossRef]
- Deak, N.A.; Johnson, L.A. Effects of extraction temperature and preservation method on functionality of soy protein. J. Am. Oil Chem. Soc. 2007, 84, 259–268. [Google Scholar] [CrossRef]
- Garg, D.; Chakraborty, S.; Gokhale, J.S. Optimizing the extraction of protein from Prosopis cineraria seeds using response surface methodology and characterization of seed protein concentrate. LWT 2020, 117, 108630. [Google Scholar] [CrossRef]
- Gerliani, N.; Hammami, R.; Aïder, M. Assessment of the extractability of protein-carbohydrate concentrate from soybean meal under acidic and alkaline conditions. Food Biosci. 2019, 28, 116–124. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Deleu, L.J.; Lambrecht, M.A.; Van De Vondel, J.; Delcour, J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: Consideration of environmental implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Yaqub, M.; Woo, C.; Lee, W. Optimization of hypochlorous acid generation by HCl electrolysis through response surface methodology and artificial neural networks. J. Environ. Chem. Eng. 2021, 9, 105826. [Google Scholar] [CrossRef]
- Mourad, K.A.; Hobro, S. Developing chlorine-based antiseptic by electrolysis. Sci. Total Environ. 2020, 709, 136108. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Yaqub, M.; Lee, W. Electrolyzed water as a disinfectant: A systematic review of factors affecting the production and efficiency of hypochlorous acid. J. Water Process Eng. 2021, 43, 102228. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC International: Washington, DC, USA, 1990; ISBN 0-935584-42-0. [Google Scholar]
- AACC. International Approved Methods of the American Association of Cereal Chemists; Minnesota University Press: St. Paul, MN, USA, 2000. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Long, G.; Ji, Y.; Pan, H.; Sun, Z.; Li, Y.; Qin, G. Characterization of thermal denaturation structure and morphology of soy glycinin by FTIR and SEM. Int. J. Food Prop. 2015, 18, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, F.; Xue, W.; Lee, L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008, 22, 568–575. [Google Scholar] [CrossRef]
- Soria-Hernández, C.; Serna-Saldívar, S.; Chuck-Hernández, C. Physicochemical and functional properties of vegetable and cereal proteins as potential sources of novel food ingredients. Food Technol. Biotechnol. 2015, 53, 269–277. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official and Tentative Methods of American Oil Chemists Society, 3rd ed.; American Oil Chemists Society: Champaign, IL, USA, 1980. [Google Scholar]
- Haque, Z.; Kito, M. Lipophilization of. alpha. s1-casein. 2. Conformational and functional effects. J. Agric. Food Chem. 1983, 31, 1231–1237. [Google Scholar] [CrossRef]
- Acosta-Domínguez, L.; Cocotle-Ronzón, Y.; Alamilla-Beltrán, L.; Hernandez-Martinez, E. Effect of a cryogenic treatment in the microstructure, functional and flow properties of soy protein isolate. Food Hydrocoll. 2021, 119, 106871. [Google Scholar] [CrossRef]
- Soria-Hernández, C.G.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Comparison of physicochemical, functional and nutritional properties between proteins of soybean and a novel mixture of soybean-maize. Appl. Sci. 2020, 10, 6998. [Google Scholar] [CrossRef]
- Tian, H.; Guo, G.; Fu, X.; Yao, Y.; Yuan, L.; Xiang, A. Fabrication, properties and applications of soy-protein-based materials: A review. Int. J. Biol. Macromol. 2018, 120, 475–490. [Google Scholar] [CrossRef]
- Suryana, A.L.; Rosiana, N.M.; Olivia, Z. Effect of drying method on the chemical properties of local soy flour. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012030. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; McGraw, B.; Hardy, R.W. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Perović, M.N.; Knežević Jugović, Z.D.; Antov, M.G. Improved recovery of protein from soy grit by enzyme-assisted alkaline extraction. J. Food Eng. 2020, 276, 109894. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Goffin, D.; Blecker, C.; Richard, G.; Richel, A.; Haubruge, E. Application of soy protein isolate and hydrocolloids-based mixtures as promising food material in 3D food printing. J. Food Eng. 2019, 261, 76–86. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Ren, X.; Huang, Y.; Huang, C.; Zhang, K. Swirling cavitation improves the emulsifying properties of commercial soy protein isolate. Ultrason. Sonochem. 2018, 42, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Wang, R.; Sui, X.; Qi, B.; Han, F.; Li, Y.; Jiang, L. Secondary structure and subunit composition of soy protein in vitro digested by pepsin and its relation with digestibility. Biomed Res. Int. 2016, 2016, 5498639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent applications of ATR FTIR spectroscopy and imaging to proteins. Biochim. Biophys. Acta 2013, 1834, 2849–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Rosa-Millán, J.; Chuck-Hernandez, C.; Serna-Saldívar, S.O. Molecular structure characteristics, functional parameters and in vitro protein digestion of pressure-cooked soya bean flours with different amounts of water. Int. J. Food Sci. Technol. 2015, 50, 2490–2497. [Google Scholar] [CrossRef]
- Bai, M.; Qin, G.; Sun, Z.; Long, G. Relationship between molecular structure characteristics of feed proteins and protein in vitro digestibility and solubility. Asian-Australas. J. Anim. Sci. 2016, 29, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Carbonaro, M.; Maselli, P.; Nucara, A. Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: A Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 2012, 43, 911–921. [Google Scholar] [CrossRef]
- Yalcin, S.; Basman, A. Effects of infrared treatment on urease, trypsin inhibitor and lipoxygenase activities of soybean samples. Food Chem. 2015, 169, 203–210. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; He, F.; Yan, W.; Tang, Y.; Yang, R.; Zhao, W. Highly effective inactivation of anti-nutritional factors (lipoxygenase, urease and trypsin inhibitor) in soybean by radio frequency treatment. Int. J. Food Sci. Technol. 2021, 56, 93–102. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Zhu, X.; Ning, C.; Li, S.; Xu, P.; Zheng, Y.; Zhou, C. Effects of l-lysine/l-arginine on the emulsion stability, textural, rheological and microstructural characteristics of chicken sausages. Int. J. Food Sci. Technol. 2018, 53, 88–96. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, T.; Liu, C.; Guo, F.; Zhao, J. L-Histidine improves solubility and emulsifying properties of soy proteins under various ionic strengths. LWT 2021, 152, 112382. [Google Scholar] [CrossRef]

| Variables | Levels | ||
|---|---|---|---|
| Low | Medium | High | |
| Temperature (°C) | 25 | 47.5 | 70 |
| Time (min) | 25 | 62.5 | 100 |
| Alkaline solution (coded) 1 | 1 | 2 | 3 |
| Component | This Study (%) | Reported Values (%) [44] |
|---|---|---|
| Fiber | 4.28 ± 0.10 | 3.0 |
| Fat | 0.87 ± 0.09 | 5.1 |
| Protein | 56.12 ± 1.01 | 53.0 |
| Ash | 6.99 ± 0.02 | 6.0 |
| Carbohydrate * | 31.72 | 26.4 |
| Moisture | 6.82 ± 0.09 | 6.5 |
| Electric Current Intensity (A) * | Sanitizer Concentration Generated (ppm) * | By-Product Solution Generated | NaOH Concentration (N) | pH |
|---|---|---|---|---|
| 46 | 100 | 1 | 0.0086 ± 0.0002 | 12.10 ± 0.0252 |
| 60 | 200 | 2 | 0.0112 ± 0.0005 | 12.29 ± 0.0200 |
| 110 | 500 | 3 | 0.0214 ± 0.0005 | 12.68 ± 0.0173 |
| Factors | Protein Yield (%) | |||||
|---|---|---|---|---|---|---|
| Run | Temperature (°C) | Time (min) | Alkaline Solutions (Coded) | Experimental * | Predicted ** | Difference |
| 1 | 25 | 100 | 2 | 31.51 ± 1.83 | 33.16 | 1.65 |
| 2 | 47.5 | 25 | 1 | 23.19 ± 1.36 | 24.80 | 1.60 |
| 3 | 25 | 62.5 | 3 | 45.56 ± 1.16 | 45.51 | 0.04 |
| 4 | 70 | 25 | 2 | 44.03 ± 2.28 | 42.38 | 1.65 |
| 5 | 47.5 | 62.5 | 2 | 41.29 ± 1.20 | 38.57 | 2.71 |
| 6 | 47.5 | 100 | 3 | 49.86 ± 2.31 | 48.26 | 1.60 |
| 7 | 25 | 62.5 | 1 | 20.39 ± 1.44 | 16.79 | 3.59 |
| 8 | 47.5 | 25 | 3 | 48.77 ± 1.96 | 46.82 | 1.94 |
| 9 | 25 | 25 | 2 | 21.04 ± 1.66 | 23.03 | 1.99 |
| 10 | 70 | 62.5 | 3 | 45.88 ± 1.42 | 49.47 | 3.59 |
| 11 | 47.5 | 100 | 1 | 33.43 ± 2.72 | 35.37 | 1.94 |
| 12 | 47.5 | 62.5 | 2 | 38.26 ± 1.11 | 38.57 | 0.30 |
| 13 | 70 | 62.5 | 1 | 43.23 ± 2.74 | 43.28 | 0.04 |
| 14 | 47.5 | 62.5 | 2 | 36.17 ± 1.58 | 38.57 | 2.40 |
| 15 | 70 | 100 | 2 | 46.25 ± 1.23 | 44.26 | 1.99 |
| Source of Variation | Degree of Freedom | Sum of Square | Mean Square | F-Value | p-Value | Determination Coefficient (R2) |
|---|---|---|---|---|---|---|
| Model | 9 | 4009.98 | 445.55 | 52.88 | 0.000 | 0.9315 |
| Linear | 3 | 3435.29 | 1145.10 | 135.89 | 0.000 | |
| Two-way interaction | 3 | 494.05 | 164.68 | 19.54 | 0.000 | |
| Temperature vs. time | 1 | 50.93 | 50.93 | 6.04 | 0.019 | |
| Temperature vs. alkaline solution | 1 | 380.52 | 380.52 | 45.16 | 0.000 | |
| Time vs. alkaline solution | 1 | 62.6 | 62.6 | 7.43 | 0.010 |
| Factors | Predicted Optimized Level | Predicted Protein Yield (%) | Confidence Interval (95%) | Experimental Protein Yield (%) |
|---|---|---|---|---|
| Temperature (°C) | 70 | 49.79 | (46.66–52.93) | 48.30 ± 0.21 |
| Time (min) | 44.7 | - | - | - |
| Alkaline solution (coded) | 3 | - | - | - |
| Sample | β-Conformations (%) | α-Helix (%) | Random Coil (%) | β:α Ratio |
|---|---|---|---|---|
| Soybean flour | 69.23 | 15.38 | 15.38 | 4.50 |
| Soybean flour [54] | 69.20 | 12.16 | 12.34 | 5.69 |
| Commercial SPI | 77.77 | 11.11 | 11.11 | 7.00 |
| SPI | 81.81 | 9.09 | 9.09 | 9.00 |
| SPI [51] | 72.00 | 12.00 | - * | 6.00 |
| Test | SPI pH 4.5 | SPI pH 7 | Reported SPI [43] |
|---|---|---|---|
| UA | - * | 0.06 ± 0.02 | 0.04 ± 0.01 |
| WSI (%) | 6.28 ± 0.10 | 81.39 ± 3.02 | 33.11 ± 1.13 |
| WAI | 3.26 ± 0.15 | - * | 7.97 ± 0.16 |
| FA (%) | 315.90 ± 15.53 | 302.30 ± 19.91 | 442.00 ± 15.57 |
| FD (%) | 12.12 ± 1.89 | 24.90 ± 1.27 | 18.46 ± 0.54 |
| FS (%) | 34.54 ± 4.72 | 88.63 ± 4.61 | 90.17 ± 2.42 |
| EC (%) | 82.78 ± 5.08 | 79.76 ± 3.72 | - * |
| ES 24 h (%) | 82.78 ± 5.08 | 79.76 ± 3.72 | 43.39 ± 0.92 |
| ES 48 h (%) | 100.00 ± 0.00 | 97.76 ± 0.10 | 76.39 ± 4.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovando, E.; Rodríguez-Sifuentes, L.; Martínez, L.M.; Chuck-Hernández, C. Optimization of Soybean Protein Extraction Using By-Products from NaCl Electrolysis as an Application of the Industrial Symbiosis Concept. Appl. Sci. 2022, 12, 3113. https://doi.org/10.3390/app12063113
Ovando E, Rodríguez-Sifuentes L, Martínez LM, Chuck-Hernández C. Optimization of Soybean Protein Extraction Using By-Products from NaCl Electrolysis as an Application of the Industrial Symbiosis Concept. Applied Sciences. 2022; 12(6):3113. https://doi.org/10.3390/app12063113
Chicago/Turabian StyleOvando, Emilio, Lucio Rodríguez-Sifuentes, Luz María Martínez, and Cristina Chuck-Hernández. 2022. "Optimization of Soybean Protein Extraction Using By-Products from NaCl Electrolysis as an Application of the Industrial Symbiosis Concept" Applied Sciences 12, no. 6: 3113. https://doi.org/10.3390/app12063113
APA StyleOvando, E., Rodríguez-Sifuentes, L., Martínez, L. M., & Chuck-Hernández, C. (2022). Optimization of Soybean Protein Extraction Using By-Products from NaCl Electrolysis as an Application of the Industrial Symbiosis Concept. Applied Sciences, 12(6), 3113. https://doi.org/10.3390/app12063113









