Analysis of a Cardiac-Necrosis-Biomarker Release in Patients with Acute Myocardial Infarction via Nonlinear Mixed-Effects Models †
Abstract
1. Introduction
2. Background
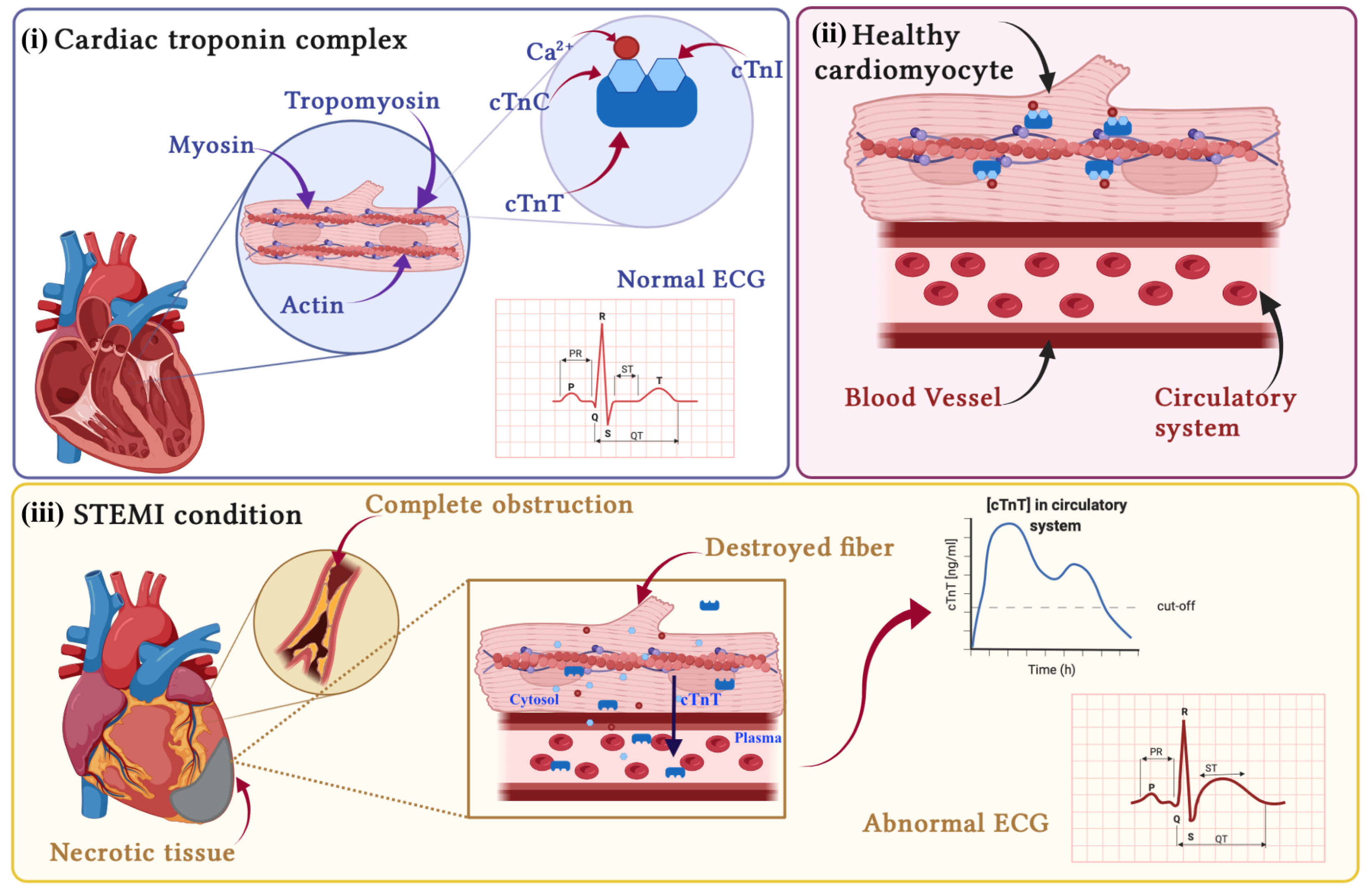
2.1. Pathophysiology of AMI
- STEMI (ST-segment elevation AMI): in this class of patients, the obstruction usually completely blocks the blood supply to the tissues downstream of it. In this case, the extension of the cardiac damage is such that it causes alterations in the electrical activity of the heart, resulting in a visible elevation of the ST-segment on the ECG, [21]; see Figure 1(iii).
- NSTEMI (non-ST-segment elevation AMI): the occlusion is only partial; therefore, a reduced blood flow is guaranteed downstream of the obstruction; these patients exhibit an ECG without visible alteration [22].
2.2. Nonlinear Mixed-Effects Models
- Fixed effects—the terms, in the parameter expressions, that remain constant over all the individuals of the analyzed population. They represent the set of the population parameters.
- Random effects—the random components of the model parameters, assumed to be different among individuals or groups of individuals;
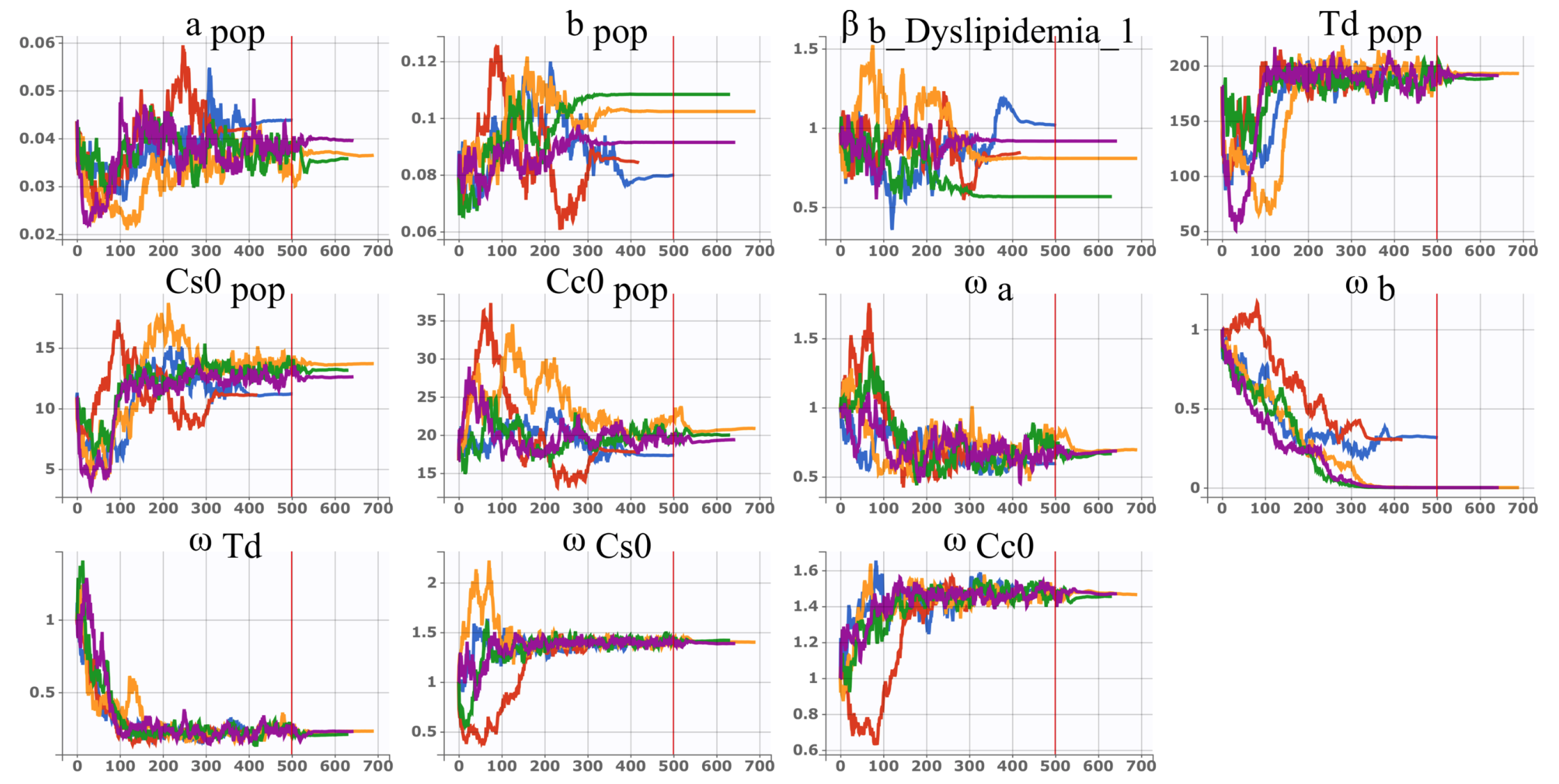
2.3. Stochastic Approximation Version of Expectation Maximisation Algorithm (SAEM)
- Expectation step (E)—during which the likelihood of the observed data is computed starting from the current estimate of the model parameters;
- Maximization step (M)—where the estimate of the parameters is updated, seeking the point in the parameter space that maximizes the likelihood.
- (i)
- The set of the individual parameters are computed by sampling a conditional distribution through Markov-chain Monte-Carlo procedure, namelywhere represents the individual parameters set for subject i, is the experimental observation vector for subject i, and is the population parameters set at SAEM iteration k.
- (ii)
- the set of the population parameters is computed starting from the individual parameter sets identified at the previous iteration and represents the initialization point for a new exploratory iteration,
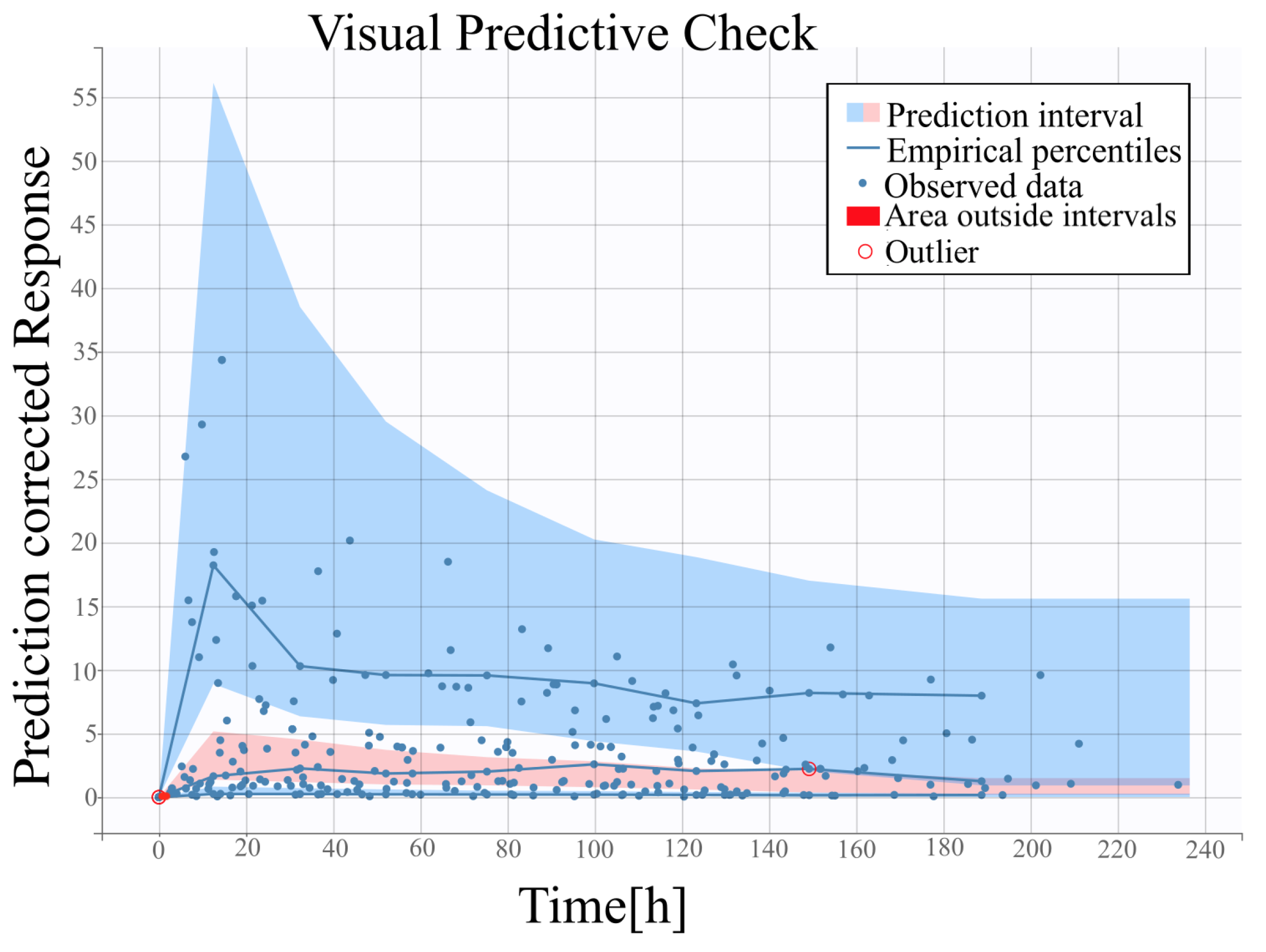
2.4. Visual Predictive Check
3. Materials and Methods
3.1. Nonlinear Model of Cardiac Troponin T (cTnT) Release in AMI Patients
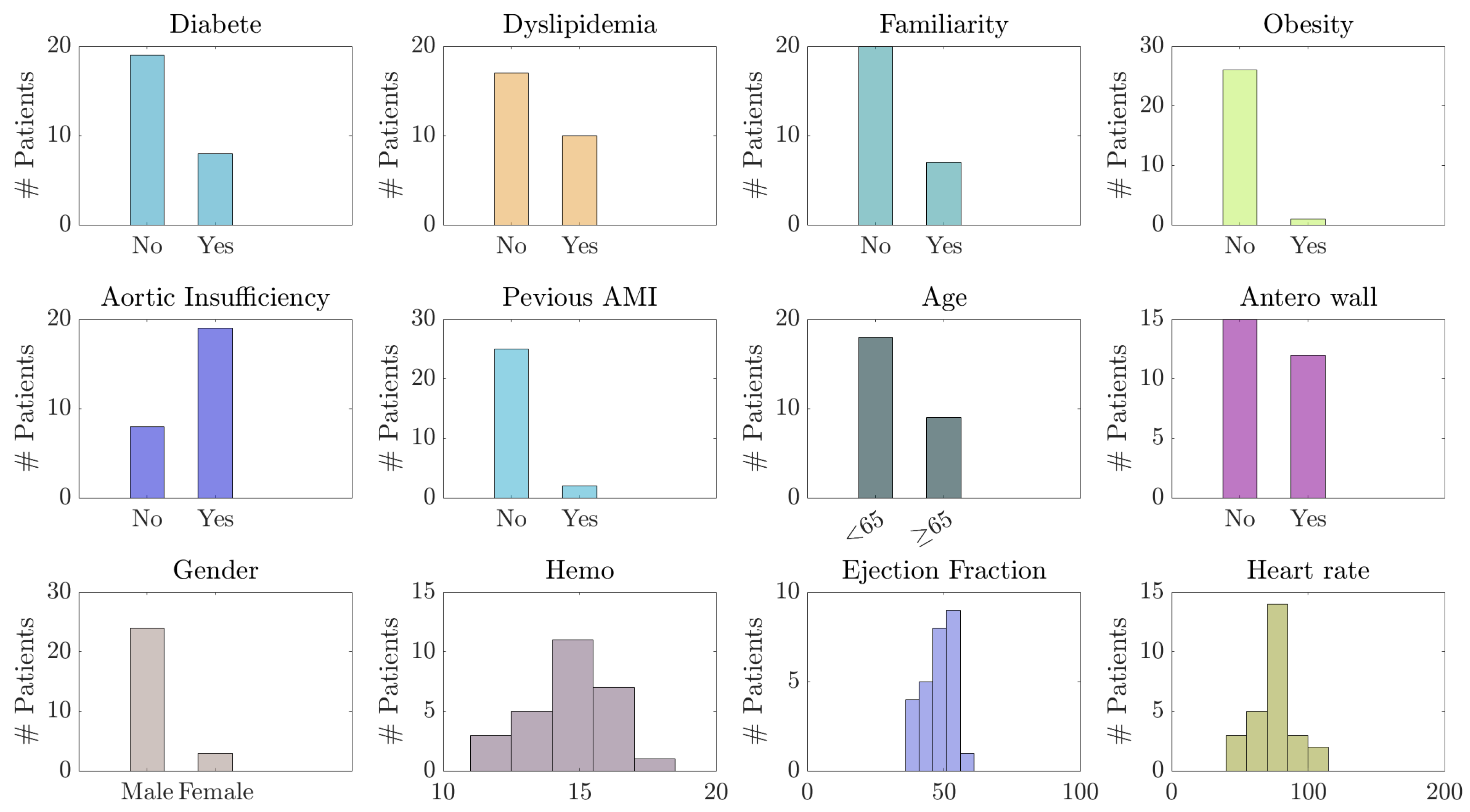
3.2. Experimental Dataset: cTnT Concentration Levels and Clinical Covariates
- Pre-hospital data: diabetes, dyslipidemia, smoke, obesity, familiarity with the disease (presence of cases of AMI in the family), and other cardiac pathologies;
- Admission data: time from onset of symptoms, gender, age;
- Post-hospitalization data: ejection fraction, thrombolysis, revascularization, biomarkers, anterior wall, and other information, along with data and time labels.
3.3. Strategy for Covariates Selection
- A first analysis was performed without the introduction of covariates, in order to initialize the parameters identification phase.
- The effect of each covariate on the model parameters was assessed; Wald’s test was used to statistically evaluate which covariate yields a significant effect and should be added to the model. Bonferroni correction was applied to avoid the problem of multiple tests.
- Finally, the models obtained by the combination of the most significant covariates were analyzed.
4. Results
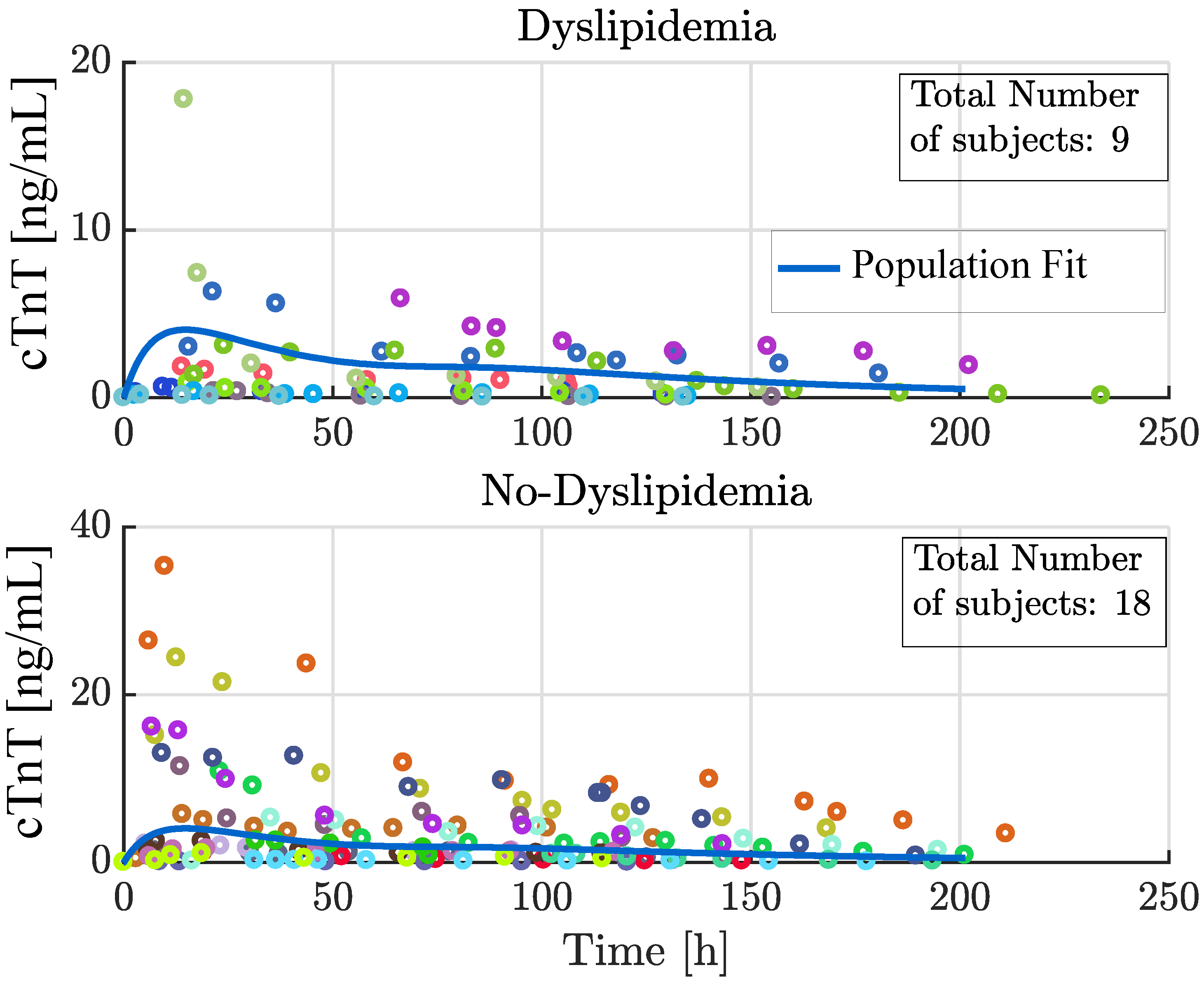
4.1. Analysis of Selected Covariates
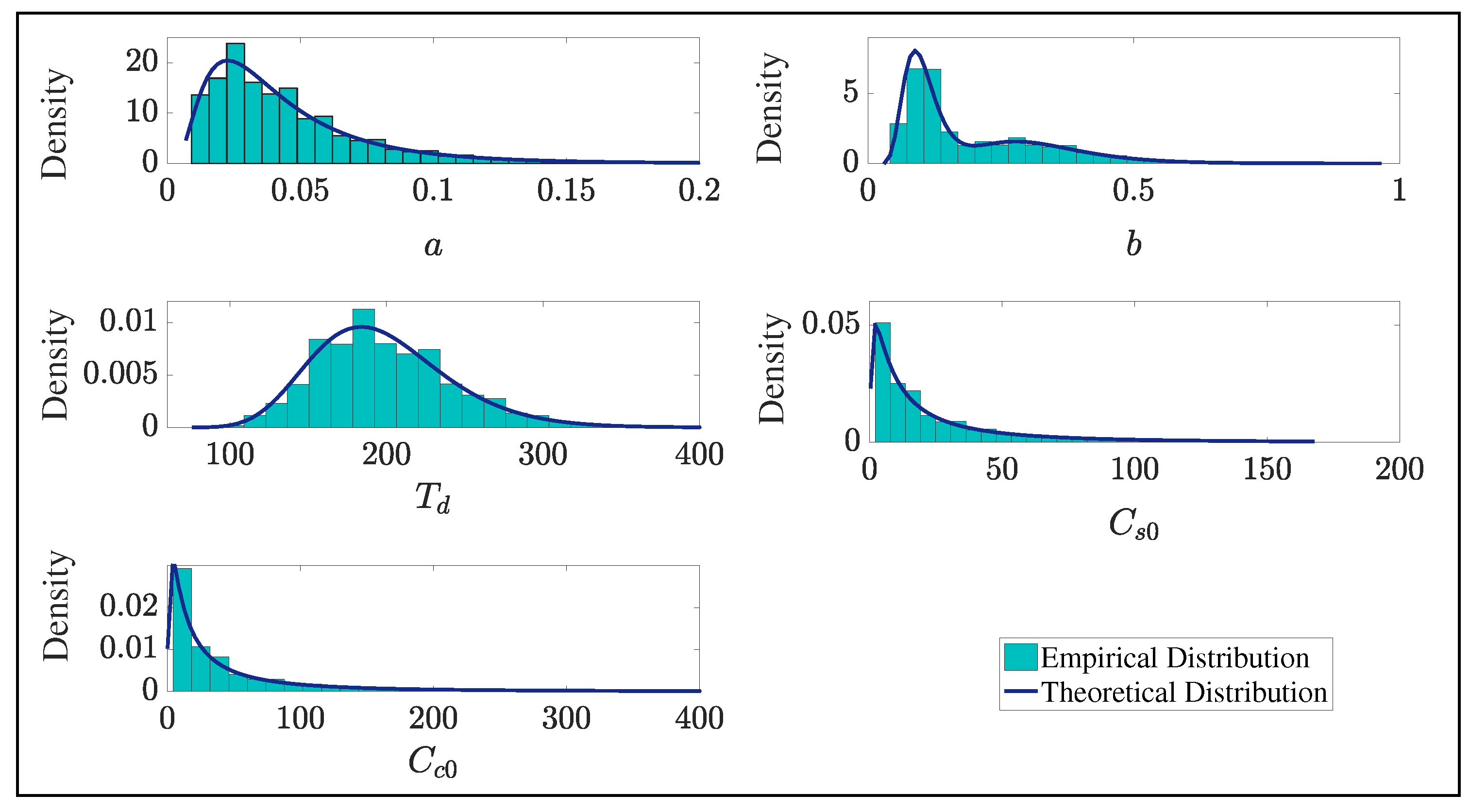
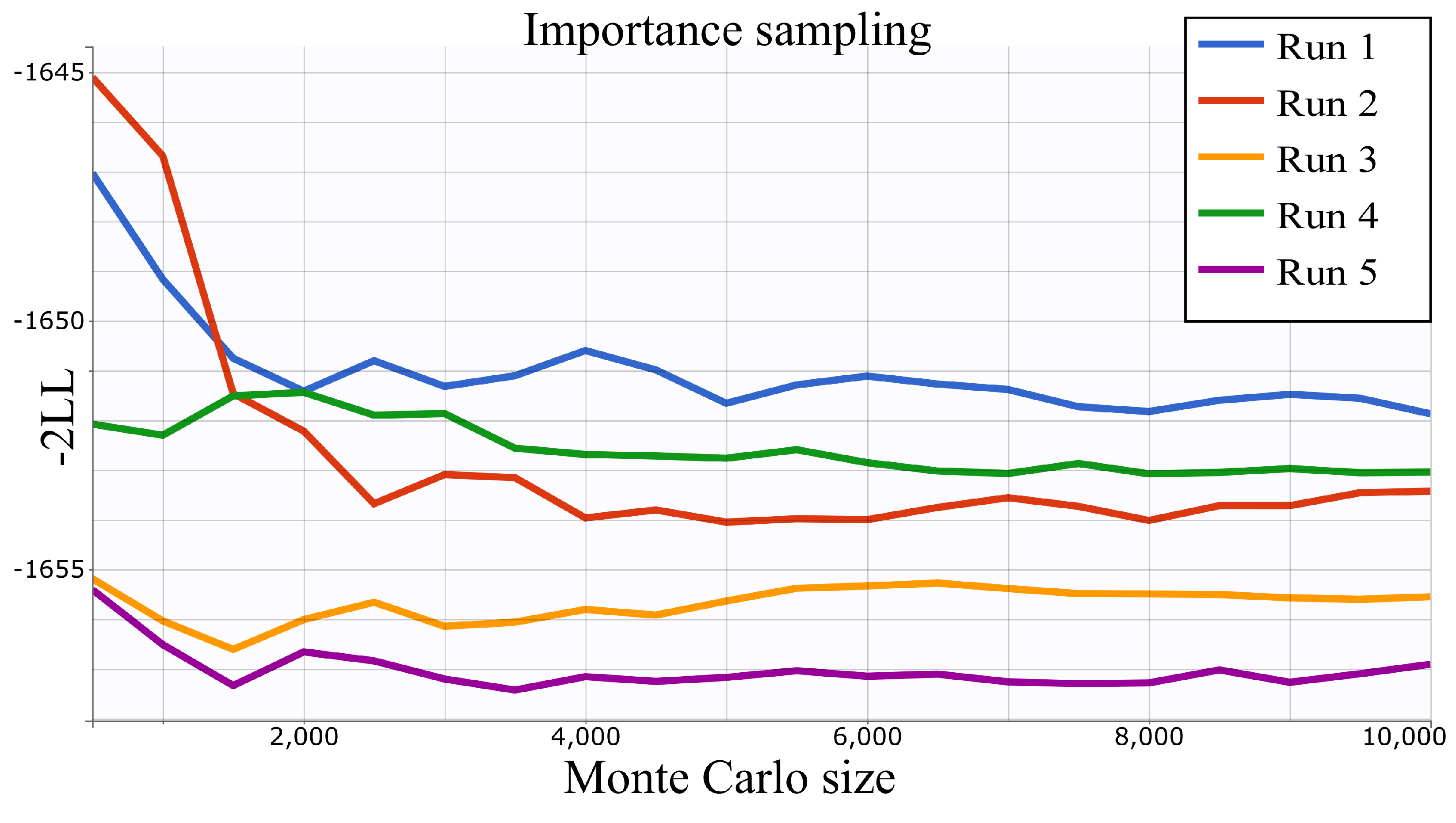
4.2. Parameter Estimation
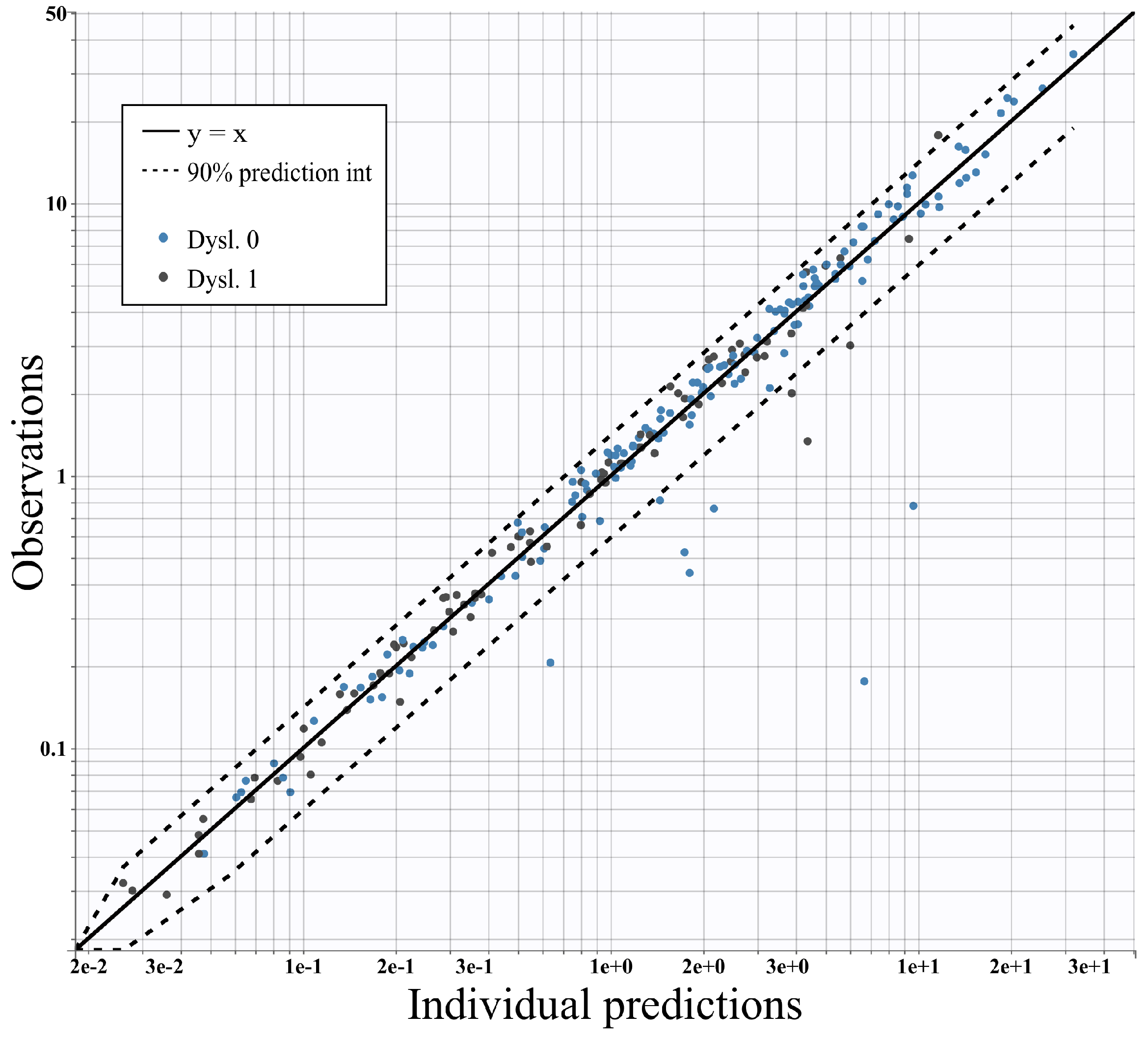
4.3. Assessment of the Model against Experimental Data
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tilea, I.; Varga, A.; Serban, R.C. Past, Present, and Future of Blood Biomarkers for the Diagnosis of Acute Myocardial Infarction—Promises and Challenges. Diagnostics 2021, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Couch, L.S.; Sinha, A.; Navin, R.; Hunter, L.; Perera, D.; Marber, M.S.; Kaier, T.E. Rapid risk stratification of acute coronary syndrome: Adoption of an adapted European Society of Cardiology 0/1-hour troponin algorithm in a real-world setting. Eur. Heart J. Open 2022, 2, oeac048. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. Biology of Cardiac Troponins: Emphasis on Metabolism. Biology 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.K.; Higgins, V.; Kavsak, P.; Hoffman, B.; Adeli, K. High-sensitivity generation 5 cardiac troponin T sex-and age-specific 99th percentiles in the CALIPER cohort of healthy children and adolescents. Clin. Chem. 2019, 65, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.; Iten, L.; Marques-Vidal, P.; Boulat, O.; Bardy, D.; Beggah, A.; Calderara, R.; Morawiec, B.; Lauriers, N.; Monney, P.; et al. Circadian rhythm of blood cardiac troponin T concentration. Clin. Res. Cardiol. 2017, 106, 1026–1032. [Google Scholar] [CrossRef]
- Lindstrom, M.J.; Bates, D.M. Newton–Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J. Am. Stat. Assoc. 1988, 83, 1014–1022. [Google Scholar]
- Lindstrom, M.J.; Bates, D.M. Nonlinear mixed effects models for repeated measures data. Biometrics 1990, 46, 673–687. [Google Scholar] [CrossRef]
- Montefusco, F.; Pedersen, M.G. Explicit theoretical analysis of how the rate of exocytosis depends on local control by Ca2+ channels. Comput. Math. Methods Med. 2018, 2018, 5721097. [Google Scholar] [CrossRef]
- Sawlekar, R.; Montefusco, F.; Kulkarni, V.V.; Bates, D.G. Biomolecular implementation of a quasi sliding mode feedback controller based on DNA strand displacement reactions. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 949–952. [Google Scholar] [CrossRef]
- Montefusco, F.; Procopio, A.; Bulai, I.M.; Amato, F.; Pedersen, M.G.; Cosentino, C. Interacting with COVID-19: How population behavior, feedback and memory shaped recurrent waves of the epidemic. IEEE Control. Syst. Lett. 2022, 7, 583–588. [Google Scholar] [CrossRef]
- Parrotta, E.I.; Procopio, A.; Scalise, S.; Esposito, C.; Nicoletta, G.; Santamaria, G.; De Angelis, M.T.; Dorn, T.; Moretti, A.; Laugwitz, K.L.; et al. Deciphering the role of Wnt and Rho signaling pathway in iPSC-derived ARVC cardiomyocytes by in silico mathematical modeling. Int. J. Mol. Sci. 2021, 22, 2004. [Google Scholar] [CrossRef]
- Davidian, M.; Gallant, A.R. The nonlinear mixed effects model with a smooth random effects density. Biometrika 1993, 80, 475–488. [Google Scholar] [CrossRef]
- Aggrey, S. Logistic nonlinear mixed effects model for estimating growth parameters. Poult. Sci. 2009, 88, 276–280. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M.; Lindstrom, M.J. Model Building for Nonlinear Mixed Effects Models; Department of Biostatistics Madison, University of Wisconsin: Madison, WI, USA, 1995. [Google Scholar]
- Hickey, G.L.; Mokhles, M.M.; Chambers, D.J.; Kolamunnage-Dona, R. Statistical primer: Performing repeated-measures analysis. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 539–544. [Google Scholar] [CrossRef]
- Samson, A.; Lavielle, M.; Mentré, F. Extension of the SAEM algorithm to left-censored data in nonlinear mixed-effects model: Application to HIV dynamics model. Comput. Stat. Data Anal. 2006, 51, 1562–1574. [Google Scholar] [CrossRef]
- French, R. Using Mixed Effects Modeling to Quantify Difference Between Patient Groups with Diabetic Foot Ulcers. Ph.D. Thesis, WKU Mahurin College, Bowling Green, KY, USA, 2017. [Google Scholar]
- Ieva, F.; Paganoni, A.M.; Secchi, P. Mining administrative health databases for epidemiological purposes: A case study on acute myocardial infarctions diagnoses. In Advances in Theoretical and Applied Statistics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 417–426. [Google Scholar]
- Procopio, A.; De Rosa, S.; Covello, C.; Merola, A.; Sabatino, J.; De Luca, A.; Liebetrau, C.; Hamm, C.W.; Indolfi, C.; Amato, F.; et al. Estimation of the Acute Myocardial Infarction Onset Time Based on Time-Course Acquisitions. Ann. Biomed. Eng. 2021, 49, 477–486. [Google Scholar] [CrossRef]
- Procopio, A.; Merola, A.; Cosentino, C.; De Rosa, S.; Canino, G.; Sabatino, J.; Ielapi, J.; Indolfi, C.; Amato, F. Analysis and classification of patients with acute myocardial infarction by using nonlinear mixed-effects modeling. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Virtual, 6–9 September 2021; pp. 569–573. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Mair, J.; Artner-Dworzak, E.; Lechleitner, P.; Smidt, J.; Wagner, I.; Dienstl, F.; Puschendorf, B. Cardiac troponin T in diagnosis of acute myocardial infarction. Clin. Chem. 1991, 37, 845–852. [Google Scholar] [CrossRef]
- Wu, A.H. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Lavielle, M.; Mentré, F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 2007, 34, 229–249. [Google Scholar] [CrossRef]
- Moon, T.K. The expectation-maximization algorithm. IEEE Signal Process. Mag. 1996, 13, 47–60. [Google Scholar] [CrossRef]
- Delyon, B.; Lavielle, M.; Moulines, E. Convergence of a stochastic approximation version of the EM algorithm. Ann. Stat. 1999, 27, 94–128. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; De Rosa, S.; Covello, C.; Merola, A.; Sabatino, J.; De Luca, A.; Indolfi, C.; Amato, F.; Cosentino, C. Mathematical model of the release of the CTNT and CK-MB cardiac biomarkers in patients with acute myocardial infarction. In Proceedings of the 18th European Control Conference, Naples, Italy, 25–28 June 2019; pp. 1653–1658. [Google Scholar]
- Procopio, A.; De Rosa, S.; García, M.R.; Covello, C.; Merola, A.; Sabatino, J.; De Luca, A.; Indolfi, C.; Amato, F.; Cosentino, C. Experimental Modeling and Identification of Cardiac Biomarkers Release in Acute Myocardial Infarction. IEEE Trans. Control. Syst. Technol. 2020, 28, 183–195. [Google Scholar] [CrossRef]
- Procopio, A.; De Rosa, S.; Montefusco, F.; Canino, G.; Merola, A.; Sabatino, J.; Ielapi, J.; Indolfi, C.; Amato, F.; Cosentino, C. CBRA: Cardiac biomarkers release analyzer. Comput. Methods Programs Biomed. 2021, 204, 106037. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Procopio, A.; Bilotta, M.; Merola, A.; Amato, F.; Cosentino, C.; De Rosa, S.; Covello, C.; Sabatino, J.; De Luca, A.; Indolfi, C. Predictive mathematical model of cardiac troponin release following acute myocardial infarction. In Proceedings of the 2017 IEEE 14th International Conference on Networking, Sensing and Control (ICNSC), Calabria, Italy, 16–18 May 2017; pp. 643–648. [Google Scholar]
- Villaverde, A.F.; Barreiro, A.; Papachristodoulou, A. Structural Identifiability of Dynamic Systems Biology Models. PLoS Comput. Biol. 2016, 12, e1005153. [Google Scholar] [CrossRef]
- Villaverde, A.F. Observability and Structural Identifiability of Nonlinear Biological Systems. Complexity 2019, 2019, 8497093. [Google Scholar] [CrossRef]
- Chan, P.L.; Jacqmin, P.; Lavielle, M.; McFadyen, L.; Weatherley, B. The use of the SAEM algorithm in MONOLIX software for estimation of population pharmacokinetic-pharmacodynamic-viral dynamics parameters of maraviroc in asymptomatic HIV subjects. J. Pharmacokinet. Pharmacodyn. 2011, 38, 41–61. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef]
- Takeda, N.; Maemura, K. Circadian clock and cardiovascular disease. J. Cardiol. 2011, 57, 249–256. [Google Scholar] [CrossRef]
- Knapp, T.R. Bimodality revisited. J. Mod. Appl. Stat. Methods 2007, 6, 3. [Google Scholar] [CrossRef]
- Nylander, J.A.; Wilgenbusch, J.C.; Warren, D.L.; Swofford, D.L. AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24, 581–583. [Google Scholar] [CrossRef]
- Mueller, M.; Vafaie, M.; Biener, M.; Giannitsis, E.; Katus, H.A. Cardiac Troponin T–From Diagnosis of Myocardial Infarction to Cardiovascular Risk Prediction. Circ. J. 2013, 77, 1653–1661. [Google Scholar] [CrossRef]
- Gore, M.O.; Seliger, S.L.; Defilippi, C.R.; Nambi, V.; Christenson, R.H.; Hashim, I.A.; Hoogeveen, R.C.; Ayers, C.R.; Sun, W.; McGuire, D.K.; et al. Age-and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J. Am. Coll. Cardiol. 2014, 63, 1441–1448. [Google Scholar] [CrossRef]


| M | Cov | Cov | Cov | −2LL | BIC | SE | PM(Cov: ) |
|---|---|---|---|---|---|---|---|
| 1 | −1613.80 | −1576.76 | 0.655 | ||||
| 2 | D | −1651.87 | −1569.93 | 0.427 | b (D: ) | ||
| 3 | D | AI | −1655.09 | −1604.58 | 1.575 | b(D: ), T(AI: ) | |
| 4 | D | A | −1560.24 | −1507.51 | 0.142 | b(D,A: , ), (A: ), (A: ) | |
| 5 | A | AI | −1586.06 | −1536.63 | 0.42 | b(A,AI: , ), (A: ), (A: ) | |
| 6 | A | AI | D | −1574.81 | −1505.38 | 0.183 | b(D: ), (A,AI: ) |
| Dyslipidemia Yes | Dyslipidemia No | |
|---|---|---|
| mean | 1.5 | 4.1 |
| std | 2.4 | 5.7 |
| median | 0.62 | 1.8 |
| Fixed Effects | |||
| Parameter | Value | S.E. | R.S.E(%) |
| 0.0361 | 0.00675 | 18.7 | |
| 0.0986 | 0.0144 | 14.6 | |
| 1.16 | 0.241 | 20.9 | |
| 193 | 17.9 | 9.26 | |
| 15.1 | 4.3 | 28.4 | |
| 23.5 | 7.34 | 31.2 | |
| Standard Deviation of the Random Effect | |||
| Parameter | Value | S.E. | R.S.E(%) |
| 0.682 | 0.165 | 24.2 | |
| 0.329 | 0.109 | 33.2 | |
| 0.221 | 0.0611 | 27.7 | |
| 1.4 | 0.188 | 13.5 | |
| 1.47 | 0.204 | 13.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procopio, A.; De Rosa, S.; Montefusco, F.; Canino, G.; Merola, A.; Sabatino, J.; Critelli, C.; Indolfi, C.; Amato, F.; Cosentino, C. Analysis of a Cardiac-Necrosis-Biomarker Release in Patients with Acute Myocardial Infarction via Nonlinear Mixed-Effects Models. Appl. Sci. 2022, 12, 13038. https://doi.org/10.3390/app122413038
Procopio A, De Rosa S, Montefusco F, Canino G, Merola A, Sabatino J, Critelli C, Indolfi C, Amato F, Cosentino C. Analysis of a Cardiac-Necrosis-Biomarker Release in Patients with Acute Myocardial Infarction via Nonlinear Mixed-Effects Models. Applied Sciences. 2022; 12(24):13038. https://doi.org/10.3390/app122413038
Chicago/Turabian StyleProcopio, Anna, Salvatore De Rosa, Francesco Montefusco, Giovanni Canino, Alessio Merola, Jolanda Sabatino, Claudia Critelli, Ciro Indolfi, Francesco Amato, and Carlo Cosentino. 2022. "Analysis of a Cardiac-Necrosis-Biomarker Release in Patients with Acute Myocardial Infarction via Nonlinear Mixed-Effects Models" Applied Sciences 12, no. 24: 13038. https://doi.org/10.3390/app122413038
APA StyleProcopio, A., De Rosa, S., Montefusco, F., Canino, G., Merola, A., Sabatino, J., Critelli, C., Indolfi, C., Amato, F., & Cosentino, C. (2022). Analysis of a Cardiac-Necrosis-Biomarker Release in Patients with Acute Myocardial Infarction via Nonlinear Mixed-Effects Models. Applied Sciences, 12(24), 13038. https://doi.org/10.3390/app122413038












