A Comparative Study of Different Sorbents in the Context of Direct Air Capture (DAC): Evaluation of Key Performance Indicators and Comparisons
Abstract
:1. Introduction
2. Mathematical Modelling
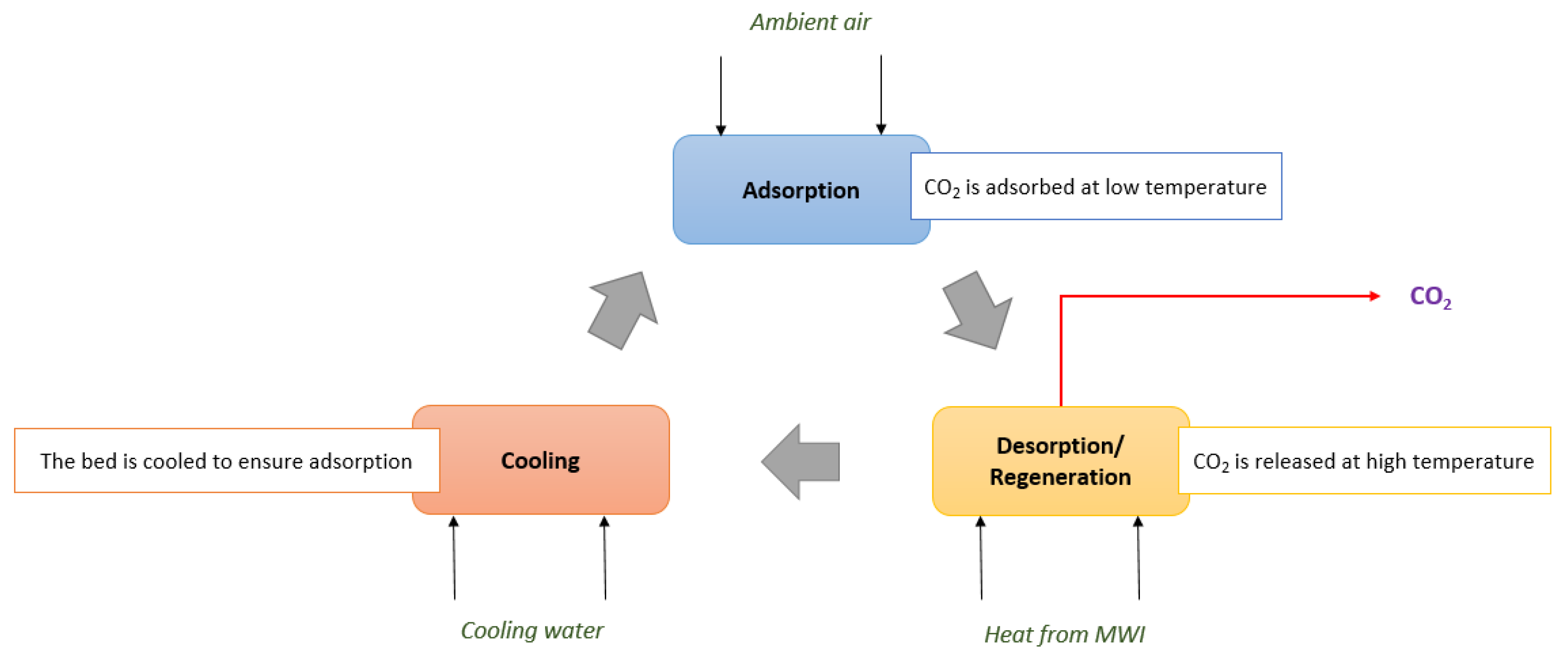
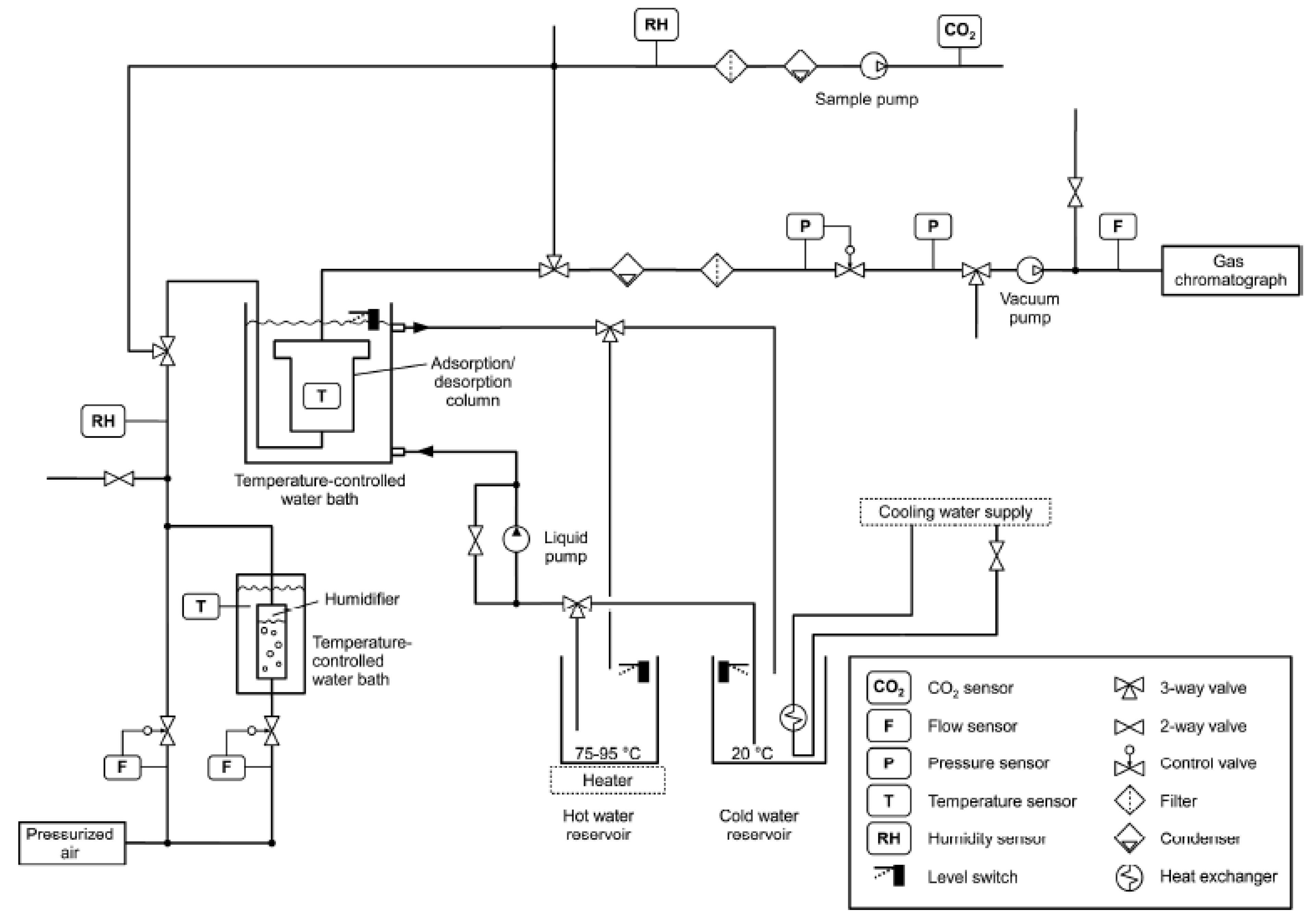
2.1. Sorbents and Adsorbent Bed
2.2. Model Equations
2.3. Energy Consumption
2.4. Economic Analysis
3. Results and Discussion
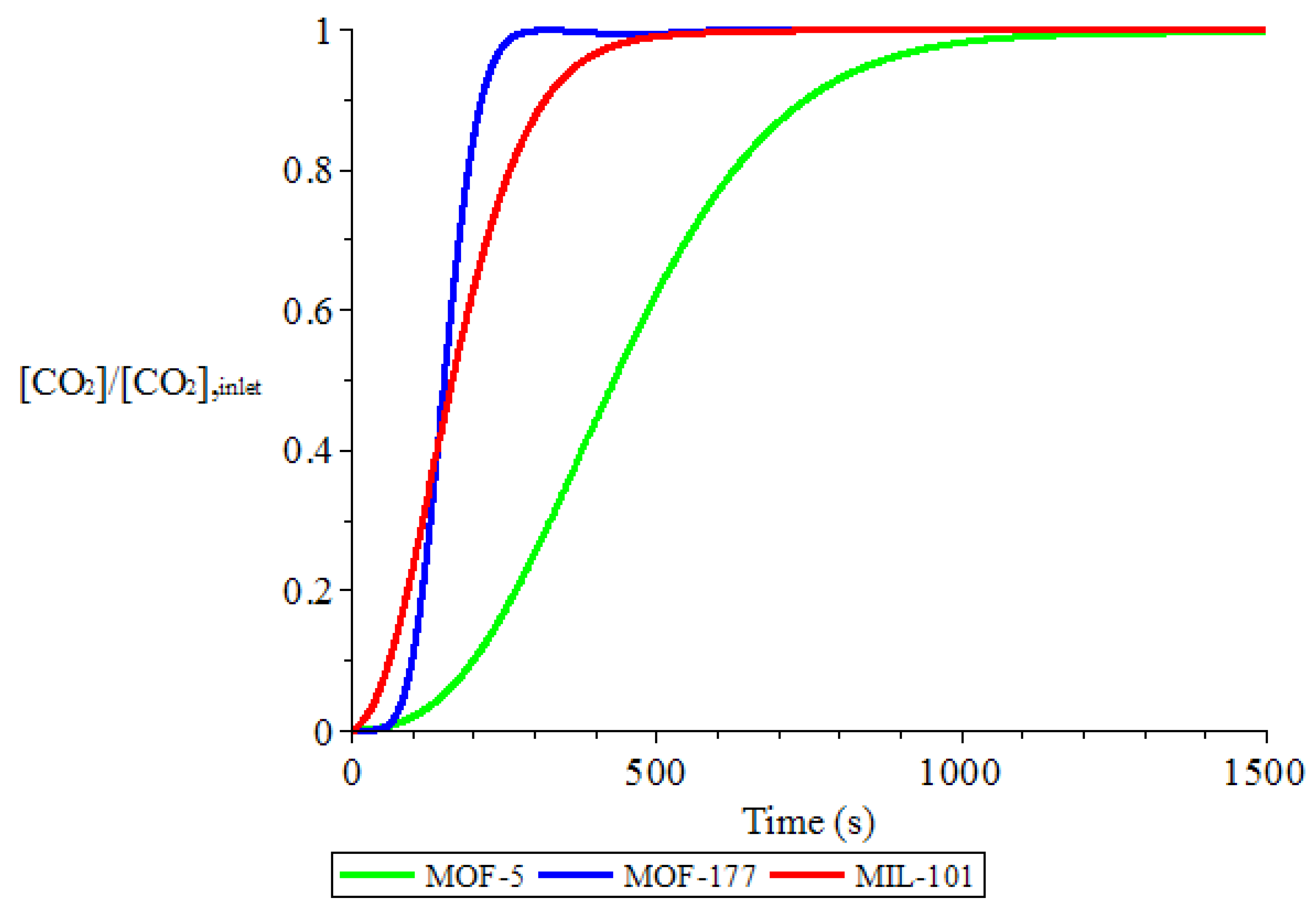
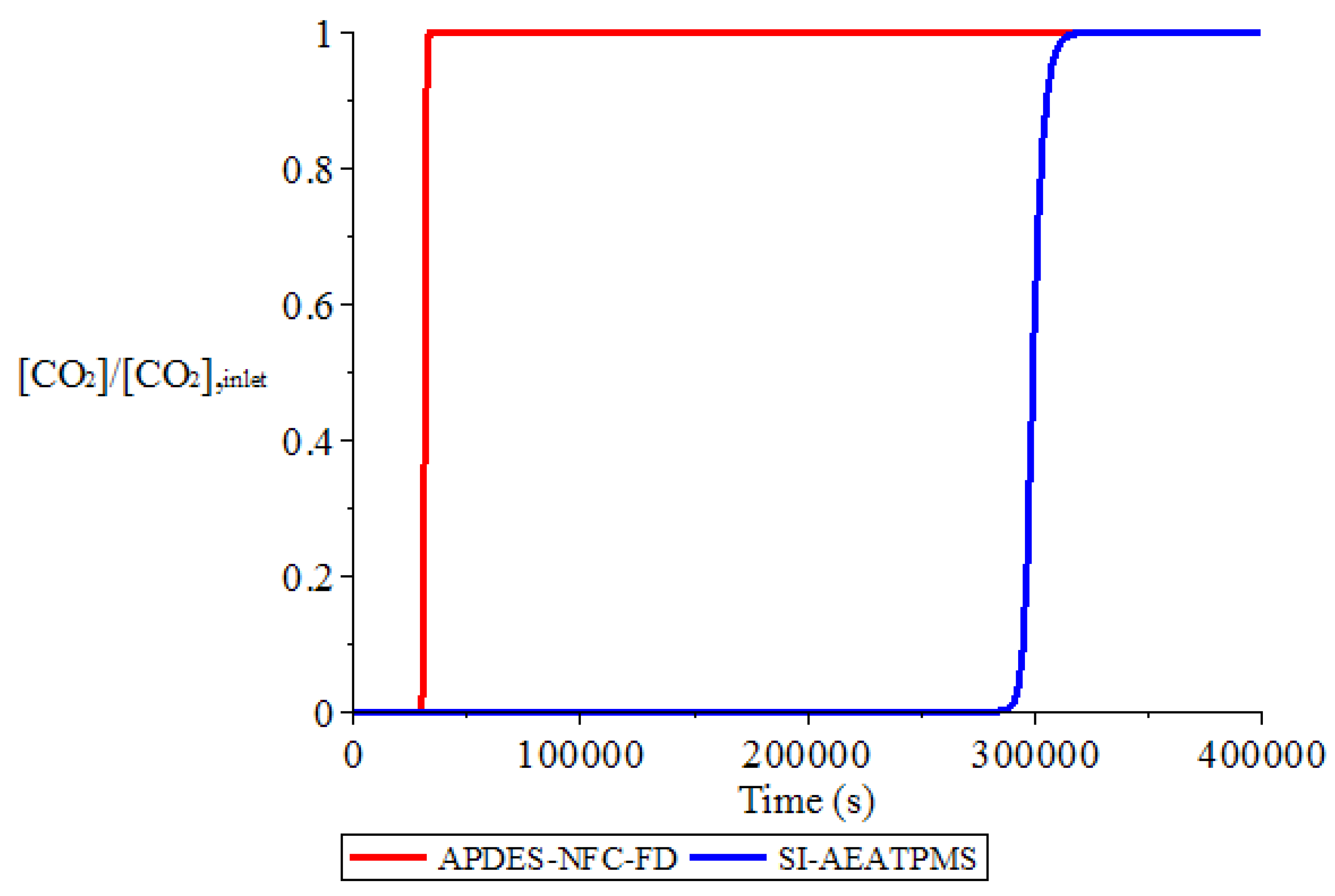
3.1. Adsorption and Desorption Curves of DAC Systems
3.2. KPI: CO2 Productivity of DAC Systems
3.3. KPIs: Energy Consumption and Cost of DAC Systems
3.4. Comparison with Climeworks Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. 2020. Available online: https://www.iea.org/data-and-statistics/?country=WORLD&fuel=CO2%20emissions&indicator=TotCO2 (accessed on 1 November 2020).
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Wijesiri, R.P.; Knowles, G.P.; Yeasmin, H.; Hoadley, A.F.A.; Chaffee, A.L. Technoeconomic Evaluation of a Process Capturing CO2 Directly from Air. Processes 2019, 7, 503. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Pérez, E.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, T.; Yang, F.; Wang, R. Design of steam-assisted temperature vacuum-swing adsorption processes for efficient CO2 capture from ambient air. Renew. Sustain. Energy Rev. 2020, 137, 110651. [Google Scholar] [CrossRef]
- Lackner, K.S.; Ziock, H.J.; Grimes, P. Carbon Dioxide Extraction from Air: Is It an Option? Los Alamos National Lab.: Los Alamos, NM, USA, 1999. [Google Scholar]
- Fuss, S.; Lamb, W.F.; Callaghan, M.W.; Hilaire, J.; Creutzig, F.; Amann, T.; Beringer, T.; de Oliveira Garcia, W.; Hartmann, J.; Khanna, T.; et al. Negative emissions—Part 2: Costs, potentials and side effects, Environ. Res. Lett. 2018, 13, 063002. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Sen, R.; Goeppert, A.; Prakash, G.K.S. Integrative CO2 Capture and Hydrogenation to Methanol with Reusable Catalyst and Amine: Toward a Carbon Neutral Methanol Economy. J. Am. Chem. Soc. 2018, 140, 1580–1583. [Google Scholar] [CrossRef]
- Bos, M.J.; Kersten, S.R.A.; Brilman, D.W.F. Wind power to methanol: Renewable methanol production using electricity, electrolysis of water and CO2 air capture. Appl. Energy 2020, 264, 114672. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Sabatino, F.; Mehta, M.; Grimm, A.; Gazzani, M.; Gallucci, F.; Kramer, G.J.; Annaland, M.V.S. Evaluation of a Direct Air Capture Process Combining Wet Scrubbing and Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2020, 59, 7007–7020. [Google Scholar] [CrossRef]
- Eisaman, M.D.; Schwartz, D.E.; Amic, S.; Larner, D.L.; Zesch, J.; Torres, F.; Littau, K. Energy-Efficient Electrochemical CO2 Capture from the Atmosphere. In Proceedings of the 2009 Clean Technology Conference and Trade Show, Houston, TX, USA, 3−7 May 2009. [Google Scholar]
- Kelemen, P.B.; McQueen NWilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- von Hippel, T. Thermal Removal of Carbon Dioxide from the Atmosphere: Energy Requirements and Scaling Issues. Clim. Change 2018, 148, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Adamu, A.; Abegão, F.R.; Boodhoo, K. Process intensification technologies for CO2 capture and conversion—A review. BMC Chem. Eng. 2020, 2, 2. [Google Scholar] [CrossRef]
- Realmonte, G. Direct Air Capture and Negative Emission Technologies in Deep Mitigation Pathways. Master’s Thesis, Politecnico of Milano, Milan, Italy, 2017. [Google Scholar]
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Appl. Energy 2018, 230, 1093–1107. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Capturing CO2 from ambient air using a polyethyleneimine–silica adsorbent in fluidized beds. Chem. Eng. Sci. 2014, 116, 306–316. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Chen, Z.; Guillerm, V.; Cairns, A.; Adil, K.; Eddaoudi, M. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat. Commun. 2014, 5, 4228. [Google Scholar] [CrossRef] [Green Version]
- Bahamon, D.; Díaz-Márquez Pablo Gamallo, A.; Vega, L.F. Energetic evaluation of swing adsorption processes for CO2 capture in selected MOFs and zeolites: Effect of impurities. Chem. Eng. J. 2018, 342, 458–473. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, Y.; Han, S. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. J. Environ. Chem. Eng. 2021, 9, 105523. [Google Scholar] [CrossRef]
- Demir, H.; Keskin, S. Hypothetical yet Effective: Computational Identification of High-performing MOFs for CO2 Capture. Comput. Chem. Eng. 2022, 55, 101811. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; Al-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, S.-J.; Han, S. CO2 capture using amine-functionalized bimetallic MIL-101 MOFs and their stability on exposure to humid air and acid gases. Microporous Mesoporous Mater. 2018, 277, 253–260. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Qasem, N.A.A.; Antar, M.A. Carbon dioxide adsorption separation from dry and humid CO2/N2 mixture. Comput. Chem. Eng. 2018, 117, 221–235. [Google Scholar] [CrossRef]
- NovoMOF. 2021. Available online: https://blog.novomof.com/blog/gas-separation-zeolites-vs-mofs (accessed on 1 March 2021).
- Yu, J.; Chuang, S.S.C. The Role of Water in CO2 Capture by Amine. Ind. Eng. Chem. Res. 2017, 56, 6337–6347. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Amine-bearing mesoporous silica for CO2 removal from dry and humid air. Chem. Eng. Sci. 2010, 65, 3695–3698. [Google Scholar] [CrossRef]
- Goeppert, A.; Zhang, H.; Czaun, M.; May, R.B.; Prakash, G.K.S.; Olah, G.A.; Narayanan, S.R. Easily Regenerable Solid Adsorbents Based on Polyamines for Carbon Dioxide Capture from the Air. ChemSusChem 2014, 7, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.T.; Sakwa-Novak, M.A.; Pang, S.; Sujan, A.R.; Ping, E.W.; Jones, C.W. Aminopolymer-Impregnated Hierarchical Silica Structures: Unexpected Equivalent CO2 Uptake under Simulated Air Capture and Flue Gas Capture Conditions. Chem. Mater. 2019, 31, 5229–5237. [Google Scholar] [CrossRef]
- Sarazen, M.L.; Sakwa-Novak, M.A.; Ping, E.W.; Jones, C.W. Effect of different acid initiators on branched poly(propyleneimine) synthesis and CO2 sorption performance. ACS Sustain. Chem. Eng. 2019, 7, 7338–7345. [Google Scholar] [CrossRef]
- Wagner, A.; Steen, B.; Johansson, G.; Zanghellini, E.; Jacobsson, P.; Johansson, P. Ran Carbon Dioxide Capture from Ambient Air Using Amine-Grafted Mesoporous Adsorbents. Int. J. Spectrosc. 2013, 2013, 690186. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.E.; Cho, K.M.; Lee, J.J.; Jones, C.W. Role of Alumina Basicity in CO2 Uptake in 3-Aminopropylsilyl-Grafted Alumina Adsorbents. ChemSusChem 2017, 10, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, K.A.S.; Deepthi, T.; Sadhana, R.A.; Benny, K.G. Functionalized Polysilsesquioxane-Based Hybrid Silica Solid Amine Sorbents for the Regenerative Removal of CO2 from Air. ACS Appl. Mater. Interfaces 2015, 7, 17969–17976. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Hwang, S.Y.; Ryu, D.W.; Lim, K.S.; Han, S.S.; Moon, D.; Choi, J.; Hong, C.S. Diamine-Functionalized Metal−organic Framework: Exceptionally High CO2 Capacities from Ambient Air and Flue Gas, Ultrafast CO2 Uptake Rate, and Adsorption Mechanism. Energy Environ. Sci. 2014, 7, 744–751. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Madden, D.G.; Lusi, M.; Chen, K.-J.; Daniels, E.A.; Curtin, T.; Perry, J.J.; Zaworotko, M.J. Direct Air Capture of CO2 by Physisorbent Materials. Angew. Chem. Int. Ed. 2015, 54, 14372–14377. [Google Scholar] [CrossRef]
- Choi, S.; Watanabe, T.; Bae, T.-H.; Sholl, D.S.; Jones, C.W. Modification of the Mg/DOBDC MOF with Amines to Enhance CO2 Adsorption from Ultradilute Gases. J. Phys. Chem. Lett. 2012, 3, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Pirngruber, G.D.; Hamon, L.; Bourrelly, S.; Llewellyn, P.L.; Lenoir, E.; Guillerm, V.; Serre, C.; Devic, T. A Method for Screening the Potential of MOFs as CO2 Adsorbents in Pressure Swing Adsorption Processes. ChemSusChem 2012, 5, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Meth, S.; Goeppert, A.; Prakash, G.K.S.; Olah, G.A. Silica Nanoparticles as Supports for Regenerable CO2 Sorbents. Energy Fuels 2012, 26, 3082–3090. [Google Scholar] [CrossRef]
- Brilman, D.W.F.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents. Energy Procedia 2013, 37, 6070–6078. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.H.; Jue, M.L.; Leisen, J.; Jones, C.W.; Lively, R.P. PIM-1 as a solutionprocessable “molecular basket” for CO2 capture from dilute sources. ACS Macro Lett. 2015, 4, 1415–1419. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Khunsupat, R.; Chen, T.T.; Jones, C.W. Poly(allylamine)–Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 2011, 50, 14203–14210. [Google Scholar] [CrossRef]
- Gebald, C.; Wurzbacher, J.A.; Borgschulte, A.; Zimmermann, T.; Steinfeld, A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48, 2497–2504. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Chen, X.-W.; Liu, S.-Y.; Li, X.-Y.; Xu, Y.-T.; Tang, M.; Rui, Z.; Ji, H.; Zhang, J.-P.; Chen, X.-M. Putting an ultrahigh concentration of amine groups into a metal–organic framework for CO2 capture at low pressures. Chem. Sci. 2016, 7, 6528–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, Y.C.; Yang, L.; Jovanovic, Z.R. The Development and Validation of a Closed-Loop Experimental Setup for Investigating CO2 and H2O Coadsorption Kinetics under Conditions Relevant to Direct Air Capture. Ind. Eng. Chem. Res. 2018, 57, 13987–13998. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef]
- Shekhah, O.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Cairns, A.J.; Eddaoudi, M. A facile solvent-free synthesis route for the assembly of a highly CO2 selective and H2S tolerant NiSIFSIX metal–organic framework. Chem. Commun. 2015, 51, 13595–13598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Vakili, R.; Kalumpasut, P.; Gorgojo, P.; Siperstein, F.R.; Fan, X.; McCloskey, P. Velocity variation effect in fixed bed columns: A case study of CO2 capture using porous solid adsorbents. AIChE J. 2018, 64, 2189–2197. [Google Scholar] [CrossRef] [Green Version]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-economic assessment of CO2 direct air capture plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]
- Viebahn, P.; Scholz, A.; Zelt, O. The Potential Role of Direct Air Capture in the German Energy Research Program—Results of a Multi-Dimensional Analysis. Energies 2019, 12, 3443. [Google Scholar] [CrossRef] [Green Version]
- Kintisch, E. Can Sucking CO2 Out of the Atmosphere Really Work? 2019. Available online: https://www.technologyreview.com/s/531346/can-sucking-co2-out-of-the-atmosphere-really-work/ (accessed on 25 July 2019).
- Sabatino, F.; Grimm, A.; Gallucci, F.; van Sint Annaland, M.; Kramer, G.J.; Gazzani, M. A comparative energy and costs assessment and optimization for direct air capture technologies. Joule 2021, in press. [Google Scholar]
- Schellevis, H.; van Schagen, T.; Brilman, D. Process optimization of a fixed bed reactor system for direct air capture. Int. J. Greenh. Gas Control 2021, 110, 103431. [Google Scholar] [CrossRef]
- Miao, Y.; He, Z.; Zhu, X.; Izikowitz, D.; Li, J. Operating temperatures affect direct air capture of CO2 in polyamine-loaded mesoporous silica. Chem. Eng. J. 2021, 426, 131875. [Google Scholar] [CrossRef]
- van Schagen, T.; van der Wal, P.; Brilman, D. Development of a novel, through-flow microwave-based regenerator for sorbent-based direct air capture. Chem. Eng. J. Adv. 2021, 9, 100187. [Google Scholar] [CrossRef]
- Drechsler, C.; Agar, D.W. Simulation and optimization of a novel moving belt adsorber concept for the direct air capture of carbon dioxide. Comput. Chem. Eng. 2019, 126, 520–534. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, L.; Zhao, L.; Deng, S.; Li, S.; Zhang, Y.; Li, H. Thermodynamic exploration of temperature vacuum swing adsorption for direct air capture of carbon dioxide in buildings. Energy Convers. Manag. 2019, 183, 418–426. [Google Scholar] [CrossRef]
- Qasem, N.; Ben-Mansour, R.; Habib, M.A. An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Appl. Energy 2018, 210, 317–326. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Aghamiri, S.F.; Talaie, M.R.; Tangestaninejad, S. A comparative investigation of CO2 adsorption on powder and pellet forms of MIL-101. J. Taiwan Inst. Chem. Eng. 2017, 72, 45–52. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, J. Adsorption and Diffusion of Carbon Dioxide on Metal−Organic Framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Poirier, E.; Chahine, R.; Bénard, P.; Lafi, L.; Dorval-Douville, G.; Chandonia, P.A. Hydrogen Adsorption Measurements and Modeling on Metal-Organic Frameworks and Single-Walled Carbon Nanotubes. Langmuir 2006, 22, 8784–8789. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.P.; Al-Betar, A.R.; Al-Mousa, A.H. Conductivity Enhancement of Mofs via Development of Mofpolymer Composite Material. U.S. Patent 20200291045A1, 17 September 2020. [Google Scholar]
- Zacharia, R.; Fernando Gomez, L.; Chahine, R.; Cossement, D.; Benard, P. Thermodynamics and kinetics of CH4/CO2 binary mixture separation by metal-organic frameworks from isotope exchange and adsorption breakthrough. Microporous Mesoporous Mater. 2018, 263, 165–172. [Google Scholar] [CrossRef]
- Viditha, V.; Rao, M.V.; Srilatha, K.; Himabindu, V.; Yerramilli, A. A study on metal organic framework (MOF-177) synthesis, characterization and hydrogen adsorption –desorption cycles. Int. J. Energy Environ. 2013, 4, 127–132. [Google Scholar]
- Saha, D.; Deng, S. Synthesis, characterization and hydrogen adsorption in mixed crystals of MOF-5 and MOF-177. Int. J. Hydrogen Energy 2009, 34, 2670–2678. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Chena, C.; Ahn, W.S. Chromium terephthalate metal–organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500. [Google Scholar] [CrossRef]
- Wurzbacher, J.A. Development of a Temperature-Vacuum Swing Process for CO2 Capture from Ambient Air. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2015. [Google Scholar]
- Eyitope. Modelling of CO2 Using Metal Organic Frameworks. Master’s Thesis, King Fahd University, Dhahran, Arabia Saudita, 2015. [Google Scholar]
- Kaskel, S. The Chemistry of Metal Organic Frameworks; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Goyal, P.; Purdue, M.; Farooq, S. Adsorption and Diffusion of N2 and CO2 and Their Mixture on Silica Gel. Ind. Eng. Chem. Res. 2019, 58, 19611–19622. [Google Scholar] [CrossRef]
- Climeworks. 2020. Available online: www.climeworks.com (accessed on 1 December 2020).
- Gebald, C.; Piatkowski, N.; Ruesch, T.; Wurzbacher, J.A. Low-Pressure Drop Structure of Particle Adsorbent Bed for Adsorption Gas Separation Process. WO Patent 2014/170184 Al, 23 October 2014. [Google Scholar]
- Buijs, W.; de Flart, S. Direct Air Capture of CO2 with an Amine Resin: A Molecular Modeling Study of the CO2 Capturing Process. Ind. Eng. Chem. Res. 2017, 56, 12297–12304. [Google Scholar] [CrossRef]
- Knox, J.C.; Ebner, A.D.; LeVan, M.D.; Coker, R.F.; Ritter, J.A. Limitations of Breakthrough Curve Analysis in Fixed-Bed Adsorption. Ind. Eng. Chem. Res. 2016, 55, 4734–4748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Ospino, R.; Santiago, R.G.; Siqueira, R.M.; de Azevedo, D.C.S.; Bastos-Neto, M. Assessment of CO2 desorption from 13X zeolite for a prospective TSA process. Adsorption 2020, 26, 813–824. [Google Scholar] [CrossRef]
- Perry, R.H. Perry’s Chemical Engineerings’ Handbook; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Poundsterlinglive. 2021. Available online: https://www.poundsterlinglive.com/bank-of-england-spot/historical-spot-exchange-rates/usd/USD-to-CHF-1993 (accessed on 1 July 2021).
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, S.; Xian, S.; Xi, H.; Li, Z. Adsorption Equilibrium and Kinetics of CO2 on Chromium Terephthalate MIL-101. Energy Fuels 2011, 25, 835–842. [Google Scholar] [CrossRef]
- Keller, J.U. Gas Adsorption Equilibria: Experimental Methods and Adsorptive Isotherms; Springer: New York, NY, USA, 2005. [Google Scholar]
- Yu, Q.; Brilman, D.W.F. Design strategy for CO2adsorption from ambient air using a supported amine based sorbent in a fixed bed reactor. Energy Procedia 2017, 114, 6102–6114. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, Y.; Cakstins, J.; Sun, C.-G.; Snape, C. Parametric study on the regeneration heat requirement of an amine-based solid adsorbent process for post-combustion carbon capture. Appl. Energy 2016, 168, 394–405. [Google Scholar] [CrossRef]
- Wurzbacher, J.A.; Gebald, C.; Steinfeld, A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel. Energy Environ. Sci. 2011, 4, 3584–3592. [Google Scholar] [CrossRef]
- Huck, J.M.; Lin, L.-C.; Berger, A.H.; Shahrak, M.N.; Martin, R.L.; Bhown, A.S.; Haranczyk, M.; Reuter, K.; Smit, B. Evaluating different classes of porous materials for carbon capture. Energy Environ. Sci. 2014, 7, 4132–4146. [Google Scholar] [CrossRef]
- Peter, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineering; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Matches. 2020. Available online: http://www.matche.com/equipcost/Default.html (accessed on 1 December 2020).
- Towerling Skills. 2021. Available online: https://www.toweringskills.com/financial-analysis/cost-indices/ (accessed on 1 July 2021).
- Danaci, D.; Bui, M.; Mac Dowell, N.; Petit, C. Exploring the limits of adsorption-based CO2 capture using MOFs with PVSA—from molecular design to process economics. Mol. Syst. Des. Eng. 2019, 5, 212–231. [Google Scholar] [CrossRef]
- Bauer, C.; Cox, B.; Heck, T.; Zhang, X. Potentials, Costs and Environmental Assessment of Electricity Generation Technologies; PSI, Technology Assessment Group: Villigen, Switzerland, 2019. [Google Scholar]
- SDH. 2017. Available online: https://www.solar-district-heating.eu/switzerland-plans-for-solar-district-heating-pilot-system/ (accessed on 13 May 2021).
- Gebelman, A. Adsorption Basics: Part 1. 2017. Available online: https://www.aiche.org/sites/default/files/docs/pages/adsorption_basics_part_1.pdf (accessed on 1 December 2020).
- Papurello, D.; Gandiglio, M.; Lanzini, A. Experimental Analysis and Model Validation on thePerformance of Impregnated Activated Carbons for the Removal of Hydrogen Sulfide (H2S) from Sewage Biogas. Processes 2019, 7, 548. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.M. Evaluation of Mass Transfer Rate in Column of Small Lilsx Particle. Master’s Thesis, Cleveland State University, Cleveland, OH, USA, 2017. [Google Scholar]
- Bahrun, M.H.V.; Bono, A.; Dzilrazman, N.K.; Kamin, Z. Recovery of Minor Palm Oil Compounds Using Packed Bed Adsorption Column. J. Bahan Alam Terbarukan 2020, 9, 21–29. [Google Scholar] [CrossRef]
- Naksusuk, S.; Tangsathitkulchai, C. Carbon Dioxide Capture in a Fixed Bed of Coconut Shell Activated Carbon Impregnated With Sodium Hydroxide: Effects of Carbon Pore Texture and Alkali Loading. Eng. J. 2019, 23, 29–48. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Leinekugel-Le-Cocq, D. Design of a Pressure Swing Adsorption Process for Postcombustion CO2 Capture. Ind. Eng. Chem. Res. 2013, 52, 5985–5996. [Google Scholar] [CrossRef]
- Smith, R. Chemical Process Design and Integration; McGraw Hill: New York, NY, USA, 2005. [Google Scholar]
- Wurzbacher, J.A.; Gebald, C.; Piatkowski, N.; Steinfeld, A. Concurrent Separation of CO2 and H2O from Air by a Temperature-Vacuum Swing Adsorption/Desorption Cycle. Environ. Sci. Technol. 2012, 46, 9191–9198. [Google Scholar] [CrossRef] [PubMed]
- Elfvinga, J.; Kauppinena, J.; Jegoroffa, M.; Ruuskanenb, V.; Järvinenb, L.; Sainioc, T. Experimental comparison of regeneration methods for CO2 concentration from air using amine-based adsorbent. Chem. Eng. J. 2021, 404, 126337. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design; BH: Oxford, UK, 2012. [Google Scholar]
- Primo, J. 2012. Available online: https://pdhonline.com/courses/m385/m385content.pdf (accessed on 1 December 2020).
- House, K.Z.; Baclig, A.C.; Ranjan, M.; van Nierop, E.A.; Wilcox, J.; Herzog, H.J. Economic and energetic analysis of capturing CO2 from ambient air. Proc. Natl. Acad. Sci. USA 2011, 108, 20428–20433. [Google Scholar] [CrossRef] [Green Version]
- Krekel, D.; Samsun, R.C.; Peters, R.; Stolten, D. The separation of CO2 from ambient air—A techno-economic assessment. Appl. Energy 2018, 218, 361–381. [Google Scholar] [CrossRef]
- Carbonbrief. 2020. Available online: www.carbonbrief.org/swiss-company-hoping-capture-1-global-co2-emissions-2025 (accessed on 1 December 2020).
- Narula, K.; Filho, F.D.O.; Chambers, J.; Romano, E.; Hollmuller, P.; Patel, M.K. Assessment of techno-economic feasibility of centralised seasonal thermal energy storage for decarbonising the Swiss residential heating sector. Renew. Energy 2020, 161, 1209–1225. [Google Scholar] [CrossRef]
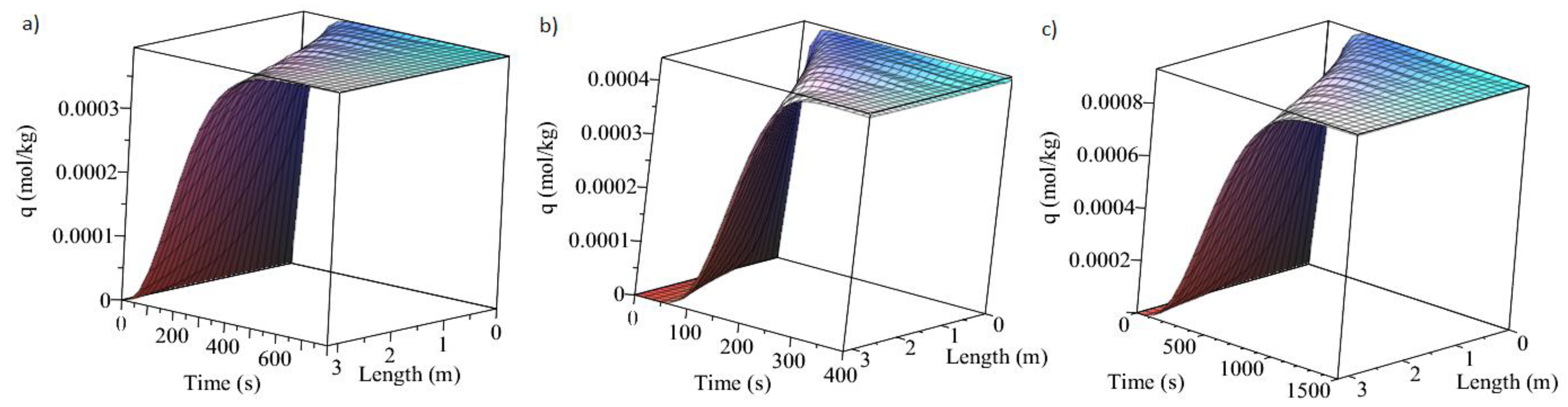
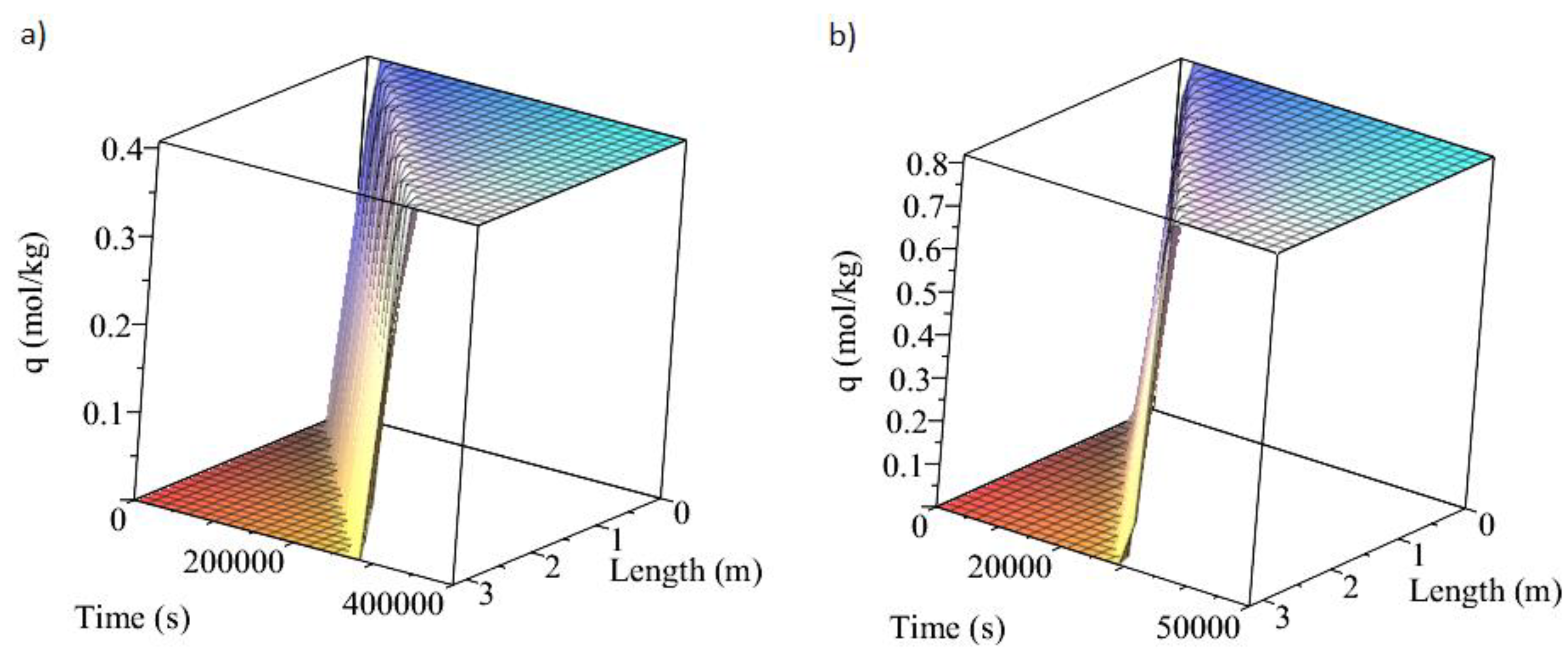
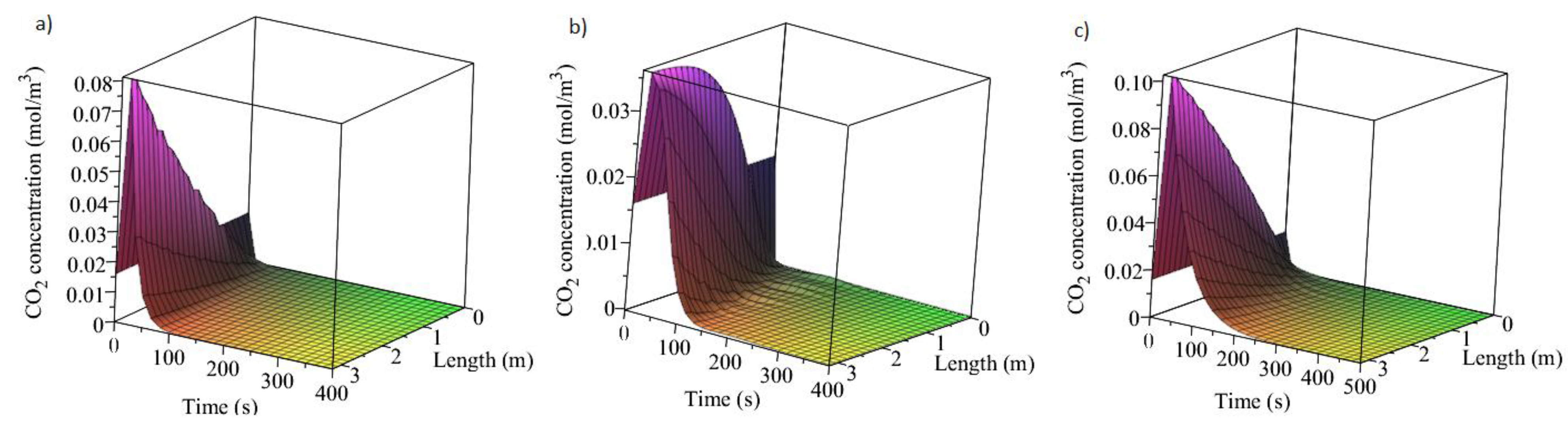
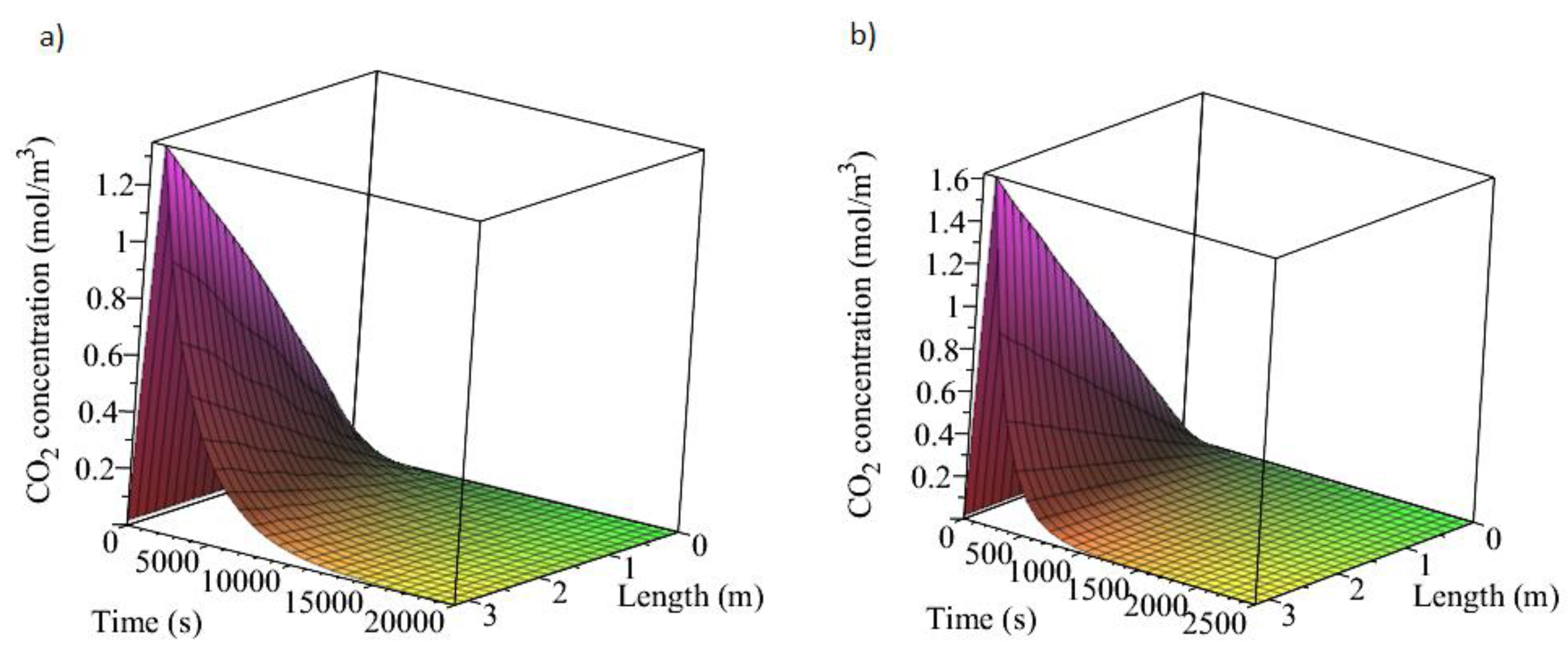
| Support | Amine | Conditions | Con. CO2 (ppm) | Adsorption Capacity (molCO2/kgsorbent) | Reference |
|---|---|---|---|---|---|
| Class I | |||||
| Hierarchical bimodal meso/microporous silica | PEI | 303 K, 19% relative humidity | 400 | 3.36 | Kwon et al. [28] |
| Fumed silica | PEI + PEG | 323 K | 360 | 6.8 × 10−1 | Meth et al. [38] |
| Silica and polymethylmethacrylate | TEPA | 308 K | 400 | 2.50 | Brilman and Veneman [39] |
| SBA-15 | PPI prepared by HClO4 | 303 K | 400 | 3.1 × 10−1 | Sarazen et al. [29] |
| SBA-15 | PPI prepared by HBr | 303 K | 400 | 2.5 × 10−1 | Sarazen et al. [29] |
| SBA-15 | PPI prepared by HCl | 303 K | 400 | 1.5 × 101 | Sarazen et al. [29] |
| SBA-15 | PPI prepared by Ch3SO3H | 303 K | 400 | 1.7 × 10−1 | Sarazen et al. [29] |
| Microporous polymer | PEI | 308 K | 400 | 2 × 10−1 | Pang et al. [40] |
| Mesocellular foam | PAA | 298 K | 400 | 6.3 × 10−1 | Chaikittisilp et al. [41] |
| Mesocellular foam | PEI | 298 K | 400 | 6.1 × 10−1 | Chaikittisilp et al. [41] |
| Mesocellular foam | PEI | 298 K | 400 | 4.4 × 10−1 | Chaikittisilp et al. [41] |
| Class II | |||||
| Nanofibrillated cellulose | APDES | 296 K, relative humidity | 400 | 2.13 | Gebald et al. [42] |
| Mg2(dobpdc) | H2N4 | 298 K | 400 | 3.89 | Liao et al. [43] |
| MCM-41 | TRI | 298 K, 67% relative humidity | 300 | 1.40 | Belmabkhout et al. [26] |
| Nanofibrillated cellulose | AEAPDMS | 298 K, 40% relative humidity | 506 | 1.39 | Ng et al. [44] |
| Mg/DOBDC | ED | 298 K | 400 | 1.50 | Choi et al. [36] |
| Class III | |||||
| SBA-15 | Aziridine | 298 K | 400 | 1.78 | Choi et al. [45] |
| Hybrid silica materia | APTMS | 303 K, relative humidity | 400 | 1.68 | Abhilash et al. [32] |
| Physisorbent | Adsorption Capacity (mol/kgsorbent) | Reference | |
|---|---|---|---|
| CO2 | H2O | ||
| SIFSIX-3-Ni | 1.8 × 10−1 | 5.17 | Shekhah et al. [50] |
| HKUST-1 | 5 × 10−2 | 9.89 | Kumar et al. [39] |
| Mg-MOF-74 | 1.4 × 10−1 | 9.50 | Caskey et al. [51] |
| Literature Work/Team | Actual Cost (USD/tonneCO2) | Future Cost (USD/tonneCO2) |
|---|---|---|
| Fasihi et al. [53] | 6.3 × 101–8.6 × 101 | |
| Kulkarni and Sholl [54] | 4.3 × 101–4.94 × 102 | |
| Zhang et al. [18] | 9.1 × 101–2.27 × 102 | |
| Sinha et al. [55] | 6 × 101–1.9 × 102 | |
| Viebahn [56] (Climeworks) | 6 × 102 | 1 × 102 |
| Kintisch [57] (Global Thermostat) | 1.5 × 101–5 × 101 |
| MIL 101 | |||
| Particle diameter | 3.8 × 10−3 | m | Eyitope [75] |
| Particle density | 6.2 × 102 | kg/m3 | Ferey et al. [72] |
| Bed porosity | 4.4 × 10−1 | ||
| MOF-177 | |||
| Particle diameter | 3.8 × 10−3 | m | Eyitope [75] |
| Particle density | 4.3 × 102 | kg/m3 | Kaskel [76] |
| Bed porosity | 4.4 × 10−1 | Qasem et al. [64] | |
| MOF-5 | |||
| Particle diameter | 3.8 × 10−3 | m | Eyitope [75] |
| Particle density | 6.21 × 102 | kg/m3 | Qasem et al. [64] |
| Bed porosity | 4.4 × 10−1 | ||
| SI-AEATPMS | |||
| Particle diameter | 2 × 10−3 | m | Goyal et al. [77] |
| Particle density | 1.31 × 103 | kg/m3 | Goyal et al. [77] |
| Bed porosity | 6 × 10−1 | ||
| APDES-NFC-FD | |||
| Particle diameter | 5 × 10−3 | m | Wurzbacher [73] |
| Particle density | 5.54 × 101 | kg/m3 | Wurzbacher [73] |
| Bed porosity | 5 × 10−1 | ||
| Area footprint excl. options | 2 × 101 | m2 |
| Height | 3.2 | m |
| CO2 flow rate, inlet | 1.8 × 102 | kg/day |
| YCO2, inlet | 4 × 10−4 | |
| Air flow rate, inlet | 2.86 | m3/s |
| MOF-177 | |||
| qsat | 1.03 × 101 | mol/kg | Mason et al. [84] |
| bo | 2.51 × 10−12 | 1/Pa | Mason et al. [84] |
| ΔHCO2 | −1.4 × 104 | J/mol | Qasem et al. [64] |
| MOF-5 | |||
| qsat | 4.8 × 10 | mol/kg | Eyitope [75] |
| bo | 8.06 × 10−10 | 1/Pa | Eyitope [75] |
| ΔHCO2 | −3.4 × 104 | J/mol | Eyitope [75] |
| MIL-101 | |||
| qsat (298 K) | 3.32 × 101 | mol/kg | Montazerolghaem et al. [65] |
| b (298 K) | 3 × 10−2 | 1/bar | Montazerolghaem et al. [65] |
| qsat (373 K) | 3 × 10−5 | mol/kg | Montazerolghaem et al. [65] |
| b (373 K) | 7.32 × 102 | 1/bar | Montazerolghaem et al. [65] |
| ΔHCO2 | −1.63 × 104 | J/mol | Zhang et al. [85] |
| bo | 2.25 × 104 | 1/bar |
| ΔHCO2 | 6 × 104 | J/mol |
| to | 4.22 × 10−1 | |
| α | 9.49 × 10−1 | |
| nso | 1.97 | mol/kg |
| χ | 2.37 |
| MOF-5 | 1.95 | USD/kg | Danaci et al. [95] |
| MOF-177 | 1.95 | USD/kg | Danaci et al. [95] |
| MIL-101 | 1.95 | USD/kg | Danaci et al. [95] |
| SI-AEATPMS | 1.30 | USD/kg | |
| APDES-NFC-FD | 1.16 | USD/kg | |
| Cooling water | 8 × 10−2 | USD/m3 | Danaci et al. [95] |
| Electricity from MWI | 1 × 10−1 | USD/kWh | Bauer et al. [96] |
| Waste heat from MWI | 2.4 × 10−2 | USD/kWh | SDH [97] |
| Sorbent | MIL-101 | MOF-177 | MOF-5 | SI-AEATPMS | APDES-NFC-FD |
|---|---|---|---|---|---|
| Mass of the sorbent (kg) | 2.22 × 104 | 1.54 × 104 | 2.23 × 104 | 3.36 × 104 | 1.77 × 103 |
| Average single pass recovery | 5.2 × 10−1 | 5.9 × 10−1 | 5.3 × 10−1 | 8.6 × 10−1 | 8.9 × 10−1 |
| Adsorption time (s) | 3.2 × 102 | 2.14 × 102 | 7.49 × 102 | 3.06 × 105 | 3.26 × 104 |
| Desorption time (s) | 9.9 × 101 | 1.6 × 102 | 3.15 × 102 | 1.67 × 104 | 1.85 × 103 |
| Full cycle time (h) | 1.2 × 10−1 | 9 × 10−2 | 2.9 × 10−1 | 8.92 × 101 | 9.53 |
| Number of cycles/day | 2.07 × 102 | 2.71 × 102 | 8.15 × 101 | 3 × 10−1 | 2.5 |
| Captured CO2 (kg/h) | 3.96 | 4.5 | 4.00 | 6.43 | 6.66 |
| Captured CO2 (kg/cycle) | 3.5 × 10−1 | 2.6 × 10−1 | 8.3 × 10−1 | 546 × 102 | 6.03 × 101 |
| Captured CO2 (kg/day) | 7.3 × 101 | 7.2 × 101 | 6.8 × 101 | 1.47 × 102 | 1.52 × 102 |
| Sorbent | MIL-101 | MOF-177 | MOF-5 | SI-AEATPMS | APDES-NFC-FD |
|---|---|---|---|---|---|
| Regeneration mode | TSA | TSA | TSA | TSA | TSA |
| Regeneration temperature (K) | 3.73 × 102 | 3.73 × 102 | 3.73 × 102 | 3.83 × 102 | 3.73 × 102 |
| Pressure drop (Pa) | 1.94 × 103 | 1.94 × 103 | 1.94 × 103 | 7.34 × 102 | 6.36 × 102 |
| Electrical energy (kWhel/tonneCO2) | 1.54 × 103 | 1.35 × 103 | 1.52 × 103 | 3.57 × 102 | 2.99 × 102 |
| Working capacity (mol/kg) | 3.5 × 10−4 | 3.9 × 10−4 | 8.4 × 10−4 | 3.67 × 10−1 | 7.71 × 10−1 |
| Regeneration energy (kWhth/tonneCO2) | 1.07 × 106 | 1.3 × 106 | 4.94 × 105 | 1.88 × 103 | 1.4 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonzio, G.; Fennell, P.S.; Shah, N. A Comparative Study of Different Sorbents in the Context of Direct Air Capture (DAC): Evaluation of Key Performance Indicators and Comparisons. Appl. Sci. 2022, 12, 2618. https://doi.org/10.3390/app12052618
Leonzio G, Fennell PS, Shah N. A Comparative Study of Different Sorbents in the Context of Direct Air Capture (DAC): Evaluation of Key Performance Indicators and Comparisons. Applied Sciences. 2022; 12(5):2618. https://doi.org/10.3390/app12052618
Chicago/Turabian StyleLeonzio, Grazia, Paul S. Fennell, and Nilay Shah. 2022. "A Comparative Study of Different Sorbents in the Context of Direct Air Capture (DAC): Evaluation of Key Performance Indicators and Comparisons" Applied Sciences 12, no. 5: 2618. https://doi.org/10.3390/app12052618
APA StyleLeonzio, G., Fennell, P. S., & Shah, N. (2022). A Comparative Study of Different Sorbents in the Context of Direct Air Capture (DAC): Evaluation of Key Performance Indicators and Comparisons. Applied Sciences, 12(5), 2618. https://doi.org/10.3390/app12052618







