Shotokuseki Extract Promotes Keratinocyte Differentiation Even at a Low Calcium Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Atomic Absorption Spectroscopy
2.3. Cell Culturing
2.4. MTT Assay
2.5. Trypan Blue Exclusion Test
2.6. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Western Blotting Analysis
2.8. Quantification of Intracellular Calcium Concentration
2.9. Statistical Analysis
3. Results
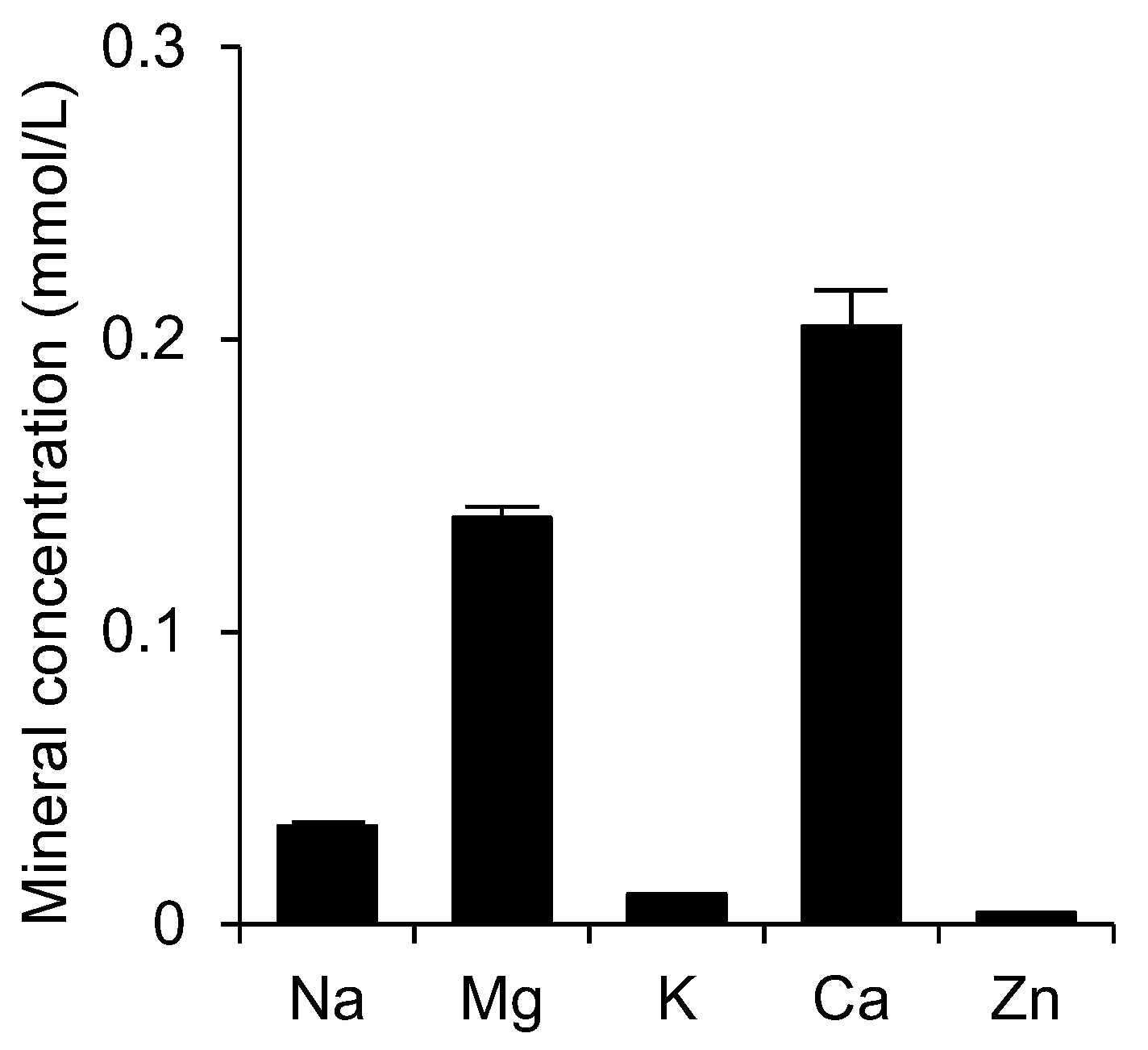
3.1. Concentrations of Minerals in Shotokuseki Extract
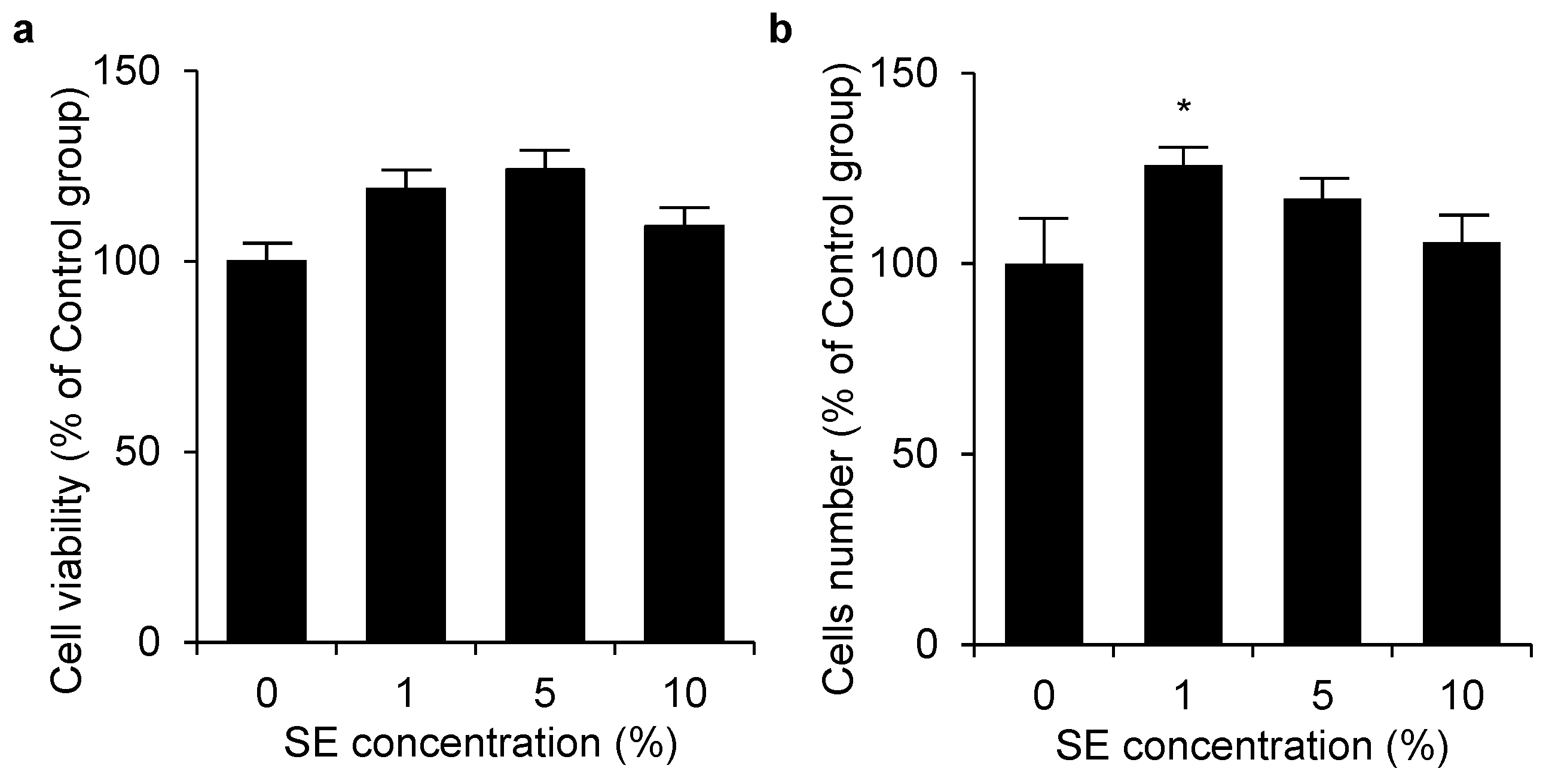
3.2. Cell Viability and Cell Number of Shotokuseki Extract toward NHEKs
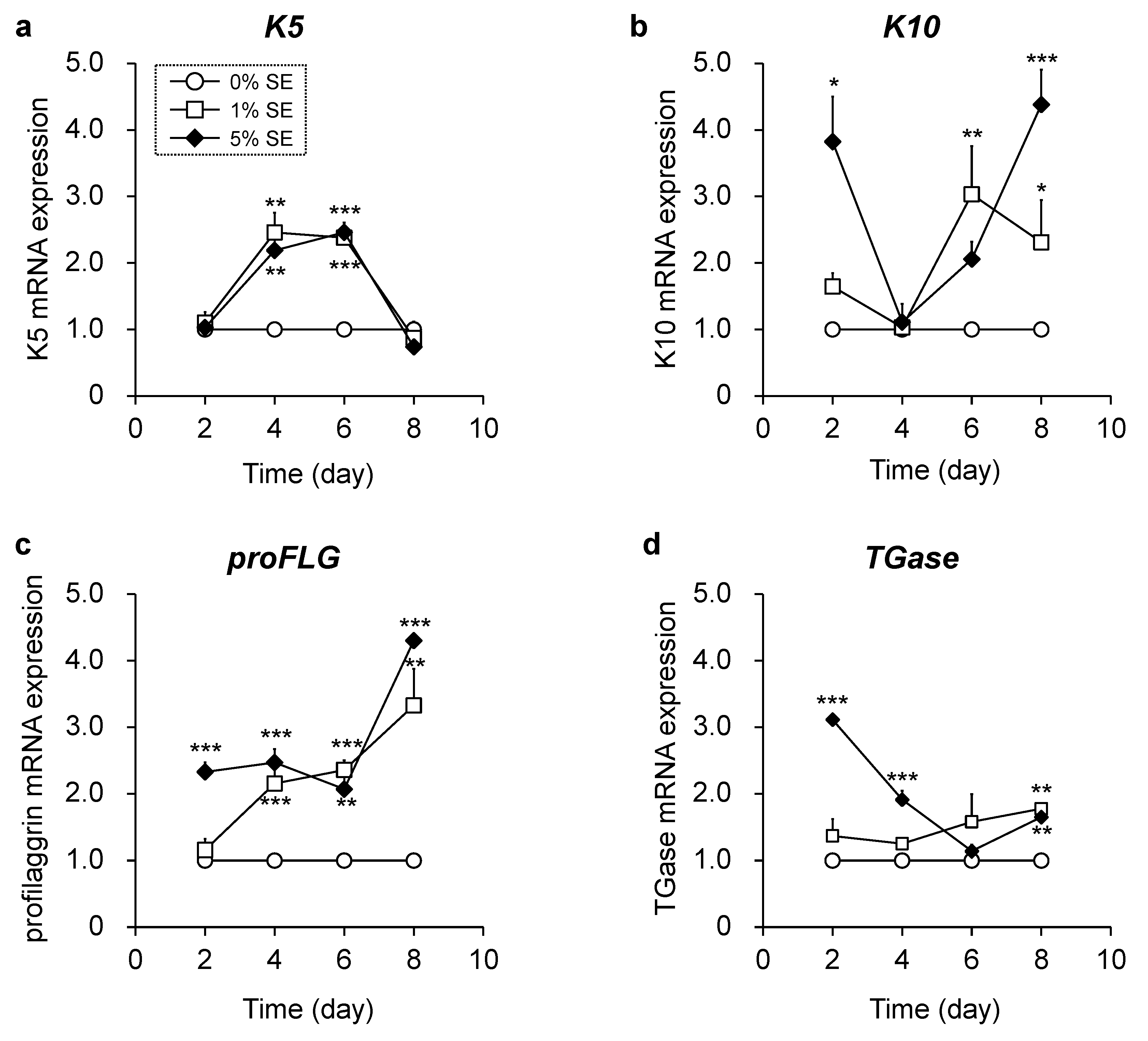
3.3. Effects of Shotokuseki Extract on Epidermal Differentiation in NHEKs
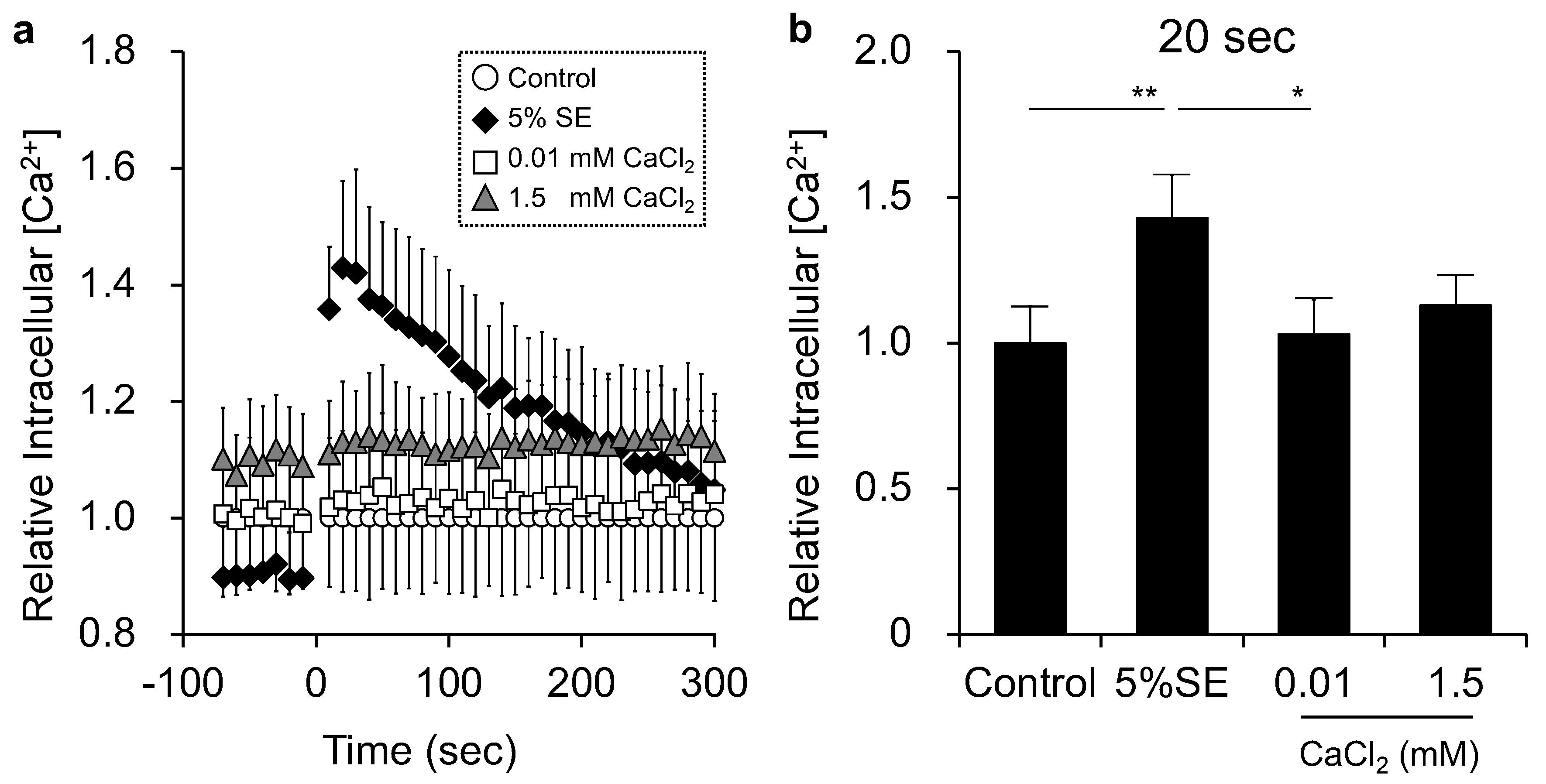
3.4. Effects of Shotokuseki Extract on Intracellular Calcium Levels in NHEKs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, E.; Green, H. Changes in keratin gene-expression during terminal differentiation of the keratinocyte. Cell 1980, 19, 1033–1042. [Google Scholar] [CrossRef]
- Porter, R.M.; Lane, E.B. Phenotypes, genotypes and their contribution to understanding keratin function. Trends Genet. 2003, 19, 278–285. [Google Scholar] [CrossRef]
- Rice, R.H.; Green, H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: Activation of the cross-linking by calcium ions. Cell 1979, 18, 681–694. [Google Scholar] [CrossRef]
- Eckert, R.L.; Yaffe, M.B.; Crish, J.F.; Murthy, S.; Rorke, E.A.; Welter, J.F. Involucrin-structure and role in envelope assembly. J. Investig. Dermatol. 1993, 100, 613–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, M.; Green, H. The glutamine residues reactive in transglutaminase-catalyzed cross-linking of involucrin. J. Biol. Chem. 1988, 263, 18093–18098. [Google Scholar] [CrossRef]
- Marchese, C.; Rubin, J.; Ron, D.; Faggioni, A.; Torrisi, M.R.; Messina, A.; Frati, L.; Aaronson, S.A. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: Differentiation response distinguishes KGF from EGF family. J. Cell Physiol. 1990, 144, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Ng, D.; Tu, C.L.; Oda, Y.; Xie, Z. Calcium and vitamin D-regulated keratinocyte differentiation. Mol. Cell Endocrinol. 2001, 177, 161–171. [Google Scholar] [CrossRef]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef]
- Lee, S.E.; Jun, J.E.; Choi, E.H.; Ahn, S.K.; Lee, S.H. Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin. Exp. Dermatol. 2010, 35, 650–657. [Google Scholar] [CrossRef]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Emri, E.; Miko, E.; Bai, P.; Boros, G.; Nagy, G.; Rózsa, D.; Juhász, T.; Hegeds, C.; Horkay, I.; Remenyik, É.; et al. Effects of non-toxic zinc exposure on human epidermal keratinocytes. Metallomics 2015, 7, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodak, E.; Gottlieb, A.B.; Segal, T.; Politi, Y.; Maron, L.; Sulkes, J.; David, M. Climatotherapy at the Dead Sea is a remittive therapy for psoriasis: Combined effects on epidermal and immunologic activation. J. Am. Acad. Dermatol. Sci. 2003, 49, 451–457. [Google Scholar] [CrossRef]
- Seite, S. Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, J.P.; Kim, Y.M.; Son, J.; Kim, C.J.; Kim, E.H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement. Altern. Med. 2012, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Chun, S.Y.; Lee, M.G.; Kim, S.; Jang, T.J.; Nam, K.S. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018, 97, 1331–1340. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hohl, D.; Lichti, U.; Breitkreutz, D.; Steinert, P.M.; Roop, D.R. Transcription of the human loricrin gene in vitro is induced by calcium and cell density and suppressed by retinoic acid. J. Investig. Dermatol. 1991, 96, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Fleckman, F.; Dale, B.A.; Holbrook, K.A. Profilaggrin, a high-molecular-weight precursor of filaggrin in human epidermis and cultures keratinocytes. J. Investig. Dermatol. 1985, 85, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Menon, G.K.; Grayson, S.; Elias, P.M. Ionic calcium reservoirs in mammalian epidermis: Ultrastructural localization by ion-capture cytochemistry. J. Investig. Dermatol. 1985, 84, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Menon, G.K.; Elias, P.M. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch. Dermatol. 1991, 127, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Ahn, S.K.; Brown, B.E.; Crumrine, D.; Feingold, K.R. Origin of the epidermal calcium gradient: Regulation by barrier status and role of active vs passive mechanisms. J. Investig. Dermatol. 2002, 119, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuspa, S.H.; Kilkenny, A.E.; Steinert, P.M.; Roop, D.R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J. Cell Biol. 1989, 109, 1207–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, S.; Bikle, D.D.; Hincenbergs, M.; Elias, P.M. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J. Cell Physiol. 1988, 134, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Ratnam, A.; Mauro, T.; Harris, J.; Pillai, S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J. Clin. Investig. 1996, 97, 1085–1093. [Google Scholar] [CrossRef]
- Tu, C.L.; Chang, W.; Bikle, D.D. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J. Biol. Chem. 2001, 276, 41079–41085. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.J.; Pang, Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Bíró, T. TRP channels in the skin. Br. J. Pharmacol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.C.; Lee, C.H. TRP channels in skin: From physiological implications to clinical significances. Biophysics 2015, 11, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Denda, M.; Fujiwara, S.; Hibino, T. Expression of voltage-gated calcium channel subunit alpha1C in epidermal keratinocytes and effects of agonist and antagonists of the channel on skin barrier homeostasis. Exp. Dermatol. 2006, 5, 455–460. [Google Scholar] [CrossRef]
- Tu, C.L.; Crumrine, D.A.; Man, M.Q.; Chang, W.; Elalieh, H.; You, M.; Elias, P.M.; Bikle, D.D. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J. Investig. Dermatol. 2012, 132, 2350–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef] [Green Version]
- Dascalu, A.; Matithyou, A.; Oron, Y.; Korenstein, R. A hyperosmotic stimulus elevates intracellular calcium and inhibits proliferation of a human keratinocyte cell line. J. Investig. Dermatol. 2000, 115, 714–718. [Google Scholar]
- Lehen’kyi, V.; Vandenberghe, M.; Belaubre, F.; Julié, S.; Castex-Rizzi, N.; Skryma, R.; Prevarskaya, N. Acceleration of keratinocyte differentiation by transient receptor potential vanilloid (TRPV6) channel activation. J. Eur. Acad. Dermatol. Venereol. 2011, 1, 12–18. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukui, K.; Kakiuchi, T.; Sakurai, H.; Tokudome, Y. Shotokuseki Extract Promotes Keratinocyte Differentiation Even at a Low Calcium Concentration. Appl. Sci. 2022, 12, 2270. https://doi.org/10.3390/app12052270
Tsukui K, Kakiuchi T, Sakurai H, Tokudome Y. Shotokuseki Extract Promotes Keratinocyte Differentiation Even at a Low Calcium Concentration. Applied Sciences. 2022; 12(5):2270. https://doi.org/10.3390/app12052270
Chicago/Turabian StyleTsukui, Kei, Takuya Kakiuchi, Hidetomo Sakurai, and Yoshihiro Tokudome. 2022. "Shotokuseki Extract Promotes Keratinocyte Differentiation Even at a Low Calcium Concentration" Applied Sciences 12, no. 5: 2270. https://doi.org/10.3390/app12052270
APA StyleTsukui, K., Kakiuchi, T., Sakurai, H., & Tokudome, Y. (2022). Shotokuseki Extract Promotes Keratinocyte Differentiation Even at a Low Calcium Concentration. Applied Sciences, 12(5), 2270. https://doi.org/10.3390/app12052270






