Malignant Knee Joint Effusion—A New Dimension of Laboratory Diagnostics
Abstract
:1. Introduction
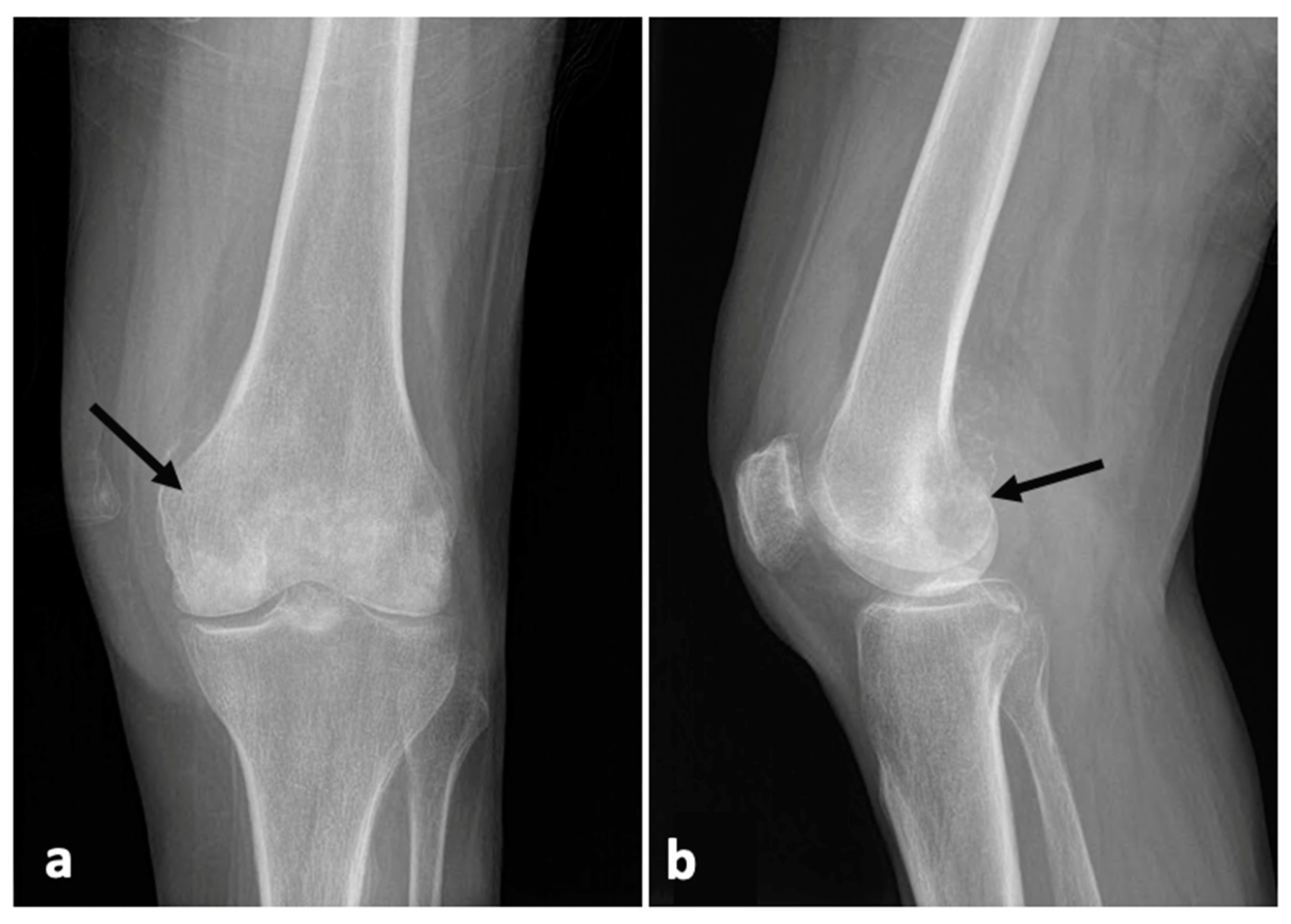
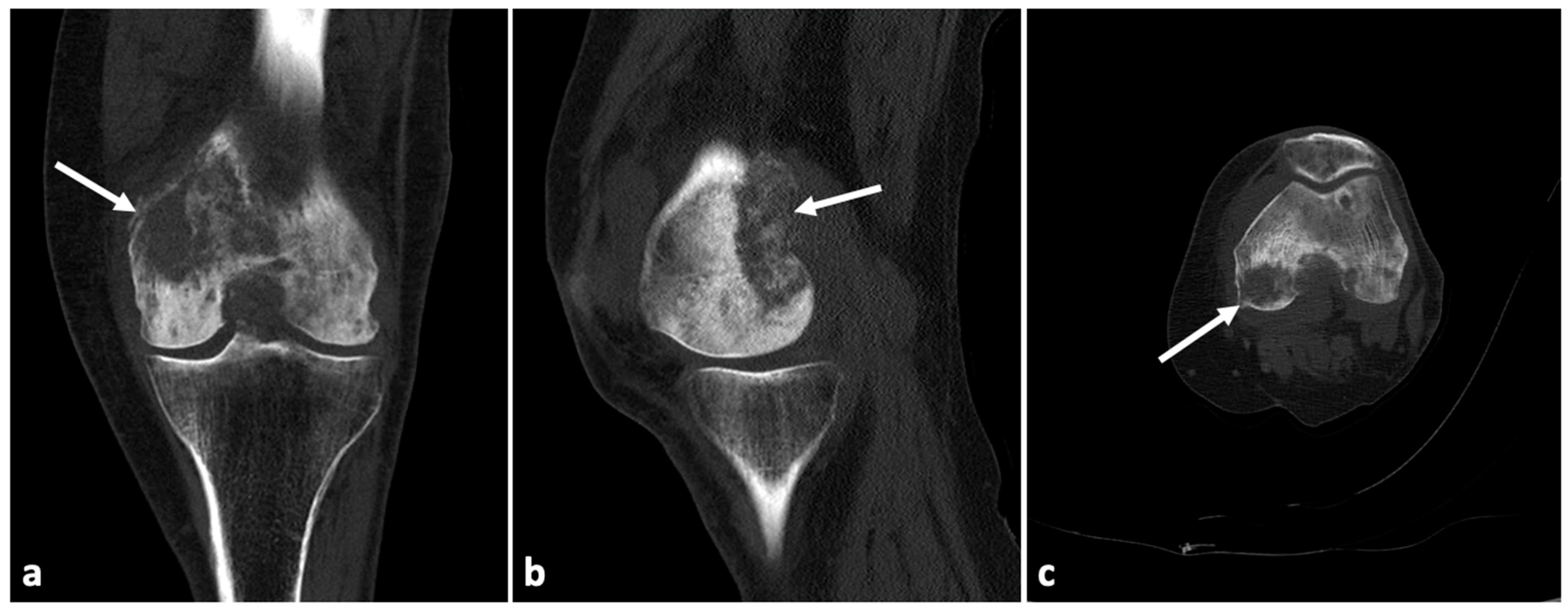
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ene, R.; Sinescu, R.D.; Ene, P.; Cîrstoiu, M.M.; Cîrstoiu, F.C. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom. J. Morphol. Embryol. 2015, 56, 169–173. [Google Scholar] [PubMed]
- Burke, C.J.; Alizai, H.; Beltran, L.S.; Regatte, R.R. MRI of synovitis and joint fluid. J. Magn. Reson. Imaging 2019, 49, 1512–1527. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Paul, S.; Kundu, C.A.; Kalonia, T. Monoarticular synovitis of knee: Dealing with the dilemma. SICOT J. 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W. Acute knee effusions: A systematic approach to diagnosis. Am. Fam. Physician 2000, 61, 2391–2400. [Google Scholar] [PubMed]
- Jokic, A.; Kopcinovic, L.M.; Culej, J.; Kocijan, I.; Bozovic, M. Laboratory testing of extravascular body fluids: National recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Part II—Synovial fluid. Biochem. Med. 2020, 30, 030501. [Google Scholar] [CrossRef]
- Hamilton, W. Cancer diagnosis in primary care. Br. J. Gen. Pract. 2010, 60, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Munn, R.K.; Pierce, S.T.; Sloan, D.; Weeks, J.A. Malignant joint effusions secondary to solid tumor metastasis. J. Rheumatol. 1995, 22, 973–975. [Google Scholar] [PubMed]
- Ferguson, J.L.; Turner, S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician 2018, 98, 205–213. [Google Scholar]
- Ibrahim, T.; Mercatali, L.; Amadori, D. Bone and cancer: The osteoncology. Clin. Cases Miner. Bone Metab. 2013, 10, 121–123. [Google Scholar]
- Ruparelia, N.; Ahmadi, H.; Cobiella, C. Metastatic adenocarcinoma of the colon presenting as a monarthritis of the hip in a young patient. World J. Surg. Oncol. 2006, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Capovilla, M.; Durlach, A.; Fourati, E.; Beucher, A.-B.; Eschard, J.-P.; Dehoux, E.; Le Noach, J.; Cucherousset, J. Chronic monoarthritis and previous history of cancer: Think about synovial metastasis. Clin. Rheumatol. 2007, 26, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.S.; Reyes, C.V.; Jensen, J.; Gattuso, P.; Sacks, R. Synovial metastasis: Diagnosis by fine-needle aspiration cytologic investigation. Diagn. Cytopathol. 1996, 15, 334–337. [Google Scholar] [CrossRef]
- Fam, A.G.; Cross, E.G. Hypertrophic osteoarthropathy, phalangeal and synovial metastases associated with bronchogenic carcinoma. J. Rheumatol. 1979, 6, 680–686. [Google Scholar] [PubMed]
- Zuber, T.J. Knee joint aspiration and injection. Am. Fam. Physician 2002, 66, 1497–1507. [Google Scholar]
- Kelbich, P.; Hejčl, A.; Selke Krulichová, I.; Procházka, J.; Hanuljaková, E.; Peruthová, J.; Koudelková, M.; Sameš, M.; Krejsek, J. Coefficient of energy balance, a new parameter for basic investigation of the cerebrospinal fluid. Clin. Chem. Lab. Med. 2014, 52, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Kelbich, P.; Hejčl, A.; Procházka, J.; Sameš, M.; Krejsek, J. Comment on the study ‘Cerebrospinal fluid lactate: Measurement of an adult reference interval’ by Sally D Slack, Paul Turley, Victoria Allgar and Ian B Holbrook. Ann. Clin. Biochem. 2016, 53, 180–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelbich, P.; Hejčl, A.; Staněk, I.; Svítilová, E.; Hanuljaková, E.; Sameš, M. Principles of the cytological-energy analysis of the extravascular body fluids. Biochem. Mol. Biol. J. 2017, 3, 6. [Google Scholar]
- Kelbich, P.; Malý, V.; Matuchová, I.; Čegan, M.; Staněk, I.; Král, J.; Karpjuk, O.; Moudrá-Wünschová, I.; Kubalík, J.; Hanuljaková, E.; et al. Cytological-energy analysis of pleural effusions. Ann. Clin. Biochem. 2019, 56, 630–637. [Google Scholar] [CrossRef]
- Matuchova, I.; Kelbich, P.; Kubalik, J.; Hanuljakova, E.; Stanek, I.; Maly, V.; Karpjuk, O.; Krejsek, J. Cytological-energy analysis of pleural effusions with predominance of neutrophils. Ther. Adv. Respir. Dis. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Kelbich, P.; Hejčl, A.; Krejsek, J.; Radovnický, T.; Matuchová, I.; Lodin, J.; Špička, J.; Sameš, M.; Procházka, J.; Hanuljaková, E.; et al. Development of the Cerebrospinal Fluid in Early Stage after Hemorrhage in the Central Nervous System. Life 2021, 11, 300. [Google Scholar] [CrossRef]
- Bielsa, S.; García-Zamalloa, A.; Monteagudo, P.; González-Sans, D.; Ascanio, D.; Esquerda, A.; Taboada-Gómez, J.; Porcel, J.M. Detection of Pleural Fluid Biochemistry Changes in Two Consecutive Thoracenteses for Differentiating Malignant from Benign Effusions. Cambios en los parámetros bioquímicos del líquido pleural entre 2 toracocentesis consecutivas para diferenciar derrames malignos de benignos. Arch. Bronconeumol. 2018, 54, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Hayem, G.; Brissaud, P.; Grossin, M.; Kahn, M.F.; Meyer, O. Monoarthritis secondary to joint metastasis. Two case reports and literature review. Jt. Bone Spine 2002, 69, 495–498. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanaskova, E.; Kelbich, P.; Cegan, M.; Novotny, T. Malignant Knee Joint Effusion—A New Dimension of Laboratory Diagnostics. Appl. Sci. 2022, 12, 994. https://doi.org/10.3390/app12030994
Vanaskova E, Kelbich P, Cegan M, Novotny T. Malignant Knee Joint Effusion—A New Dimension of Laboratory Diagnostics. Applied Sciences. 2022; 12(3):994. https://doi.org/10.3390/app12030994
Chicago/Turabian StyleVanaskova, Eliska, Petr Kelbich, Martin Cegan, and Tomas Novotny. 2022. "Malignant Knee Joint Effusion—A New Dimension of Laboratory Diagnostics" Applied Sciences 12, no. 3: 994. https://doi.org/10.3390/app12030994
APA StyleVanaskova, E., Kelbich, P., Cegan, M., & Novotny, T. (2022). Malignant Knee Joint Effusion—A New Dimension of Laboratory Diagnostics. Applied Sciences, 12(3), 994. https://doi.org/10.3390/app12030994






