Pain Relief and Antimicrobial Activity in Alveolar Osteitis after Platelet-Rich Fibrin Application—A Non-Randomized Controlled Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Method
2.1. Study Design
2.2. Patient Selection
2.3. Intervention
2.4. Assessing Impact of PRF on Pain Relief in Dry Socket
2.5. Assessing the Impact of PRF on Bacterial Activity in Extraction Wounds
2.6. Statistical Methods
3. Results
3.1. Patient Characteristics
3.2. Influence of Treatment on Intensity of Pain
3.3. Influence of Treatment on Bacteria Levels in Wounds
3.3.1. Bacterial Concentrations
3.3.2. Differences in Bacteria Concentration
3.4. Influence of Other Factors
3.5. Summary of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taberner-Vallverdú, M.; Nazir, M.; Sánchez-Garcés, M.Á.; Gay-Escoda, C. Efficacy of different methods used for dry socket management: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e633–e639. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Joshi, S.; Garad, A.; Mhatre, B.; Bagade, S.; Jain, R. A Study to Evaluate the Efficacy of Honey in the Management of Dry Socket. Contemp. Clin. Dent. 2019, 10, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Lone, P.A.; Ahmed, S.W.; Prasad, V.; Ahmed, B. Role of turmeric in management of alveolar osteitis (dry socket): A randomised clinical study. J. Oral Biol. Craniofacial Res. 2018, 8, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Mamoun, J. Dry Socket Etiology, Diagnosis, and Clinical Treatment Techniques. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Aggarwal, N.; Rastogi, S.; Choudhury, R.; Tripathi, S. Effectiveness of platelet-rich fibrin in the management of pain and delayed wound healing associated with established alveolar osteitis (dry socket). Eur. J. Dent. 2017, 11, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Shi, P.; Zhang, P.; Shen, J.; Kang, J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Olech, E.; Miloro, M. Alveolar osteitis: A comprehensive review of concepts and controversies. Int. J. Dent. 2010, 2010, 249073. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Salman, B.; Abdul Razak, N.H.; Qabbani, A.A.; Samsudin, A.R. The Efficacy of Concentrated Growth Factor in the Healing of Alveolar Osteitis: A Clinical Study. Int. J. Dent. 2020, 2020, 9038629. [Google Scholar] [CrossRef]
- Faizel, S.; Thomas, S.; Yuvaraj, V.; Prabhu, S.; Tripathi, G. Comparision between neocone, alvogyl and zinc oxide eugenol packing for the treatment of dry socket: A double blind randomised control trial. J. Maxillofac. Oral Surg. 2015, 14, 312–320. [Google Scholar] [CrossRef]
- Rastogi, S.; Choudhury, R.; Kumar, A.; Manjunath, S.; Sood, A.; Upadhyay, H. Versatility of platelet rich fibrin in the management of alveolar osteitis—A clinical and prospective study. J. Oral Biol. Craniofacial Res. 2018, 8, 188–193. [Google Scholar] [CrossRef]
- Chakravarthi, S. Platelet rich fibrin in the management of established dry socket. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 160–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamal, A.; Salman, B.; Razak, N.H.A.; Samsudin, A.B.R. A Comparative Clinical Study between Concentrated Growth Factor and Low-Level Laser Therapy in the Management of Dry Socket. Eur. J. Dent. 2020, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.S.; Singh, B.P.; Verma, V. Comparative evaluation of zinc oxide eugenol versus gelatin sponge soaked in plasma rich in growth factor in the treatment of dry socket: An initial study. Contemp. Clin. Dent. 2013, 4, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Keshini, M.P.; Shetty, S.K.; Sundar, S.; Chandan, S.N.; Manjula, S. Assessment of Healing Using Alvogyl and Platelet Rich Fibrin in Patients with Dry Socket—An Evaluative Study. Ann. Maxillofac. Surg. 2020, 10, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamed, F.S.; Tawfik, M.A.-M.; Abdelfadil, E.; Al-Saleh, M.A.Q. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Martinasso, G.; Pol, R.; Polastri, C.; Cristiano, A.; Muzio, G.; Canuto, R. The impact of plasma rich in growth factors on clinical and biological factors involved in healing processes after third molar extraction. J. Biomed. Mater. Res. A 2010, 95, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Haraji, A.; Lassemi, E.; Motamedi, M.H.; Alavi, M.; Adibnejad, S. Effect of plasma rich in growth factors on alveolar osteitis. Natl. J. Maxillofac. Surg. 2012, 3, 38–41. [Google Scholar] [CrossRef]
- Chatterjee, A.; Debnath, K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J. Indian Soc. Periodontol. 2019, 23, 322–328. [Google Scholar] [CrossRef]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Serafini, G.; Lopreiato, M.; Lollobrigida, M.; Lamazza, L.; Mazzucchi, G.; Fortunato, L.; Mariano, A.; D’abusco, A.S.; Fontana, M.; De Biase, A. Platelet Rich Fibrin (PRF) and Its Related Products: Biomolecular Characterization of the Liquid Fibrinogen. J. Clin. Med. 2020, 9, 1099. [Google Scholar] [CrossRef]
- Massagué, J.; Xi, Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Lollobrigida, M.; Fortunato, L.; Mazzucchi, G.; Lamazza, L.; Di Nardo, D.; Vozza, I.; Riminucci, M.; De Biase, A. Postextractive Alveolar Ridge Preservation Using L-PRF: Clinical and Histological Evaluation. Case Rep. Dent. 2020, 2020, 5073519. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alonso, R.; Girbau, C.; Aguirre, J.J.; Muruzabal, F.; Orive, G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin. Exp. Dermatol. 2012, 37, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Bortolin, M.; Vassena, C.; Taschieri, S.; Del Fabbro, M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013, 13, 47. [Google Scholar] [CrossRef]
- Badade, P.S.; Mahale, S.A.; Panjwani, A.A.; Vaidya, P.D.; Warang, A.D. Antimicrobial effect of platelet-rich plasma and platelet-rich fibrin. Indian J. Dent. Res. 2016, 27, 300–304. [Google Scholar] [CrossRef]
- Nagaoka, S.; Murata, S.; Kimura, K.; Mori, T.; Hojo, K. Antimicrobial activity of sodium citrate against Streptococcus pneumoniae and several oral bacteria. Lett. Appl. Microbiol. 2010, 51, 546–551. [Google Scholar] [CrossRef]
- Kour, P.; Pudakalkatti, P.S.; Vas, A.M.; Das, S.; Padmanabhan, S. Comparative Evaluation of Antimicrobial Efficacy of Platelet-rich Plasma, Platelet-rich Fibrin, and Injectable Platelet-rich Fibrin on the Standard Strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp. Clin. Dent. 2018, 9 (Suppl. 2), S325–S330. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Therapeutics of stem cells in periodontal regeneration. J. Nat. Sci. Biol. Med. 2011, 2, 38–42. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Mean | Standard Deviation | Median | Min | Max | Range | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|
| Nipas | 1.07 | 1.17 | 1 | 0 | 4 | 4 | 0 | 1.75 |
| PRF | 2.10 | 1.12 | 2 | 0 | 4 | 4 | 1 | 3.00 |
| Treatment | Mean | Standard Deviation | Median | Min | Max | Range | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|
| Nipas | 1.1 | 0.96 | 1 | 0 | 3 | 3 | 0 | 2 |
| PRF | 2.0 | 1.49 | 2 | 0 | 5 | 5 | 1 | 3 |
| Type of Bacteria | Time of Measurement | Treatment | Mean | Standard Deviation | Median | Min | Max | Range | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|---|---|
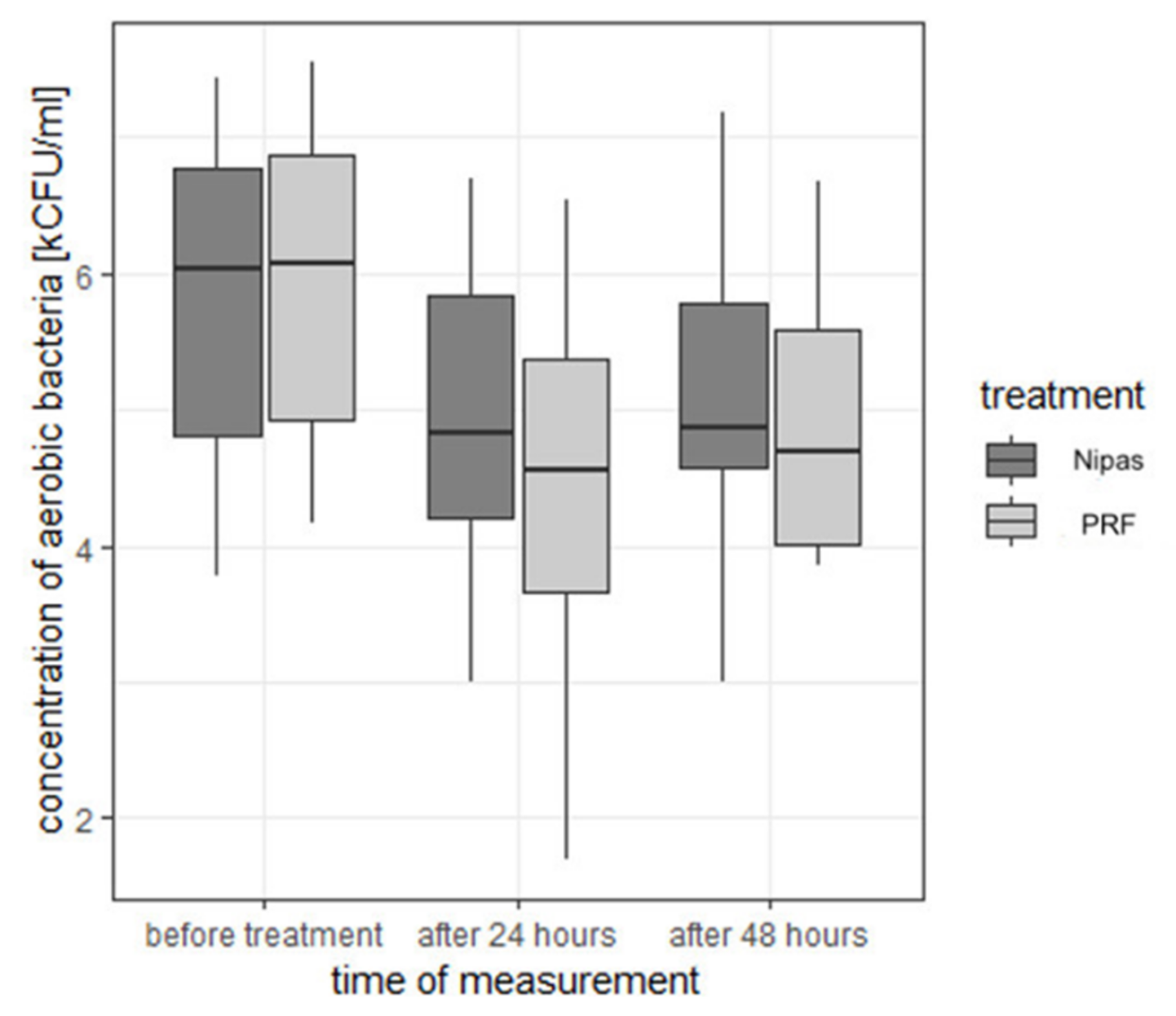
| aerobic bacteria | before treatment | Nipas | 5.83 | 1.080 | 6.03 | 3.78 | 7.43 | 3.65 | 4.81 | 6.77 |
| PRF | 5.88 | 1.051 | 6.07 | 4.16 | 7.55 | 3.39 | 4.92 | 6.86 | ||
| 24 h after treatment | Nipas | 4.96 | 1.040 | 4.83 | 3.00 | 6.70 | 3.70 | 4.21 | 5.83 | |
| PRF | 4.50 | 1.220 | 4.56 | 1.70 | 6.54 | 4.84 | 3.67 | 5.37 | ||
| 48 h after treatment | Nipas | 5.12 | 1.030 | 4.87 | 3.00 | 7.18 | 4.18 | 4.57 | 5.78 | |
| PRF | 4.85 | 0.853 | 4.68 | 3.85 | 6.68 | 2.83 | 4.00 | 5.58 | ||
| anaerobic bacteria | before treatment | Nipas | 6.07 | 0.963 | 6.16 | 4.48 | 7.68 | 3.20 | 5.17 | 6.75 |
| PRF | 5.94 | 0.989 | 6.05 | 4.33 | 7.53 | 3.20 | 5.01 | 6.80 | ||
| 24 h after treatment | Nipas | 4.96 | 1.010 | 4.93 | 3.00 | 6.48 | 3.48 | 4.05 | 5.75 | |
| PRF | 4.66 | 0.998 | 4.45 | 3.00 | 6.54 | 3.54 | 3.92 | 5.42 | ||
| 48 h after treatment | Nipas | 5.46 | 0.966 | 5.40 | 3.30 | 7.15 | 3.85 | 4.98 | 6.00 | |
| PRF | 4.95 | 0.764 | 4.68 | 4.00 | 6.52 | 2.52 | 4.30 | 5.70 | ||
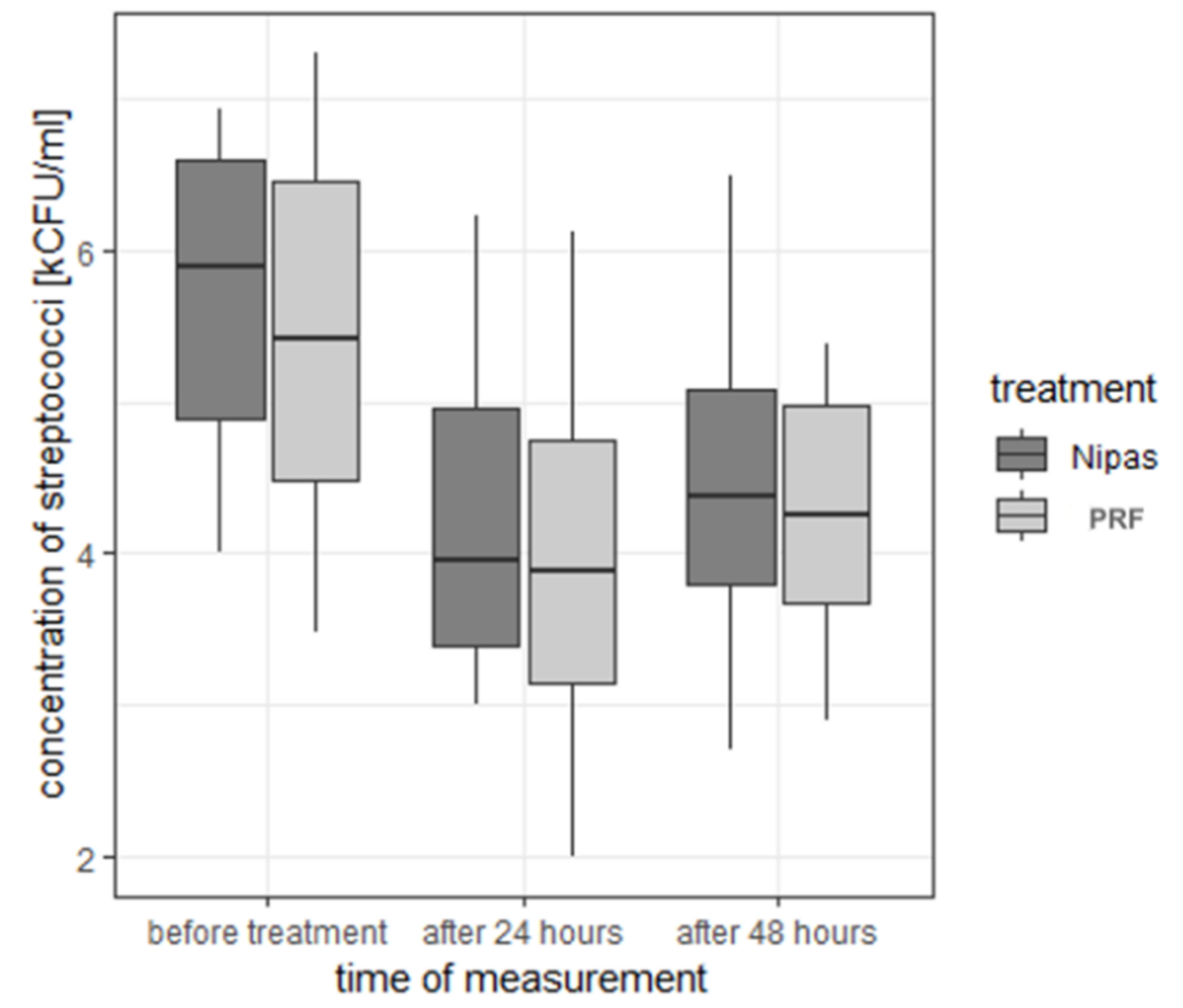
| streptococci | before treatment | Nipas | 5.74 | 0.938 | 5.89 | 4.00 | 6.92 | 2.92 | 4.89 | 6.58 |
| PRF | 5.52 | 1.069 | 5.42 | 3.48 | 7.30 | 3.82 | 4.49 | 6.45 | ||
| 24 h after treatment | Nipas | 4.27 | 1.028 | 3.96 | 3.00 | 6.23 | 3.23 | 3.39 | 4.95 | |
| PRF | 3.96 | 1.148 | 3.88 | 2.00 | 6.12 | 4.12 | 3.13 | 4.74 | ||
| 48 h after treatment | Nipas | 4.41 | 0.882 | 4.37 | 2.70 | 6.49 | 3.79 | 3.79 | 5.07 | |
| PRF | 4.29 | 0.750 | 4.25 | 2.89 | 5.38 | 2.49 | 3.68 | 4.97 | ||
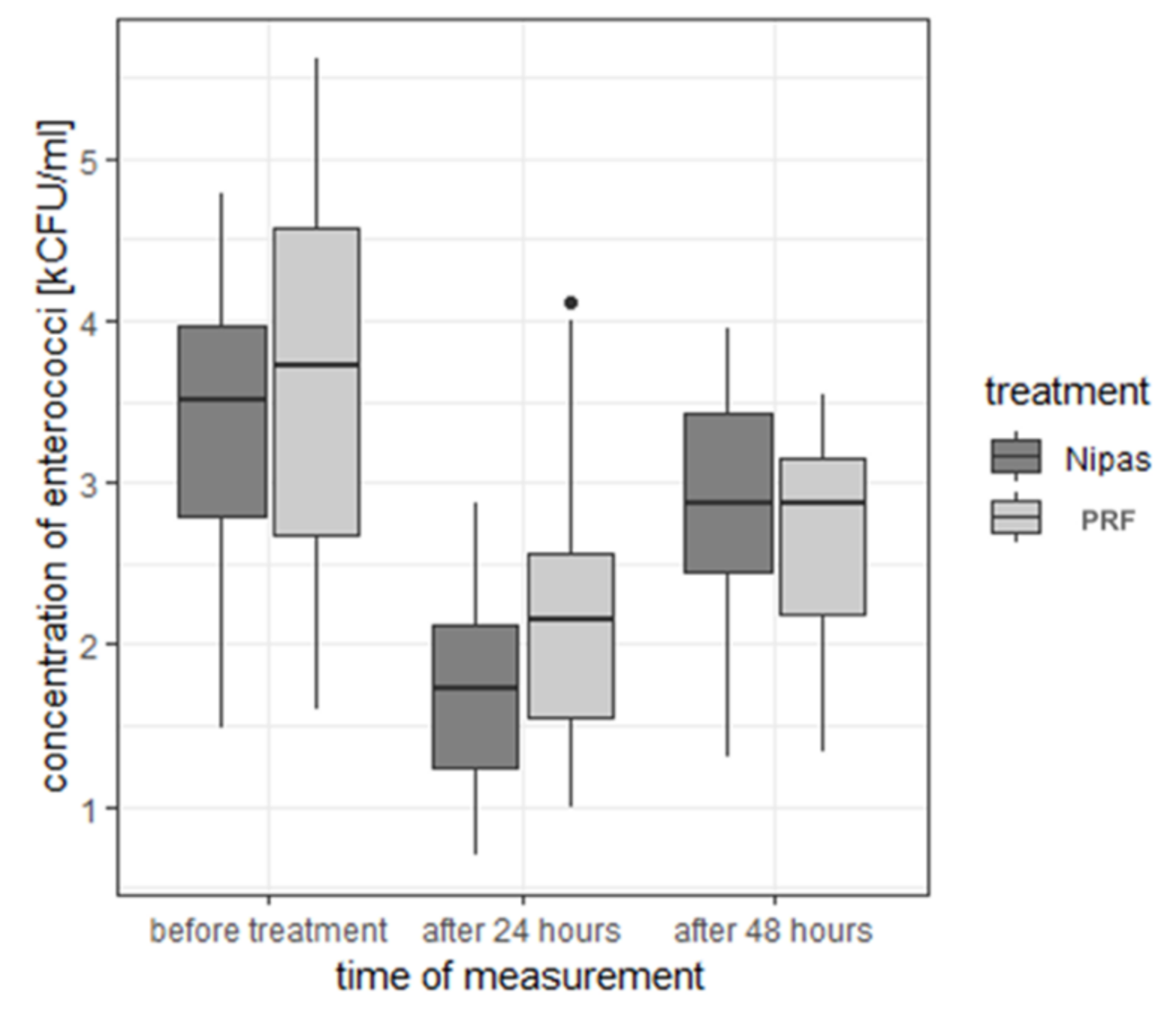
| enterococci | before treatment | Nipas | 3.31 | 0.810 | 3.50 | 1.48 | 4.78 | 3.30 | 2.79 | 3.97 |
| PRF | 3.64 | 1.240 | 3.73 | 1.60 | 5.62 | 4.02 | 2.67 | 4.57 | ||
| 24 h after treatment | Nipas | 1.71 | 0.621 | 1.73 | 0.70 | 2.88 | 2.18 | 1.23 | 2.12 | |
| PRF | 2.21 | 0.778 | 2.16 | 1.00 | 4.11 | 3.11 | 1.55 | 2.56 | ||
| 48 h after treatment | Nipas | 2.85 | 0.627 | 2.88 | 1.30 | 3.95 | 2.65 | 2.45 | 3.42 | |
| PRF | 2.67 | 0.606 | 2.87 | 1.33 | 3.54 | 2.21 | 2.19 | 3.16 | ||
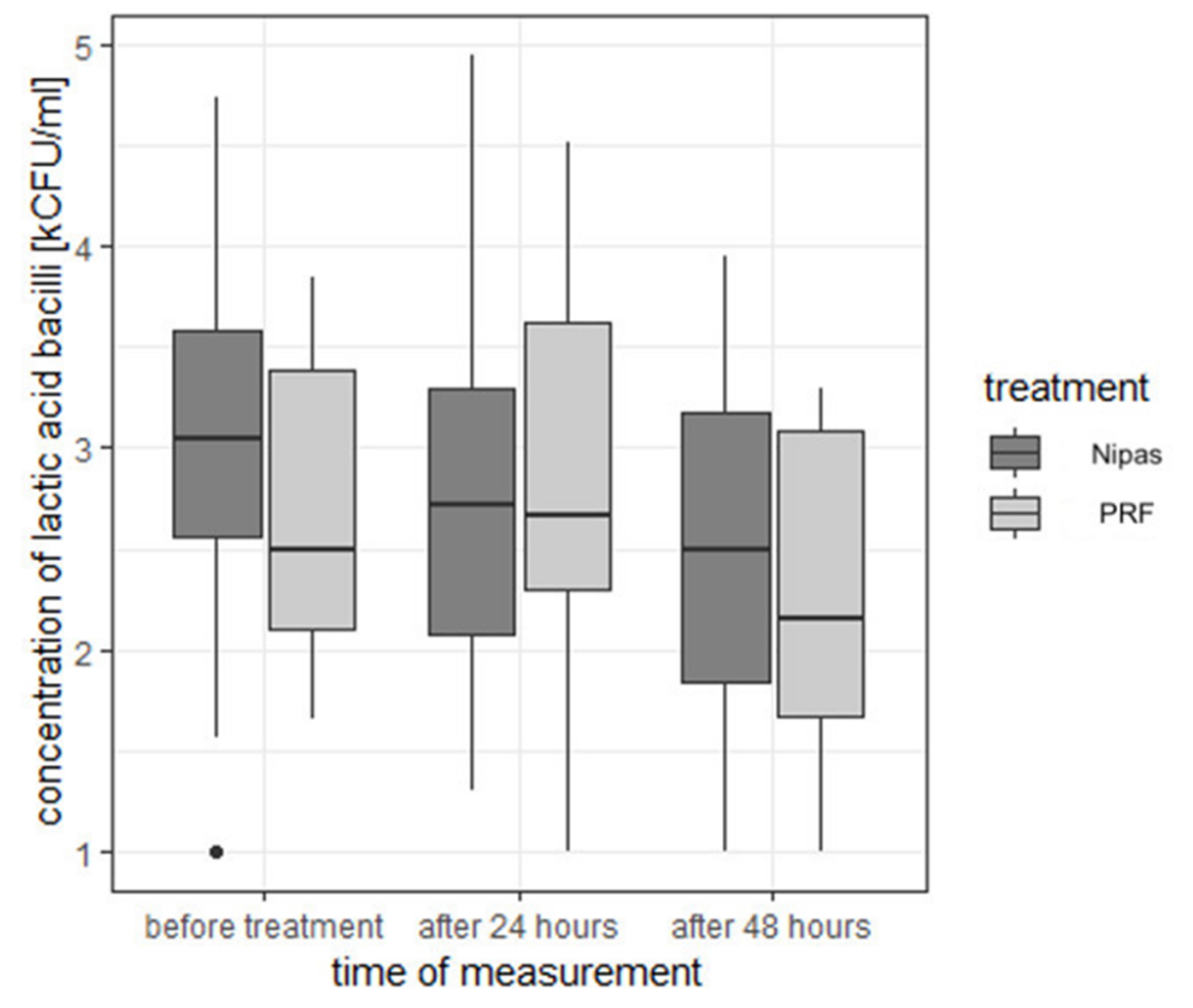
| lactic acid bacilli | before treatment | Nipas | 3.05 | 0.914 | 3.04 | 1.00 | 4.74 | 3.74 | 2.56 | 3.59 |
| PRF | 2.69 | 0.722 | 2.50 | 1.65 | 3.85 | 2.20 | 2.10 | 3.38 | ||
| 24 h after treatment | Nipas | 2.75 | 0.897 | 2.72 | 1.30 | 4.95 | 3.65 | 2.07 | 3.29 | |
| PRF | 2.86 | 0.933 | 2.67 | 1.00 | 4.51 | 3.51 | 2.30 | 3.62 | ||
| 48 h after treatment | Nipas | 2.53 | 0.835 | 2.50 | 1.00 | 3.95 | 2.95 | 1.84 | 3.18 | |
| PRF | 2.22 | 0.734 | 2.15 | 1.00 | 3.29 | 2.29 | 1.67 | 3.08 |
| Type of Bacteria | Interval | Treatment | Mean | Standard Deviation | Median | Min | Max | Range | Q1 | Q3 |
|---|---|---|---|---|---|---|---|---|---|---|
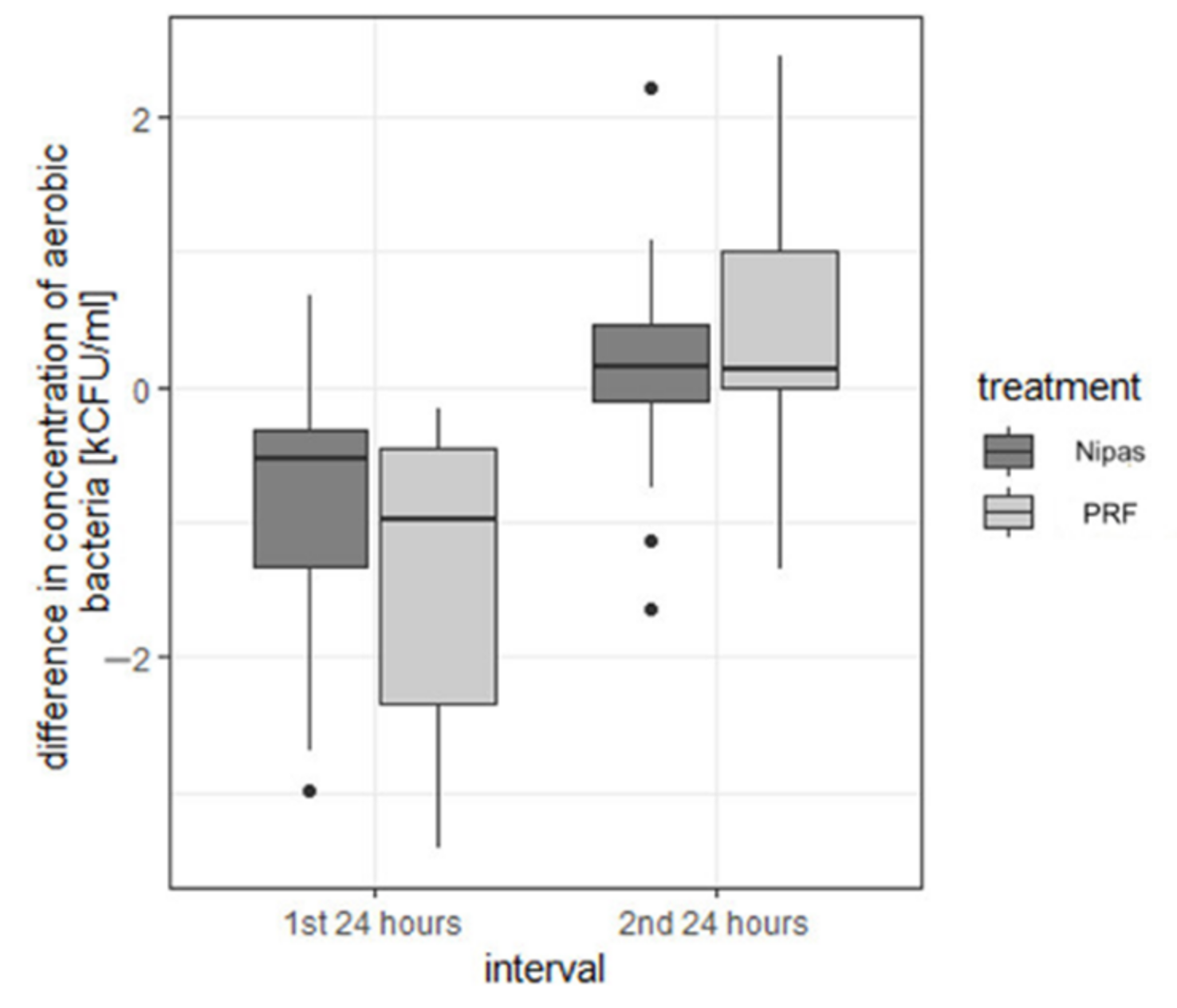
| aerobic bacteria | first 24 h | Nipas | −0.876 | 0.874 | −0.540 | −2.98 | 0.67 | 3.65 | −1.320 | −0.323 |
| PRF | −1.379 | 1.079 | −0.975 | −3.41 | −0.16 | 3.25 | −2.350 | −0.447 | ||
| second 24 h | Nipas | 0.168 | 0.710 | 0.145 | −1.65 | 2.22 | 3.87 | −0.097 | 0.458 | |
| PRF | 0.347 | 0.963 | 0.130 | −1.35 | 2.45 | 3.80 | −0.007 | 1.008 | ||
| anaerobic bacteria | first 24 h | Nipas | −1.105 | 0.779 | −0.920 | −2.80 | −0.11 | 2.69 | −1.360 | −0.545 |
| PRF | −1.279 | 1.109 | −0.680 | −3.83 | 0.10 | 3.93 | −1.900 | −0.485 | ||
| second 24 h | Nipas | 0.492 | 0.795 | 0.730 | −1.02 | 2.33 | 3.35 | 0.035 | 0.990 | |
| PRF | 0.284 | 0.556 | 0.210 | −0.85 | 2.18 | 3.03 | 0.050 | 0.440 | ||
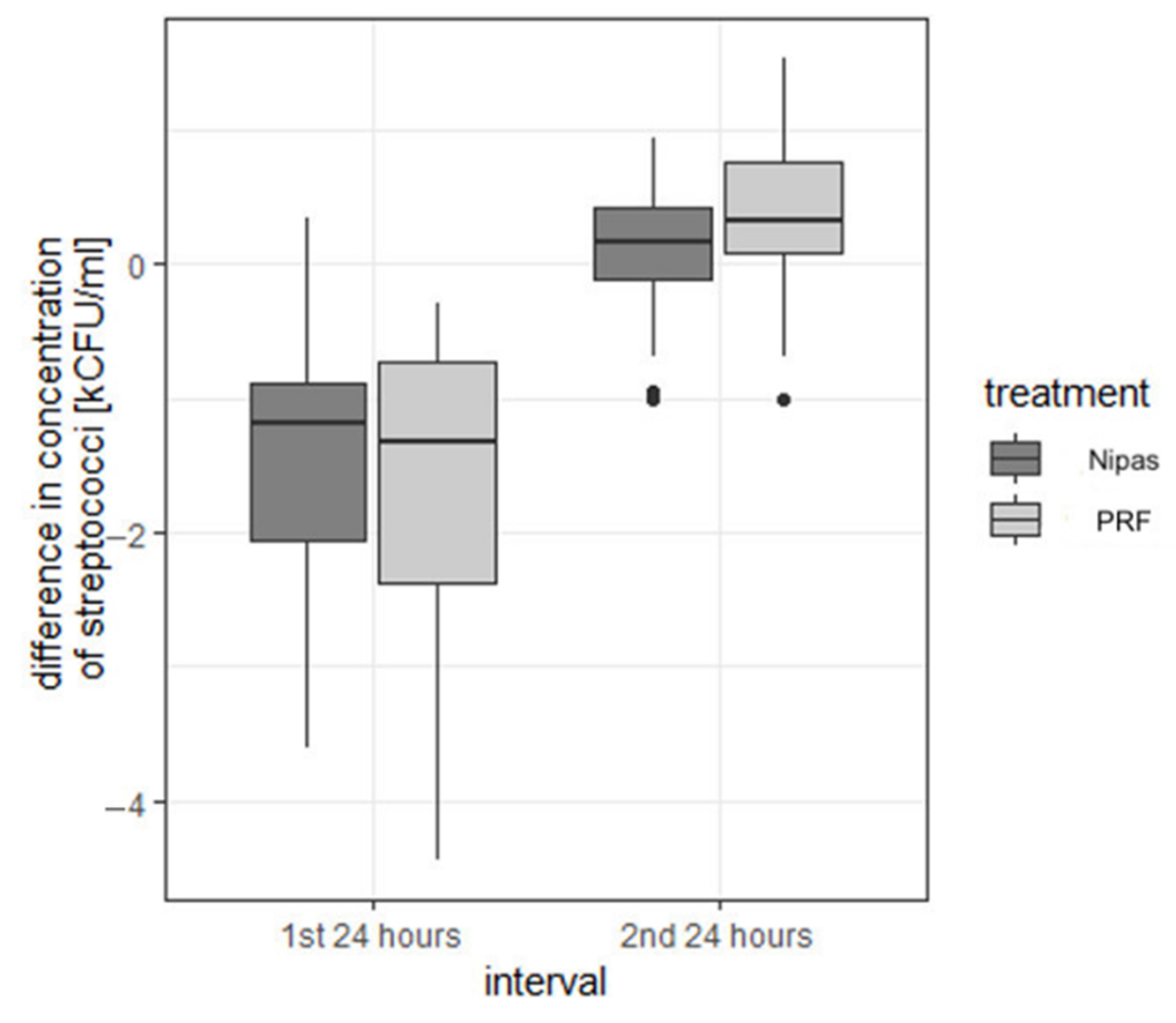
| streptococci | first 24 h | Nipas | −1.469 | 0.884 | −1.180 | −3.60 | 0.34 | 3.94 | −2.058 | −0.895 |
| PRF | −1.559 | 1.052 | −1.330 | −4.44 | −0.30 | 4.14 | −2.383 | −0.735 | ||
| second 24 h | Nipas | 0.141 | 0.493 | 0.170 | −1.00 | 0.94 | 1.94 | −0.115 | 0.432 | |
| PRF | 0.325 | 0.529 | 0.320 | −1.01 | 1.54 | 2.55 | 0.085 | 0.767 | ||
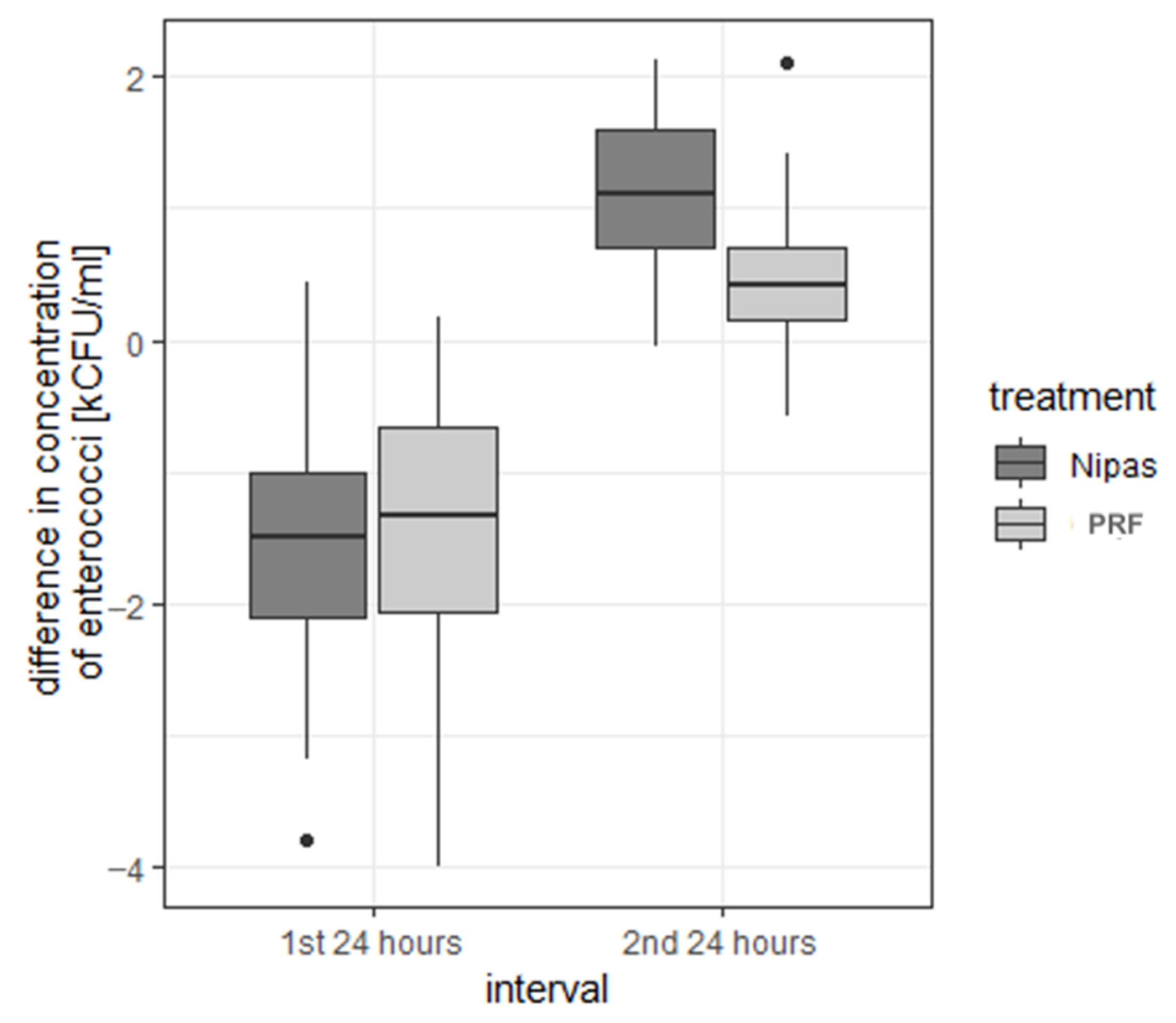
| enterococci | first 24 h | Nipas | −1.597 | 0.932 | −1.495 | −3.78 | 0.45 | 4.23 | −2.095 | −0.995 |
| PRF | −1.433 | 1.090 | −1.320 | −3.99 | 0.17 | 4.16 | −2.065 | −0.663 | ||
| second 24 h | Nipas | 1.137 | 0.514 | 1.120 | −0.04 | 2.13 | 2.17 | 0.697 | 1.590 | |
| PRF | 0.459 | 0.509 | 0.420 | −0.57 | 2.10 | 2.67 | 0.160 | 0.698 | ||
| lactic acid bacilli | first 24 h | Nipas | −0.306 | 0.575 | −0.155 | −2.74 | 0.71 | 3.45 | −0.302 | −0.103 |
| PRF | 0.175 | 0.411 | 0.210 | −1.10 | 1.40 | 2.50 | 0.025 | 0.272 | ||
| second 24 h | Nipas | −0.218 | 0.256 | −0.135 | −1.03 | 0.16 | 1.19 | −0.240 | −0.100 | |
| PRF | −0.642 | 0.575 | −0.655 | −2.33 | 0.67 | 3.00 | −0.848 | −0.335 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chybicki, D.; Janas-Naze, A. Pain Relief and Antimicrobial Activity in Alveolar Osteitis after Platelet-Rich Fibrin Application—A Non-Randomized Controlled Study. Appl. Sci. 2022, 12, 1324. https://doi.org/10.3390/app12031324
Chybicki D, Janas-Naze A. Pain Relief and Antimicrobial Activity in Alveolar Osteitis after Platelet-Rich Fibrin Application—A Non-Randomized Controlled Study. Applied Sciences. 2022; 12(3):1324. https://doi.org/10.3390/app12031324
Chicago/Turabian StyleChybicki, Damian, and Anna Janas-Naze. 2022. "Pain Relief and Antimicrobial Activity in Alveolar Osteitis after Platelet-Rich Fibrin Application—A Non-Randomized Controlled Study" Applied Sciences 12, no. 3: 1324. https://doi.org/10.3390/app12031324
APA StyleChybicki, D., & Janas-Naze, A. (2022). Pain Relief and Antimicrobial Activity in Alveolar Osteitis after Platelet-Rich Fibrin Application—A Non-Randomized Controlled Study. Applied Sciences, 12(3), 1324. https://doi.org/10.3390/app12031324






