Penetration Depth of Initiated and Non-Initiated Diode Lasers in Bovine Gingiva
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. Histological Processing Method
2.3. Statistical Analysis
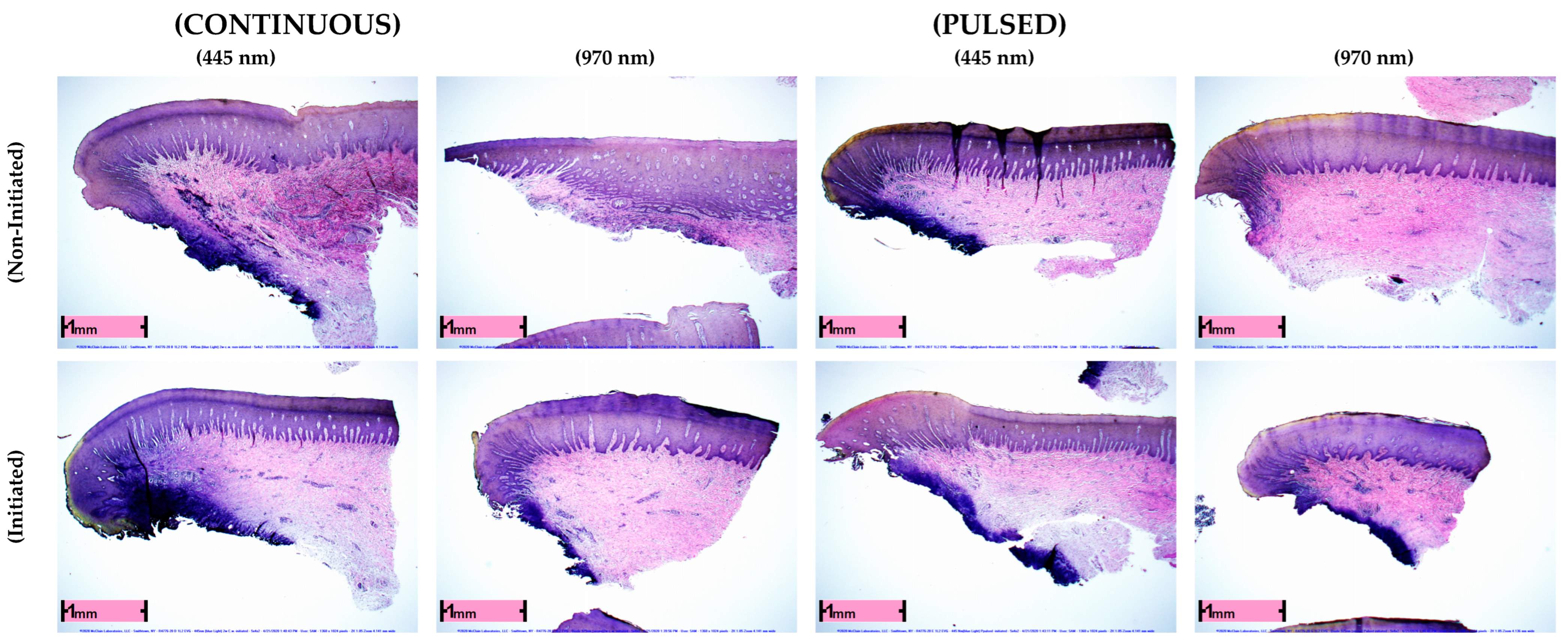
3. Results
Evaluation of Penetration Depths among Treatment Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontology 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Aoki, A.; Sasaki, K.M.; Watanabe, H.; Ishikawa, I. Lasers in nonsurgical periodontal therapy. Periodontology 2000 2004, 36, 59. [Google Scholar] [CrossRef]
- Romanos, G.E. Diode laser soft-tissue surgery: Advancements aimed at consistent cutting, improved clinical outcomes. Compend. Contin. Educ. Dent. 2013, 34, 752–757, quiz 758. [Google Scholar] [PubMed]
- Kranendonk, A.; Van der Reijden, W.; Van Winkelhoff, A.; Van der Weijden, G. The bactericidal effect of a Genius® Nd:YAG laser. Int. J. Dent. Hyg. 2010, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Nammour, S.; El Mobadder, M.; Maalouf, E.; Namour, M.; Namour, A.; Rey, G.; Matamba, P.; Matys, J.; Zeinoun, T.; Grzech-Leśniak, K. Clinical Evaluation of Diode (980 nm) Laser-Assisted Nonsurgical Periodontal Pocket Therapy: A Randomized Comparative Clinical Trial and Bacteriological Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, J.; Winter, J.; Meister, J.; Frentzen, M.; Kraus, D. A novel blue light laser system for surgical applications in dentistry: Evaluation of specific laser-tissue interactions in monolayer cultures. Clin. Oral Investig. 2017, 21, 985–994. [Google Scholar] [CrossRef]
- Romanos, G.; Nentwig, G.H. Diode laser (980 nm) in oral and maxillofacial surgical procedures: Clinical observations based on clinical applications. J. Clin. Laser Med. Surg. 1999, 17, 193–197. [Google Scholar] [CrossRef]
- Romanos, G.E.; Belikov, A.V.; Skrypnik, A.V.; Feldchtein, F.I.; Smirnov, M.Z.; Altshuler, G.B. Uncovering dental implants using a new thermo-optically powered (TOP) technology with tissue air-cooling. Lasers Surg. Med. 2015, 47, 411–420. [Google Scholar] [CrossRef]
- Romanos, G.E.; Sacks, D.; Montanaro, N.; Delgado-Ruiz, R.; Calvo-Guirado, J.L.; Javed, F. Effect of initiators on thermal changes in soft tissues using a diode laser. Photomed. Laser Surg. 2018, 36, 386–390. [Google Scholar] [CrossRef]
- Romanos, G.E. Advanced Laser Surgery in Dentistry; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Estrin, N.E.; Lesniewski, A.; McClain, S.; Hou, W.; Romanos, G.E. Thermal Penetration Depth of Pulsed Lasers in Gingival Tissues: An In-Vitro Study. Photobiomodul. Photomed. Laser Surg. 2022, 40, 410–416. [Google Scholar] [CrossRef]
- Matys, J.; Flieger, R.; Dominiak, M. Effect of diode lasers with a wavelength of 445 and 980 nm on a temperature rise when uncovering implants for second stage surgery: An ex-vivo study in pigs. Adv. Clin. Exp. Med. 2017, 26, 687–693. [Google Scholar] [CrossRef]
- Boulnois, J.-L. Photophysical processes in recent medical laser developments: A review. Lasers Med. Sci. 1986, 1, 47–66. [Google Scholar] [CrossRef]
- Lamont, R.J.; Chan, A.; Belton, C.M.; Izutsu, K.T.; Vasel, D.; Weinberg, A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 1995, 63, 3878–3885. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Romanos, G.E.; Henze, M.; Banihashemi, S.; Parsanejad, H.R.; Winckler, J.; Nentwig, G.-H. Removal of epithelium in periodontal pockets following diode (980 nm) laser application in the animal model: An in vitro study. Photomed. Laser Surg. 2004, 22, 177–183. [Google Scholar] [CrossRef]
- Goldman, H.M. A Rationale for the Treatment of the Intrabony Pocket; One Method of Treatment, Subgingival Curettage. J. Periodontol. 1949, 20, 83–91. [Google Scholar] [CrossRef]
- Gold, S.I.; Vilardi, M.A. Pulsed laser beam effects on the gingiva. J. Clin. Periodontol. 1994, 21, 391–396. [Google Scholar] [CrossRef]
- Harris, D.M.; Gregg, R.H.; McCarthy, D.K.; Colby, L.E.; Tilt, L.V. Laser-assisted new attachment procedure in private practice. Gen. Dent. 2004, 52, 396–403. [Google Scholar]
- Nevins, M.L.; Camelo, M.; Schupbach, P.; Kim, S.-W.; Kim, D.M.; Nevins, M. Human clinical and histologic evaluation of laser-assisted new attachment procedure. Int. J. Periodontics Restor. Dent. 2012, 32, 497–507. [Google Scholar]
- Nguyen, D.D.; Pang, J.Y.; Madill, C.; Novakovic, D. Effects of 445-nm Laser on Vessels of Chick Chorioallantoic Membrane with Implications to Microlaryngeal Laser Surgery. Laryngoscope 2021, 131, E1950–E1956. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 176–198. [Google Scholar] [CrossRef]
- Kamma, J.; Romanos, G.E.; Vasdekis, V. The effect of diode laser (980 nm) treatment on aggressive Periodontitis. Evaluation of microbial and clinical parameters. Photomed. Laser Surg. 2009, 27, 11–19. [Google Scholar] [CrossRef] [PubMed]
| Experimental Group | N | Mean | Std. Dev. | Min. | Max. | p-Value |
|---|---|---|---|---|---|---|
| 970 nm C.W. Non-Initiated | 96 | 0.131 | 0.161 | 0 | 0.46 | <0.001 |
| 445 nm C.W. Non-Initiated | 96 | 0.22 | 0.15 | 0 | 0.6 | |
| 970 nm C.W. Initiated | 96 | 0.20 | 0.14 | 0 | 0.8 | <0.001 |
| 445 nm C.W. Initiated | 96 | 0.35 | 0.20 | 0.09 | 0.8 | |
| 445 nm Pulsed Initiated | 96 | 0.13 | 0.14 | 0 | 0.48 | 0.7939 |
| 445 nm Pulsed Non-Initiated | 96 | 0.13 | 0.15 | 0 | 0.5 | |
| 970 nm Pulsed Initiated | 96 | 0.10 | 0.10 | 0 | 0.46 | <0.001 |
| 970 nm Pulsed Non-Initiated | 96 | 0.01 | 0.05 | 0 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanos, G.E.; Estrin, N.E.; Lesniewski, A.; McClain, S.; Hou, W. Penetration Depth of Initiated and Non-Initiated Diode Lasers in Bovine Gingiva. Appl. Sci. 2022, 12, 12771. https://doi.org/10.3390/app122412771
Romanos GE, Estrin NE, Lesniewski A, McClain S, Hou W. Penetration Depth of Initiated and Non-Initiated Diode Lasers in Bovine Gingiva. Applied Sciences. 2022; 12(24):12771. https://doi.org/10.3390/app122412771
Chicago/Turabian StyleRomanos, Georgios E., Nathan E. Estrin, Agata Lesniewski, Steve McClain, and Wei Hou. 2022. "Penetration Depth of Initiated and Non-Initiated Diode Lasers in Bovine Gingiva" Applied Sciences 12, no. 24: 12771. https://doi.org/10.3390/app122412771
APA StyleRomanos, G. E., Estrin, N. E., Lesniewski, A., McClain, S., & Hou, W. (2022). Penetration Depth of Initiated and Non-Initiated Diode Lasers in Bovine Gingiva. Applied Sciences, 12(24), 12771. https://doi.org/10.3390/app122412771







