One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrochemical Measurements
2.3. Electrochemical Preparation of Nickel/Graphene-Modified Electrodes (GCE/ERGO-NiNPs)
2.4. Sample Preparation and Detection
3. Results and Discussion
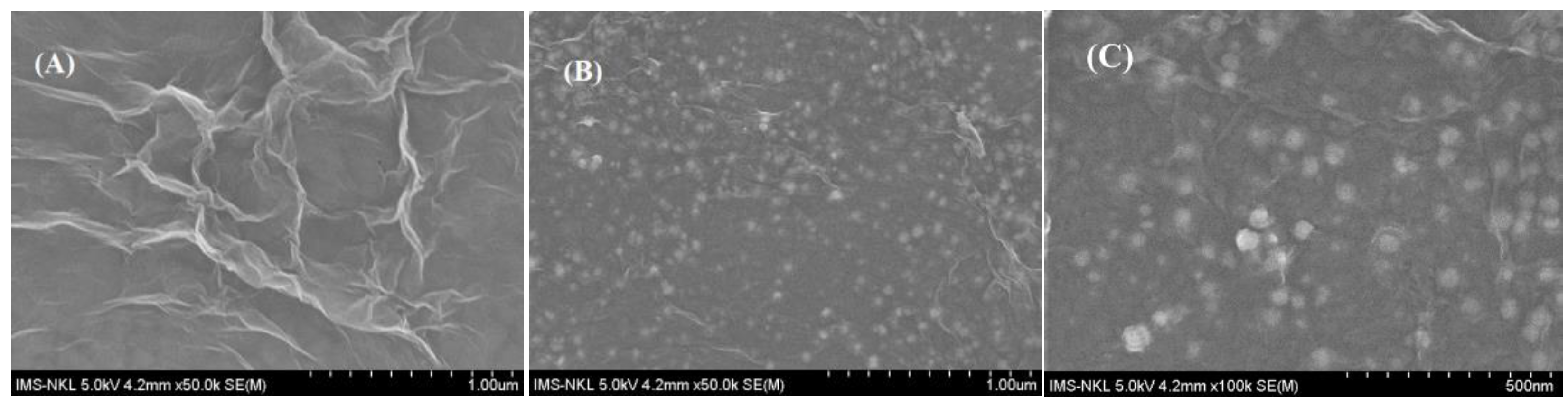
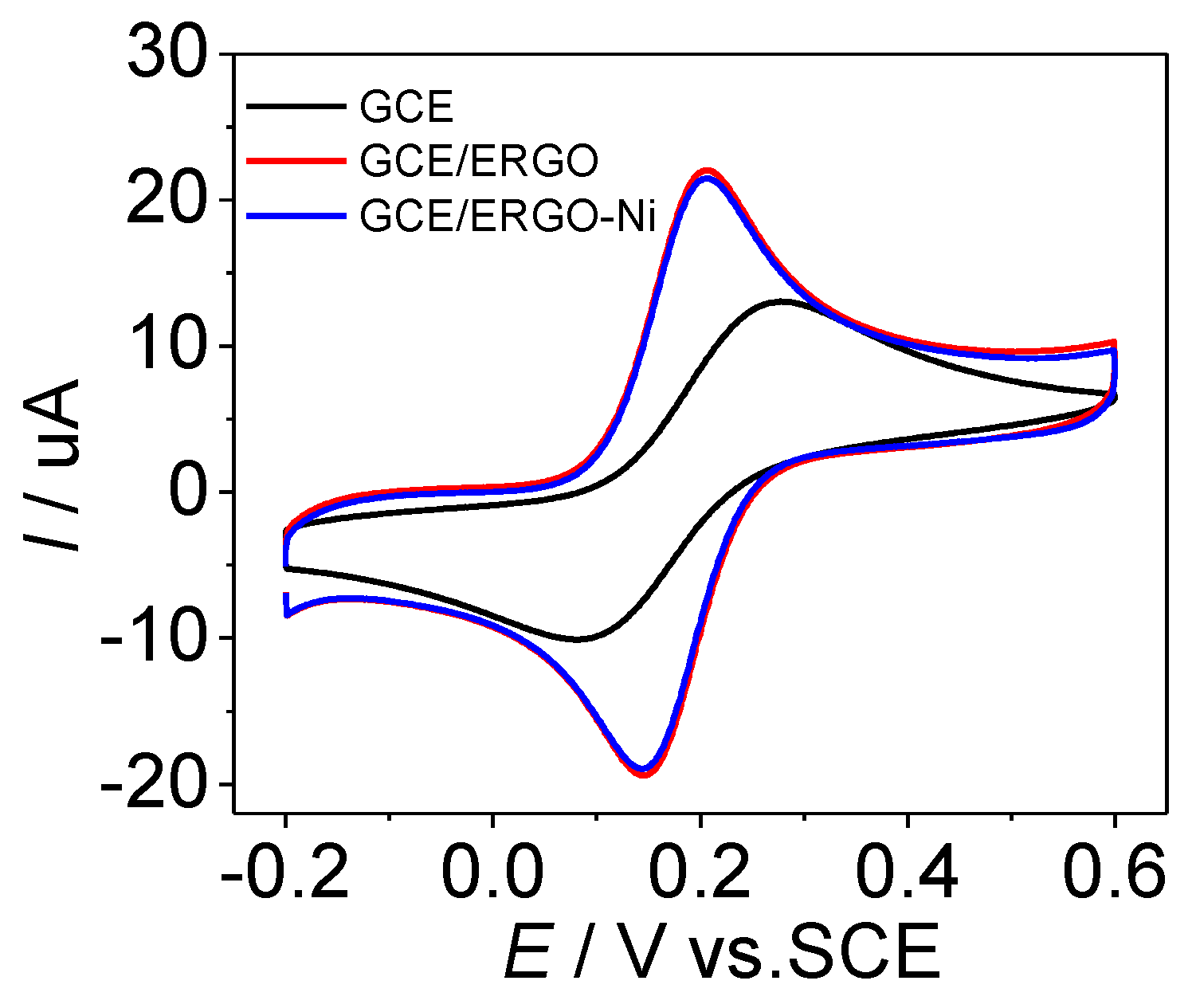
3.1. Preparation and Characterization of ERGO-NiNP Film-Modified Electrode
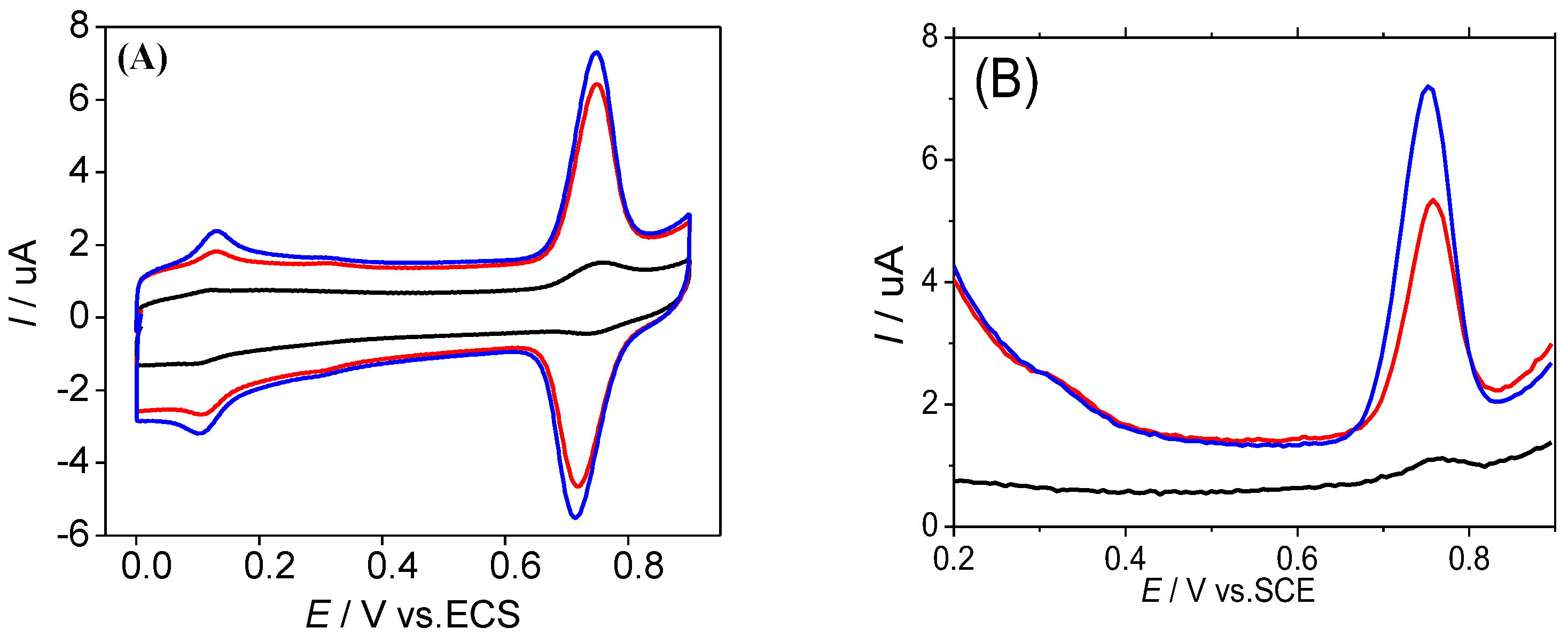
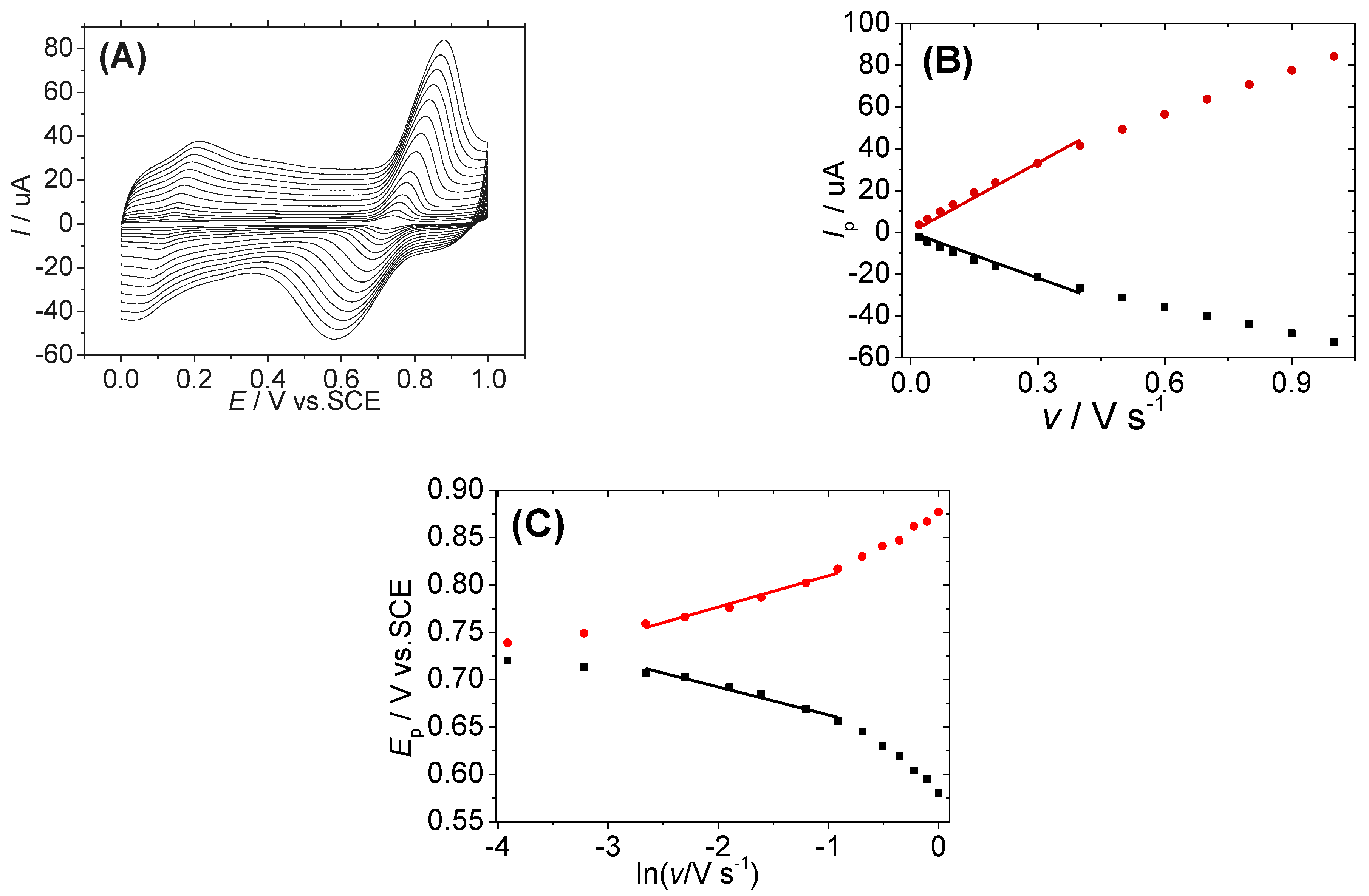
3.2. Electrochemical Behaviors of the SY onto GCE/ERGO-NiNPs Surface
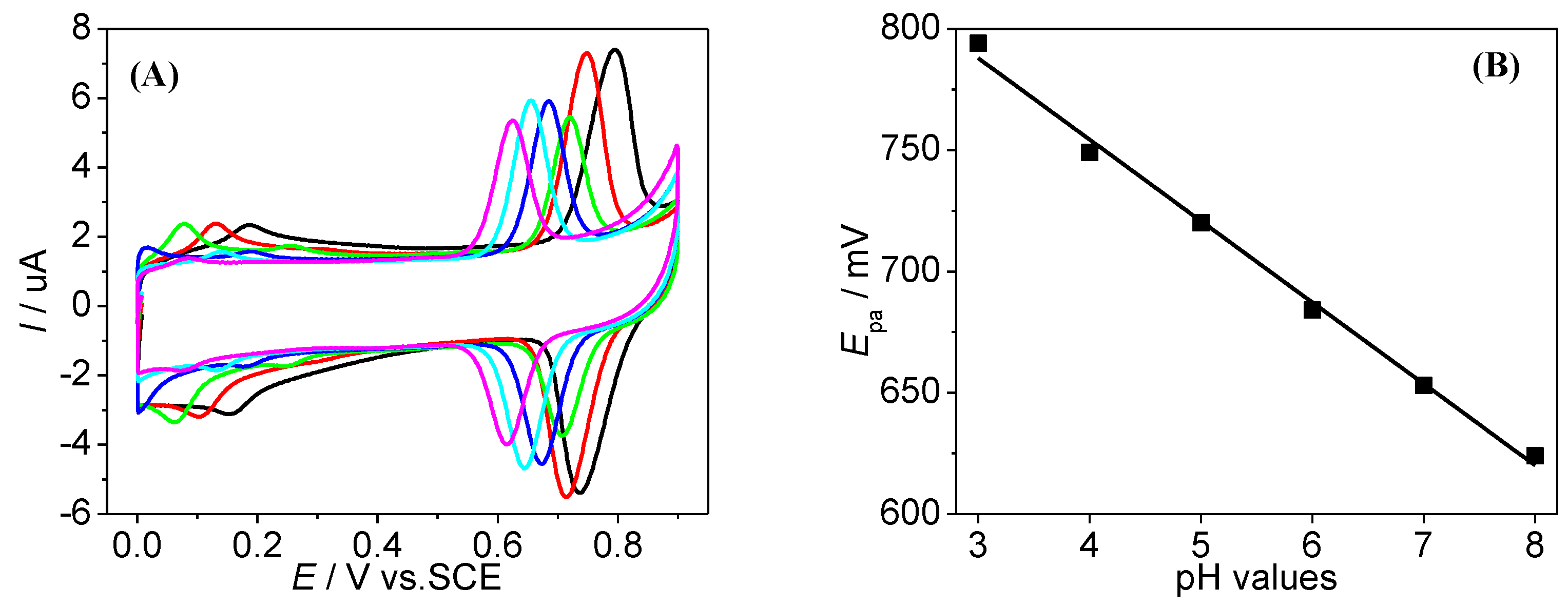
3.3. Optimisation of DPV Parameters for SY Detection
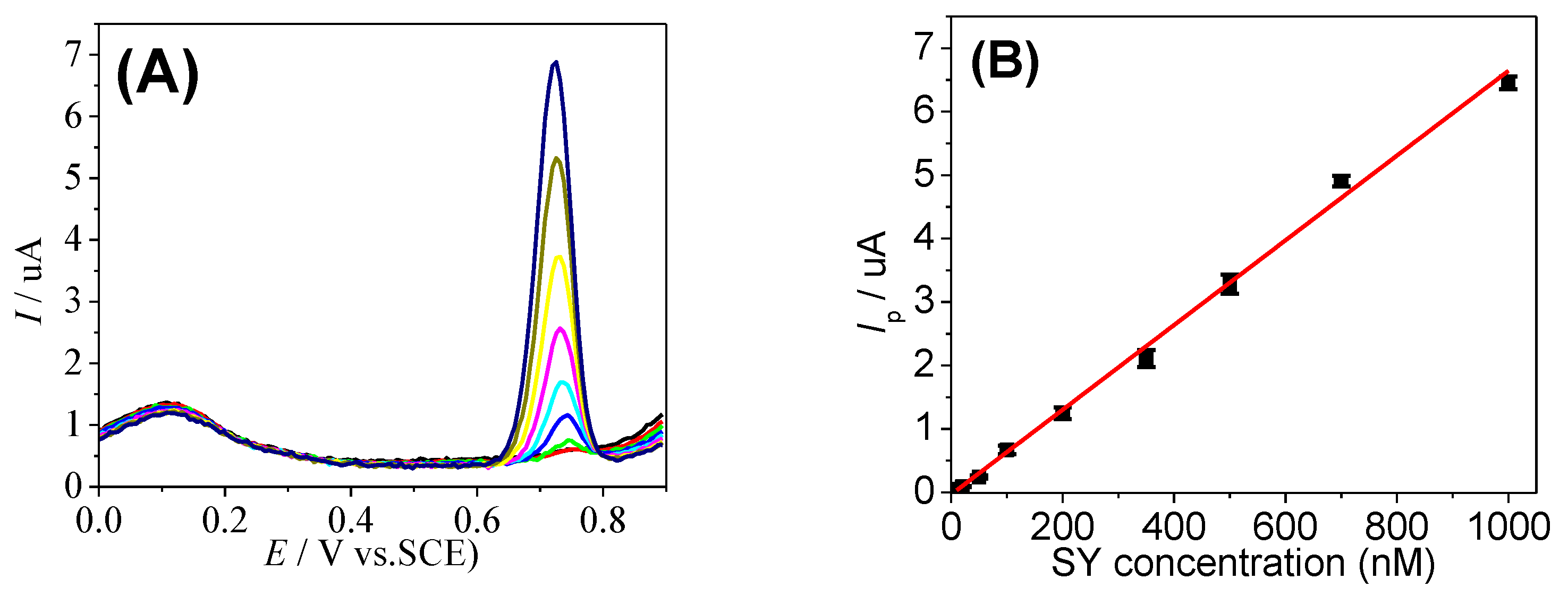
3.4. SY Detection Using GCE/ERGO-NiNPs Electrode
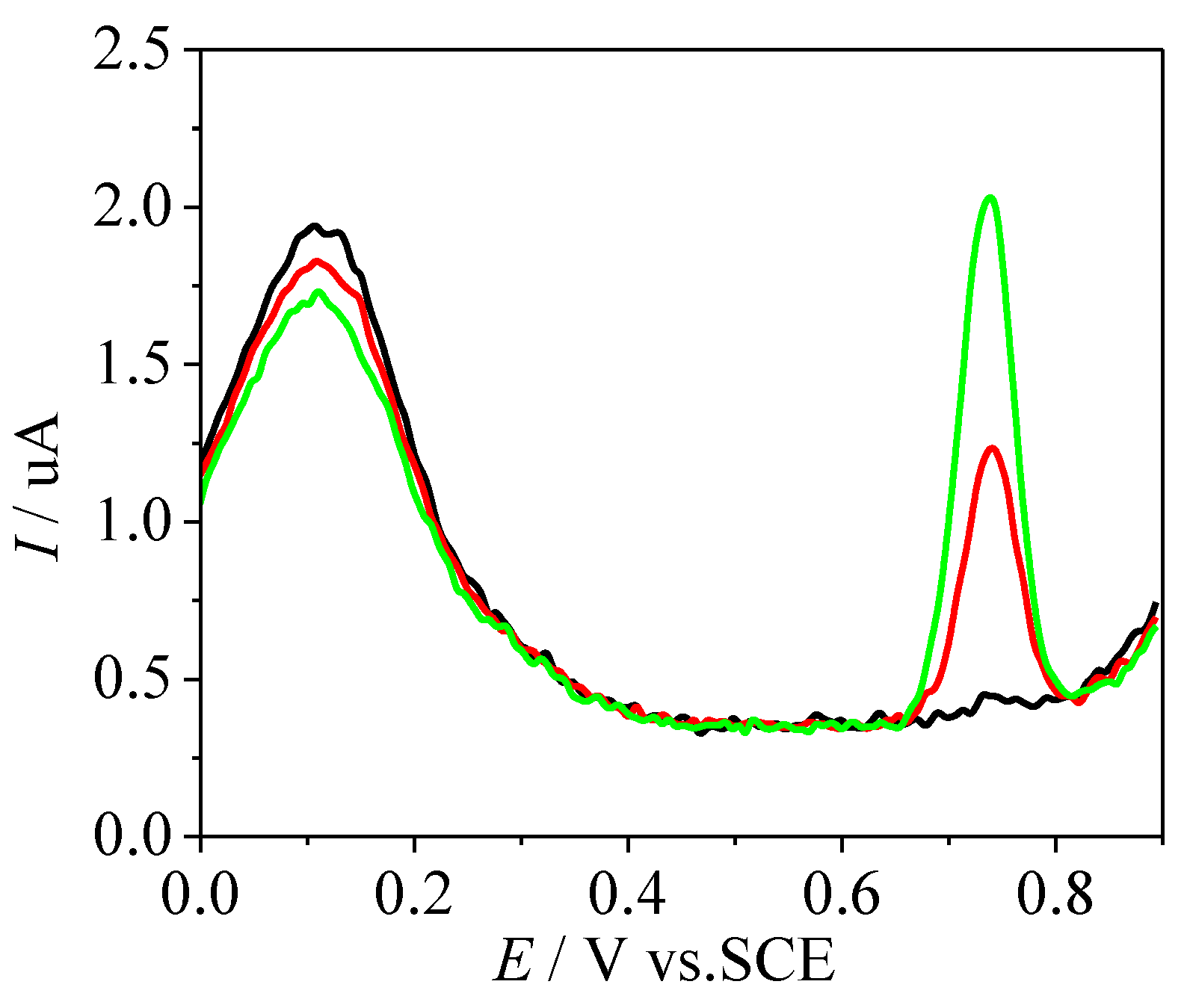
3.5. Stability and Anti-Interference Ability
3.6. Practical Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci. 2018, 4, 568–586. [Google Scholar]
- Dwivedi, K.; Kumar, G. Genetic Damage Induced by a Food Coloring Dye (Sunset Yellow) on Meristematic Cells of Brassica campestris L. J. Environ. Public Health 2015, 2015, 319727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, K.M.S.; de Oliveira, M.V.G.A.; Carvalho, F.R.; Menezes, C.C.; Peron, A.P. Citotoxicity of food dyes Sunset Yellow (E-110), Bordeaux Red (E-123), and Tatrazine Yellow (E-102) on Allium cepa L. root meristematic cells. Food Sci. Technol. 2013, 33, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Llamas, N.E.; Garrido, M.; di Nezio, M.S.; Band, B.S.F. Second order advantage in the determination of amaranth, sunset yellow FCF and tartrazine by UV-vis and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 2009, 655, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Zeynali, K.A.; Manafi-Khoshmanesh, S. Simultaneous spectrophotometric determination of sunset yellow and quinoline yellow in a single step. J. Chin. Chem. Soc. 2015, 62, 772–779. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, X.; Qiao, M.; Zhu, J.; Liu, S.; Yang, J.; Hu, X. Determination of sunset yellow in soft drinks based on fluorescence quenching of carbon dot. Spectrochim. Acta A 2016, 167, 106–110. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Yang, L. Designing of the functional paper-based surface-enhanced raman spectroscopy substrates for colorants detection. Mater. Res. Bull. 2015, 63, 199–204. [Google Scholar] [CrossRef]
- Alp, H.; Bas, D.; Yas, A.; Yaylı, N.; Ocak, M. Simultaneous Determination of Sunset Yellow FCF, Allura Red AC, Quinoline Yellow WS, and Tartrazine in Food Samples by RP-HPLC. J. Chem. 2018, 2018, 6486250. [Google Scholar] [CrossRef]
- Zou, T.; He, P.; Yasen, A.; Li, Z. Determination of seven synthetic dyes in animal feeds and meat by high performance liquid chromatography with diode array and tandem mass detectors. Food Chem. 2013, 138, 1742–1748. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Yang, J.; Hu, P.; Yu, G. Perspective of graphene-based electronic devices: Graphene synthesis and diverse applications. APL Mater. 2019, 7, 020901. [Google Scholar] [CrossRef] [Green Version]
- Lemine, A.S.; Zagho, M.M.; Altahtamouni, T.M.; Bensalah, N. Graphene a promising electrode material for supercapacitors—A review. Int. J. Energy Res. 2018, 42, 4284–4300. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F. Graphene-based screen-printed electrochemical (bio)sensors and their applications: Efforts and criticisms. Biosens. Bioelectron. 2017, 89, 107–122. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Kung, C.; Lin, P.; Xue, Y.; Akolkar, R.; Dai, L.; Yu, X. Three dimensional graphene foam supported platinum e ruthenium bimetallic nanocatalysts for direct methanol and direct ethanol fuel cell applications. J. Power Sources 2014, 256, 329–335. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, G.; Zhang, F.; Xia, J.; Gui, R.; Yang, M.; Bi, S.; Xia, L.; Li, Y.; Xia, L.; et al. Electrochimica Acta Amphoteric surfactant promoted three-dimensional assembly of graphene micro / nanoclusters to accomodate Pt nanoparticles for methanol oxidation. Electrochim. Acta 2015, 160, 288–295. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Valiyaveettil, S. Cationic surfactant mediated exfoliation of graphite into graphene flakes. Carbon 2009, 47, 3288–3294. [Google Scholar] [CrossRef]
- Yu, L.; Shi, M.; Yue, X.; Qu, L. A novel and sensitive hexadecyltrimethyl ammonium bromide functionalized graphene supported platinum nanoparticles composite modified glassy carbon electrode for determination of sunset yellow in soft drinks. Sens. Actuators B Chem. 2015, 209, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J. Facile synthesis of Au supported on ionic liquid functionalized reduced graphene oxide for simultaneous determination of Sunset yellow and Tartrazine in drinks. Sens. Actuators B Chem. 2015, 216, 578–585. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Preparation and application of sunset yellow imprinted ionic liquid polymer—Ionic liquid functionalized graphene composite film coated glassy carbon electrodes. Electrochim. Acta 2014, 115, 247–254. [Google Scholar] [CrossRef]
- Jia, L.; Wang, H. Chemical Preparation and application of a highly sensitive nonenzymatic ethanol sensor based on nickel nanoparticles/Nafion/graphene composite film. Sens. Actuators B Chem. 2013, 177, 1035–1042. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V. Graphene—And Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications. Int. J. Mol. Sci. 2019, 20, 2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, B.; Wang, H.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on gold nanoparticles/graphene electrode. Anal. Chim. Acta 2015, 893, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L. Direct electrodeposition of graphene enabling the one-step synthesis of graphene-metal nanocomposite films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef]
- Lee, C.; Yu, S.H.; Kim, T.H. One-Step Electrochemical Fabrication of Reduced Graphene Oxide/Gold Nanoparticles Nanocomposite-Modified Electrode for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. Nanomaterials 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, B.; Zhang, K.; Bin, D.; Shiraishi, Y.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2016, 481, 229–235. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. Development of an Amperometric Cholesterol Biosensor Based on Graphene—Pt Nanoparticle Hybrid Material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Palanisamy, S.; Karuppiah, C.; Chen, S.M. Direct electrochemistry and electrocatalysis of glucose oxidase immobilized on reduced graphene oxide and silver nanoparticles nanocomposite modified electrode. Colloids Surf. Biointerfaces 2014, 114, 164–169. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P. Novel Electrochemical Sensors Based on Cuprous Oxide-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrode toward Sensitive. Molecules 2018, 23, 2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.W.; Hsu, T.K.; Sun, C.L.; Nien, Y.T.; Pu, N.W.; Ger, M.D. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of Graphene Oxide Based CuO Nanoparticles Composite Electrode for Highly Enhanced Nonenzymatic Glucose Detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Wang, G.; Li, G.; Deng, D.; Qu, L. One-pot synthesis of Mn3O4 nanoparticles decorated with nitrogen-doped reduced graphene oxide for sensitive nonenzymatic glucose sensing. J. Electroanal. Chem. 2015, 755, 15–21. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-Arporn, R.P. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater. Sci. Eng. C 2017, 76, 398–405. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef]
- He, Q.; Liu, J.; Liu, X.; Xia, Y.; Li, G.; Deng, P.; Liang, J.; Chen, D. Sensitive and Selective Detection of Tartrazine Based on TiO2-Electrochemically Reduced Graphene Oxide Composite-Modified Electrodes. Sensor 2018, 18, 1911. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, S.; Kim, S.J. An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO2-reduced graphene oxide nanocomposite. RSC Adv. 2015, 5, 12937–12943. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. A hybrid functional nanoscaffold based on reduced graphene oxide–ZnO for the development of an amperometric biosensing platform. RSC Adv. 2013, 3, 25858–25864. [Google Scholar] [CrossRef]
- Vilian, A.E.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M. Direct electrochemistry of glucose oxidase immobilized on ZrO2 nanoparticles-decorated reduced graphene oxide sheets for a glucose biosensor. RSC Adv. 2014, 4, 30358–30367. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Wang, F.; Sheng, Z.; Xia, X. A facile approach to the synthesis of highly electroactive Pt nanoparticles on graphene as an anode catalyst for direct methanol fuel cells. Chem. Commun. 2010, 46, 5951–5953. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Guo, S.; Fang, Y.; Han, L.; Wang, E.; Dong, S. One-Step Electrochemical Approach to the Synthesis of Graphene/MnO2 Nanowall Hybrids. Nano Res. 2011, 4, 648–657. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Zhang, D.; Sun, X.; Lin, H.; Wang, C. One-Step Electrophoretic Deposition of Reduced Graphene Oxide and Ni(OH)2 Composite Films for Controlled Syntheses Supercapacitor Electrodes. J. Phys. Chem. B 2013, 117, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Pan, D.; Lin, M.; Han, H.; Li, F. One-Step Electrochemical Deposition of Reduced Graphene Oxide-Bismuth Nanocomposites for Determination of Lead. ECS Electrochem. Lett. 2015, 4, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Moozarm, P.; Meng, P.; Alias, Y. Facile one-step electrochemical deposition of copper nanoparticles and reduced graphene oxide as nonenzymatic hydrogen peroxide sensor. Appl. Surf. Sci. 2017, 413, 56–65. [Google Scholar] [CrossRef]
- Henni, A.; Harfouche, N.; Karar, A.; Zerrouki, D.; Perrin, F.X.; Rosei, F. Synthesis of graphene–ZnO nanocomposites by a one-step electrochemical deposition for efficient photocatalytic degradation of organic pollutant. Solid State Sci. 2019, 98, 106039. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, F.; Sun, Y.; Shi, Y.; Li, Z. Assembly of Ni(OH)2 nanoplates on reduced graphene oxide: A two dimensional nanocomposite for enzyme-free glucose sensing. J. Mater. Chem. 2011, 21, 16949–16954. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Sun, Y.; Shi, Y.; Dai, H.; Ni, P.; Hu, J.; Li, Z.; Song, Y.; Wang, L. Electrochemical deposition of nickel nanoparticles on reduced graphene oxide film for nonenzymatic glucose sensing. Electroanalysis 2013, 25, 959–966. [Google Scholar] [CrossRef]
- Yuan, B.; Xu, C.; Deng, D.; Xing, Y.; Liu, L.; Pang, H.; Zhang, D. Graphene oxide/nickel oxide modified glassy carbon electrode for supercapacitor and nonenzymatic glucose sensor. Electrochim. Acta 2013, 88, 708–712. [Google Scholar] [CrossRef]
- Li, S.J.; Xia, N.; Lv, X.L.; Zhao, M.M.; Yuan, B.Q.; Pang, H. A facile one-step electrochemical synthesis of graphene/NiO nanocomposites as efficient electrocatalyst for glucose and methanol. Sens. Actuators B Chem. 2014, 190, 809–817. [Google Scholar] [CrossRef]
- Xue, Z.; Yin, B.; Li, M.; Rao, H.; Wang, H.; Zhou, X.; Liu, X.; Lu, X. Direct electrodeposition of well dispersed electrochemical reduction graphene oxide assembled with nickel oxide nanocomposite and its improved electrocatalytic activity toward 2,4,6-Trinitrophenol. Electrochim. Acta 2016, 192, 512–520. [Google Scholar] [CrossRef]
- Tran, Q.T.; Phung, T.T.; Nguyen, Q.T.; Le, T.G.; Lagrost, C. Highly sensitive and rapid determination of sunset yellow in drinks using a low-cost carbon material-based electrochemical sensor. Anal. Bioanal. Chem. 2019, 411, 7539–7549. [Google Scholar] [CrossRef]
- Tao, S.; Yang, F.; Schuch, J.; Jaegermann, W.; Kaiser, B. Electrodeposition of Nickel Nanoparticles for the Alkaline Hydrogen Evolution Reaction: Correlating Electrocatalytic Behavior and Chemical Composition. ChemSusChem 2018, 11, 948–958. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Wang, M.; Sun, Q.; Gao, Y.; Yang, X.; Zhao, J. Determination of Sunset yellow in foods based on a facile electrochemical sensor. Anal. Methods 2014, 6, 8760–8766. [Google Scholar] [CrossRef]
- Sierra-Rosales, P.; Berríos, C.; Squella, J.A. Experimental and theoretical insights into the electrooxidation pathway of azo-colorants on glassy carbon electrode. Electrochim. Acta 2018, 290, 556–567. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronides Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food Chem. 2017, 227, 73–77. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Liu, G.; Sun, D. Determination of sunset yellow and tartrazine using silver and poly (L-cysteine) composite film modified glassy carbon electrode. Indian J. Chem. 2016, 55, 298–303. [Google Scholar]
- Gan, T.; Sun, J.; Wu, Q.; Jing, Q.; Yu, S. Graphene decorated with nickel nanoparticles as a sensitive substrate for simultaneous determination of sunset yellow and tartrazine in food samples. Electroanalysis 2013, 25, 1505–1512. [Google Scholar] [CrossRef]


| Electrode | Linear Range (nM) | LOD (nM) | Reference |
|---|---|---|---|
| GCE/ZnO-cysteic acid | 100–3000 | 30 | [61] |
| GCE/GO-MWCNT | 90–8000 | 25 | [62] |
| GCE/poly(L-cysteine)-Ag | 100–10.000 | 50 | [63] |
| GCE/ERGO-AuNPs | 2–110.000 | 2 | [27] |
| GCE/GN-Ni | 7.4–442 | 2.2 | [64] |
| GCE/GN-CTAB-Pt | 80–10.000 | 4.2 | [21] |
| GCE/rGO/Au-Pd | 686–331.000 | 1.5 | [30] |
| GCE/ERGO-NiNPs | 10–1.000 | 3.7 | This work |
| Sample | Added (nM) | Expected (nM) | Found (nM) | RSD (%) (n = 3) | Recovery (%) | By HPLC (nM) | Relative Error (%) |
|---|---|---|---|---|---|---|---|
| Mirinda | 127.8 | 2.50 | 127.6 | 0.2 | |||
| 100 | 227.6 | 235.4 | 3.85 | 103.3 | |||
| Samurai | 120.5 | 4.45 | 128.8 | −6.4 | |||
| 100 | 228.8 | 229.3 | 4.03 | 100.2 | |||
| Number 1 | 111.2 | 5.84 | 109.40 | 1.6 | |||
| 100 | 211.2 | 228.9 | 6.48 | 108.4 | |||
| Sting | 86.4 | 3.93 | 90.00 | −6.1 | |||
| 100 | 186.4 | 171.1 | 4.10 | 91.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.-T.; Le, T.-G.; Bergonzo, P.; Tran, Q.-T. One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Appl. Sci. 2022, 12, 2614. https://doi.org/10.3390/app12052614
Nguyen Q-T, Le T-G, Bergonzo P, Tran Q-T. One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Applied Sciences. 2022; 12(5):2614. https://doi.org/10.3390/app12052614
Chicago/Turabian StyleNguyen, Quang-Trung, Truong-Giang Le, Philippe Bergonzo, and Quang-Thuan Tran. 2022. "One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks" Applied Sciences 12, no. 5: 2614. https://doi.org/10.3390/app12052614
APA StyleNguyen, Q.-T., Le, T.-G., Bergonzo, P., & Tran, Q.-T. (2022). One-Step Fabrication of Nickel-Electrochemically Reduced Graphene Oxide Nanocomposites Modified Electrodes and Application to the Detection of Sunset Yellow in Drinks. Applied Sciences, 12(5), 2614. https://doi.org/10.3390/app12052614







