Abstract
The purpose of this study was to observe the effects of audible and inaudible binaural beat stimuli on alpha power elicitation and compare the differences in triggering effects depending on sound perception. Experiments were conducted on healthy male and female subjects (11 males and 10 females, mean age of 24.6 ± 1.8). To induce alpha waves, audible (250 Hz) or non-audible baseline sound frequencies (18,000 Hz) were presented to the left ear, and a frequency 10 Hz higher than the baseline was presented to the right ear. There were two experimental phases: a rest phase (5 min) in which no stimulus was presented and a stimulation phase (5 min) in which the binaural beat stimulus was presented. An electroencephalogram was measured at a sampling rate of 500 Hz, and relative alpha power values were calculated for each phase in each brain area. In the central regions, both baseline frequencies (audible and inaudible) increased the relative alpha power during the stimulation phase compared with the rest phase, and there were no differences between the two baseline frequencies. In the frontal and central regions, there was a greater increase in relative alpha power in the audible case compared with the inaudible case.
1. Introduction
A binaural beat (BB) is a sound that is capable of evoking specific brain-wave states based on differences in the frequencies of auditory stimuli [1,2]. When two different frequencies of sound are presented to both ears, the brain recognizes the difference between the two sounds. For instance, when a baseline frequency of 250 Hz is presented to one ear and a frequency of 10 Hz higher than the baseline frequency is presented to the other ear, the human brain will be tuned to the beat frequency of 10 Hz, which is the difference in frequency.
Previous studies have shown that specific brain waves can be induced depending on the difference in the BB frequency, and different effects from cognitive and emotional perspectives have been noted depending on the state of the induced brain waves [3,4,5,6,7]. Therefore, alpha activity increased from the onset of the BB stimulus that induced alpha waves [8], and the state–trait anxiety inventory measure decreased in subjects exposed to this type of BB stimulus [9]. In addition, it was found that tension, confusion, and fatigue were reduced in subjects whose delta and theta waves were induced by BB stimuli [10]. In subjects whose beta waves were induced by the BB stimulus, improvements were evident in long- and short-term memory and cognitive abilities [11], as well as in the ability to recall memories and improve moods [12].
Various baseline frequencies have been tested in previous studies; however, they fell within the range of audible frequencies. One study used only baseline sinusoidal sounds, whereby a constant sound was presented at a single frequency [13]. Another study used the sound of music as a baseline frequency on top of which the BB stimulus was overlaid [11,14]. The brainwave induced by the audible BB stimulus and the cognitive and emotional effects may be affected by both the sound perception (baseline frequency) and the frequency difference. Thus, BB can be effectively applied only when a few studies that evaluate the effects of such sound perception can validate scientifically the effectiveness of BB. There are reports indicating that auditory stimulation in the inaudible frequency range could affect human recognition and psychology. This phenomenon is called the hypersonic effect. Moreover, auditory stimulation, including that in the inaudible frequency ranges, can increase the alpha-wave and comfortable listening levels [15].
This study verifies the effects of inaudible binaural beat (BB) by comparing it with audible BB. When analyzing the effect of audible BB, the effect of auditory perception at the baseline frequency cannot be excluded. Therefore, the study aimed to investigate whether inaudible BB has similar effects as audible BB by comparing the two. Thus, this study examined the effects of inducing brainwaves with BB and an inaudible baseline frequency. It was determined that a specific brainwave (α) was induced when a BB with a baseline frequency of 18,000 Hz and a frequency difference of 10 Hz was presented. Furthermore, the effect of BB based on the commonly used audible baseline frequency (250 Hz) on inducing the brainwave (α) was also observed to compare the effects of audible and inaudible baseline frequencies.
2. Materials and Methods
2.1. Subjects
The experiments were conducted on a total of 21 healthy male and female subjects (11 males with a mean age of 25.9 ± 1.4 years and 10 females with a mean age of 23.3 ± 1.7 years). The subjects did not have any history of hearing deficiency or hearing loss, and they were unaware of the purpose of the study. The subjects were instructed not to move during the experiment to minimize the noise due to movements. In addition, they were instructed to look at a white cross (+) on a black background displayed on the monitor with their eyes open during the experiment. This study was conducted with the approval of the Konkuk University institutional review board (7001355-202105-HR-439). Prior to the experiments, participants were informed about the study and provided written informed consent.
2.2. Binaural Beats
An auditory stimulator (company G product Q) induced alpha waves by presenting a sound with a baseline frequency (250 or 18,000 Hz) to the left ear and a sound with a frequency 10 Hz higher than the baseline frequency to the right ear through earphones. The experimental procedure consisted of a rest phase (5 min) without any stimuli presented, followed by a stimulation phase (5 min) which involved BB stimuli (Figure 1). All subjects took part in two experiments using audible (250 Hz) and inaudible (18,000 Hz) baseline frequencies, and the order of the experiments was counterbalanced. Moreover, after the end of the experiment, a survey was conducted to assess if they could actually hear the inaudible BB, and all the subjects answered that they could not hear.
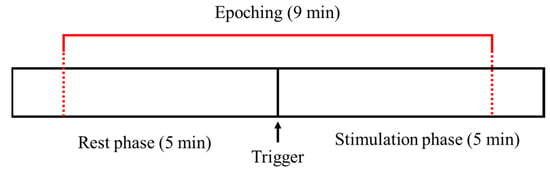
Figure 1.
Experimental design and analysis section.
2.3. Encephalogram Measurement
An electroencephalogram (EEG) was recorded using Enobio20 (NeuroElectrics, Barcelona, Spain) at a sampling rate of 500 Hz. The electrodes were placed at 19 positions (including frontal (Fp1, Fp2, F3, F4, F7, F8, and Fz), central (C3, C4, and Cz), parietal (P3, P4, P7, P8, and Pz), temporal (T7 and T8), and occipital (O1 and O2)) according to 10–20 systems. The reference electrode was positioned on the right mastoid and the ground electrode on the right earlobe. The impedance between the electrode and the scalp was maintained at values below 5 kΩ. The experiment was conducted in a shielded room to block external electromagnetic waves that may affect the experiment.
2.4. EEG Analysis
The EEG data were analyzed using MATLAB 2017 (MathWorks, Boston, MA, USA). Artifacts caused by eye blinking and movement were removed through visual inspection. Initially, the noise artifact due to eye blinking and motions was manually removed. Next, noise due to the power supply and electromagnetic noise was removed using a 0.5–50 Hz bandpass filter. The noise that may occur at the beginning and end of the experiment was reduced by capturing only the EEG signals from the rest (270 s) and stimulation (270 s) phases based on the trigger signal (Figure 1).
A power spectral analysis was performed to observe the average alpha power of the rest and stimulation phases. Each phase was subjected to a fast Fourier transform and converted into power values based on its frequency. The relative power of the alpha band was calculated for each data point to reduce variability between subjects. Therefore, the alpha frequency range (8–13 Hz) was divided by the total frequency range (0.5–50 Hz) to calculate the ratio.
The relative power values for each of the five brain regions (frontal, central, parietal, temporal, and occipital) were averaged, followed by a two-way analysis of variance (ANOVA), with the phase (phase: rest/stimulation) and audible state (state: audible/inaudible) as the independent variables. Additionally, an independent t-test was performed to compare the relative power values of the two types of baseline frequencies (audible and inaudible) for each brain region (SPSS, version 25, IBM, Armonk, NY, USA).
3. Results
Two-way ANOVA results showed that the increase in relative alpha power was more significant in the stimulation phase than in the rest phase in all brain regions, as shown in Table 1. However, there was no difference between the two baseline frequencies (states). There was an interaction effect between the frontal and central regions. Therefore, the variation according to the phase of the two baseline frequencies differed. To interpret the interaction effect, a simple effect test was conducted on the frontal and central regions(Table 2). As a result, in the case of audible BB in the frontal region, alpha power significantly increased during the stimulation phase compared to the rest phase (p = 0.017). In the central region, alpha power significantly increased during the stimulation phase compared to the rest phase for both audible and inaudible BBs (audible p = 0.002, inaudible p = 0.012). In addition, no differences were recognized for both frontal and central states (Figure 2). The increase in relative alpha power caused by the stimulus was greater with audible sounds than with inaudible sounds (Figure 2). This was verified by an independent t-test analysis of the difference in the rate of increase (stimulation-rest/rest × 100) in relative power between the two baseline frequencies (audible and inaudible). The rate of increase in the relative alpha power was greater in both regions when the sound was audible (Figure 3).

Table 1.
Results of the relative alpha power of a two-way analysis of variance (ANOVA) by using phase and state as independent variables at the five brain areas.

Table 2.
Simple-effect test results for phase and state in two brain regions.
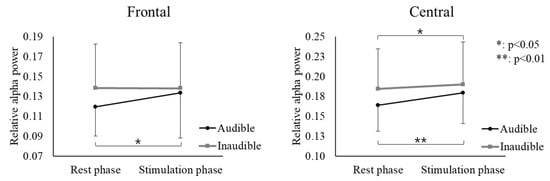
Figure 2.
Interactive effect between phase and state on the relative alpha power at the frontal and central areas (bars represent the standard deviation).
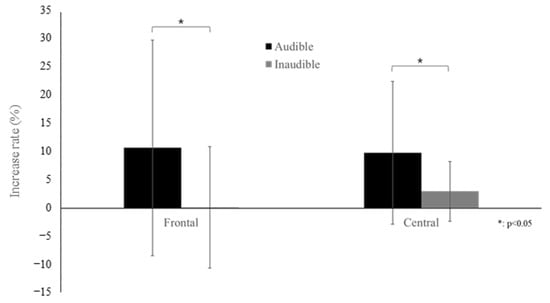
Figure 3.
Increase rate of relative alpha power between audible and inaudible states at the frontal and central areas (bars represent the standard deviation).
4. Discussion
This study examined the effects of audible and inaudible BB stimuli on alpha power elicitation, with differences observed according to the availability of sound perception.
In five brain regions, the increase in relative alpha power was significant with the BB stimulus during the stimulation phase compared with the rest phase, both with and without sound perception, and there were no differences between the states. Thus, both baseline frequencies influenced the induction of alpha waves. However, the increase in relative alpha power was greater during the audible phase than during the inaudible phase in the frontal and central areas.
It has been reported that single frequency sound alone influences EEG activation. Thus, when stimuli with different single frequency sounds were presented, the brain responded differently to each stimulus [16]. In addition, there have been reports that the sounds in the inaudible frequency range affect human recognition and psychology. The alpha-wave and comfortable listening levels increased when exposed to auditory stimulation in inaudible and audible frequency ranges, rather than just the audible frequency range [17]. For BB stimuli with equal difference frequency but different baseline frequency, there was a difference in the activated brain region and the degree of activation [11,13,18]. Particularly, previous studies comparing low and high baseline frequencies (400 Hz versus 3200 Hz, 250 Hz versus 1000 Hz) with equal frequency differences [19,20] exhibited a greater effect from BB at the lower baseline frequency compared with that at a higher baseline frequency. This can be attributed to the larger evocation of the auditory steady-state response (ASSR) at the lower baseline frequencies.
In this study, the difference in the reference frequency was considerably large; therefore, an extreme case with the presence or absence of sound perception was compared, and a difference in activation was observed, as in previous studies. Particularly, it could be expected that the case of sound perception induced ASSR, further highlighting the BB effect in both frontal and central regions. This showed similar results to previous studies. Yamsa-ard revealed that pure-tone audible alpha BB increases alpha waves in the frontal and central regions [21].
This study concludes that an increase in the alpha-power value can be induced only owing to frequency differences, even when the sound is not audible. Consequently, the pure effect of BB without the effect of sound was verified. Additionally, it has been observed that the sound factor can alter the BB effect.
The ages of the subject-group participants were a limitation of this study. Considering this limitation, future research is necessary, particularly considering that ASSR is affected by age. Additionally, new research on the distinct mechanisms that influence the BB effect is necessary for optimizing the BB effect by comparing various baseline frequencies. In particular, it is necessary to study the cause of the EEG-induced effect observed only in specific brain regions (frontal and central) and not in other regions. Finally, research on the effects of the various components (delta, theta, and beta) beyond the alpha component is required.
Author Contributions
Conceptualization, J.-H.Y. and S.-C.C.; methodology, J.-H.Y. and S.-C.C.; software, M.-H.C.; validation, K.-B.K.; formal analysis, Y.-J.K.; investigation, K.-B.K., Y.-J.K., and J.-S.K.; resources, H.-S.K.; data curation, M.-H.C.; writing—original draft preparation, J.-H.Y. and S.-C.C.; writing—review and editing, J.-H.Y. and S.-C.C.; visualization, M.-H.C.; supervision, S.-C.C.; project administration, S.-C.C.; funding acquisition, J.-H.Y. and S.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The authors declare that this study was approved by the Konkuk University institutional review board (7001355-202105-HR-439). All subjects provided written informed consent in accordance with the Declaration of Helsinki and received a monetary reward for their participation in the experiment. The privacy rights of human subjects in this study must always be observed.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C2009136).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lim, H.M.; Ku, J.H. Flickering exercise video produces mirror neuron system (MNS) activation and steady state visually evoked potentials (SSVEPs). J. Biomed. Eng. Lett. 2017, 7, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wan, F.; Wong, C.M.; da Cruz, J.N.; Hu, Y. Objective evaluation of fatigue by EEG spectral analysis in steady-state visual evoked potential-based brain-computer interfaces. J. Biomed. Eng. Online 2014, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- da Silva Junior, M.; de Freitas, R.C.; dos Santos, W.P.; da Silva, W.W.A.; Rodrigues, M.C.A.; Conde, E.F.Q. Exploratory study of the effect of binaural beat stimulation on the EEG activity pattern in resting state using artificial neural networks. J. Cogn. Syst. Res. 2019, 54, 1–20. [Google Scholar] [CrossRef]
- Jirakittayakorn, N.; Wongsawat, Y. Brain responses to a 6-Hz binaural beat: Effects on general theta rhythm and frontal midline theta activity. J. Front. Neurosci. 2017, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Argibay, M.; Santed, M.A.; Reales, J.M. Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. J. Psychol. Res. 2019, 83, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Wilpert, E.C.; Reber, T.P.; Fell, J. Auditory beat stimulation and its effects on cognition and mood states. J. Front. Psychiatry. 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Puzi, N.M.; Jailani, R.; Norhazman, H.; Zaini, N.M. Alpha and Beta Brainwave Characteristics to Binaural Beat Treatment. In Proceedings of the IEEE 9th International Colloquium on Signal Processing and its Applications, Kuala Lumpur, Malaysia, 8–10 March 2013; IEEE Publications: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Park, J.; Kwon, H.; Kang, S.; Lee, Y. The effect of binaural beat-based audiovisual stimulation on brain waves and concentration. In Proceedings of the International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Republic of Korea, 17–19 October 2018; pp. 420–423. [Google Scholar] [CrossRef]
- Padmanabhan, R.; Hildreth, A.J.; Laws, D. A prospective, randomised, controlled study examining binaural beat audio and pre-operative anxiety in patients undergoing general anaesthesia for day case surgery. J. Anaesth. 2005, 60, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, H.; Calabrese, C.; Zwickey, H.; Zajdel, D. Binaural beat technology in humans: A pilot study to assess neuropsychologic, physiologic, and electroencephalographic effects. J. Altern. Complement. Med. 2007, 13, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Beauchene, C.; Abaid, N.; Moran, R.; Diana, R.A.; Leonessa, A. The effect of binaural beats on verbal working memory and cortical connectivity. J. Neural Eng. 2017, 14, 026014. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Kasian, S.J.; Owens, J.E.; Marsh, G.R. Binaural auditory beats affect vigilance performance and mood. Physiol. Behav. 1998, 63, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Solcà, M.; Mottaz, A.; Guggisberg, A.G. Binaural beats increase interhemispheric alpha-band coherence between auditory cortices. J. Hear. Res. 2016, 332, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Munro, B.A.; Searchfield, G.D. The short-term effects of recorded ocean sound with and without alpha frequency binaural beats on tinnitus perception. Complement. Ther. Med. 2019, 44, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Oohashi, T.; Kawai, N.; Nishina, E.; Honda, M.; Yagi, R.; Nakamura, S.; Shibasaki, H. The role of biological system other than auditory air-conduction in the emergence of the hypersonic effect. Brain Res. 2006, 1073, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Sudirman, R.; Seow, S.C. Electroencephalographic based hearing identification using back-propagation algorithm. In Proceedings of the IEEE Toronto International Conference Science and Technology for Humanity (TIC-STH), Toronto, ON, Canada, 26–27 September 2009; Volume 991–995. [Google Scholar] [CrossRef]
- Oohashi, T.; Nishina, E.; Honda, M.; Yonekura, Y.; Fuwamoto, T.; Kawai, N.; Shibasaki, H. Inaudible high-frequency sounds effect brain activity: Hypersonic effect. J. Neurophysiol. 2000, 83, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, C. Influence of binaural beats on EEG signal. J. Acta Phys. Pol. A. 2011, 119, 986–990. [Google Scholar] [CrossRef]
- Schwarz, D.W.; Taylor, P. Human auditory steady state responses to binaural and monaural beats. J. Clin. Neurophysiol. 2005, 116, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Pratt, H.; Starr, A.; Michalewski, H.J.; Dimitrijevic, A.; Bleich, N.; Mittelman, N. A comparison of auditory evoked potentials to acoustic beats and to binaural beats. J. Hear. Res. 2010, 262, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Yamsa-ard, T.; Wongsawat, Y. The relationship between EEG and binaural beat stimulation in meditation. In Proceedings of the 7th 2014 Biomedical Engineering International Conference, Fukuoka, Japan, 26–28 November 2014; IEEE: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).