Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Mentha arvensis (MA) Leaf Extract
2.3. Preparation of Green Synthesized ZnO Particles
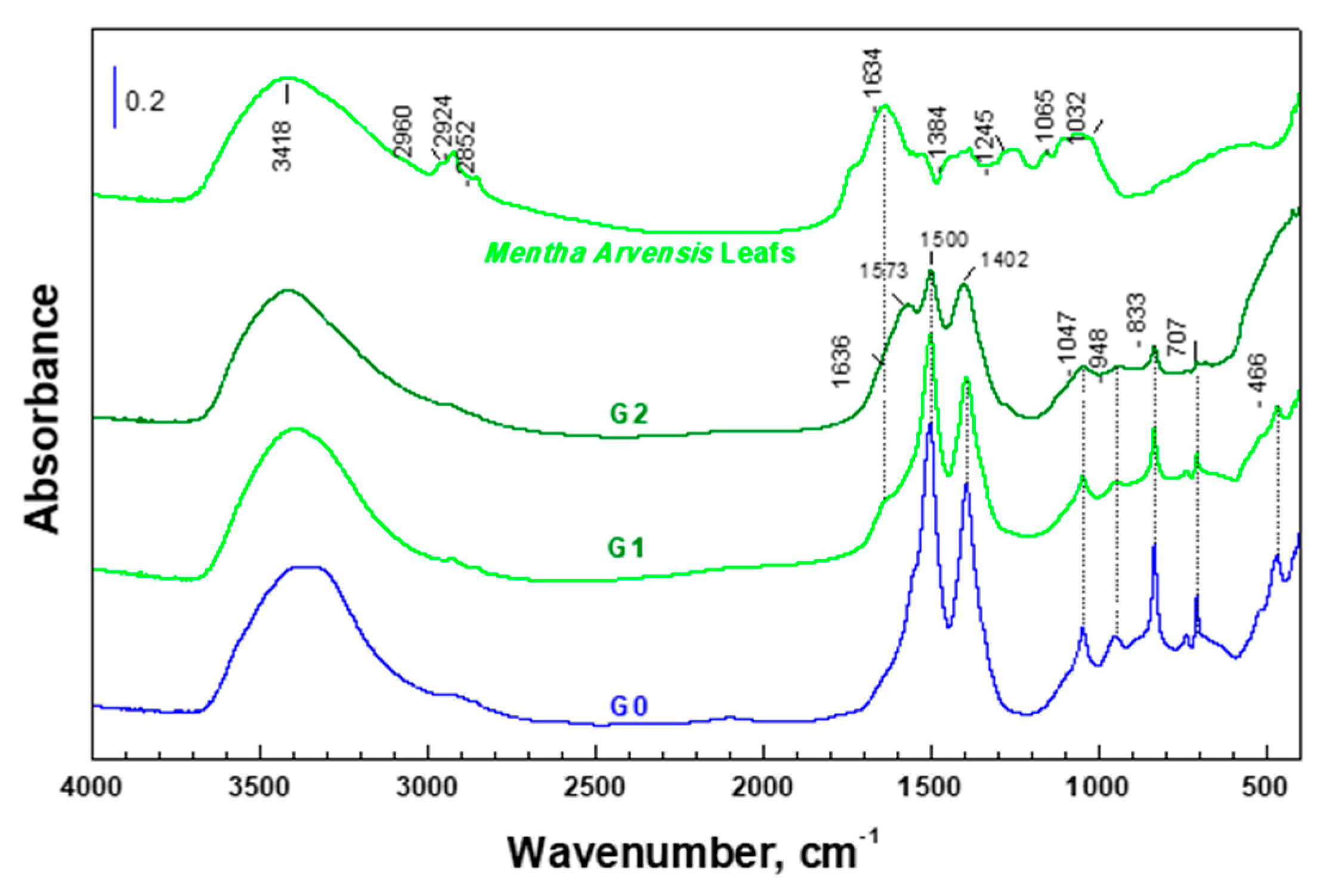
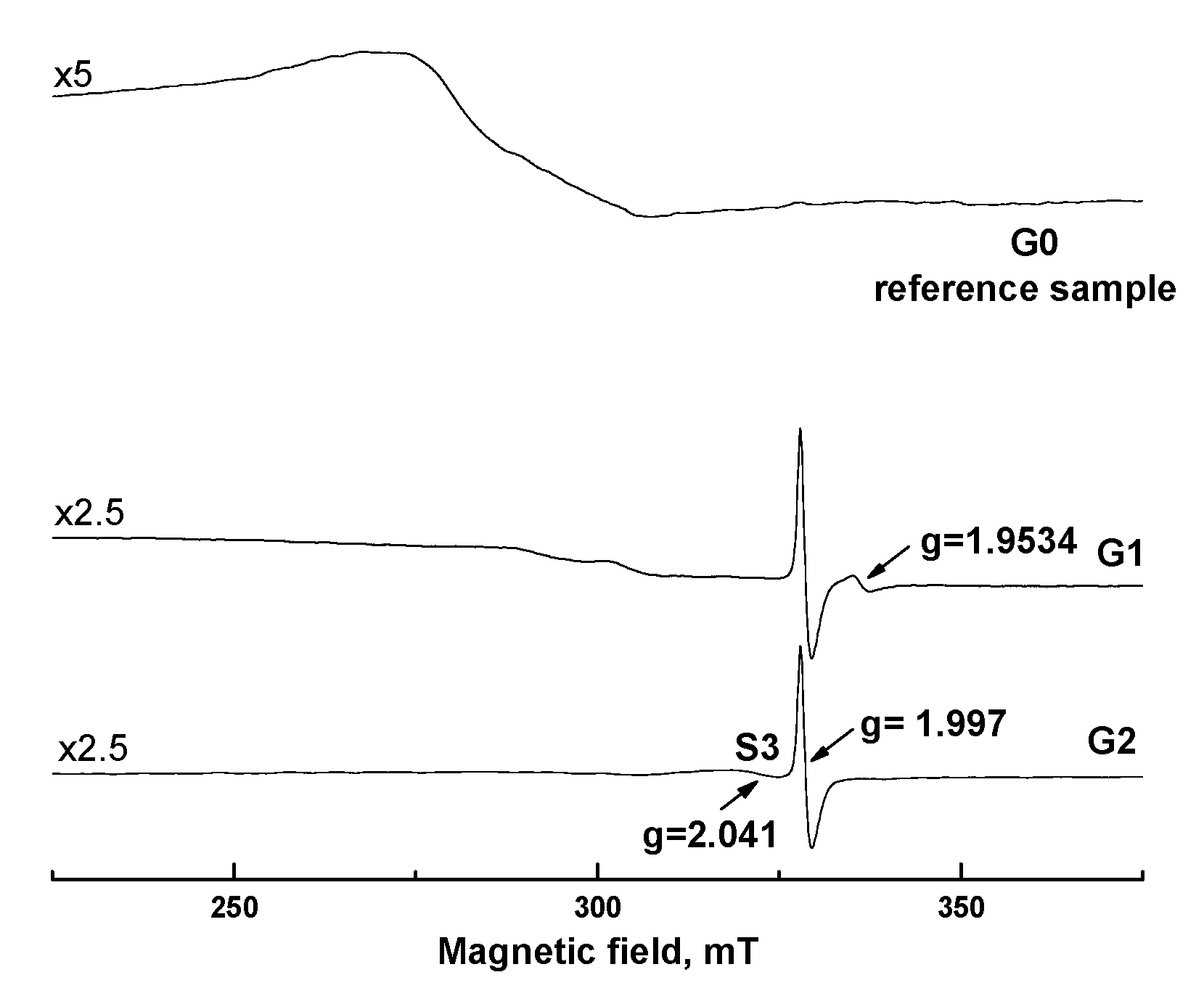
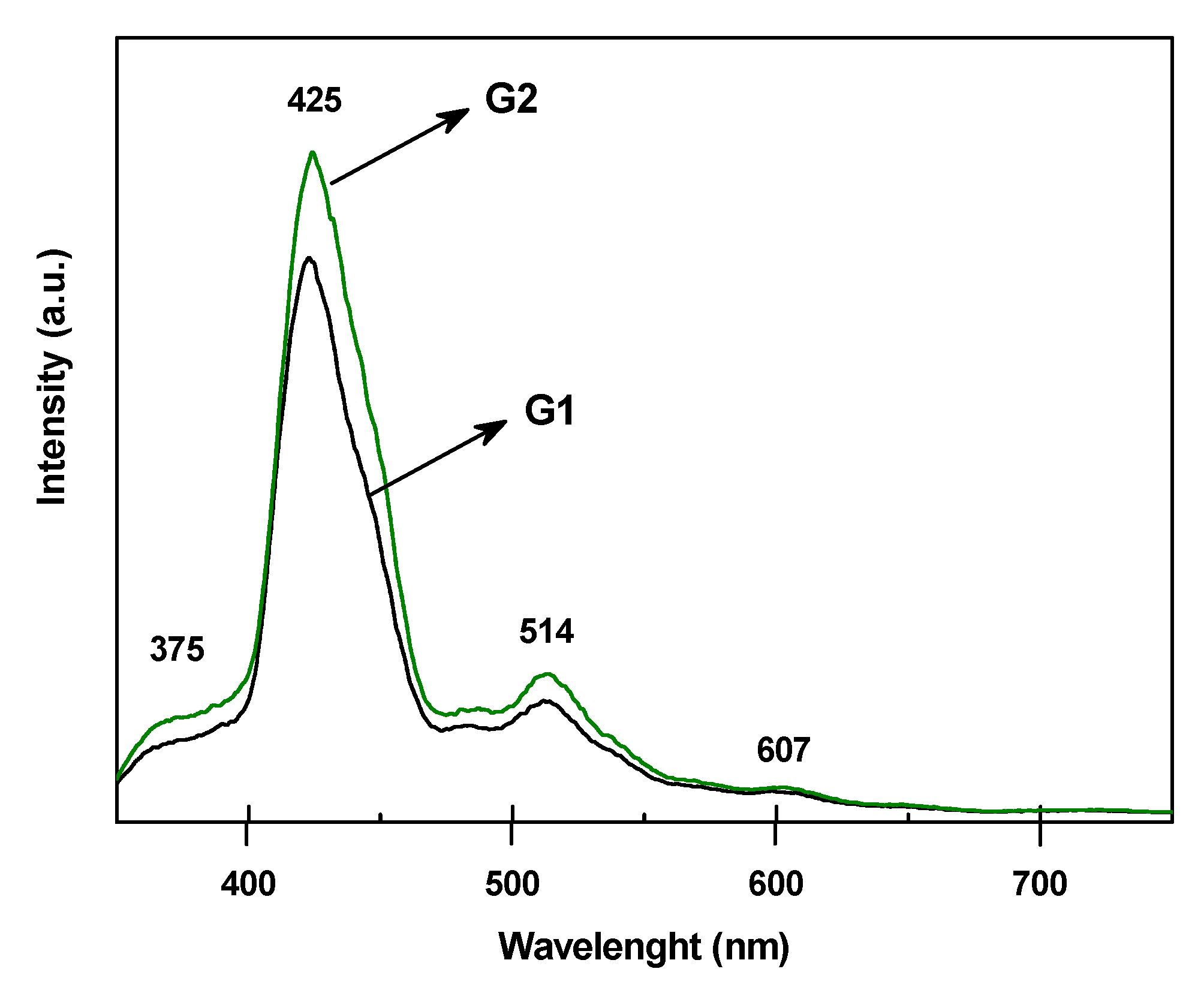
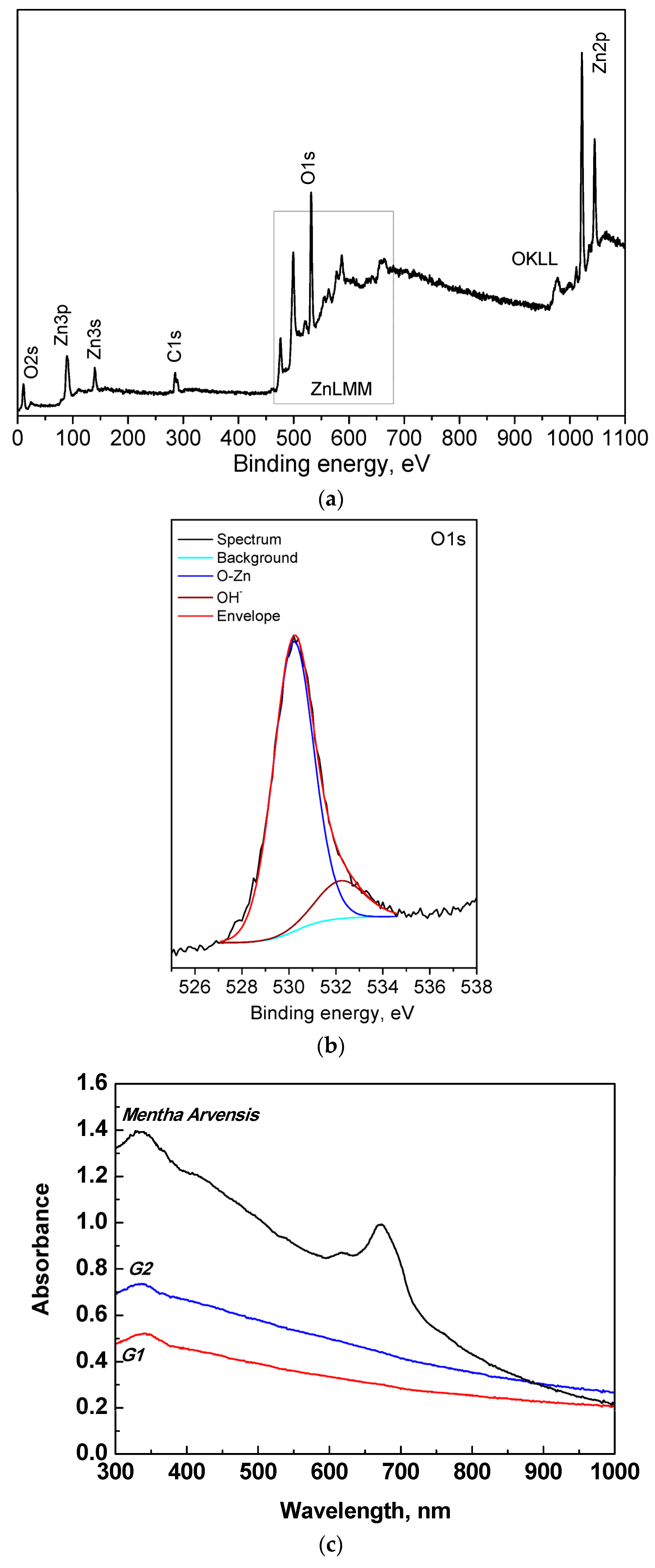
2.4. Characterization of the Samples
2.5. Photocatalytic Activity Tests
2.6. Recyclability Tests
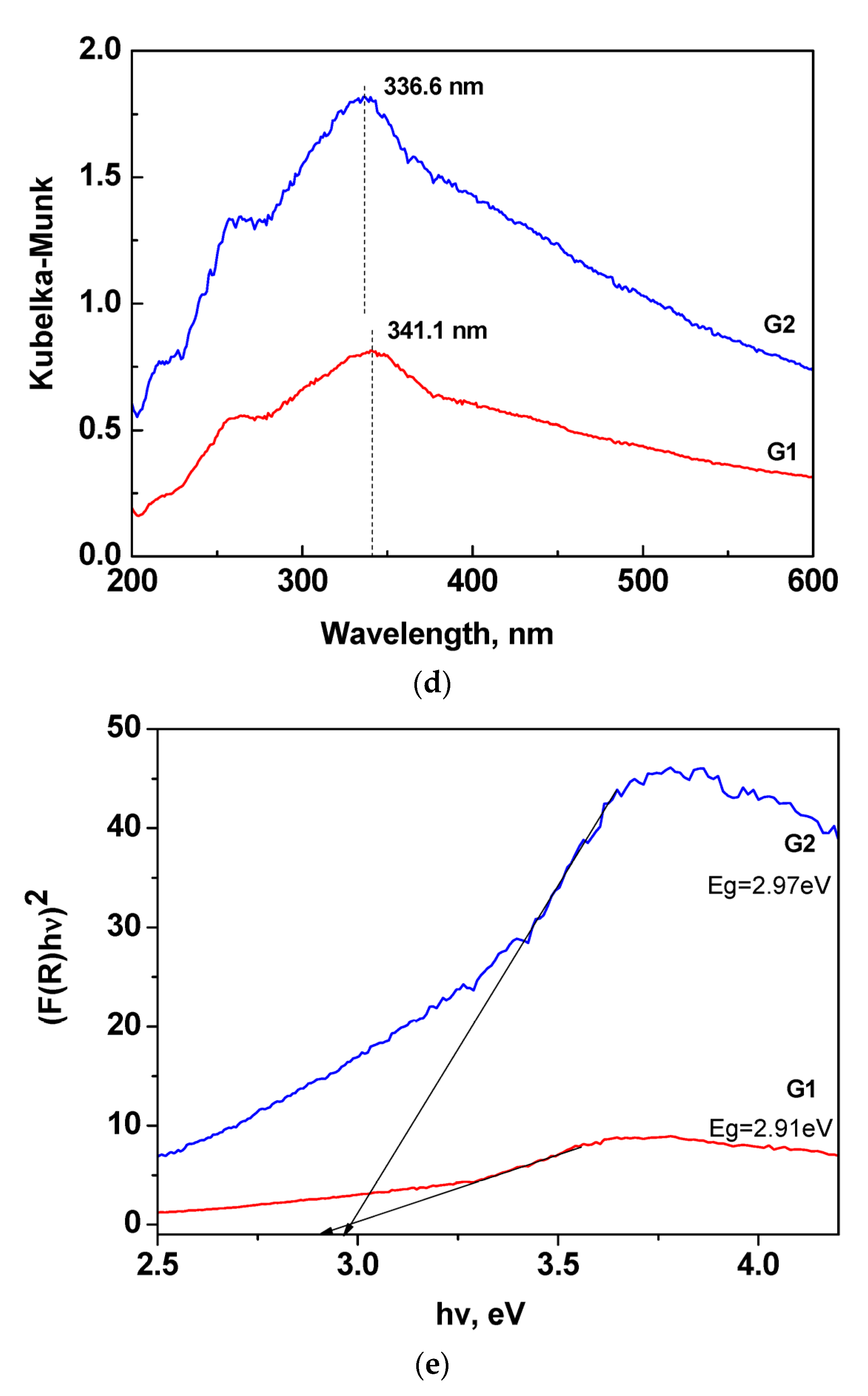
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi B 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Djurišić, A.; Ng, A.M.C.; Chen, X. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Bu, D.; Batmunkh, M.; Zhang, Y.; Li, Y.; Qian, B.; Lan, Y.; Hou, X.; Li, S.; Jia, B.; Song, X.-M.; et al. Rechargeable sunlight-promoted Zn-air battery constructed by bifunctional oxygen photoelectrodes: Energy-band switching between ZnO/Cu2O and ZnO/CuO in charge-discharge cycles. Chem. Eng. J. 2021, 133559. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Z.; Wu, M.; Liu, H.; Zhang, X.; Wang, Z.; Wang, Z.L.; Li, L. Enhanced Piezo-Photoelectric Catalysis with Oriented Carrier Migration in Asymmetric Au−ZnO Nanorod Array. Small 2020, 16, e1907603. [Google Scholar] [CrossRef]
- Gao, G.; Yu, L.; Vinu, A.; Shapter, J.G.; Batmunkh, M.; Shearer, C.J.; Yin, T.; Huang, P.; Cui, D. Synthesis of ultra-long hierarchical ZnO whiskers in a hydrothermal system for dye-sensitised solar cells. RSC Adv. 2016, 6, 109406–109413. [Google Scholar] [CrossRef]
- Laurent, S.; Boutry, S.; Muller, R. Chapter 1—Metal Oxide Particles and Their Prospects for Applications. In Iron Oxide Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–42. [Google Scholar] [CrossRef]
- Samad, U.A.; Alam, M.A.; Anis, A.; Sherif, E.M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of Incorporated ZnO Nanoparticles on the Corrosion Performance of SiO2 Nanoparticle-Based Mechanically Robust Epoxy Coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceram. Int. 2013, 39, 9803–9808. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Cordero-Arias, L.; Virtanen, S.; Misra, S.K.; Valsami-Jones, E.; Tuchscherr, L.; Rutkowski, B.; Górecki, K.; Bała, P.; Czyrska-Filemonowicz, A.; et al. Electrophoretic deposition of organic/inorganic composite coatings containing ZnO nanoparticles exhibiting antibacterial properties. Mater. Sci. Eng. C 2017, 77, 780–789. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Goudouri, O.; Misra, S.; Gilabert, J.; Valsami-Jones, E.; Sanchez, E.; Virtanen, S.; Boccaccini, A. Electrophoretic deposition of ZnO/alginate and ZnO-bioactive glass/alginate composite coatings for antimicrobial applications. Mater. Sci. Eng. C 2015, 55, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Penchev, H.; Zaharieva, K.; Milenova, K.; Ublekov, F.; Dimova, S.; Budurova, D.; Staneva, M.; Stambolova, I.; Sinigersky, V.; Blaskov, V. Novel meta- and AB-Polybenzimidazole/Zinc oxide polymer hybrid nanomaterials for photocatalytic degradation of organic dyes. Mater. Lett. 2018, 230, 187–190. [Google Scholar] [CrossRef]
- Dimova, S.; Zaharieva, K.; Ublekov, F.; Kyulavska, M.; Stambolova, I.; Blaskov, V.; Nihtianova, D.; Markov, P.; Penchev, H. Novel dye degradation photocatalyst nanocomposite powders based on polydiphenylacetylene-zinc oxide in polystyrene matrix. Mat. Lett. 2020, 269, 127683. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2020, 609, 125741. [Google Scholar] [CrossRef]
- McCluskey, M.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Stambolova, I.; Blaskov, V.; Stoyanova, D.; Avramova, I.; Dimitrov, L.; Milenova, K.; Balashev, K.; Simeonova, S.; Tzonev, A.; Aleksandrov, L.; et al. Dependence of the textural properties and surface species of ZnO photocatalytic materials on the type of precipitating agent used in the hydrothermal synthesis. Bull. Mater. Sci. 2017, 40, 483–492. [Google Scholar] [CrossRef][Green Version]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C.; Zhang, W.; Cai, S.; Yang, Z.; Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J. Hazard. Mater. 2007, 141, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Zhu, H.W.; Tang, W.; Li, P.G. Propeller-Shaped ZnO Nanostructures Obtained by Chemical Vapor Deposition: Photoluminescence and Photocatalytic Properties. J. Nanomater. 2012, 2012, 594290. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Jhun, C. ZnO Nanospheres Fabricated by Mechanochemical Method with Photocatalytic Properties. Catalysts 2021, 11, 572. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, Y. Photocatalytic degradation for methylene blue using zinc oxide prepared by codeposition and sol–gel methods. J. Hazard. Mater. 2008, 152, 172–175. [Google Scholar] [CrossRef]
- Lee, Y.; Fujimoto, T.; Yamanaka, S.; Kuga, Y. Evaluation of photocatalysis of Au supported ZnO prepared by the spray pyrolysis method. Adv. Powder Technol. 2021, 32, 1619–1626. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2021, 160, 110353. [Google Scholar] [CrossRef]
- Blaskov, V.; Stambolova, I.; Dimitrov, L.; Shipochka, M.; Stoyanova, D.; Eliyas, A. Nanosized Zn2SnO4 powders synthesized by coprecipitation and consecutive hydrothermal treatment in two different alkaline media. Bulg. Chem. Commun. 2017, 50, 58–62. [Google Scholar]
- Basnet, P.; Chatterjee, S. Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis—A systematic review. Nano-Struct. Nano-Objects 2020, 22, 100426. [Google Scholar] [CrossRef]
- Huang, Y.; Haw, C.Y.; Zheng, Z.; Kang, J.; Zheng, J.; Wang, H. Biosynthesis of Zinc Oxide Nanomaterials from Plant Extracts and Future Green Prospects: A Topical Review. Adv. Sustain. Syst. 2021, 5, 2000266. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-Mediated Biosynthesis and Photocatalysis Activities of Zinc Oxide Nanoparticles: A Prospect towards Dyes Mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Azab, W.I.M.E.; Ali, H.R.; Mansour, M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045012. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.-S.; Watanabe, A. Green synthesis of zinc oxide nanoparticles using aqueous extract of Garcinia mangostana fruit pericarp and their photocatalytic activity. Bull. Mater. Sci. 2018, 41, 50. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P.; Li, X.; Batoo, K.M.; Imran, A.; Kulshrestha, S.; Kumar, R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. 2019, 12, 444–457. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Bhushana, N.; Sharma, S. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Ahmad, W.; Kumar, K.; Soni, S. Green synthesis if titanium dioxide (TiO2) nanoparticles by using Mentha Arvenis leafs extract and its antimicrobial properties. Inorg. Nanometal. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Babu, P.J.; Sharma, P.; Borthakur, B.B.; Das, R.K.; Nahar, P.; Bora, U. Synthesis of Gold Nanoparticles Using Mentha arvensis Leaf Extract. Int. J. Green Nanotechnol. Biomed. 2010, 2, 62–68. [Google Scholar] [CrossRef]
- Manjula, R.; Prasad, B.D.; Vidya, Y.; Nagabhushana, H.; Anantharaju, K. Mentha Arvensis mediated synthesis and characterization of zinc oxide nanoparticles for energy applications. Mater. Today Proc. 2021, 46, 6051–6055. [Google Scholar] [CrossRef]
- Mohammadi, F.M.; Ghasemi, N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J. Nanostruct. Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Raj, G.A. Bio-approach: Plant mediated synthesis of ZnO nanoparticles and their catalytic reduction of methylene blue and antimicrobial activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder Cell for Windows, Federal Institute for Materials Research and Testing; International Union of Crystallography: Berlin, Germany, 2000. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A. Synthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Lippia adoensis (Koseret) and Evaluation of Its Antibacterial Activity. J. Chem. 2020, 2020, 7459042. [Google Scholar] [CrossRef]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolia leaf extract: Characterization and their application as anti-bacterial agents. J. Mater. Sci. Mater. Electron. 2018, 29, 13596–13605. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.; Garadkar, K.M. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Pramila, D.M.; Xavier, R.; Marimuthu, K.; Kathiresan, S.; Khoo, M.L.; Senthilkumar, M.; Sathya, K.; Sreeramanan, S. Phytochemical analysis and antimicrobial potential of methanolic leaf extract of peppermint (Mentha piperita: Lamiaceae). J. Med. Plants Res. 2012, 6, 331–335. [Google Scholar] [CrossRef]
- Music, S.; Popović, S.; Maljković, M.; Dragčević, D. Influence of synthesis procedure on the formation and properties of zinc oxide. J. Alloys Compd. 2002, 347, 324–332. [Google Scholar] [CrossRef]
- Liewhiran, C.; Seraphin, S.; Phanichphant, S. Synthesis of nano-sized ZnO powders by thermal decomposition of zinc acetate using Broussonetia papyrifera (L.) Vent pulp as a dispersant. Curr. Appl. Phys. 2006, 6, 499–502. [Google Scholar] [CrossRef]
- Jakes, P.; Erdem, E. Finite size effects in ZnO nanoparticles: An electron paramagnetic resonance (EPR) analysis. Phys. Status solidi (RRL) Rapid Res. Lett. 2011, 5, 56–58. [Google Scholar] [CrossRef]
- Krasil’nikov, V.; Dyachkova, T.; Tyutyunnik, A.; Gyrdasova, O.; Melkozerova, M.; Baklanova, I.; Perevozchikova, Y.A.; Emelyanova, S.; Weber, H.; Marchenkov, V. Magnetic and optical properties as well as EPR studies of polycrystalline ZnO synthesized from different precursors. Mater. Res. Bull. 2017, 97, 553–559. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, X.; Qin, G.; Li, H.; Xiong, Y.; Ye, L.; Ruan, H.; Tong, C.; Kong, C.; et al. Non-axial NO-VZn shallow acceptor complexes in nitrogen implanted p-type ZnO thin films. Appl. Surf. Sci. 2020, 529, 147168. [Google Scholar] [CrossRef]
- Smith, J.; Vehse, W. ESR of electron irradiated ZnO confirmation of the F+ center. Phys. Lett. A 1970, 31, 147–148. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Bazta, O.; Urbieta, A.; Piqueras, J.; Fernández, P.; Addou, M.; Calvino, J.; Hungría, A. Influence of yttrium doping on the structural, morphological and optical properties of nanostructured ZnO thin films grown by spray pyrolysis. Ceram. Int. 2018, 45, 6842–6852. [Google Scholar] [CrossRef]
- Rainey, K.; Chess, J.; Eixenberger, J.; Tenne, D.A.; Hanna, C.B.; Punnoose, A. Defect induced ferromagnetism in undoped ZnO nanoparticles. J. Appl. Phys. 2014, 115, 17D727. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Kuo, S.; Liu, C.; Huang, J.; Yao, W.; Wu, Y. Tuning of crystal quality and optical properties of hydrothermally synthesized ZnO nanorods by magnetic field. Mater. Chem. Phys. 2014, 148, 1113–1118. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.H.; Djurišić, A.B.; Liu, Z.T.; Xie, M.H.; Shi, S.L.; Xu, S.; Chan, W.K. Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- Hanrahan, P.; Krueger, W. Reflection from layered surfaces due to subsurface scattering. In Proceedings of the 20th Annual Conference on Computer Graphics and Interactive Techniques, Anaheim, CA, USA, 2–6 August 1993; pp. 165–174. [Google Scholar]
- Suwanboon, S. Structural and Optical Properties of Nanocrystalline ZnO Powder from Sol-Gel Method. Sci. Asia 2008, 34, 031–034. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Alharthi, M.; Ismail, I.; Bellucci, S.; Khdary, N.; Salam, M.A. Biosynthesis Microwave-Assisted of Zinc Oxide Nanoparticles with Ziziphus jujuba Leaves Extract: Characterization and Photocatalytic Application. Nanomaterials 2021, 11, 1682. [Google Scholar] [CrossRef]
- Perez-Estrada, L.A.; Aguera, A.; Hernando, M.D.; Malato, S.; Fernnandez-Alba, A.R. Photodegradation of malachite green under natural sunlight irradiation: Kinetic and toxicity of the transformation products. Chemosphere 2008, 70, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, A.; Zhang, M.; Chen, J.; Zhang, Q. Photocatalytic Degradation Mechanism of Malachite Green under Visible Light Irradiation over Novel Biomimetic Photocatalyst HMS-FePcs. Chin. J. Catal. 2008, 29, 426–430. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Chandrashekar, B.N.; Ramakrishna, S.; et al. Novel Green Biomimetic Approach for Synthesis of ZnO-Ag Nanocomposite; Antimicrobial Activity against Food-borne Pathogen, Biocompatibility and Solar Photocatalysis. Sci. Rep. 2019, 9, 8303. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Pai, S.; Varadavenkatesan, T.; Pugazhendhi, A.; Selvaraj, R. Characterization and photocatalytic activity of ZnO nanoflowers synthesized using Bridelia retusa leaf extract. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Hu, J.; Weng, S.; Zheng, Z.; Huang, M.; Liu, P. Defect and its dominance in ZnO films: A new insight into the role of defect over photocatalytic activity. Appl. Catal. B Environ. 2013, 142–143, 736–743. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Benmami, M.; Chhor, K.; Kanaev, A. High photocatalytic activity of monolayer nanocoatings prepared from non-crystalline titanium oxide sol nanoparticles. Chem. Phys. Lett. 2006, 422, 552–557. [Google Scholar] [CrossRef]
- Stoyanova, D.; Stambolova, I.; Blaskov, V.; Zaharieva, K.; Avramova, I.; Dimitrov, O.; Vassilev, S.; Eliyas, A.; Nedyalkov, N. Mechanical milling of hydrothermally obtained CaTiO3 powders—Morphology and photocatalytic activity. Nano-Struct. Nano-Objects 2019, 18, 100301. [Google Scholar] [CrossRef]
- Guo, S.; Han, S.; Mao, H.; Dong, S.; Wu, C.; Jia, L.; Chi, B.; Pu, J.; Li, J. Structurally controlled ZnO/TiO2 heterostructures as efficient photocatalysts for hydrogen generation from water without noble metals: The role of microporous amorphous/crystalline composite structure. J. Power Sources 2014, 245, 979–985. [Google Scholar] [CrossRef]
- Domaschke, M.; Zhou, X.; Worgen, L.; Romeis, S.; Miehlich, M.; Meyer, K.; Peukert, W.; Schmuki, P. Magneli phases in anatse strongly promote co-catalyst–free photocatalytic hydrogen evolution. ACS Catal. 2019, 9, 3627–3632. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L. Controllable One-Pot Synthesis and Enhanced Photocatalytic Activity of Mixed-Phase TiO2 Nanocrystals with Tunable Brookite/Rutile Ratios. J. Phys. Chem. C 2009, 113, 1785–1790. [Google Scholar] [CrossRef]
- Stambolova, I.; Stoyanova, D.; Shipochka, M.; Blaskov, V.; Nihtianova, D.; Markov, P.; Eliyas, A.; Mladenova, R.; Dimitrov, L.; Abrashev, M.; et al. Enhanced effect of combination of new hybrid TiO2 phase and phosphorus dopant on the physicochemical properties and UV/Visible light photocatalytic activity. Mater. Charact. 2021, 172, 110775. [Google Scholar] [CrossRef]
- Soto-Robles, C.; Luque, P.; Gómez-Gutiérrez, C.; Nava, O.; Vilchis-Nestor, A.; Lugo, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Anwar, F.; Abbas, A.; Mehmood, T.; Gilani, A.; Rehman, N. Mentha: A genus rich in vital nutra-pharmaceuticals—A review. Phytother. Res. 2019, 33, 2548–2570. [Google Scholar] [CrossRef]
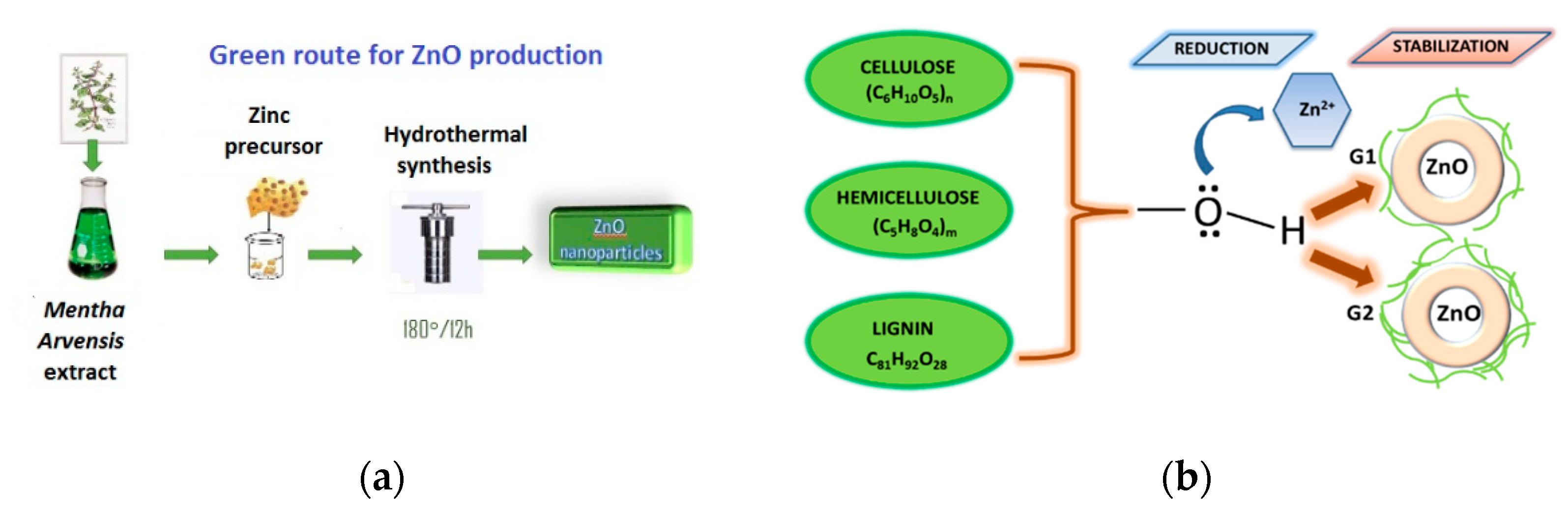

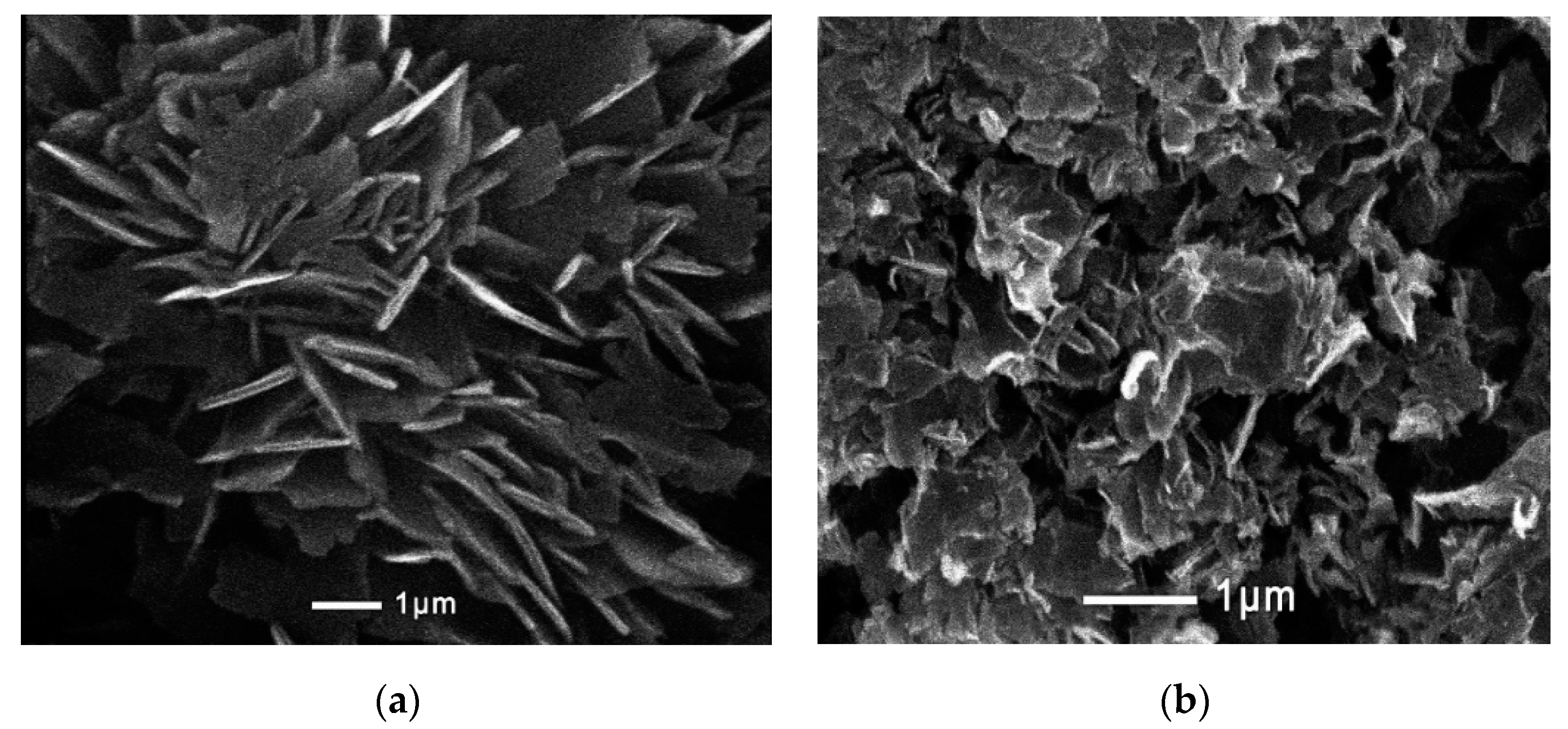

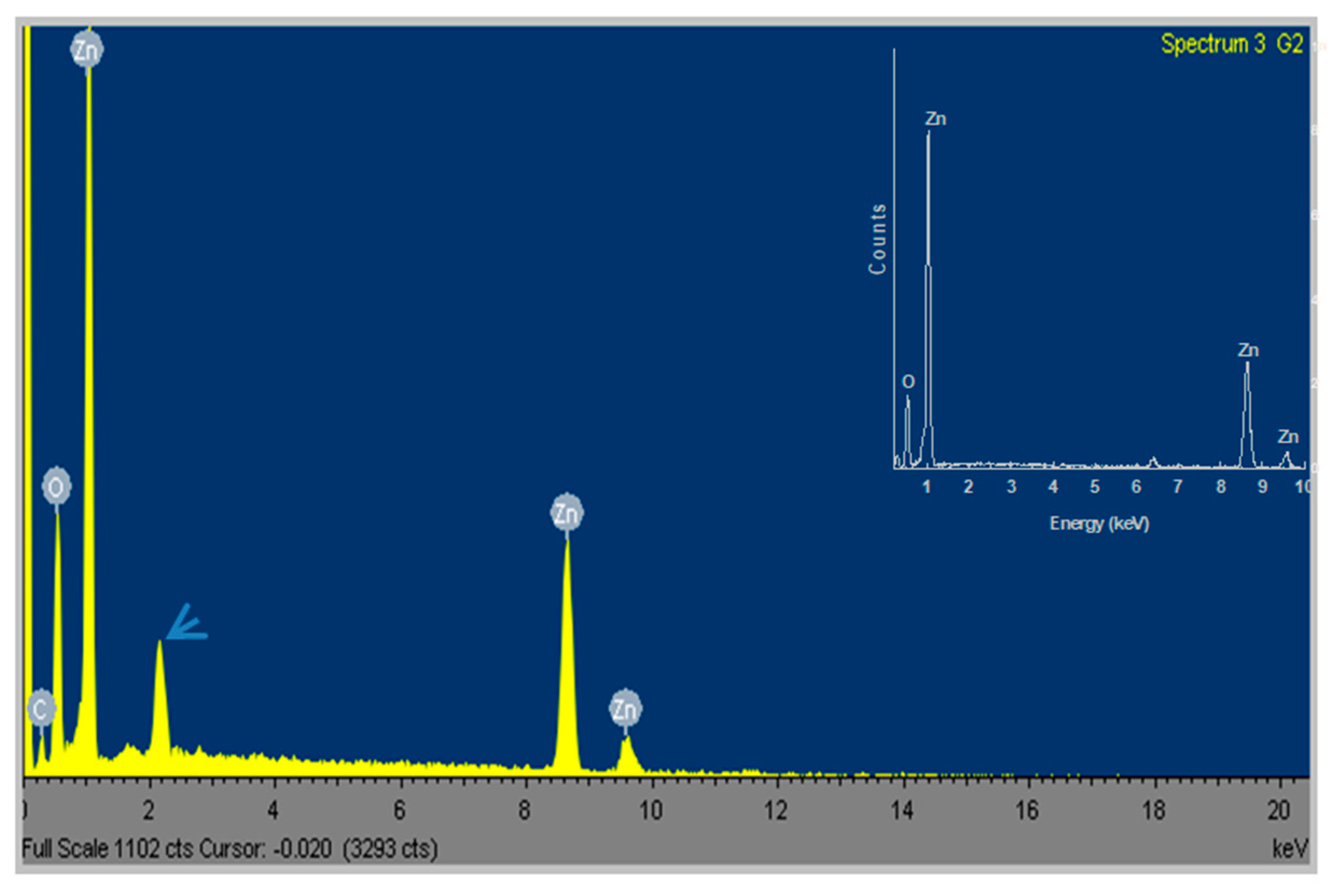
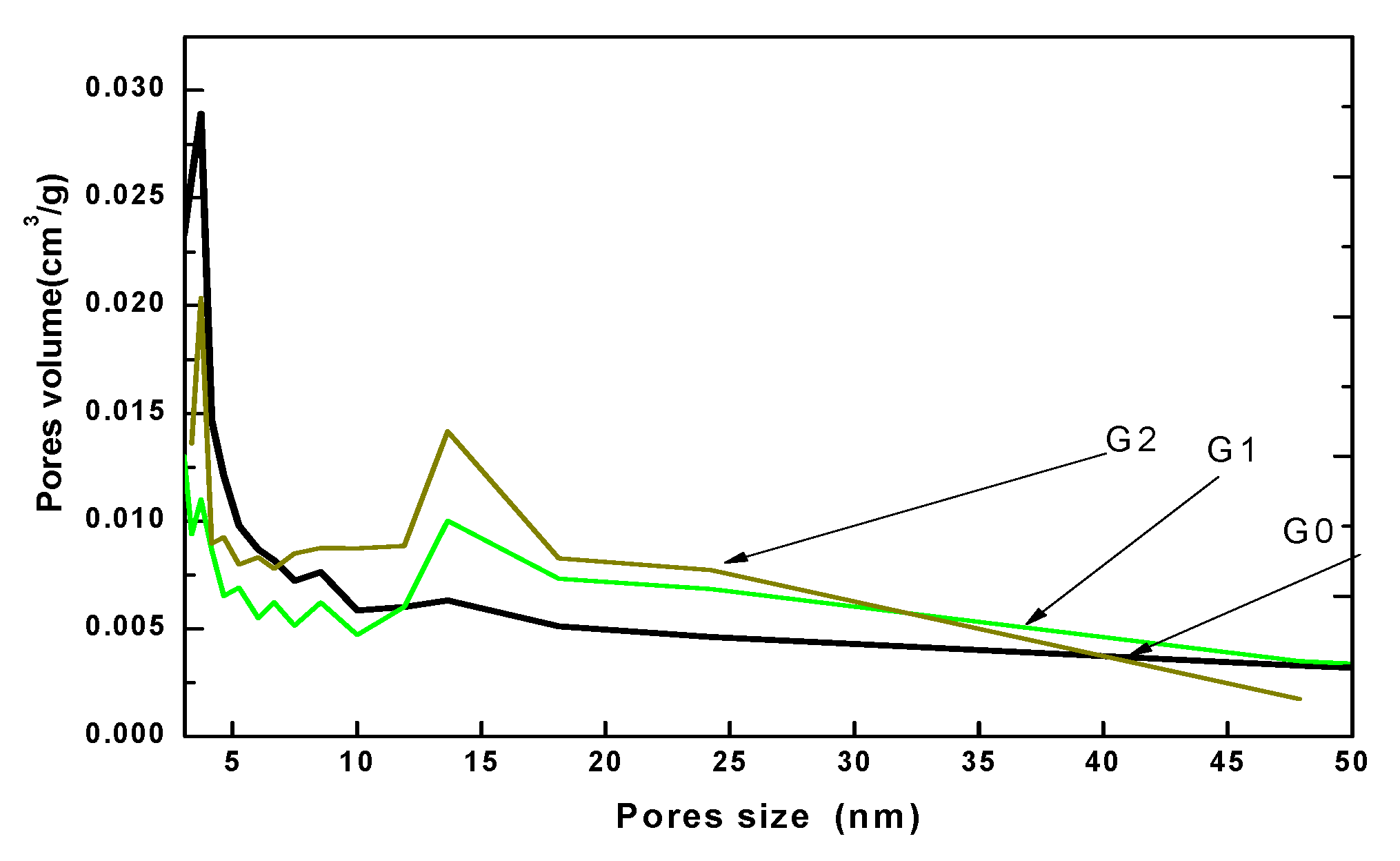


| Sample | Phase | D, nm | ε × 10−3, a.u. | a, Å |
|---|---|---|---|---|
| G0 | Wurtzite | 14.8 | 1.1 | 3.249 |
| G1 | Wurtzite | 9.8 | 4.2 | 3.257 |
| Hydrozincite | 11.5 | 3.6 | 13.714 | |
| G2 | Wurtzite | 8.6 (left) | 3.3 | 3.245 |
| Hydrozincite | 7.6 | 4.2 | 13.720 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, D.; Stambolova, I.; Blaskov, V.; Georgieva, P.; Shipochka, M.; Zaharieva, K.; Dimitrov, O.; Markov, P.; Dyakova, V.; Kostova, Y.; et al. Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties. Appl. Sci. 2022, 12, 1096. https://doi.org/10.3390/app12031096
Stoyanova D, Stambolova I, Blaskov V, Georgieva P, Shipochka M, Zaharieva K, Dimitrov O, Markov P, Dyakova V, Kostova Y, et al. Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties. Applied Sciences. 2022; 12(3):1096. https://doi.org/10.3390/app12031096
Chicago/Turabian StyleStoyanova, Daniela, Irina Stambolova, Vladimir Blaskov, Petya Georgieva, Maria Shipochka, Katerina Zaharieva, Ognian Dimitrov, Pavel Markov, Vanya Dyakova, Yoanna Kostova, and et al. 2022. "Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties" Applied Sciences 12, no. 3: 1096. https://doi.org/10.3390/app12031096
APA StyleStoyanova, D., Stambolova, I., Blaskov, V., Georgieva, P., Shipochka, M., Zaharieva, K., Dimitrov, O., Markov, P., Dyakova, V., Kostova, Y., Mladenova, R., Tzvetkov, G., Boshkova, N., & Boshkov, N. (2022). Modified Approach Using Mentha arvensis in the Synthesis of ZnO Nanoparticles—Textural, Structural, and Photocatalytic Properties. Applied Sciences, 12(3), 1096. https://doi.org/10.3390/app12031096








