Abstract
Machine learning is a branch of artificial intelligence that allows computer systems to learn directly from examples, data, and experience. Statistical modeling is more about finding connections between variables and consequently the impact of these relationships, while also catering for prediction. It should be clear that these two methodologies are different in terms of their purpose, despite the fact that they use similar means to get there. The evaluation of the machine learning algorithm uses a set of tests to validate its accuracy. Although, for a statistical model, the analysis of regression parameters by confidence intervals, significance tests and other tests can be used to assess the legitimacy of the model. To demonstrate the applications and usefulness of this theory, an experimental study was conducted on zebrafish exposed to mycotoxin. Methods: Patulin (70 µg/L) and kojic acid (100 mg/L, 204 mg/L, and 284 mg/L) were administered by immersion to zebrafish once daily for a period of 7 days before the behavior testing. The following behavioral tests were performed: a novel tank test (NTT) (to assess the explorative behavior and anxiety); and a Y-maze test (which measures the spontaneous explorative behavior). Behavioral tests were performed on separate days. For the behavior tests, the statistical analysis was performed using ANOVA variation analysis (two-way ANOVA). All results are expressed as the mean ± standard error of the mean. The values of the general index F for which p < 0.05 were considered statistically significant. Results: Y-maze—patulin exposure led to an intensification of the locomotor activity and an increased traveled distance and number of arm entries. By increasing the spontaneous alternation between the aquarium’s arms, patulin has shown a stimulating effect on spatial memory. In the case of zebrafish exposed to 100 mg/L kojic acid, the traveled distance was shorter by 27% than the distance attained by those in the control group. The higher doses of kojic acid (204 mg/L and 284 mg/L) led to an increased locomotor activity, distance traveled, number of arm entries, and the spontaneous alternation. The increase in spontaneous alternation demonstrates that 204 mg/L and 284 mg/L kojic acid doses had a stimulating effect on spatial memory. Novel tank test—compared to the control group, the traveled distance of the patulin-exposed fish is slightly reduced. Compared to the control group, the traveled distance of the kojic acid-exposed fish is reduced, due to a shorter mobile time (by 25–27% in the case of fish exposed to 204 mg/L and 284 mg/L kojic acid). Patulin and kojic acid exhibit toxic effects on zebrafish liver, kidney, and myocardium and leads to severe alteration. We continued the analysis by trying some machine learning algorithms on the classification problems in the case of the two behavioral tests MAZE and NTT, after which we concluded that the results were better in the case of the NTT test relative to the MAZE test and that the use of decision tree algorithms leads to amazing results, knowing that their hierarchical structure allows them to learn signals from both classes. Conclusions: The groups exposed to patulin and kojic acid show histological changes in the liver, kidneys, and myocardial muscle tissue. The novel tank test, which assesses exploratory behavior, has been shown to be conclusive in the behavioral analysis of fish that have been given toxins, demonstrating that the intoxicated fish had a decreased explorative behavior and increased anxiety. We were able to detect a machine learning algorithm in the category of decision trees, which can be trained to classify the behavior of fish that were given a toxin in the category of those used in the experiment, only by analyzing the characteristic features of the NTT Behavior Test.
1. Introduction
In the field of statistical learning, there is a wide range of techniques for deciphering data. Two types of such tools exist: supervised and unsupervised. In general, through supervised statistical learning it is understood that a statistical model is created, which estimates an output based on inputs. Many sectors such as business, medicine, astrophysics, and public policy can be seen to present such a problem. In typical unsupervised statistical learning, there are no supervisory outputs; however, relationships and structures can be learned from such data. Even though statistical learning is a relatively new domain, many of the concepts underpinning it are old. Gauss and Legendre wrote works on the method of least squares, which is the oldest form of what is known now as linear regression. This method was originally used for various astronomical problems. Through linear regression, numerical quantities are predicted, such as a person’s income. Linear discriminant analysis was invented by Fisher in 1936 for the prediction of quantitative values such as the life-or-death probability of a patient, or whether or not stock markets would rise or fall. Some scholars proposed the alternate approach of logistic regression in the 1940s. Nelder and Wedderburn invented the notion of “generalized linear models” in the early 1970s to describe families of statistical learning approaches that included both linear and logistic regression. Many additional strategies for such learning from data became available by the end of the 1970s. However, because fitting non-linear connections was not computationally feasible at that time, they were all linear approaches. Thanks to advances in computer technology, by the 1980s non-linear approaches were no longer impossible. Breiman, Friedman, Olshen and Stone introduced various types of regression and classification trees in the mid-1980s and were the first to show the potential of a full implementation of this method, including model selection through cross-validation. In 1986, the concept of “generalized additive models” was invented by Hastie and Tibshirani to describe groups of non-linear extensions to generalized linear models, as well as the software implementations associated with them. Meanwhile, statistical learning became a fully fledged topic in statistics, focused on supervised and unsupervised modeling and prediction, aided by machine learning and other disciplines. The fact that powerful and user-friendly software is now readily available, such as the popular and free R system, has aided statistical learning in recent years. Through this, the field can change from a collection of approaches developed by statisticians to a useful toolkit used by a much larger community [1].
The evaluation of the machine learning algorithm uses a set of tests to validate its accuracy. Although, for a statistical model, the analysis of regression parameters by confidence intervals, significance tests and other tests can be used to assess the legitimacy of the model.
Contrary to popular belief, machine learning has been around for decades. Initially, it was avoided due to its high computing needs and therefore the limitations of computing power that existed at that time. However, machine learning has been reborn in recent years due to the superiority of knowledge stemming from the knowledge explosion.
A big difference between machine learning and statistics is their purpose. However, to say that machine learning refers to accurate predictions, while statistical models are designed for inference, is almost a pointless statement if you are not well versed in these concepts. First of all, we need to understand that statistics and statistical models are not equivalents. Statistics is the mathematical study of knowledge. You cannot make statistics unless you have data. A statistical model can be a model for data that is used either to assume something about the relationships within the data, or to make a model that is ready to predict future values. Often these two go hand in hand. So, there are two things we would like to discuss: firstly, how machine learning statistics are different, and secondly, how machine learning statistical models are different. To make this a little more explicit, there are many statistical models that will make predictions, but predictive accuracy is not their strength. Additionally, machine learning models offer varying degrees of interpretability, from highly interpretable lasso regression to impenetrable neural networks, but usually sacrifice interpretability for predictive power.
Machine learning is focused on results, while statistical modeling is more about finding connections between variables and therefore the significance of these relationships, while also taking into account prediction. The fundamentals of artificial intelligence (AI) are based on mathematical operational procedures such as group theory (group isomorphisms and automorphism; isometry by compacting dynamic variables, although compacting group parameters either between dynamic variables or group parameters known as transients; compaction type; common invariant functions on isomorphic groups; integral invariant function). All these types of procedures are manifested both structurally and functionally as databases, a situation in which the implementation of the principle of maximum information energy allows the selection of only certain dynamics compatible with the external restriction. These types of situations can often be found in complex system dynamics such as plasma physics [2,3,4,5,6] or polymer-drug release systems [7,8].
These two approaches are different in their purpose, despite the fact that they use similar means to get there. The evaluation of the machine learning algorithm uses a set of tests to validate its accuracy. Taking into account, for a statistical model, the analysis of regression parameters by confidence intervals, significance tests and other tests can be used to assess the legitimacy of the model. Because these methods produce an equivalent result, it is easy to understand why they could be assumed to be equivalent. Machine learning is based on the theory of statistical learning, which remains supported by this axiomatic notion of probability spaces. This theory was developed in the 1960s and expands upon old-fashioned statistics.
The major difference between statistics and machine learning is that statistics are uniquely based on probability spaces. You will get the full statistics from pure math, which discusses how numbers will be grouped into categories, called sets, and then apply a measure to this set to ensure that the sum of all of them is one. We call this the probability space. Statistics make no assumptions about the universe other than these conceptions of sets and measures.
Paralleling machine learning and statistical models may be a bit more difficult. Whichever you employ depends largely on what your purpose is. If you just want to create an algorithm which will predict housing prices to a high accuracy or use data to work out whether someone is probably going to contract certain sorts of diseases, machine learning is probably going to be the superior approach. To try a relationship between variables or make inferences from data, a statistical model will probably be the best approach.
To demonstrate the applications and utility of this theory, we performed an experimental study on zebrafish exposed to mycotoxin.
Mycotoxins are considered one of the most important environmental pollutants synthesized on grains, nuts, and other plant materials. Acquisition of the toxins even in smaller amounts through ingestion, inhalation, or contact may cause serious health problems. The occurrence of toxigenic fungi in the food chain poses a potential danger to human health.
Patulin (PAT) is a common mycotoxin contaminant of fruit juices, produced by fungi belonging to several genera, including Penicillium, Aspergillus, and Byssochlamys species. Although patulin can occur in many moldy fruits, cereals, and other foods, the major sources of patulin exposure are apple products. Patulin can act as a phytotoxin and is active against pathogenic fungi. Patulin was initially suggested as a treatment of the common cold, as it is anti-viral in addition to being antibacterial, but the trials on humans were quickly stopped because of the toxicity of patulin [9]. Patulin has been reported to be toxic, teratogenic, and cardiotoxic; it influences the permeability of the colic epithelium and renal function and is immunotoxic [10]. Singh N et al. conducted a study where normal intestinal cells were exposed to non-toxic levels of PAT for longer durations and found that PAT exposure induced pre-cancerous cells in normal intestinal cells [11]. The study by Pillay Y et al. showed that patulin altered the transcription and translation of α1-AR in HEK293 cell-induced renal toxicity [12]. Kojic acid (KA) is a mycotoxin produced by various fungal or bacterial strains, such as Aspergillus strains, Penicillium, or Acetobacter species. KA possesses weak antimicrobial properties and is active against several common bacterial strains. It is widely used as a food ingredient and dermatological skin lightening agent. Kojic acid has a complex toxicological profile. It was reported to be non-toxic after a single intraperitoneal, oral, or topical dose in rodents [13] but genotoxic in several in vitro tests. Chronic oral administration of kojic acid has been reported to produce thyroid adenomas in rats, although no increase in the incidence of malignant tumors due to continuous serum thyroid-stimulating hormone (TSH) stimulation by a non-genotoxic mechanism [14]. Novel kojic acid derivatives showed the best inhibitory effects on diphenolase activity and monophenolase activity with anti-melanogenic activity confirmed by assessing the inhibition of melanin content and intracellular tyrosinase activity in zebrafish models and could provide new insights into the development of novel anti-melanogenic agents [15].
While most behavioral tests have been conducted on rodents and most histological research (involving mycotoxins) had zebrafish embryos as animal models, this study uses adult zebrafish. Zebrafish were chosen because of their small size, social behavior, resistance to environmental stress, and due to the histological, physiological, histopathological, and genetic similarity with mammals and humans [16]. Thus, the costs, time, and space requirements are lower compared to those required in the case of rodents.
The main goal of the research is to assess the impact of patulin and kojic acid exposure on the explorative behavior and internal organs of the zebrafish. By using NTT, Y-maze test, and statistics for analyzing the behavior and microscopy for detecting histopathological alterations, we can observe how these two mycotoxins affect the life of zebrafish. We have also aimed to detect an automatic learning algorithm which can be trained to classify the behavior of patulin-/kojic acid-exposed fish, only by analyzing the characteristic features of the NTT behavioral test.
2. Materials and Methods
2.1. Test Chemicals and Test Solutions Preparation
Kojic acid was obtained from Carl Roth GmbH + Co KG (Karlsruhe, Germany) through Amex Export-Import SRL (București).
Patulin was obtained from Romer Labs Diagnostic GmbH (Tulln, Austria).
Adult zebrafish (Danio rerio) were kept in aquariums for 7 days to accommodate to laboratory conditions.
After this period, the animals were divided into 5 groups: control (nothing was administered), patulin 70 μg/L, kojic acid 100 mg/L, kojic acid 204 mg/L, and kojic acid 284 mg/L.
Patulin (70 µg/L) and kojic acid (100 mg/L, 204 mg/L, and 284 mg/L) were administered by immersion (both toxins are water soluble) to zebrafish once daily for 7 days before the behavior testing.
To measure the desired mycotoxin quantities, we have used an AND HR-202 4 digit analytical balance.
2.2. Test Organisms
Adult zebrafish (with a body weight ranging between 0.48 and 0.74 g) were kept in a ZebTEC (Tecniplast) (12 sliding trays, each containing 10 tanks; a dedicated tray inlet valve guaranteed water recirculation through tanks) recirculating system that allowed a continuous flow of water, at “Ion Ionescu de la Brad” University of Agricultural Sciences and Veterinary Medicine of Iasi.
Two trays (containing 20 × 1 L water tanks) were assigned for each group, trays were labeled with the toxin and administered dose. Culture water was obtained through reverse osmosis and activated carbon filtration of tap water, complemented with salt (Instant Ocean Synthetic SeaSalt, Spectrum Brands), and automatically adjusted for pH and conductivity. Water temperature was 26 ± 1 °C, conductivity 800 ± 50 ms, pH 7.5 ± 0.5, and dissolved oxygen equal or above 95% (7.6 mg/L) saturation. A 14:10 h light–dark photoperiod was maintained. The adult fish were fed twice a day (at 8:00 and 20:00) with a commercially available artificial diet for tropical fish, Norwin Norvital.
The breeding and use of laboratory animals were in accordance with the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA).
One third of the water volume was changed every 3 days.
2.3. Behavior Tests
Fish were randomly selected for behavioral tests to avoid bias. Disturbances (e.g., noise) were reduced to a minimum during recordings. The following behavioral tests were performed: a novel tank test (NTT) (to assess the explorative behavior) [17] (Beckmann, Biro, 2013); and a Y-maze test (to assess the spontaneous explorative behavior). Behavioral tests were performed on separate days. In our studies, a Logitech HD Webcam C922 Pro Stream camera recorded zebrafish behavior, and the videos were analyzed using ANY-maze® software (Stoelting CO, Wood Dale, IL, USA).
2.3.1. Novel Tank Test (NTT)
This test was based on the work of Wong et al. [18] and aimed to evaluate the explorative behavior and the anxiety level of fish when placed in a new environment. The position (bottom × upper levels) was considered an index of anxiety, similar to the position near the wall versus the center of an open field with rodents [19]. Using a net, fish were transferred individually to a test aquarium (24 cm × 8 cm × 20 cm; width × depth × height) and filmed for 6 min. In the NTT test, 7 characteristics were monitored: “No. entries in top”, “Total distance traveled” (m), “Total imob. time” (s), “Total mobile time” (s), “Latency to enter in the top zone” (s), “Time in top zone” (s), and “Time in bottom zone” (s).
After testing, each fish was temporarily put in a tank with untreated water (as the water from the control group aquarium). After all the fish from one group were tested, they were taken from the temporary tank and put back in their original tank.
2.3.2. Y-Maze Test
The Y-maze test was used to assess spatial memory in zebrafish, like in rodents [20]. Each fish was transferred and tested individually during a 5 min session [21] in a Y-shaped tank with 3 identical arms (10 cm × 15 cm × 7 cm; height × length × width) filled with water (8 cm—depth) [22]. Each arm was noted: A, B, C. In the Y-maze test, 3 characteristics were monitored: “Number of arm entries”, “Spontaneous alternation” (%), and “Distance traveled” (m). All explorations were related to the type and amount of toxins administered, as mentioned above.
After testing, each fish was temporarily put in a tank with untreated water (as the water from the control group aquarium). After all the fish from one group were tested, they were taken from the temporary tank and put back in their original tank.
Spontaneous alternation (%) was calculated with the following formula:
An entry is considered valid when the head of the fish enters one of the arms.
Total no. of alternations = No. ABC sequences + No. ACB sequences + No. BAC sequences + No. BCA sequences + No. CAB sequences + No. CBA sequences
Histological Examination
First, the fish were euthanized by rapid cooling [23]: 8 zebrafish from each group were transferred into an ice water bath (the temperature was maintained at 0–4 °C) using a net.
Immediately after euthanasia, the fish were fixed in formaldehyde solution 10% for 48 h. After that, for demineralization, the fish were immersed in Bouin solution for 24 h. For the sectioning, the fish were fully embedded in paraffin, using histologic processor Leica TP 1020. Starting from the median axis, 5 µm thick histological sections were made longitudinally. We used Masson’s trichromic stain (hematoxylin–eosin–methylene blue) presented by Diaconiță et al. (1953) [24] and Șincai (2000) [25]. Histological examination was made under a light microscope Leica DM 750, the images being captured with a Leica HD 5 megapixels digital camera. Histological alterations of the control and patulin-treated group were examined and compared, with emphasis on liver, kidney, and myocardium.
2.4. Statistical Analysis
For the behavior tests, the statistical analysis was performed using ANOVA variation analysis (two-way ANOVA). All results are expressed as the mean ± standard error of the mean. The values of the general index F for which p < 0.05 were considered statistically significant. Significant differences between the analyzed groups were determined using the Tukey post hoc test. Then, we realized the descriptive statistics calculation with standard statistical packages (Microsoft Excel). The results were expressed as means ± standard error. The process was started with a standard statistical analysis on the two behavioral tests (NTT and MAZE), using the ANOVA test and the PCA mathematical technique to reduce the dimensionality of the system, by reducing to the main components, using the software AnalyseIt statistical analysis and data visualization for Microsoft Excel [26]. Continuing the analysis, we used the machine learning algorithms SVM, KNN, XGBoost, and Random Forest [27]. The main existing machine learning frameworks, such as TensorFlow [28], Keras [29], PyTorch [30], and the Python programming language [31], are maturing and offer a lot of functionalities to streamline the above-mentioned process. There are also other excellent toolkits for deep learning. One of these is the Google Colaboratory environment. This environment, based on Python Jupyter notebooks, gives the user free access to Tesla K80 GPUs [32].
We built a program in order to try some machine learning algorithms on the classification problems present in the case of the two behavioral tests. We started with some pre-processing (data scanning, basic imports) and then we covered the classification algorithms enumerated above. Each time we calculated the accuracy of the model (accuracy) for both the test and the training data set (to discover a possible overfitting) and we calculated the Confusion Matrix and the Area under the curve—AUC. As a starting point, we used the following notebook [33]. We repeated the same analysis for the situation when the treatment administered to the groups of fish was reduced to two components, control—0 and toxin administered—1, to find out how the model behaves in case of reducing the variable “Treatment” to a categorical dichotomous variable. We used PCA to reduce the 4 dimensions to 2 or 3, so that we can better read and understand these data. I used this notebook [34] as a starting point.
Unbalanced classes push “classification accuracy” out of acceptable values. This is a surprisingly common situation in machine learning problems (and especially in classification problems), which occurs in datasets with a disproportionate ratio of observations for each class. Standard accuracy no longer reliably measures performance, making model training much more difficult. The case of reducing the treatment administered to batches of fish to two components (binary analysis), control—0 and toxin administered—1 is in the situation of unbalanced classes. For the unbalanced classes encountered in the NTT and MAZE analysis, we explored 5 efficient ways to manage the situation: over-sampling the minority class; majority class sub-sampling; changing the performance evaluation; use of penalty algorithms (cost-sensitive training); the use of decision tree algorithms. We used the following notebook [35] as a starting point.
3. Results
3.1. Behavior Tests
Y-Maze
Patulin exposure led to an intensification of the locomotor activity, an increased traveled distance and number of arm entries (Figure 1A) (see detailed data in Appendix A).
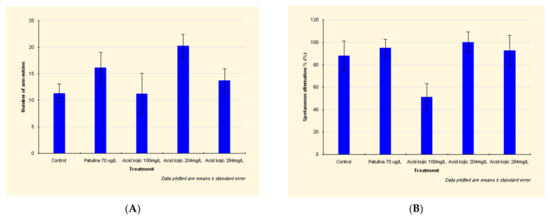
Figure 1.
Effects of patulin (PAT, 70 µg/L) and kojic acid (KA, 100 mg/L, 204 mg/L, and 284 mg/L) on the behavioral parameters ((A) number of arm entries; (B) spontaneous alternations %), in zebrafish submitted to Y-maze test. Data are expressed as mean ± S.E.M. (one-way ANOVA, n = 20). For Tukey’s post hoc analyses: a: PAT (70 µg/L) vs. KA (100 mg/L): p < 0.001 and b: KA (204 mg/L) vs. KA (284 mg/L): p < 0.01.
By increasing the spontaneous alternation between the aquarium’s arms, patulin has shown a stimulating effect on spatial memory (Figure 1A).
In the case of zebrafish exposed to 100 mg/L kojic acid, the traveled distance was shorter by 27% than the distance attained by those in the control group. Additionally, the spontaneous alternation decreased by 42% (Figure 1B), which means the 100 mg/L kojic acid dose significantly decreases spatial memory.
The higher doses of kojic acid (204 mg/L and 284 mg/L) led to an increased locomotor activity, distance traveled, number of arm entries, and the spontaneous alternation (Figure 1B).
The increase in spontaneous alternation demonstrates that 204 mg/L and 284 mg/L kojic acid doses had a stimulating effect on spatial memory.
3.2. Novel Tank Test
Compared to the control group, the traveled distance of the patulin-exposed fish is slightly reduced (Figure 2A) due to a shorter mobile time and a significantly reduced number of entries in the top zone (see detailed data in Appendix A).
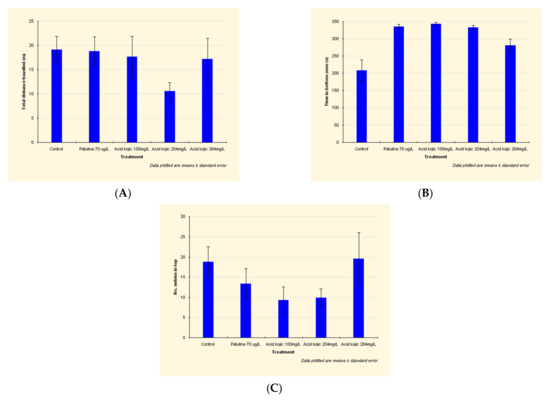
Figure 2.
Effects of patulin (PAT, 70 µg/L) and kojic acid (KA, 100 mg/L, 204 mg/L and 284 mg/L) on the behavioral parameters ((A) Total distance traveled; (B) Time in bottom zone; (C) No. of entries in top zone) in zebrafish submitted to a novel tank diving test. Data are expressed as mean ± S.E.M. (one-way ANOVA, n = 20).
In the patulin-exposed individuals, time in the bottom zone is highly increased (by 61%) (Figure 2B).
Compared to the control group, the traveled distance of the kojic acid-exposed fish is reduced, due to a shorter mobile time (by 25–27% in the case of fish exposed to 204 mg/L and 284 mg/L kojic acid) and a significantly reduced number of entries in the top zone (by almost 50% in individuals exposed to 100 mg/L and 204 mg/L kojic acid) (Figure 2C).
The group exposed to 204 mg/L kojic acid exhibited the lowest traveled distance: by 45% lower than control group (Figure 2A). This, and the increased amount of time in the bottom zone demonstrates the anxiogenic effect of the 204 mg/L kojic acid dose.
The anxiogenic effect of kojic acid is also manifested at 100 mg/L dose, the individuals of this group registering the minimum number of entries in the top zone (51% lower than the control group) (Figure 2C). Increased time in the bottom zone, the decrease in distance traveled and mobility time, show an anxiogenic effect, but lower compared to the group exposed to 204 mg/L.
The fish from the 100 mg/L and 204 mg/L kojic acid-exposed groups register the lowest number of entries in the top zone (approx. 50% of the control group) (Figure 2C), exhibiting a pronounced anxiety.
At the maximum kojic acid dose (284 mg/L), the number of entries in the top zone is slightly increased compared to the control group, and the distance traveled is close to that recorded in non-intoxicated individuals (lower by only 11%).
For the individuals in this group, we have also registered a 34% increase in time in the bottom zone (Figure 2B).
The reduced mobility time (by 25% compared to the fish of the control group) and the increased time in the bottom zone prove that this dose leads to anxious behavior.
Histological Alterations
After examining the zebrafish from the control group, we observed there were no histological alterations of the liver, kidney, brain, or myocardium.
Patulin exhibits toxic effects on zebrafish liver and leads to severe alteration (Figure 3A): swollen hepatocytes, cytoplasm vacuolization, nuclear hyperhydration, triglyceride loading (empty intracytoplasmic vacuoles), loss of hepatocyte integrity, hydropic and lipid dystrophy (Figure 3B), and cytoplasm and nucleus hyperhydration.
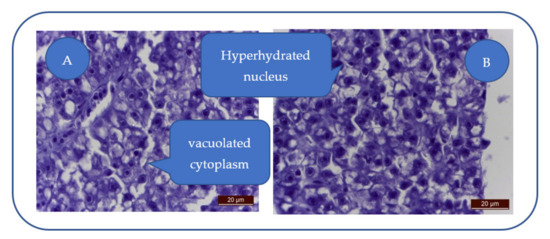
Figure 3.
Severe hepatocyte alteration. (A) Swollen hepatocytes with vacuolated cytoplasm and triglyceride loading; (B) Hydropic and lipidic dystrophy hepatocytes; hyperhydrated nucleus.
Lymphocyte infiltration, hepatocyte necrosis, sinusoids dilation/irregularities, or nuclear abnormalities were absent in the liver of patulin-treated individuals.
At 100 mg/L kojic acid, major liver degeneration is characterized by hepatocyte hyperhydration and loss of cellular integrity (Figure 4A). The degenerative aspect is characteristic of hydropic dystrophy, this change being characteristic of toxic aggression that led to altered cell membranes permeability, leading to hepatocyte hyperhydration and the loss of cell integrity. Cell membranes are fragmented, and some hepatocytes are empty (lacking nuclei and cytoplasm).
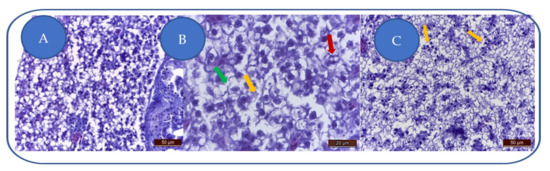
Figure 4.
Kojic acid effects on the liver: (A) 100 mg/L; (B) 204 mg/L; (C) 284 mg/L. Vacuolation (→), cell membrane fragmentation (→), karyolysis/nuclear pycnosis (→).
At 204 mg/L kojic acid, the severe toxic degeneration is represented by hepatocytes vacuolation, cell membranes fragmentation, and karyolysis (Figure 4B).
At 284 mg/L kojic acid, due to severe/brutal toxic alteration, the hepatocytes appear disintegrated, with fragmented cell membranes (Figure 4C). Some hepatocytes show karyolysis or nuclear pycnosis.
The uniform degenerative appearance of the liver is translated by swollen hepatocytes, with fragmented membranes, but also cellular debris (nuclei, cell membranes, cytoplasmic blocks). The inflammatory component is absent.
Patulin exposure leads to a medium-severe degeneration of urinary epithelium, swelling of the nephrocytes, cytoplasm vacuolation, nuclear hyperhydration (Figure 5A) while some nephrocytes lose integrity. The apoptosis of the renal cells was absent.
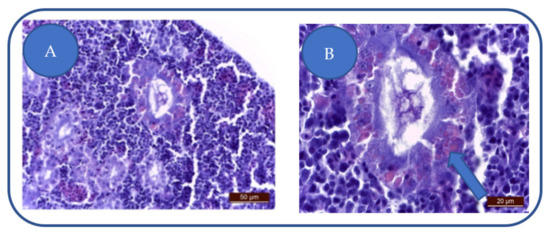
Figure 5.
(A) degeneration of urinary epithelium: nephrocytes swelling, cytoplasm vacuolation, nuclear hyperhydration, interstitial capillaries congestion, monocyte infiltration of the urinary epithelium. (B) tubular cell hyalinosis.
Interstitial capillaries of the patulin-treated zebrafish are overloaded with erythrocytes, thus leading to a medium congestion.
Due to a monocyte infiltration of the urinary epithelium, the patulin-treated zebrafish exhibit a renal inflammatory process.
In the nephrocytes, we have observed the presence of large, vitreous casts (Figure 5B). The presence of increased numbers of hyaline casts can lead to kidney failure.
The Hyalinosis presence in the tubular epithelium does not imply a nephrocyte lesion but the saturation of the physiological mechanism.
Following exposure to 100 mg/L kojic acid, we have remarked a moderate degeneration of the proximal and distal urinary tract, noted by moderate alteration of the epithelium (Figure 6A). Nephrocytes are slightly swollen, have a granular cytoplasm, and the nuclei are hyperhydrated. Tubular lumens are slightly reduced.
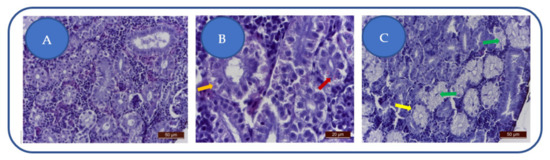
Figure 6.
Effects of kojic acid on the kidneys: (A) 100 mg/L; (B) 204 mg/L; (C) 284 mg/L. Nephrocytes degeneration: granular cytoplasm (→), hyaline casts (→), swelling of the tubular epithelium, with tubular lumens disappearance (→), prominent nucleoli (→).
The 204 mg/L kojic acid dose leads to the degeneration of the proximal tubules nephrocytes, distinguished by granular, non-homogeneous cytoplasm and the presence of hyaline casts (Figure 6B). Hyalin denotes altered glomerular filtration, resulting in massive proteinuria. Hyalin is the result of intracytoplasmic accumulation of pinocyte proteins in the primary urinary filtrate.
The 284 mg/L kojic acid-treated group exhibits moderate kidney toxicosis: swelling of the tubular epithelium and tubular lumens disappearance (Figure 6C). The nephrocytes are swollen with abundant cytoplasm, hypertrophied nuclei and prominent nucleoli.
Patulin exhibits toxic effects on the zebrafish heart, characterized by myocardial edema, enlarged interfibrillar spaces, swollen myocardiocytes and sarcoplasm hyperhydration (Figure 7). These effects can alter the cardiac function and lead to a decrease of the heartbeat rate.
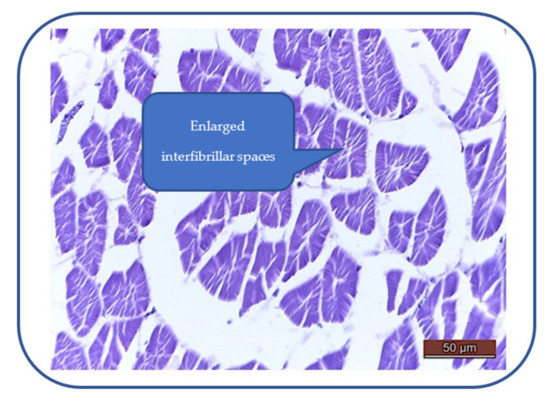
Figure 7.
Myocardial edema: enlarged interfibrillar spaces, swollen myocardiocytes, hyperhydrated sarcoplasm hyperhydration.
After 100 mg/L kojic acid exposure, we have noticed cardiac muscle fibers degeneration and perinuclear sarcoplasm vacuolization (Figure 8). There is also a moderate to severe myocardial congestion, characterized by ectasia and erythrocyte overload of the interstitial capillaries.
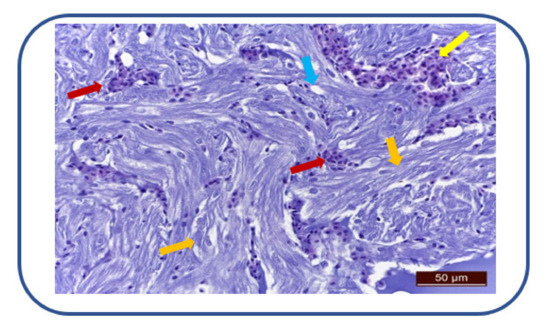
Figure 8.
Cardiac muscle fibers degeneration (→), sarcoplasm vacuolization (→). Myocardial congestion (→), ectasia and erythrocyte overload of interstitial capillaries (→).
At the beginning of analysis, the ANOVA test was used to compare the batch averages of variables present in the MAZE test. For all batches of variables, the result “H0 is not rejected, the difference is insignificant at the significance threshold of 95%” was obtained.
According to the table of main components (PCA), also in the case of the MAZE test, the first two main components represent almost 80% of the variance between the four initial variables, while the first three components represent almost 96% (Table 1).

Table 1.
With PCA-Y-Maze test.
The relationships between the variables described above using the scatter plots are easier to visualize in the monoplot and indicate the same lack of correlation of the “Code” variable with any of the other test variables (Figure 9).
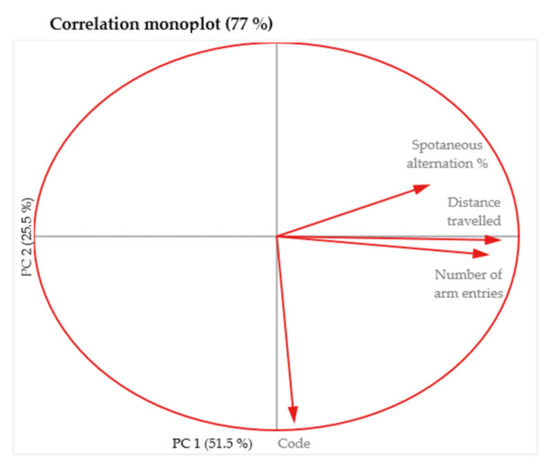
Figure 9.
Two dimensional monoplot of correlation of the coefficients for the first two principal components—MAZE test.
The ANOVA test launched to compare the batch averages of the variables in the NTT test, obtained for all batches of variables the result “H0 is not rejected, the difference is insignificant at the significance threshold of 95%”, except for the variables “Time in top zone” and “Time in bottom zone” for which the result was obtained “H0 is rejected with the significance threshold of 99.9%—at least two averages differ significantly”. Here, we found out about the existence of a highly significant difference between the media, without specifying which pair of media differs significantly, but it is enough for us to know that the NTT test contains variables that correlate with the toxin administered.
According to the PCA table, the first two main components of the NTT test represented almost 70% of the variance between the eight original variables, while the first three components represented almost 80% (Table 2).

Table 2.
With variances—NTT test.
Following the monoplot analysis, it resulted that “No. entries in top”, “Total distance traveled” and “Code” are not very well represented as indicated by the short length of their vectors, therefore it is not wise to refer to the relationships involving these variables due to their weakness representations (Figure 10).
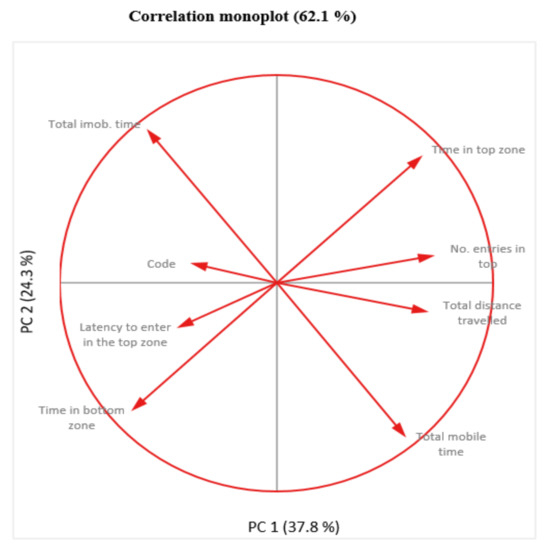
Figure 10.
Two dimensional monoplot of correlation of the coefficients for the first two principal components—NTT test.
We continued the analysis trying some machine learning algorithms on the classification problems in the case of the two behavioral tests MAZE and NTT.
- MAZE ML
There are 68 samples in the training set and 30 samples in the test set.
The accuracy of the SVM classifier on training data is 0.34.
The accuracy of the SVM classifier on test data is 0.23.
[[3 0 1 3 0]
[2 0 1 2 0]
[2 0 2 2 0]
[3 0 0 2 0]
[2 0 4 1 0]]
The accuracy of the Knn classifier on training data is 0.47.
The accuracy of the Knn classifier on test data is 0.17.
[[2 1 2 0 2]
[2 1 1 1 0]
[2 0 2 0 2]
[2 3 0 0 0]
[2 2 2 1 0]]
The accuracy of the XGBoost classifier on training data is 0.91.
The accuracy of the XGBoost classifier on test data is 0.20.
[[2 1 1 0 3]
[0 1 2 2 0]
[1 0 2 2 1]
[1 2 0 0 2]
[4 1 1 0 1]]
The accuracy of the Decision Tree classifier on training data is 0.93.
The accuracy of the Decision Tree classifier on test data is 0.23.
[[2 1 1 1 2]
[0 1 2 2 0]
[1 1 2 1 1]
[1 2 1 1 0]
[2 1 1 2 1]]
The accuracy of the Random Forest classifier on training data is 0.93.
The accuracy of the Random Forest classifier on test data is 0.33.
[[1 2 1 0 3]
[0 3 2 0 0]
[1 0 3 1 1]
[1 0 0 2 2]
[3 1 2 0 1]]
MAZE test: the lowest overfitting was recorded for SVM and the highest value of accuracy was recorded for Random Forest run on the training data.
- MAZE ML reduced
There are 67 samples in the training set and 30 samples in the test set.
The accuracy of the SVM classifier on training data is 0.82.
The accuracy of the SVM classifier on test data is 0.77.
[[0 7]
[0 23]]
[1]
0.5
The accuracy of the Knn classifier on training data is 0.87.
The accuracy of the Knn classifier on test data is 0.73.
[[0 7]
[1 22]]
[0 1]
0.4782608695652174
The accuracy of the XGBoost classifier on training data is 0.94.
The accuracy of the XGBoost classifier on test data is 0.57.
[[0 7]
[6 17]]
[0 1]
0.3695652173913043
The accuracy of the Decision Tree classifier on training data is 0.99.
The accuracy of the Decision Tree classifier on test data is 0.57.
[[1 6]
[7 16]]
[0 1]
0.4192546583850931
The accuracy of the Random Forest classifier on training data is 0.97.
The accuracy of the Random Forest classifier on test data is 0.73.
[[1 6]
[2 21]]
[0 1]
0.5279503105590062
Significantly better results of the accuracy of the classification algorithms were recorded on the reduced data set. SVM records the lowest overfitting and a relatively acceptable accuracy, but erroneously predicts the existence of only one class, the majority one, which shows that the algorithm is sensitive (gives erroneous results) to unbalanced classes. The Random Forest algorithm gave the best results (as expected). Decision Tree obtained a very high classification accuracy, only with a high overfitting rate and a low AUC value. It should be noted that the confusion matrix has the most errors in the case of the minority class, so we will be forced to manage this situation (the presence of unbalanced classes) in order to obtain correct results.
For the NTT test, the lowest overfitting was recorded for KNN, and the highest accuracy values were recorded for the XGBoost, Decision Tree, and Random Forest algorithms run on the drive data, only with higher overfitting values.
- NTT ML
OUT: There are 42 samples in the training set and 18 samples in the test set.
OUT: The accuracy of the SVM classifier on training data is 0.62.
The accuracy of the SVM classifier on test data is 0.11.
[[1 2 0 0 2]
[0 1 0 0 0]
[0 3 0 2 1]
[0 4 0 0 0]
[0 1 0 1 0]]
The accuracy of the Knn classifier on training data is 0.48.
The accuracy of the Knn classifier on test data is 0.28.
[[2 2 0 0 1]
[0 1 0 0 0]
[0 3 0 2 1]
[0 2 0 2 0]
[0 0 0 2 0]]
The accuracy of the XGBoost classifier on training data is 1.00.
The accuracy of the XGBoost classifier on test data is 0.17.
[[1 1 2 0 1]
[0 0 0 0 1]
[0 2 1 3 0]
[1 0 0 0 3]
[0 0 0 1 1]]
The accuracy of the Decision Tree classifier on training data is 1.00.
The accuracy of the Decision Tree classifier on test data is 0.22.
[[1 1 0 1 2]
[0 0 0 0 1]
[0 2 1 3 0]
[0 0 0 2 2]
[0 0 0 2 0]]
The accuracy of the Random Forest classifier on training data is 0.98.
The accuracy of the Random Forest classifier on test data is 0.28.
[[2 1 0 1 1]
[0 0 0 0 1]
[0 1 2 1 2]
[1 0 1 1 1]
[0 1 0 1 0]]
Significantly better accuracy results were also recorded on the reduced data set in the case of the NTT test. SVM and KNN recorded the lowest overfitting and a fairly high accuracy, as well as the AUC value. In this case, the Random Forest and Decision Tree algorithms gave good results in accuracy, overfitting, and AUC. Overall, however, XGBoost held supremacy in all classification tests: accuracy, overfitting rate, and AUC value. It should be noted that the confusion matrix presented the most errors also for the minority class, only in this case with much lower values.
- NTT ML-reduced
There are 41 samples in the training set and 18 samples in the test set.
The accuracy of the SVM classifier on training data is 0.90.
The accuracy of the SVM classifier on test data is 0.89.
[[2 2]
[0 14]]
[0 1]
0.75
The accuracy of the Knn classifier on training data is 0.88.
The accuracy of the Knn classifier on test data is 0.89.
[[2 2]
[0 14]]
[0 1]
0.75
The accuracy of the XGBoost classifier on training data is 0.95.
The accuracy of the XGBoost classifier on test data is 0.89.
[[2 2]
[0 14]]
[0 1]
0.75
The accuracy of the Decision Tree classifier on training data is 1.00.
The accuracy of the Decision Tree classifier on test data is 0.67.
[[2 2]
[4 10]]
[0 1]
0.6071428571428572
The accuracy of the Random Forest classifier on training data is 1.00.
The accuracy of the Random Forest classifier on test data is 0.78.
[[1 3]
[1 13]]
[0 1]
0.5892857142857143
Next, we used PCA in Python to reduce the size to 2 or 3, so that we can browse and better understand the data.
Using the variance explained ratio attribute to reduce the size of the data in the MAZE test, the first main component contained 67.96% of the variance and the second main component 26.25% of the variance, together, the two components containing 94.21% of the information. We can also notice from the graph that the classes are not well separated from each other. The same can be seen from the graph represented for the case of reduced data (Figure 11 and Figure 12).
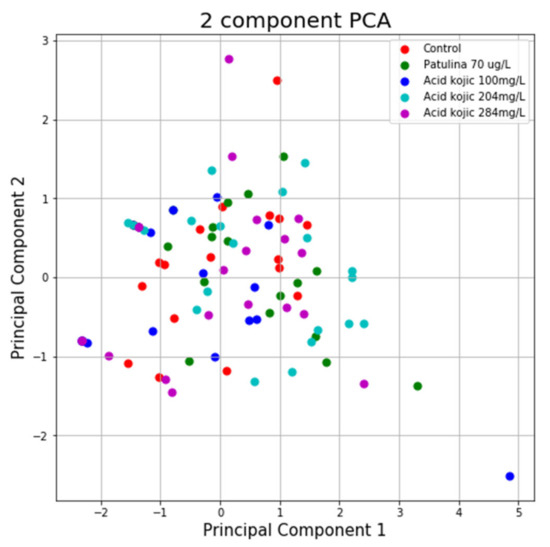
Figure 11.
PCA applied on variables from the MAZE test.
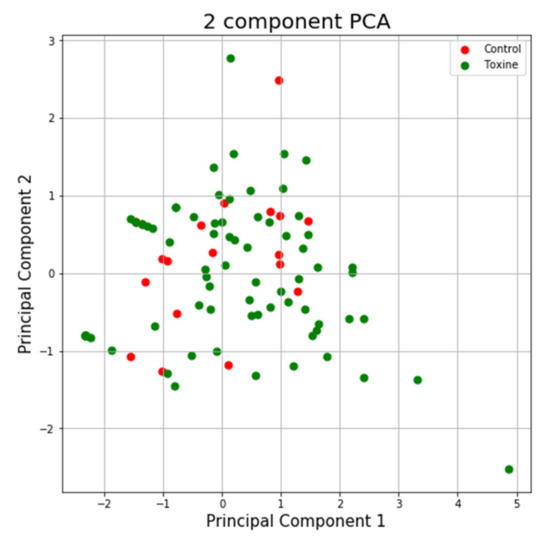
Figure 12.
PCA applied on reduced variables from the MAZE test.
Using the same program, we can see in the case of the NTT test the first main component contains 43.44% of the variance and the second main component contains 26.79% of the variance. Together, the two components contain 70.23% of the information. We can also notice from the graph that most classes are not well separated from each other, only the “Control” class seems to be slightly separated from the others. In the case of reduced data, a clearer separation of the “Control” class from the “Toxins” class is observed, with some aberrant values present in both classes (Figure 13 and Figure 14).
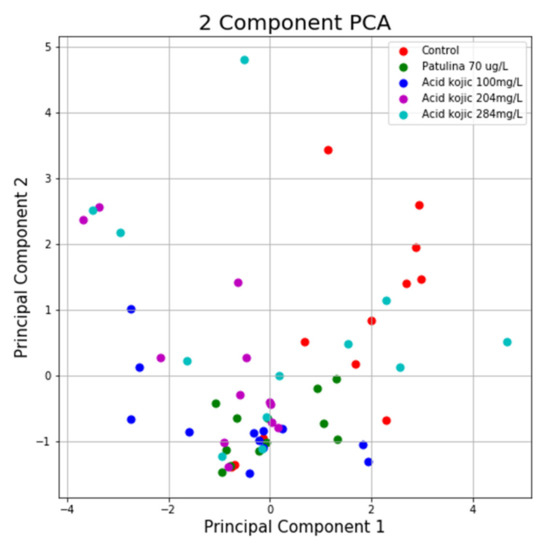
Figure 13.
PCA applied on the variables of the NTT test.
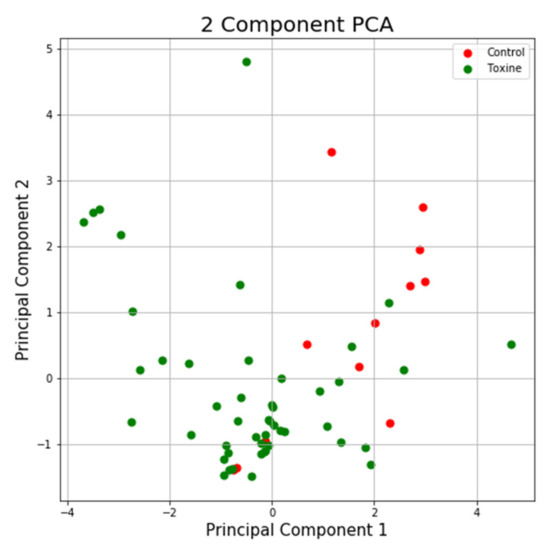
Figure 14.
PCA applied on reduced variables of test NTT.
In the end, for the unbalanced classes encountered in the NTT and MAZE analysis, we explored 5 efficient ways to manage the situation: minority class over-sampling, majority class sub-sampling, performance evaluation modification, use of penalty algorithms (cost-sensitive training), the use of decision tree algorithms, after which we concluded that the results were better in the case of the NTT test relative to the MAZE test and that the use of decision tree algorithms leads to amazing results, knowing that their hierarchical structure allows them to learn signals from both classes (majority and minority).
4. Discussion
4.1. Y-Maze Discussion
The dorso-lateral area of the fish telencephalon plays a similar role to mammal and bird hippocampus, which is involved in learning, memory, spatial, and temporal associations [36].
By its effect on spatial memory, we conclude that both patulin and kojic acid act on the dorso-lateral area of the zebrafish’s telencephalon.
Locomotion intensifying and memory improvement in the mycotoxin-exposed groups, will increase the fish ability to explore the environment, to find food, partners, and breeding places, all of which are essential for the survival of individuals and the species.
4.2. NTT Discussion
Inhibition of aggressive and/or reproductive behavior, the reduction of feeding, locomotor activity and environmental exploration is characteristic of beta (subordinate) individuals [37].
Zebrafish, which show a state of anxiety after exposure to both patulin and kojic acid, exhibit the behavior of a beta individual.
Because growth hormone (GH) has led to an increase in the aggressive behavior and dominance of individuals exposed to this hormone [38,39], we can deduce that subordinate/anxious individuals have a low level of GH. From the obtained data, it results that the patulin, through the anxiogenic effect, affects the pituitary gland and the GH production of zebrafish, the level of this hormone being low in intoxicated individuals.
The dorso-medial area of the fish’s telencephalon has functions similar to the cerebral amygdala of mammals, being involved in the processing of fear. Ablation of this area prevents the appearance of fear [36,40]. The anxiogenic effect demonstrates that both patulin and kojic acid acts on the dorso-medial area of the zebrafish telencephalon. The anxiogenic effect of patulin and kojic acid decreases the fish ability to explore the environment, to find food, partners and breeding places, all of which are essential for the survival of individuals and the species.
4.3. Histological Alterations Discussion
The presence of vacuolated hepatocytes in the patulin-treated group is confirmed by a study on rats [41]. Vacuolization is considered a side effect of cytotoxic factors and a mechanism that can reduce stress and increase the survival of the cells [42]. Hepatocyte damage and loss of cell outline has also been observed in mice, after 2 weeks of patulin exposure [43]. The hepatocytes of the patulin-treated group showed hydropic and lipid dystrophy. Additionally, in the case of mice, patulin exposure led to fatty degeneration [44], but there is a difference between the hyperhydration observed in zebrafish hepatocytes and mice hepatocytes, which had granular cytoplasm [43].
While in the case of patulin-treated rats [41] or mice [44], hepatocyte necrosis, sinusoids dilation/irregularities or nuclear abnormalities were observed, these alterations were absent in the liver of patulin-treated zebrafish. Another difference is that Lymphocyte infiltration in the liver of patulin-exposed rats and mice [45] was absent in patulin-treated zebrafish. Following the administration of a diet containing 0.5–2% kojic acid for 26 weeks, a carcinogenic effect (hepatocellular adenoma) was observed on the liver of mice. Cellular alteration, necrosis and inflammatory processes have been observed [46]. These effects have also been observed in mice exposed to kojic acid for 26 weeks [47]. Long-term exposure (13 weeks) will increase liver mass [48].
The exposure to kojic acid for 4 weeks (through diet—2%) did not cause noticeable histological alterations in the liver [49], which shows that mice are less sensitive to the effects of kojic acid compared to zebrafish. Rats’ exposure to kojic acid (2% of diet, 55 weeks) caused hepatocytes vacuolation, cell necrosis (isolated cases), granulomas, and bile ducts proliferation [50]. Human liver tumor cells also suffer from nuclear alterations after exposure to high doses (6–8 g/L) of kojic acid: the increase in the frequency of micronuclei is accompanied by a severe toxic alteration of liver cells [51]. The absence of the inflammatory component shows that, in the liver, the immune system of zebrafish has not been activated following short-term exposure to kojic acid, unlike in rodents.
At all the applied doses, liver damage will lead to the alteration of the metabolism, acid–base balance, thermoregulation, blood clotting, bile, and enzyme synthesis. Due to cell destruction, liver enzymes (transaminases, alkaline phosphatase, gamma-glutamyltransferase, lactate dehydrogenase) will reach the bloodstream. Altering bile secretion will reduce the digestive capacity of lipids.
Additionally, the antitoxic function of the liver will be affected: toxic elements in water and food, metabolic products of intestinal bacteria will reach the bloodstream causing severe intoxications.
The apoptosis of the renal cells was absent, in contrast with a study on mice [52]. The similarity between the effects on zebrafish and rodents is sustained by the morphological alterations of the glomeruli and renal tubules shown in the case of patulin-exposed zebrafish embryos [53] and mice [44]. In the case of zebrafish embryos, the morphological alterations of the glomeruli are followed by a reduction of the filtration rate [53], thus we can make the assumption that glomeruli degeneration also alters the filtration rate of the adult zebrafish. Interstitial capillaries of the patulin-treated zebrafish are overloaded with erythrocytes, thus leading to a medium congestion, while kidney of the patulin treated mammals showed capillary lesions [54,55,56] and hemorrhagic lesions of the kidney [44].
The patulin-treated zebrafish exhibit a renal inflammatory process caused by monocyte infiltration, while in patulin-treated rats the inflammation was caused by a lymphocyte infiltration [45]. Additionally, in the kidney of patulin-exposed mice, there was observed an inflammatory cell infiltration [44].
The presence of vitreous casts is in line with a study on rodents: the urine of the patulin-treated rats contained hyaline and granular casts [45]. The kidneys of rodents exposed to fungi that produce kojic acid, showed a degeneration of the proximal urinary tract. The presence of hyaline cylinders was noticed, and some areas were affected by necrosis [57].
At concentrations of 200–800 mg/L, kojic acid showed a reduced cytotoxicity (below 20%) on the cells of the primate renal epithelium [58] and the doses of 1.56–100 mg/L did not change the cell viability [59]. High kojic acid doses (over 1 g/kg body weight) administered to rodents caused diuresis and kidney pallor [47]. Long-term exposure (13 weeks) will increase kidney mass [48]. Kojic acid-exposed rats (2% of food, 55 weeks) exhibited urinary cylinders and damage to the urinary tract [51]. The hyalinosis of the tubular epithelium after patulin or kojic acid exposure, does not imply a nephrocyte lesion but the saturation of the physiological mechanism, both mycotoxins leading to this effect.
The myocardial histological alterations seen in the patulin or kojic acid-treated fish can alter the cardiac function and lead to a decrease of the heartbeat rate. On a study on mice by Boussabbeh et al., patulin’s cardiotoxic effects are discussed: oxidative stress and cell apoptosis, which can also lead to alterations of the cardio-vascular system [60]. The fact that mycotoxins exhibit cardiotoxic effects on zebrafish embryos heart are shown in several studies: pericardial edema [61,62,63,64], decrease in heartbeat rate [63,64]. After being exposed to fungi that produce kojic acid, rodent myocardium exhibited hemorrhagic lesions [57]. Exposed to doses of 900 mg/kg body weight kojic acid, pregnant female mice experienced a reduction in heart mass compared to the control group [48].
In addition to inhibiting melanogenesis, kojic acid exposure led to a slight decrease in the Danio rerio embryos heart rate [65].
Exposure of zebrafish embryos to 100 mg/L–2.5 g/L kojic acid doses, led to pericardial edema and reduced heart rate [63]. An in vitro study Chaudhari et al. showed that kojic acid (56.8 mg/L) did not affect the viability of human cardiomyocytes but led to decreased ATP levels [66].
5. Conclusions
Studies have showed that acute intoxication of PAT determines agitation and convulsions, vomiting, ulceration and intestinal inflammation, edema, and DNA damage in the brain, kidneys, and liver. On the other hand, chronic intoxication determines genotoxic, teratogenic, neurotoxic, and immunotoxic outcomes in rodents. Zebrafish embryo exposed to patulin encountered severe nephrotoxicity in histological organization and biological function. By affecting the spatial memory, both patulin and kojic acid act on the posterolateral area of the zebrafish telencephalon. The anxiogenic effect shows that both mycotoxins act on the posterolateral area of the zebrafish’s telencephalon. The anxious behavior of intoxicated individuals exposed to patulin or kojic acid is more pronounced compared to individuals in the control group, fish treated with these mycotoxins exhibited a behavior characteristic of beta (subordinate) individuals.
Patulin and kojic acid, through the anxiogenic effect, affect the pituitary gland and the GH production of zebrafish, the level of this hormone being low in beta individuals. Through the induced behavioral effects, it means that both patulin and kojic acid act on the hypothalamus–pituitary–renal and hypothalamus–pituitary–gonad axes, having an impact on serotonin, vasopressin (vasotocin), and oxytocin (isotocin) levels. The anxiogenic effect of patulin and kojic acid reduces the ability of fish to explore the environment, to find food, partners, and breeding places, all of which reduce the individuals’ and species’ chances of survival. By improving memory and intensifying anxious behavior, both patulin and kojic acid have shown significant effects on the nervous system. The onset of renal degenerative processes in rodents, embryos, and zebrafish adults exposed to patulin shows a similarity between the effects of this mycotoxin on fish and rodents. The effects of kojic acid on the liver, kidneys, and heart of rodents and zebrafish demonstrate an increased accumulation of the toxin in these organs, resulting in a toxicological similarity between upper and lower vertebrates.
Individuals in the control group did not show histological changes in the brain, liver, kidneys, intestines, pancreas, or myocardium. The groups exposed to patulin and kojic acid show histological changes in the liver, kidneys, intestines, pancreas, and myocardial muscle tissue. In the case of the three groups exposed to kojic acid, tissue alterations show a progressive intensity, corresponding to an increase in the administered kojic acid dose. In the case of the nervous system, the lesions are insignificant and unsteady. Patulin and the kojic acid doses will lead to altered metabolism, acid–base balance, thermoregulation, blood clotting, bile, and enzyme synthesis. Due to cell destruction, liver enzymes will enter the bloodstream, and altered bile secretion will reduce the digestive capacity of lipids. The liver antitoxic function will be affected: toxic elements in water, food, and the metabolic products of intestinal bacteria will reach the bloodstream producing intoxications. By affecting the kidneys, the two mycotoxins have negative effects on excretion, maintaining the body’s acid–base balance, reabsorption/secretion in the urinary tract, renin and erythropoietin formation. Through the cardiotoxic effects, both patulin and kojic acid (100 mg/L) can cause a decrease in heart rate and a reduction in the amplitude of heart contractions, thus having a negative impact on locomotor, feeding, and breeding activity.
As an overall conclusion, we can state the following:
- -
- The novel tank test (NTT), which assesses anxiety, proved to be conclusive in the behavioral analysis of fish that were given toxins, proving that intoxicated fish had more anxiety problems than spatial memory.
- -
- We have managed to detect an automatic learning algorithm, from the category of decision trees, which can be trained to classify the behavior of fish that were administered a toxin from the category of those used in the experiment, only by analyzing the characteristic features of the NTT behavioral test.
Author Contributions
All authors had equal contributions. Conceptualization and project administration T.-L.M., A.M.V., M.A., A.S.P.; investigation and data curation L.M.T., B.F.T.; methodology and supervision M.A., C.L.-U.; methodology and investigation T.-L.M., M.A., A.M.V.; formal analysis and investigation A.M.V., M.A., A.S.P., C.L.-U.; writing—review and editing and validation T.-L.M., A.M.V., M.A., A.S.P.; software M.A., L.M.T., B.F.T.; supervision T.-L.M., M.A., A.S.P., A.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ap-proved by the Ethics Committee of University Hospital St. Spiridon Iasi, approval no. 3/21.01.2022.
Informed Consent Statement
Not applicable, studies not involving humans.
Data Availability Statement
The data published in this research are available on request from the first and last author and corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Y-maze test.
Table A1.
Y-maze test.
| Animal | Treatment | Code | Stage | Trial | Apparatus | Number of Arm Entries | Spotaneous Alternation % | Distance Travelled (m) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Control | A | First stage | 1 | Y-maze | 4 | 200 | 14 |
| 2 | 2 | Control | A | First stage | 1 | Y-maze | 10 | 25 | 18 |
| 3 | 3 | Control | A | First stage | 1 | Y-maze | 3 | 0 | 7 |
| 4 | 4 | Control | A | First stage | 1 | Y-maze | 2 | 2 | |
| 5 | 5 | Control | A | First stage | 1 | Y-maze | 2 | 1 | |
| 6 | 6 | Control | A | First stage | 1 | Y-maze | 6 | 50 | 3 |
| 7 | 7 | Control | A | First stage | 1 | Y-maze | 12 | 120 | 7 |
| 8 | 8 | Control | A | First stage | 1 | Y-maze | 7 | 40 | 9 |
| 9 | 9 | Control | A | First stage | 1 | Y-maze | 21 | 105 | 13 |
| 10 | 10 | Control | A | First stage | 1 | Y-maze | 20 | 133 | 11 |
| 11 | 11 | Control | A | First stage | 1 | Y-maze | 23 | 95 | 16 |
| 12 | 12 | Control | A | First stage | 1 | Y-maze | 10 | 100 | 6 |
| 13 | 13 | Control | A | First stage | 1 | Y-maze | 2 | 1 | |
| 14 | 14 | Control | A | First stage | 1 | Y-maze | 10 | 125 | 16 |
| 15 | 15 | Control | A | First stage | 1 | Y-maze | 21 | 137 | 15 |
| 16 | 16 | Control | A | First stage | 1 | Y-maze | 5 | 67 | 5 |
| 17 | 17 | Control | A | First stage | 1 | Y-maze | 17 | 107 | 15 |
| 18 | 18 | Control | A | First stage | 1 | Y-maze | 4 | 0 | 12 |
| 19 | 19 | Control | A | First stage | 1 | Y-maze | 5 | 67 | 6 |
| 22 | 22 | Control | A | First stage | 1 | Y-maze | 9 | 86 | 10 |
| 23 | 23 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 25 | 104 | 14 |
| 24 | 24 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 11 | 22 | 11 |
| 25 | 25 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 26 | 26 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 12 | 100 | 7 |
| 27 | 27 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 10 | 100 | 11 |
| 28 | 28 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 11 | 133 | 11 |
| 29 | 29 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 18 | 75 | 16 |
| 30 | 30 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 15 | 108 | 23 |
| 31 | 31 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 7 | 160 | 17 |
| 32 | 32 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 22 | 90 | 14 |
| 33 | 33 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 35 | 73 | 16 |
| 34 | 34 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 4 | 100 | 5 |
| 35 | 35 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 7 | 120 | 11 |
| 36 | 36 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 4 | 100 | 12 |
| 37 | 37 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 29 | 81 | 17 |
| 38 | 38 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 5 | 67 | 13 |
| 39 | 39 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 43 | 88 | 26 |
| 40 | 40 | Patulin 70 μg/L | B | First stage | 1 | Y-maze | 7 | 80 | 4 |
| 41 | 41 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 42 | 42 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 2 | 1 | |
| 43 | 43 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 17 | 67 | 15 |
| 44 | 44 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 7 | 120 | 9 |
| 45 | 45 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 46 | 46 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 14 | 83 | 15 |
| 47 | 47 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 18 | 125 | 11 |
| 48 | 48 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 2 | 4 | |
| 49 | 49 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 64 | 74 | 31 |
| 50 | 50 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 9 | 29 | 16 |
| 51 | 51 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 20 | 67 | 12 |
| 52 | 52 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 9 | 29 | 5 |
| 53 | 53 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 54 | 54 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 4 | 100 | 5 |
| 55 | 55 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 2 | 2 | |
| 56 | 56 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 57 | 57 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 10 | 75 | 9 |
| 58 | 58 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 59 | 59 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 1 |
| 60 | 60 | Acid kojic 100 mg/L | C | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 61 | 61 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 10 | 100 | 12 |
| 62 | 62 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 17 | 147 | 12 |
| 63 | 63 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 28 | 54 | 16 |
| 64 | 64 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 26 | 125 | 21 |
| 65 | 65 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 14 | 167 | 16 |
| 66 | 66 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 2 | 0 | |
| 67 | 67 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 33 | 103 | 21 |
| 68 | 68 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 5 | 133 | 8 |
| 69 | 69 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 26 | 83 | 19 |
| 70 | 70 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 22 | 130 | 15 |
| 71 | 71 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 13 | 55 | 8 |
| 72 | 72 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 28 | 123 | 20 |
| 73 | 73 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 34 | 100 | 18 |
| 74 | 74 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 6 | 100 | 7 |
| 75 | 75 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 11 | 67 | 10 |
| 76 | 76 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 2 | 3 | |
| 77 | 77 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 20 | 33 | 16 |
| 78 | 78 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 2 | 1 | |
| 79 | 79 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 24 | 73 | 20 |
| 80 | 80 | Acid kojic 204 mg/L | D | First stage | 1 | Y-maze | 13 | 109 | 7 |
| 81 | 81 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 82 | 82 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 83 | 83 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 7 | 80 | 14 |
| 84 | 84 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 38 | 72 | 21 |
| 85 | 85 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 11 | 0 | 10 |
| 86 | 86 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 1 | 0 | 0 |
| 87 | 87 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 8 | 67 | 19 |
| 88 | 88 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 4 | 0 | 13 |
| 89 | 89 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 13 | 55 | 10 |
| 90 | 90 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 12 | 100 | 13 |
| 91 | 91 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 23 | 86 | 18 |
| 92 | 92 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 2 | 2 | |
| 93 | 93 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 3 | 200 | 6 |
| 94 | 94 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 23 | 86 | 15 |
| 95 | 95 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 15 | 123 | 11 |
| 96 | 96 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 19 | 118 | 17 |
| 97 | 97 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 14 | 133 | 18 |
| 98 | 98 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 17 | 120 | 15 |
| 99 | 99 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 10 | 150 | 7 |
| 100 | 100 | Acid kojic 284 mg/L | E | First stage | 1 | Y-maze | 4 | 0 | 3 |

Table A2.
NTT test.
Table A2.
NTT test.
| Test | Animal | Treatment | Code | Stage | Trial | Apparatus | No. Entries in Top | Total Distance Travelled | Total Imob. Time | Total Mobile Time | Latency to Enter in the Top Zone | Time in Top Zone | Time in Bottom Zone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2 | Control | A | First stage | 1 | NTT | 9 | 8 | 134 | 226 | 66 | 275 | 85 |
| 4 | 4 | Control | A | First stage | 1 | NTT | 26 | 21 | 0 | 360 | 21 | 219 | 141 |
| 7 | 7 | Control | A | First stage | 1 | NTT | 22 | 18 | 0 | 360 | 30 | 264 | 96 |
| 8 | 8 | Control | A | First stage | 1 | NTT | 10 | 14 | 0 | 360 | 5 | 314 | 46 |
| 9 | 9 | Control | A | First stage | 1 | NTT | 30 | 20 | 0 | 360 | 30 | 116 | 244 |
| 10 | 10 | Control | A | First stage | 1 | NTT | 28 | 25 | 0 | 360 | 25 | 228 | 132 |
| 11 | 11 | Control | A | First stage | 1 | NTT | 23 | 18 | 0 | 360 | 5 | 167 | 193 |
| 14 | 14 | Control | A | First stage | 1 | NTT | 6 | 17 | 0 | 360 | 262 | 33 | 327 |
| 15 | 15 | Control | A | First stage | 1 | NTT | 11 | 16 | 0 | 360 | 147 | 47 | 313 |
| 16 | 16 | Control | A | First stage | 1 | NTT | 48 | 46 | 24 | 336 | 42 | 45 | 315 |
| 18 | 18 | Control | A | First stage | 1 | NTT | 10 | 13 | 30 | 330 | 3 | 116 | 244 |
| 24 | 24 | Control | A | First stage | 1 | NTT | 3 | 14 | 0 | 360 | 91 | 6 | 355 |
| 25 | 25 | Patulin 70 μg/L | B | First stage | 1 | NTT | 0 | 13 | 0 | 360 | 0 | 360 | |
| 26 | 26 | Patulin 70 μg/L | B | First stage | 1 | NTT | 6 | 14 | 4 | 357 | 213 | 12 | 348 |
| 29 | 29 | Patulin 70 μg/L | B | First stage | 1 | NTT | 7 | 16 | 0 | 360 | 0 | 19 | 342 |
| 30 | 30 | Patulin 70 μg/L | B | First stage | 1 | NTT | 27 | 18 | 8 | 352 | 126 | 93 | 267 |
| 33 | 33 | Patulin 70 μg/L | B | First stage | 1 | NTT | 5 | 11 | 15 | 345 | 109 | 10 | 350 |
| 35 | 35 | Patulin 70 μg/L | B | First stage | 1 | NTT | 29 | 42 | 20 | 341 | 102 | 40 | 321 |
| 36 | 36 | Patulin 70 μg/L | B | First stage | 1 | NTT | 4 | 13 | 43 | 317 | 7 | 12 | 348 |
| 39 | 39 | Patulin 70 μg/L | B | First stage | 1 | NTT | 28 | 23 | 0 | 360 | 0 | 40 | 320 |
| 41 | 41 | Patulin 70 μg/L | B | First stage | 1 | NTT | 8 | 12 | 8 | 352 | 1 | 38 | 323 |
| 44 | 44 | Patulin 70 μg/L | B | First stage | 1 | NTT | 38 | 37 | 81 | 279 | 2 | 38 | 322 |
| 47 | 47 | Patulin 70 μg/L | B | First stage | 1 | NTT | 5 | 16 | 0 | 360 | 0 | 9 | 351 |
| 48 | 48 | Patulin 70 μg/L | B | First stage | 1 | NTT | 3 | 11 | 74 | 286 | 1 | 1 | 359 |
| 53 | 53 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 18 | 16 | 0 | 360 | 100 | 47 | 313 |
| 54 | 54 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 6 | 12 | 9 | 351 | 10 | 20 | 340 |
| 61 | 61 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 1 | 4 | 109 | 251 | 352 | 4 | 356 |
| 64 | 64 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 9 | 18 | 0 | 360 | 93 | 29 | 332 |
| 65 | 65 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 4 | 14 | 0 | 360 | 2 | 21 | 339 |
| 66 | 66 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 31 | 44 | 3 | 357 | 10 | 33 | 327 |
| 68 | 68 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 32 | 49 | 0 | 360 | 8 | 19 | 341 |
| 70 | 70 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 0 | 23 | 0 | 360 | 0 | 360 | |
| 71 | 71 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 2 | 10 | 61 | 299 | 184 | 7 | 353 |
| 74 | 74 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 0 | 2 | 218 | 142 | 0 | 360 | |
| 75 | 75 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 7 | 12 | 0 | 360 | 11 | 30 | 330 |
| 76 | 76 | Acid kojic 100 mg/L | D | First stage | 1 | NTT | 1 | 8 | 166 | 194 | 213 | 2 | 358 |
| 77 | 77 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 8 | 13 | 0 | 360 | 217 | 20 | 340 |
| 79 | 79 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 0 | 1 | 348 | 12 | 0 | 360 | |
| 81 | 81 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 20 | 16 | 0 | 360 | 0 | 137 | 223 |
| 83 | 83 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 36 | 35 | 0 | 360 | 1 | 120 | 241 |
| 86 | 86 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 3 | 6 | 5 | 355 | 109 | 24 | 336 |
| 90 | 90 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 11 | 10 | 83 | 277 | 6 | 48 | 312 |
| 104 | 104 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 2 | 1 | 348 | 12 | 2 | 6 | 354 |
| 105 | 105 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 14 | 10 | 15 | 345 | 42 | 51 | 309 |
| 106 | 106 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 12 | 19 | 87 | 273 | 38 | 12 | 348 |
| 108 | 108 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 30 | 21 | 221 | 139 | 0 | 27 | 333 |
| 109 | 109 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 8 | 10 | 12 | 348 | 6 | 53 | 307 |
| 110 | 110 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 16 | 14 | 4 | 356 | 36 | 34 | 326 |
| 111 | 111 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 5 | 9 | 160 | 200 | 172 | 11 | 349 |
| 112 | 112 | Acid kojic 204 mg/L | C | First stage | 1 | NTT | 9 | 13 | 0 | 360 | 44 | 47 | 313 |
| 113 | 113 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 2 | 9 | 0 | 360 | 158 | 23 | 337 |
| 114 | 114 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 7 | 16 | 0 | 360 | 0 | 10 | 350 |
| 116 | 116 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 79 | 51 | 8 | 352 | 4 | 143 | 217 |
| 118 | 118 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 14 | 10 | 13 | 347 | 17 | 33 | 327 |
| 119 | 119 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 4 | 2 | 283 | 77 | 250 | 70 | 290 |
| 120 | 120 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 6 | 6 | 125 | 235 | 99 | 22 | 338 |
| 121 | 121 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 38 | 31 | 65 | 296 | 0 | 144 | 216 |
| 122 | 122 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 19 | 16 | 359 | 1 | 3 | 189 | 171 |
| 124 | 124 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 1 | 1 | 351 | 9 | 5 | 0 | 360 |
| 126 | 126 | Acid kojic 284 mg/L | E | First stage | 1 | NTT | 9 | 13 | 33 | 327 | 0 | 70 | 290 |
References
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. Statistical learning. In An Introduction to Statistical Learning; Springer: New York, NY, USA, 2021; pp. 15–57. [Google Scholar]
- Irimiciuc, S.A.; Nica, P.E.; Agop, M.; Focsa, C. Target properties—Plasma dynamics relationship in laser ablation of metals: Common trends for fs, ps and ns irradiation regimes. Appl. Surf. Sci. 2020, 506, 144926. [Google Scholar] [CrossRef]
- Irimiciuc, S.; Bulai, G.; Agop, M.; Gurlui, S. Influence of laser-produced plasma parameters on the deposition process: In situ space- and time-resolved optical emission spectroscopy and fractal modeling approach. Appl. Phys. A Mater. 2018, 124, 615. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Bulai, G.; Gurlui, S.; Agop, M. On the separation of particle flow during pulse laser deposition of heterogeneous materials-A multi-fractal approach. Powder Technol. 2018, 339, 273–280. [Google Scholar] [CrossRef]
- Cobzeanu, B.M.; Irimiciuc, S.; Vaideanu, D.; Grigorovici, A.; Popa, O. Possible Dynamics of Polymer Chains by Means of a Ricatti’s Procedure-an Exploitation for Drug Release at Large Time Intervals. Mater. Plast. 2017, 54, 531–534. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Agop, M.; Nica, P.; Gurlui, S.; Mihaileanu, D.; Toma, S.; Focsa, C. Dispersive effects in laser ablation plasmas. Jpn. J. Appl. Phys. 2014, 53, 116202. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Hodoroaba, B.C.; Bulai, G.; Gurlui, S.; Craciun, V. Multiple structure formation and molecule dynamics in transient plasmas generated by laser ablation of graphite. Spectrochim. Acta Part B At. Spectrosc. 2020, 165, 105774. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Gurlui, S.; Agop, M. Particle distribution in transient plasmas generated by ns-laser ablation on ternary metallic alloys. Appl. Phys. B 2021, 125, 190. [Google Scholar] [CrossRef]
- Frisvad, J.C. A critical review of producers of small lactone mycotoxins: Patulin, penicillic acid and moniliformin. World Mycotoxin J. 2018, 11, 73–100. [Google Scholar] [CrossRef]
- Mandalian, T.L.; Coprean, D.; Pașca, S. Effects of Patulin Treatment on Zebrafish (Danio rerio) Internal Organs. Rev. Chim. 2020, 71, 191–195. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, G.; Dev, I.; Shukla, K.S.; Ansari, K.M. Study of the metabolic alterations in patulin-induced neoplastic transformation in normal intestinal cells. Toxicol. Res. 2021, 10, 592–600. [Google Scholar] [CrossRef]
- Pillay, Y.; Nagiah, S.; Phulukdaree, A.; Krishnan, A.; Chuturgoon, A.A. Patulin suppresses α1-adrenergic receptor expression in HEK293 cells. Sci. Rep. 2020, 10, 20115. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Soni, M.G.; Carabin, I.G. Evaluation of health aspects of kojic acid in food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Mitsumori, K.; Onodera, H.; Fujimoto, N.; Yasuhara, K.; Takegawa, K.; Takagi, H.; Hirose, M. Dose-threshold for thyroid tumor-promoting effects of orally administered kojic acid in rats after initiation with N-bis(2-hydroxypropyl) nitrosamine. J. Toxicol. Sci. 2001, 26, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Su, W.C.; Li, C.; Shi, Y.; Chen, Q.X.; Zheng, J.; Tang, D.L.; Chen, S.M.; Wang, Q. Anti-melanogenesis of novel kojic acid derivatives in B16F10 cells and zebrafish. Int. J. Biol. Macromol. 2019, 123, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Alhewairini, S.S. Zebrafish (Danio rerio) as a Model Organism. Curr. Trends Cancer Manag. 2019, 81517, 3–18. [Google Scholar]
- Beckmann, C.; Biro, P.A. On the validity of a single (boldness) assay in personality research. Ethology 2013, 119, 937–947. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; GoodSpeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analysing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, D.L.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2018, 22, 111–122. [Google Scholar] [CrossRef]
- Cognato, G.D.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze memory task in zebrafish (Danio rerio): The role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- Aoki, R.; Tsuboi, T.; Okamoto, H. Y-maze avoidance: An automated and rapid associative learning paradigm in zebrafish. Neurosci. Res. 2015, 91, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.K.; Bright, L.A.; Marx, J.O.; Andersen, R.P.; Mullins, M.C.; Carty, A.J. Effectiveness of rapid cooling as a method of euthanasia for young zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 58–63. [Google Scholar] [PubMed]
- Diaconiţă, G.; Eskenasy, A.; Hagi-Paraschiv, A.; Iliescu, G.; Mureşan Aug Nicolescu, P.; Repciuc, E.; Roşca, V.; Teitel, A. Tehnica Histopatologică; Editura de Stat Pentru Literatura Ştiinţifică: Bucharest, Romania, 1953. [Google Scholar]
- Șincai, M. Tehnici de Citohistologie Normală si Patologică. Ghid Practice; Ed. Mirton: Timișoara, Romania, 2000; p. 6. [Google Scholar]
- Analyse-it. Software for Statistical Analysis, Data Visualization and Modelling in Microsoft Excel. Available online: https://analyse-it.com/ (accessed on 14 January 2022).
- Hearty, J. Advanced Machine Learning with Python; Packt Publishing: Birmingham, UK, 2016. [Google Scholar]
- Tensor Flow. An End-to-End Open Source Machine Learning Platform. Available online: https://www.tensorflow.org/ (accessed on 10 January 2022).
- Keras. The Python Deep Learning API. Available online: https://keras.io/ (accessed on 12 January 2022).
- PyTorch. An Open Source Machine Learning Framework That Accelerates the Path from Research Prototyping to Production Deployment. Available online: https://pytorch.org/ (accessed on 9 January 2022).
- Python Home Page. Available online: https://www.python.org/ (accessed on 12 January 2022).
- Welcome to Colaboratory. Available online: https://colab.research.google.com/ (accessed on 14 January 2022).
- Kaggle. Visualizing KNN, SVM, and XGBoost on Iris Dataset. Available online: https://www.kaggle.com/mgabrielkerr/visualizing-knn-svm-and-xgboost-on-iris-dataset (accessed on 11 January 2022).
- PCA Using Python (Scikit-Learn)—Towards Data Science. Available online: https://towardsdatascience.com/pca-using-python-scikit-learn-e653f8989e60 (accessed on 14 January 2022).
- How to Handle Imbalanced Classes in Machine Learning. Available online: https://elitedatascience.com/imbalanced-classes (accessed on 12 January 2022).
- Portavella, M.; Vargas, J.P.; Torres, B.; Salas, C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res. Bull. 2002, 57, 397–399. [Google Scholar] [CrossRef]
- Evans, D.H.; Claiborne, J.B.; Currie, S. The Physiology of Fishes, 4th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 238–247, 291–306, 334. [Google Scholar]
- Johnsson, J.I.; Björnsson, B.T. Growth hormone increases growth rate, appetite and dominance in juvenile rainbow trout, Oncorhynchus mykiss. Anim. Behav. 1994, 48, 177–186. [Google Scholar] [CrossRef]
- Jönsson, E.; Johnsson, J.I.; Björnsson, B.T. Growth hormone increases aggressive behavior in juvenile rainbow trout. Horm. Behav. 1998, 33, 9–15. [Google Scholar] [CrossRef]
- Portavella, M.; Torres, B.; Salas, C. Avoidance response in goldfish: Emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 2004, 24, 2335–2342. [Google Scholar] [CrossRef]
- El-Sawi, N.M.; Gashlan, H.M.; Younes, S.H.; Al-Massabi, R.F.; Shaker, S. Biochemical and histological studies on the effect of the Patulin mycotoxin on male rats’ liver and treatment by crude venom extracted from jelly fish. Life Sci. J. 2012, 9, 1143–1153. [Google Scholar]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863. [Google Scholar] [CrossRef]
- Gashlan, H.M. Biochemical studies of patulin on liver functions in male albino mice. J. Appl. Anim. Res. 2008, 34, 93–96. [Google Scholar] [CrossRef][Green Version]
- Al-Hazmi, M.A. Patulin in apple juice and its risk assessments on albino mice. Toxicol. Ind. Health 2014, 30, 534–545. [Google Scholar] [CrossRef]
- Escoula, L.; More, J.; Baradat, C.; Henry, G.; Brunel-Dubech, N. The toxins of Byssochlamys n1vea westling. i. acute toxicity of patulin in adult rats and mice. In Annales de Recherches Veterinaires; INRA Editions: Rabat, Morocco, 1977; Volume 8, pp. 41–49. [Google Scholar]
- Takizawa, T.; Mitsumori, K.; Tamura, T.; Nasu, M.; Ueda, M.; Imai, T.; Hirose, M. Hepatocellular tumor induction in heterozygous p53-deficient CBA mice by a 26-week dietary administration of kojic acid. Toxicol. Sci. 2003, 73, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mori, T.; Okamura, M.; Kashida, Y.; Mitsumori, K. Induction of hepatocellular proliferative lesions in CBA mice by a 26-week dietary administration of kojic acid. J. Toxicol. Pathol. 2005, 18, 159–165. [Google Scholar] [CrossRef][Green Version]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29 (Suppl. 6), 244S–273S. [Google Scholar] [CrossRef] [PubMed]
- Higa, Y.; Kawabe, M.; Nabae, K.; Toda, Y.; Kitamoto, S.; Hara, T.; Tanaka, N.; Kariya, K.; Takahashi, M. Kojic acid-absence of tumor-initiating activity in rat liver, and of carcinogenic and photo-genotoxic potential in mouse skin. J. Toxicol. Sci. 2007, 32, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Imai, T.; Onose, J.I.; Takami, S.; Cho, Y.M.; Hirose, M.; Nishikawa, A. A 55-week chronic toxicity study of dietary administered kojic acid (KA) in male F344 rats. J. Toxicol. Sci. 2009, 34, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Boussabbeh, M.; Salem, I.B.; Belguesmi, F.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S.; Bacha, H. Crocin protects the liver and kidney from patulin-induced apoptosis in vivo. Environ. Sci. Pollut. Res. 2016, 23, 9799–9808. [Google Scholar] [CrossRef]
- Wu, T.S.; Yang, J.J.; Yu, F.Y.; Liu, B.H. Evaluation of nephrotoxic effects of mycotoxins, citrinin and patulin, on zebrafish (Danio rerio) embryos. Food Chem. Toxicol. 2012, 50, 4398–4404. [Google Scholar] [CrossRef]
- Harwig, J.; Munro, I.C. Mycotoxins of possible importance in diseases of Canadian farm animals. Can. Vet. J. 1975, 16, 125. [Google Scholar]
- Stott, W.T.; Bullerman, L.B. Patulin: A mycotoxin of potential concern in foods. J. Milk Food Technol. 1975, 38, 695–705. [Google Scholar] [CrossRef]
- Bullerman, L.B. Significance of mycotoxins to food safety and human health. J. Food Prot. 1979, 42, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Kinosita, R.; Ishiko, T.; Sugiyama, S.; Seto, T.; Igarasi, S.; Goetz, I.E. Mycotoxins in fermented food. Cancer Res. 1968, 28, 2296–2311. [Google Scholar] [PubMed]
- Srisayam, M.; Weerapreeyakul, N.; Barusrux, S.; Kanokmedhakul, K. Antioxidant, antimelanogenic, and skin-protective effect of sesamol. J. Cosmet. Sci. 2014, 65, 69–79. [Google Scholar] [PubMed]
- Montazeri, M.; Emami, S.; Asgarian-Omran, H.; Azizi, S.; Sharif, M.; Sarvi, S.; Rezaei, F.; Sadeghi, M.; Gohardehi, S.; Daryani, A. In vitro and in vivo evaluation of kojic acid against Toxoplasma gondii in experimental models of acute toxoplasmosis. Exp. Parasitol. 2019, 200, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Boussabbeh, M.; Ben Salem, I.; Neffati, F.; Najjar, M.F.; Bacha, H.; Abid-Essefi, S. Crocin Prevents Patulin-Induced Acute Toxicity in Cardiac Tissues via the Regulation of Oxidative Damage and Apoptosis. J. Biochem. Mol. Toxicol. 2015, 29, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Bakos, K.; Kovács, R.; Staszny, Á.; Sipos, D.K.; Urbányi, B.; Müller, F.; Csenki, Z.; Kovács, B. Developmental toxicity and estrogenic potency of zearalenone in zebrafish (Danio rerio). Aquat. Toxicol. 2013, 136, 13–21. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, Y.; Yuan, X.; Zhang, T.; Zhao, J.; Huang, L.; Peng, S. T-2 toxin induces developmental toxicity and apoptosis in zebrafish embryos. J. Environ. Sci. 2014, 26, 917–925. [Google Scholar] [CrossRef]
- Veselinović, J.B.; Veselinović, A.M.; Ilic-Tomic, T.; Davis, R.; O’Connor, K.; Pavic, A.; Nikodinovic-Runic, J. Potent anti-melanogenic activity and favorable toxicity profile of selected 4-phenyl hydroxycoumarins in the zebrafish model and the computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 6286–6296. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Chaudhari, U.; Nemade, H.; Sureshkumar, P.; Vinken, M.; Ates, G.; Rogiers, V.; Hescheler, J.; Hengstler, J.G.; Sachinidis, A. Functional cardiotoxicity assessment of cosmetic compounds using human-induced pluripotent stem cell-derived cardiomyocytes. Arch. Toxicol. 2018, 92, 371–381. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).