Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections
Abstract
:1. Introduction
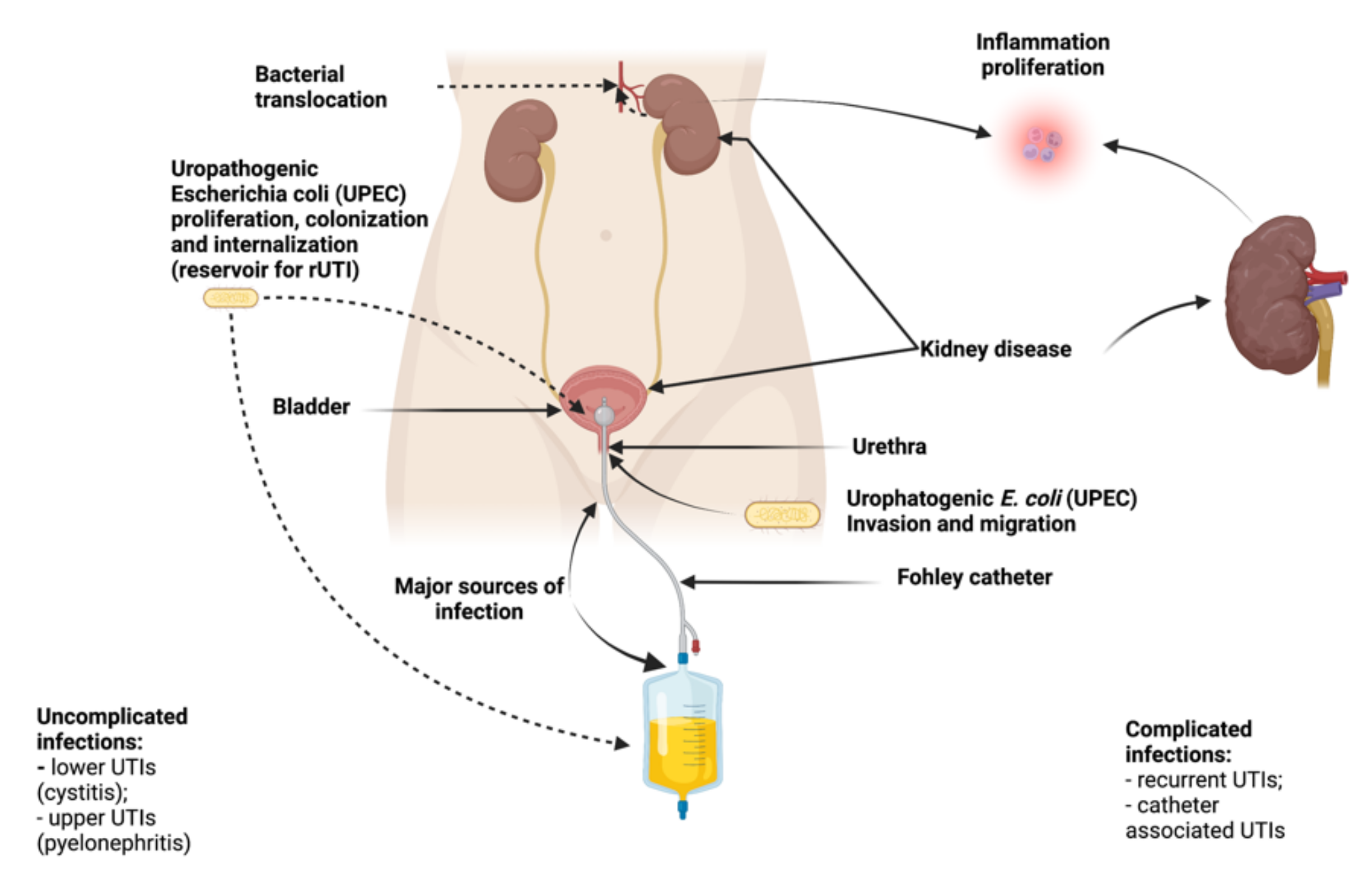
2. Pathogenesis of rUTI
3. Classification of Causes in Cases of UTI
- Uncomplicated Infection: It can be defined as the infection of a relatively healthy urinary tract patient who responds to antibiotic treatment;
- Complicated Infection: It occurs mainly in people with an abnormal urinary tract, which are often obstructed by stones or bladder-ureter reflux;
- Isolated Infections: Represent the mother infection or the infection that appears at an interval of six months, without any connection between the two episodes;
- Unresolved Infection: It is the opposite of uncomplicated infection; it does not respond to antibiotic treatment;
- Reinfection/Recurrence: Reinfection is the last stage of the evolution of urinary tract infections, described as a persistent bacterial infection. At this stage, the individual is reinfected with the same pathogen two weeks after treating a urinary tract infection.
4. In Vitro Urinary Tract Models
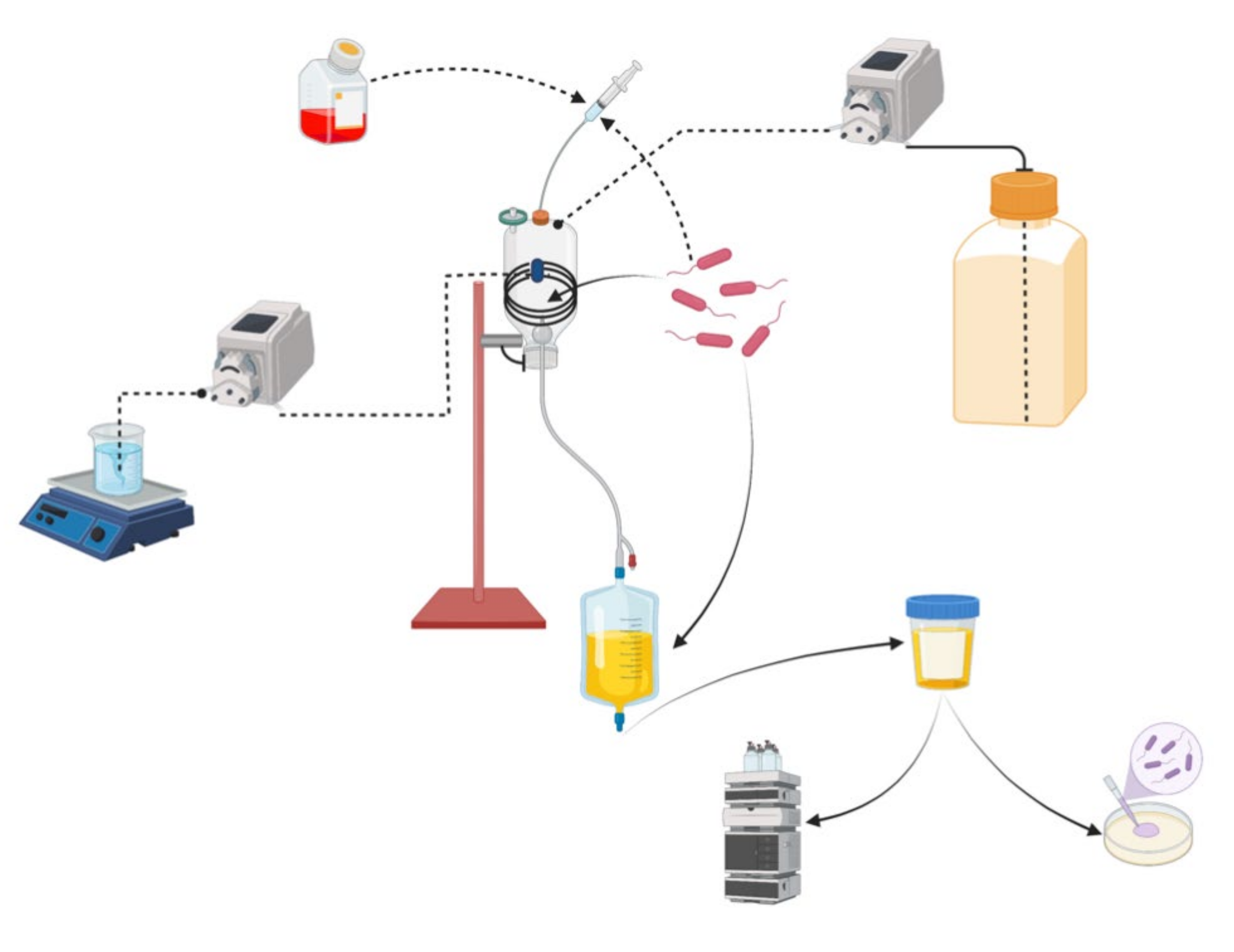
4.1. In Vitro Catheterization Model (IVCM)
- The peristaltic pump ensured the continuous supply of sterilized artificial urine at a rate of 1 L per day. The bladder temperature was maintained at 35–37 °C by a circulatory system that included a peristaltic pump and a ceramic heating plate;
- A urinary catheter was used to drain the bladder. The leftover urine contributed to the inflated balloon’s physiological stagnation in a catheterized bladder. A urine catheter was attached to a ‘drainage bag’-like effluent collection vessel;
- The bladder had rubber septa ports that facilitated the aseptic inoculation of bacteria of interest. The catheter tip was placed into the bladder and secured by inflating the balloon with 15 mL of sterile phosphate buffered saline (PBS). Urine flow was started to fill the bladder to the necessary capacity (80–100 mL) so that the balloon could be covered;
- After that, the flow of urine from the tank was turned off to allow bacteria to be inoculated into the septum port’s ‘bladder.’ To simulate the introduction of contaminating bacteria into the bladder during catheter insertion at the clinical site, approximately 5 × 107 CFU of E. coli were injected into the bladder. This would result in a contaminating bacteria concentration of 5 × 105 CFU/mL;
- To prevent bacteria from being removed immediately after inoculation, the urine flow was interrupted for one hour and then resumed [28].
4.2. In Vivo Vision of In Vitro Urinary Tract Models
5. Management of Recurrent UTI by Non-Antibiotic Plant-Based Prevention and Treatments
6. Plants Commonly Used in the Treatment of rUTI
6.1. Herbal Chinese Medicine
6.1.1. Arctostaphylos uva-ursi
6.1.2. Vaccinium macrocarpon—Cranberry
6.2. Promising Medicinal Plants to Treat rUTI
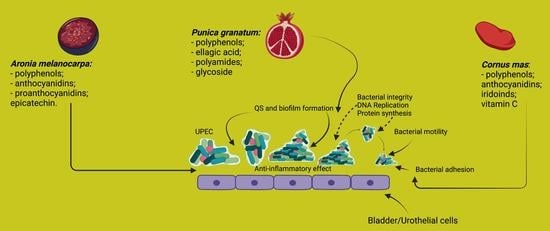
6.2.1. P. granatum L.—Pomegranate
6.2.2. A. melanocarpa (Michx.) Elliott—Black Chokeberry
6.2.3. C. mas L.—Cornelian Cherry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention, and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef]
- Murray, B.O.; Flores, C.; Williams, C.; Flusberg, D.A.; Marr, E.E.; Kwiatkowska, K.M.; Charest, J.L.; Isenberg, B.C.; Rohn, J.L. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front. Cell. Infect. Microbiol. 2021, 11, 691210. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Schreiber, H.L.; Hooton, T.M.; Hultgren, S.J. From Physiology to Pharmacy: Developments in the Pathogenesis and Treatment of Recurrent Urinary Tract Infections. Curr. Urol. Rep. 2013, 14, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, A.J.; Richards, A.C.; Mulvey, M.A. Invasion of Host Cells and Tissues by Uropathogenic Bacteria. Microbiol. Spectr. 2016, 4, 359–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzaz, B.S.F.; Fork, S.D.; Ahmadi, R.; Khameneh, B. Deep insights into urinary tract infections and effective natural remedies. Afr. J. Urol. 2021, 27, 6. [Google Scholar] [CrossRef]
- Gatea Kaabi, S.A.; Abdulrazaq, R.A.; Rasool, K.H.; Khassaf, S.A. Western herbal remedies for Urinary Tract infections. Arch. Urol. Res. 2020, 4, 49–60. [Google Scholar] [CrossRef]
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.T.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59. [Google Scholar] [CrossRef] [Green Version]
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of Catheter-Associated Urinary tract infections and In Vitro Urinary Tract Models. J. Healthc. Eng. 2018, 2018, 2986742. [Google Scholar] [CrossRef] [Green Version]
- Tamadonfar, K.O.; Omattage, S.N.; Spauldine, C.N.; Hultgren, S.J.O.N. Reaching the End of the Line: Urinary Tract Infections. In C. R. Pascale Cossart, Bacteria and Intracellularity. Microbiol. Spectr. 2019, 7, 83–99. [Google Scholar] [CrossRef]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Rev. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef]
- Storme, O.; Tirán Saucedo, J.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk factors and predisposing conditions for urinary tract infection. Therap. Adv. Urol. 2019, 11, 1756287218814382. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Malerba, G.; Cataldi, L.; Antoniazzi, F.; Franchini, M.; Monti, E.; Fanos, V. Genetic risk for recurrent urinary tract infections in humans: A systematic review. J. Biomed. Biotechnol. 2010, 2010, 321082. [Google Scholar] [CrossRef] [PubMed]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragnarsdóttir, B.; Lutay, N.; Grönberg-Hernandez, J.; Köves, B.; Svanborg, C. Genetics of innate immunity and UTI susceptibility. Nat. Rev. Urol. 2011, 8, 449–468. [Google Scholar] [CrossRef]
- Kawalec, A.; Zwolińska, D. Emerging Role of Microbiome in the Prevention of Urinary Tract Infections in Children. Int. J. Mol. Sci. 2022, 23, 870. [Google Scholar] [CrossRef]
- Yang, S.B.F. Pathophysiology of UTIs. In Female Urinary Tract Infections in Clinical Practice; Yang, S.F.B., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–10. Available online: https://link.springer.com/book/10.1007/978-3-030-27909-7 (accessed on 24 February 2022).
- Davenport, M.; Mach, E.K.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol 2017, 14, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, M.G.; Ouellette, M. Preventing antibiotic resistance through rapid genotypic identification of bacteria and of their antibiotic resistance genes in the clinical microbiology laboratory. J. Clin. Microbiol. 1998, 36, 2169–2172. [Google Scholar] [CrossRef] [Green Version]
- Mitsakakis, K.; Kaman, W.E.; Elshout, G.; Specht, M.; Hays, J.P. Challenges in identifying antibiotic resistance targets for point-of-care diagnostics in general practice. Fut. Microbiol. 2018, 13, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Stork, C.; Kovács, B.; Rózsai, B.; Putze, J.; Kiel, M.; Dorn, A.; Kovács, J.; Melegh, S.; Leimbach, A.; Kovács, T.; et al. Characterization of Asymptomatic Bacteriuria Escherichia coli Isolates in Search of Alternative Strains for Efficient Bacterial Interference against Uropathogens. Front. Microbiol. 2018, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Köves, B.; Salvador, E.; Grönberg-Hernández, J.; Zdziarski, J.; Wullt, B.; Svanborg, C.; Dobrindt, U. Rare Emergence of Symptoms during Long-Term Asymptomatic Escherichia coli 83972 Carriage without an Altered Virulence Factor Repertoire. J. Urol. 2014, 19, 191. [Google Scholar] [CrossRef]
- Ceprnja, M.; Oros, D.; Melvan, E.; Svetlicic, E.; Skrlin, J.; Barisic, K.; Starcevic, L.; Zucko, J.; Starcevic, A. Modeling of Urinary Microbiota Associated With Cystitis. Front. Cell. Infect. Microbiol. 2021, 11, 140. [Google Scholar] [CrossRef]
- Georgopoulos, N.T.; Kirkwood, L.A.; Varley, C.L.; MacLaine, N.J.; Aziz, N.; Southgate, J. Immortalisation of normal human urothelial cells compromises differentiation capacity. Eur. Urol. 2011, 60, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.C.; Shabir, S.; Southgate, J. Biomimetic urothelial tissue models for the in vitro evaluation of barrier physiology and bladder drug efficacy. Mol. Pharm. 2014, 11, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Hatina, J.; Schulz, W.A. Stem cells in the biology of normal urothelium and urothelial carcinoma. Neoplasma 2012, 59, 728–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DyStar Colours Distribution GmbH. Mixtures of Disperse Dyes. U.S. Patent US8906116B2, 9 December 2014. [Google Scholar]
- Vamanu, E.; Dinu, L.D.; Luntraru, C.M.; Suciu, A. In Vitro Coliform Resistance to Bioactive Compounds in Urinary Infection, Assessed in a Lab Catheterization Model. Appl. Sci. 2021, 11, 4315. [Google Scholar] [CrossRef]
- Chua, R.Y.R.; Lim, K.; Leong, S.S.J.; Tambyah, P.A.; Ho, B. An in-vitro urinary catheterization model that approximates clinical conditions for evaluation of innovations to prevent catheter-associated urinary tract infections. J. Hosp. Infect. 2017, 97, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.A.; Lee, S.W. Implementation of a Permeable Membrane Insert-based Infection System to Study the Effects of Secreted Bacterial Toxins on Mammalian Host Cells. J. Vis. Exp. 2016, 114, e54406. [Google Scholar] [CrossRef] [Green Version]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [Green Version]
- Machowska, A.; Stålsby Lundborg, C. Drivers of Irrational Use of Antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- Foxman, B.; Buxton, M. Alternative approaches to conventional treatment of acute uncomplicated urinary tract infection in women. Curr. Infect. Dis. Rep. 2013, 15, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Trad. Complement. Alter. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadisa, E.; Tadesse, E. Antimicrobial activity of medicinal plants used for urinary tract infections in pastoralist community in Ethiopia. BMC Complement. Med. Ther. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, G.; Akram, M.; Jabeen, F.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Riaz, M.; Tahir, I.M.; Ghauri, A.O.; Sultana, S.; et al. Therapeutic potential of medicinal plants for the management of urinary tract infection: A systematic review. Clin. Exp. Pharmacol. Physiol. 2019, 46, 613–624. [Google Scholar] [CrossRef]
- Das, S. Natural therapeutics for urinary tract infections-a review. Future Pharm. J. Sci. 2020, 6, 64. [Google Scholar] [CrossRef]
- Peng, M.M.; Fang, Y.; Hu, W.; Huang, Q. The pharmacological activities of compound salviplebeia granules on treating urinary tract infection. J. Ethnopharmacol. 2010, 129, 59–63. [Google Scholar] [CrossRef]
- Cela-López, J.M.; Camacho Roldán, C.J.; Gómez-Lizarraga, G.; Martínez, V. A Natural Alternative Treatment for Urinary Tract Infections: Itxasol©, the Importance of the Formulation. Molecules 2021, 26, 4564. [Google Scholar] [CrossRef]
- Sarecka-Hujar, B.; Szulc-Musioł, B. Herbal Medicines—Are They Effective and Safe during Pregnancy? Pharmaceutics 2022, 14, 171. [Google Scholar] [CrossRef]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med. 2012, 78, 630–640. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.B.; Foxman, B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002, 287, 3082–3083. [Google Scholar] [CrossRef] [PubMed]
- McMurdo, M.E.; Argo, I.; Phillips, G.; Daly, F.; Davey, P. Cranberry or trimethoprim for the prevention of recurrent urinary tract infections? A randomized controlled trial in older women. J. Antimicrob. Chemother. 2009, 63, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G.; et al. Analysis of the Phytochemical Composition of Pomegranate Fruit Juices, Peels and Kernels: A Comparative Study on Four Cultivars Grown in Southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; D’Souza, D.H. The pomegranate: Effects on bacteria and viruses that influence human health. Evid.-Based Complementary Altern. Med. eCAM 2013, 2013, 606212. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate juice as a functional food: A comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020, 11, 5768–5781. Available online: https://pubs.rsc.org/en/content/articlelanding/2020/fo/d0fo01251c (accessed on 24 February 2022). [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef]
- Das, S.; Panigrahi, S.; Panda, P. Antiurobacterial activity of Punica granatum L. seed extract. Eur. J. Med. Plants 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Abdel-Salam, F.F.; El_deen Moharram, Y.G.; El-Zalaki, E.M. Characterization of Wastes from Pomegranate (Punica granatum L.) Juice and Its Use as a Functional Drink. Egypt J. Food Sci. 2018, 46, 91–100. Available online: https://ejfs.journals.ekb.eg/article_46941.html (accessed on 24 February 2022).
- Zam, W.; Khaddour, A. Anti-virulence effects of aqueous pomegranate peel extract on E. coli urinary tract infection. Progr. Nutr. 2017, 19, 98–104. [Google Scholar] [CrossRef]
- AlFadel, F.; Allaham, S.A.; Alkhatib, R. The Anti-Bacterial Activity of Various Parts of Punica granatum on Antibiotics Resistance Escherichia coli. Int. J. Pharmacog. Phytochem. Res. 2014, 6, 79–85. Available online: https://brief.land/jjm/articles/56335.html (accessed on 24 February 2022).
- Dey, D.; Debnath, S.; Hazra, S.; Ghosh, S.; Ray, R.; Hazra, B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) producing Gram-negative bacilli. Food Chem. Toxicol. 2012, 50, 4302–4309. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of Bio-Pharmaceutical and Antimicrobial Properties of Pomegranate (Punica granatum L.) Leathery Exocarp Extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Duman, A.D.; Ozgen, M.; Dayisoylu, K.S.; Erbil, N.; Durgac, C. Antimicrobial Activity of Six Pomegranate (Punica granatum L.) Varieties and Their Relation to Some of Their Pomological and Phytonutrient Characteristics. Molecules 2009, 14, 1808–1817. [Google Scholar] [CrossRef] [Green Version]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Efficacy and safety of pomegranate medicinal products for cancer. eCAM 2015, 2015, 258598. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food. 2010, 13, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Valcheva-Kuzmanova, S.V.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2006, 48, 11–17. [Google Scholar]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://handle.nal.usda.gov/10113/43336 (accessed on 17 February 2022).
- Deng, H.; Zhu, J.; Tong, Y.; Kong, Y.; Tan, C.; Wang, M.; Wan, M.; Meng, X. Antibacterial characteristics and mechanisms of action of Aronia melanocarpa anthocyanins against Escherichia coli. LWT 2021, 150, 112018. [Google Scholar] [CrossRef]
- Bräunlich, M.; Økstad, O.A.; Slimestad, R.; Wangensteen, H.; Malterud, K.E.; Barsett, H. Effects of Aronia melanocarpa constituents on biofilm formation of Escherichia coli and Bacillus Cereus. Molecules 2013, 18, 14989–14999. [Google Scholar] [CrossRef] [Green Version]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef]
- Liepiņa, I.; Nicolajeva, V. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. Available online: https://americanaronia.org/antimicrobial-activity-extracts-fruits-aronia-melanocarpa-sorbus-aucuparia/ (accessed on 24 February 2022).
- Cvetanovi’c, A.; Zengin, G.; Zekovi’c, Z.; Švarc-Gaji’c, J.; Raži´c, S.; Damjanovi´c, A.; Maškovi´c, P.; Miti´c, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Dorneanu, R.; Cioanc, O.; Chifiriuc, O.; Albu, E.; Tuchilu, C.G.; Mircea, C.; Salamon, I.; Hancianu, M. Synergic benefits of Aronia melanocarpa anthocyanin–rich extracts and antibiotics used for urinary tract infections. Farmacia 2017, 65, 778–783. Available online: https://farmaciajournal.com/issue-articles/synergic-benefits-of-aronia-melanocarpa-anthocyanin-rich-extracts-and-antibiotics-used-for-urinary-tract-infections/ (accessed on 24 February 2022).
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handeland, M.; Grude, N.; Torp, T.; Slimestad, R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term--a pilot study. Nutr. Res. 2014, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)–characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [CrossRef]
- Bayram, H.M.; Ozturkcan, S.A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Dinda, S.; Kyriakopoulos, M.A.; Markopoulos, C.; Thomaidis, S.N.; Velegraki, A.; Zoumpourlis, V. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Dulger, B.; Gonuz, A. Antimicrobial activity of some Turkish medicinal plants. Pak. J. Biol. Sci. 2004, 7, 1559–1562. [Google Scholar] [CrossRef] [Green Version]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The Effects of Natural Iridoids and Anthocyanins on Selected Parameters of Liver and Cardiovascular System Functions. Oxid. Cell Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Dadkhah, N.; Shirani, M.; Etemadifar, S.; Mirtalebi, M. The effect of Cornus mas in preventing recurrent urinary tract infections in women’. Adv. Herb. Med. 2017, 3, 67–76. Available online: https://naldc.nal.usda.gov/catalog/43336 (accessed on 24 February 2022).
- Pugliese, D.; Acampora, A.; Porreca, A.; Schips, L.; Cindolo, L. Effectiveness of a novel oral combination of D-Mannose, pomegranate extract, prebiotics and probiotics in the treatment of acute cystitis in women. Arch. Ital. Urol. Androl. 2020, 92, 34–38. [Google Scholar] [CrossRef] [Green Version]

| Plant Name | Extract/Part Used | Bioactive Compounds | Name of Microorganisms Tested (E. coli or Other Uropathogens) | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Pomegranate (P. granatum L.) | aril extracts from six pomegranate varieties grown in the Mediterranean region of Turkey | polyphenols | E. coli DM | - The most acidic cultivar with the second highest phenolic content from all varities tested had the greatest inhibitory effect on E. coli. | [40] |
| leathery exocarp extract | E. coli Migula | - The higher concentration of the aqueous extract (10 mg/mL) showed a significant bactericidal effect against E. coli, equal to kanamycin, whereas the lower concentration of the extract (5 mg/mL) showed a higher bactericidal activity against E. coli than tetracycline. | [39] | ||
| peel extract | ellagic acid | E. coli | - Ellagic acid was able to inhibit biofilm formation by E. coli. | [41] | |
| fruit pericarp extract tested in combination with ciprofloxacint | polyphenols | extended-spectrum β-lactamase (ESBL)-producing E. coli, which were screened for their resistance profile against fluoroquinolone antibiotics | - This study demonstrated a synergy of a ciprofloxacin–methanolic extract combination against ESBL Gram-negative bacilli. | [38] | |
| extracts from different parts (pericarp, leaves, flowers, and seeds) | E. coli type (1), which affected calves and had shown antibiotic resistance | - The alcoholic extracts revealed different antibacterial activities against E. coli type (1). - Pericarp etanol extract had the best antibacterial activity. - The water and ether petroleum extracts had no antibacterial effectiveness. | [37] | ||
| peel extract | anthocyanins and ellangitannins | E. coli collected from urinary cultures | - The inhibitory activity was found to be dose- and pH- dependent, with a minimum inhibitory concentration (MIC) value of 0.6 mg/mL at the pH of the aqueous extract (3.5). - The assay of adhesion carried out at the MIC showed a reduction of up to 80% of the adhesion index accompanied with reductions in motility and polyamide production. | [36] | |
| seed extract | tannins, steroids, terpenes, coumarins, flavonoids, andglycosides | uropathogens E. coli, Enterococcus faecalis, S. aureus, and K. pneumonia | - This study showed urobactericidal activity against different strains that were clinically isolated from patients suffering from urinary tract infections. | [34] | |
| Black chockeberry (A. melanocarpa Michx.) | ripe fruit extracts | - anthocyanins (cyanidin 3-galactoside, cyanidin 3- glucoside, cyanidin 3-arabinoside, and cyanidin 3- xyloside) - Epicatechin and its dimers and trimers | - E. coli ATCC 13202, P. aeruginosa ATCC 27853, E.faecalis ATCC 29212, and S. aureus ATCC 29212 - 18 clinical isolates from patients with urinary tract infections (E. coli 2041, E. coli 1851, E. coli 1992, P. aeruginosa 1908, P. aeruginosa 1128, K. pneumoniae 2110, K. pneumoniae 1074, K. pneumoniae 831, Morganella morganii 2520, Acinetobacter baumanii 1908, A. baumanii 2329, Enterobacter cloacae 2951, E. faecalis 2823, E. faecium 2862, E. faecium 2980, E. faecium 2027, S. aureus 14, and S. aureus 17 | - This extract was the most active against one strain of E. coli and one of M. morganii at MIC (5 mg/mL). - It inhibited the development of biofilm in the case of an E. coli and a M. morganii. - Antibiotic disks (amikacin, tetracycline, nitrofurantoin, imipenem, and norfloxacin) supplemented with the extract slowly increased the growth inhibition zone in the cases of P. aeruginosa, E. coli, and M. morganii. | [50] |
| plant extract | anthocyanins | E. coli LWT | - This extract compromised the integrity of the bacterial cell wall and membrane, bound directly to the bacterial DNA, and interfered with protein homeostasis. | [44] | |
| crude extracts, subfractions, and compounds from aronia | epicathechin | E. coli K12 JM109, and uropathogenic E. coli CFT073 (ATCC 700928) | - These extracts inhibited bacterial growth of E. coli in vitro. - They possessed an anti-biofilm formation effect. | [45] | |
| aqueous and ethanolic extracts from fresh, dried, and frozen fruits. | E. coli MSCL 332 | - No activity against E. coli was reported. | [48] | ||
| leaves, berries, and stem extracts | E. coli | - E. coli was one of the most resistant strains. | [49] | ||
| leaf extract | E. coli, | - E. coli showed the greatest resistance and was reduced by 23% with 20 μL of aqueous ethanol extract. | [47] | ||
| leaf extract | flavonoids: quercetin derivatives, isorhamnetin derivatives, kaempferol-3-O-rutinoside, and hydroxytyrosol | E. coli | - No clear relationship was found between the influence of the plant extracts and whether the tested bacteria belonged to the Gram-positive or Gram-negative groups. - The extract had the lowest antibacterial activity on E. coli. | [46] | |
| Cornelian cherry (C. mas L.) | leaf extract | iridoids, ellagic acid, and ellagi-tannins, | - The extracts acted as bacteriostatic agents. - C. mas showed higher antimicrobial activity than A. melanocarpa extract. - C. mas leaf extract reduced the growth of all Gram-negative bacteria tested at the lowest concentration (1%). - The authors concluded that some interactions may occur between different bioactive compounds, explaining the stronger effect on Gram-negative bacteria in comparison to Gram-positive bacteria. | [46] | |
| bark extract | E. coli | - The extract did not inhibit the growth of E. coli. | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tache, A.M.; Dinu, L.D.; Vamanu, E. Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Appl. Sci. 2022, 12, 2635. https://doi.org/10.3390/app12052635
Tache AM, Dinu LD, Vamanu E. Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Applied Sciences. 2022; 12(5):2635. https://doi.org/10.3390/app12052635
Chicago/Turabian StyleTache, Adriana Mirela, Laura Dorina Dinu, and Emanuel Vamanu. 2022. "Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections" Applied Sciences 12, no. 5: 2635. https://doi.org/10.3390/app12052635
APA StyleTache, A. M., Dinu, L. D., & Vamanu, E. (2022). Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Applied Sciences, 12(5), 2635. https://doi.org/10.3390/app12052635