Rhamnolipids Mediate the Effects of a Gastropod Grazer in Regards to Carbon–Nitrogen Stoichiometry of Intertidal Microbial Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Sample Collection
2.3. Data Analysis
3. Results
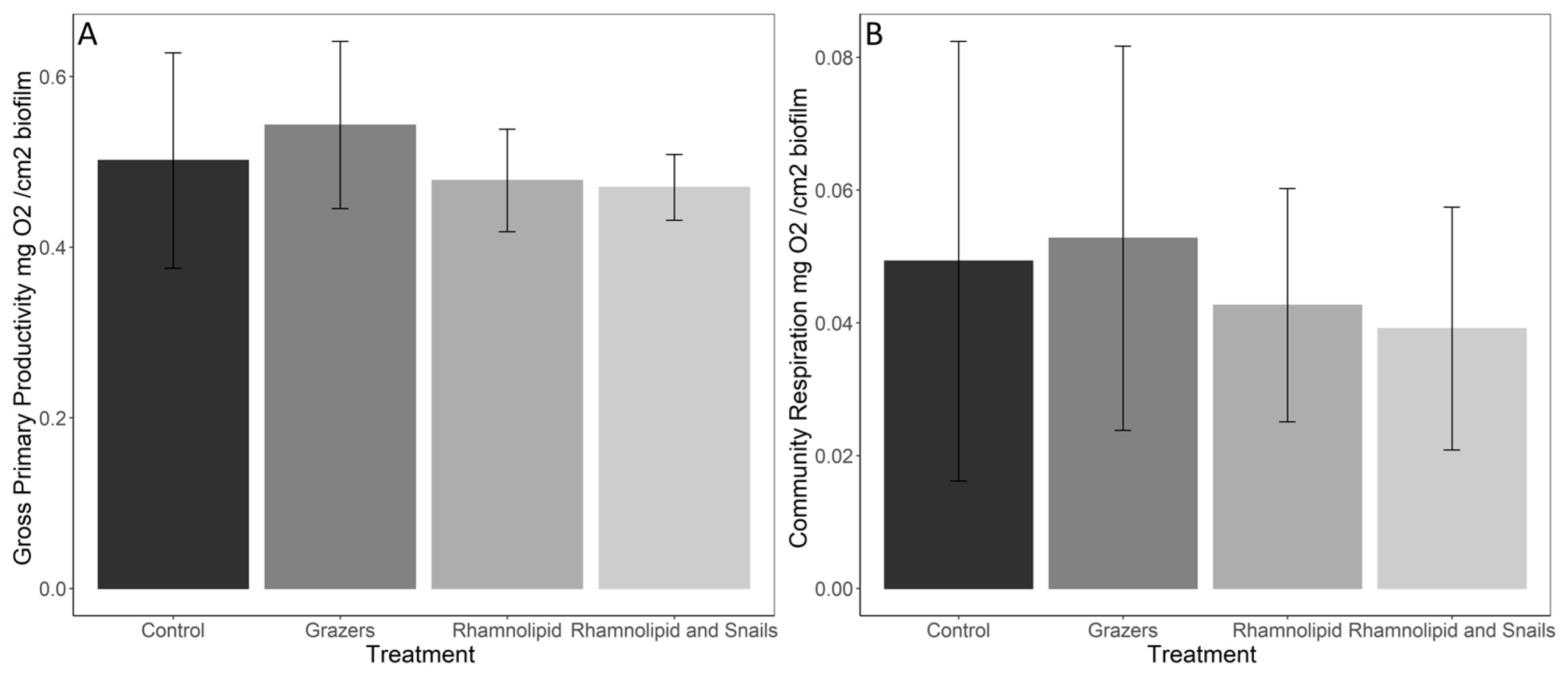
3.1. Primary Productivity, Respiration, and Biomass
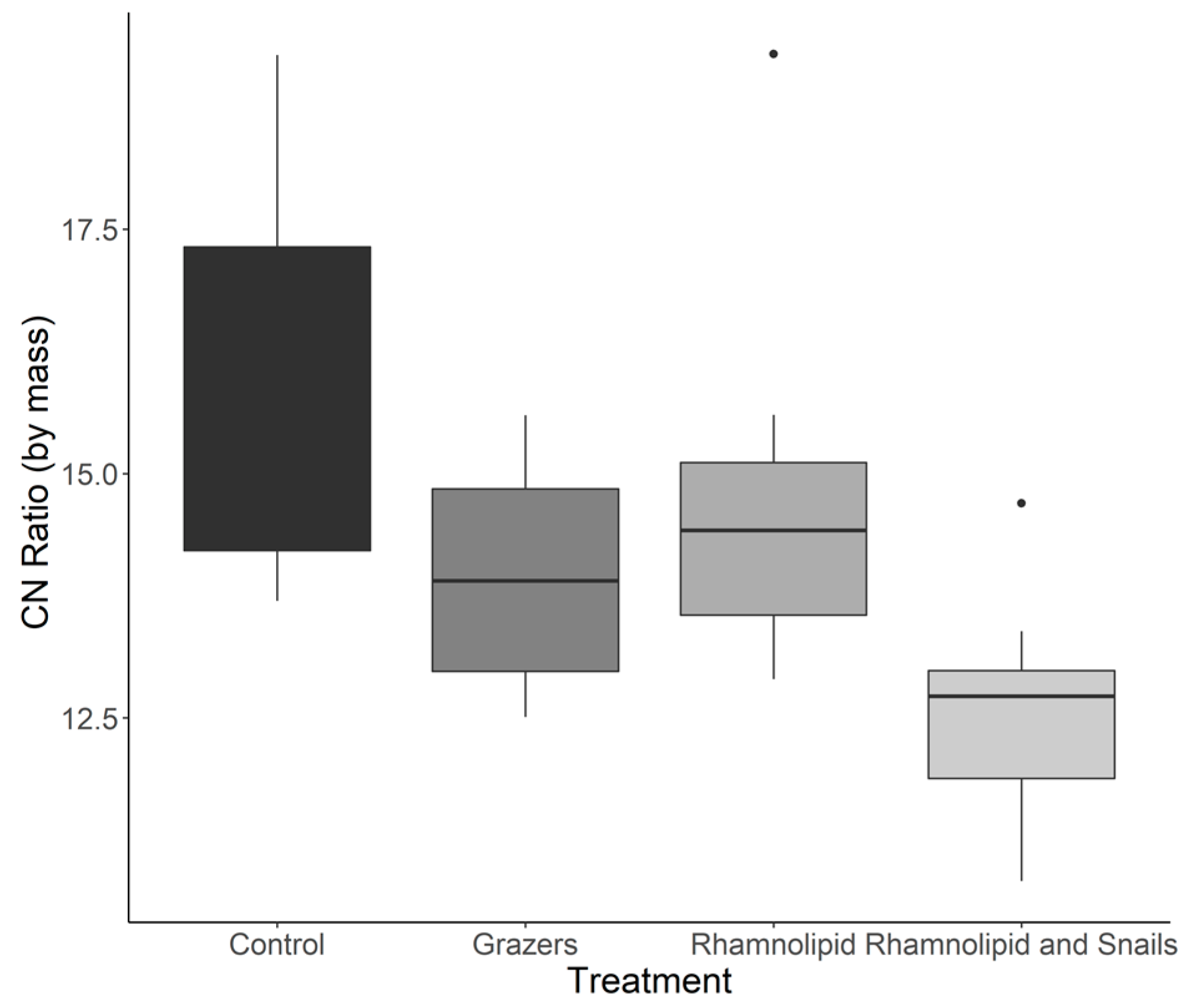
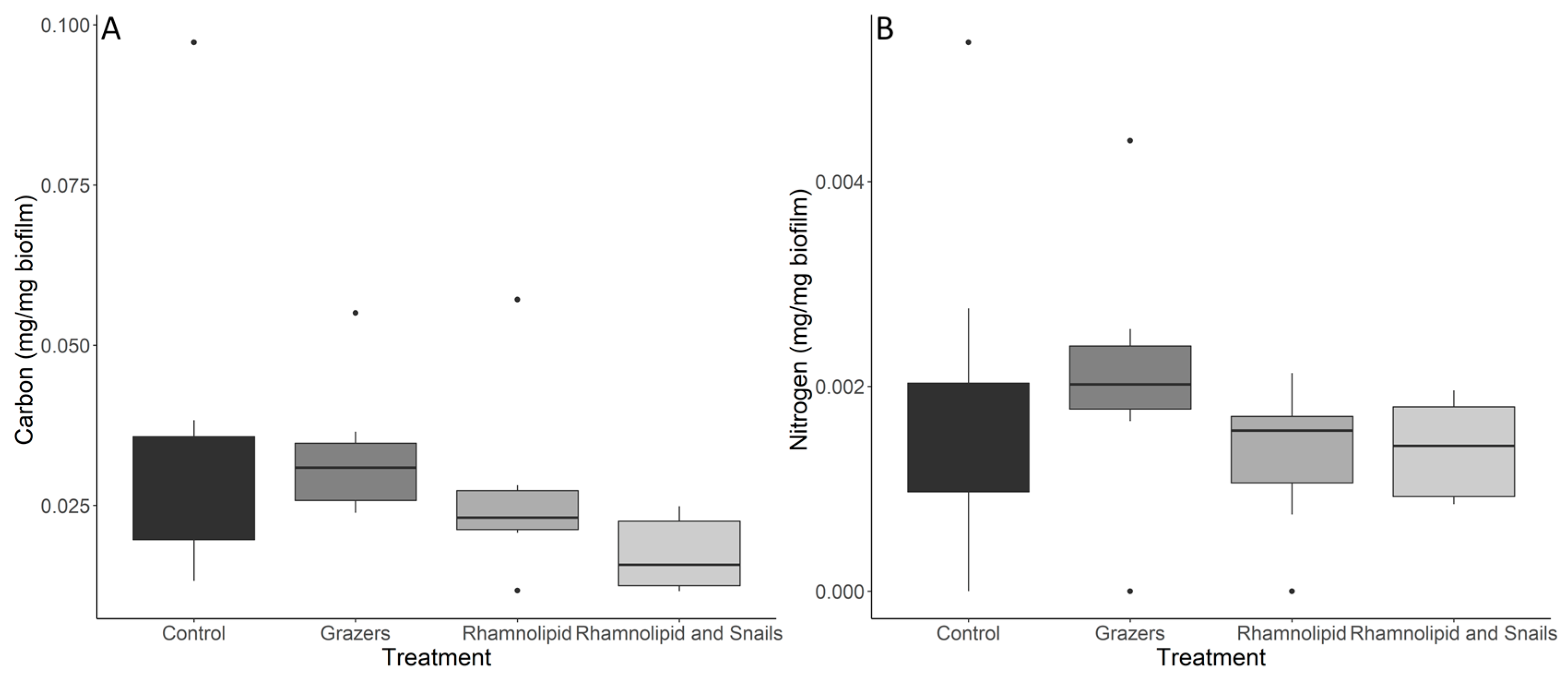
3.2. Biofilm Stoichiometry
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Ren, Z.; Zhang, H.; Zhang, M.; Zhang, Y.; Liu, X.; Peng, W. Influences of anthropogenic land use on microbial community structure and functional potentials of stream benthic biofilms. Sci. Rep. 2017, 7, 15117. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.; Guasch, H.; Munoz, I.; Ruana, J.; Vilalta, E.; Schwartz, T.; Emtiazi, F.; Sabater, S. Biofilm structure and function and possible implications for riverine DOC dynamics. Mirobiol. Ecol. 2004, 47, 316–328. [Google Scholar] [CrossRef]
- Kroukamp, O.; Wolfaardt, G.M. CO₂ Production as an indicator of biofilm metabolism. Appl. Environ. Microbiol. 2008, 75, 4391–4397. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef]
- Moore, L.J.; Fuentes, L.; Rodgers, J.H., Jr.; Bowerman, W.W.; Yarrow, G.K.; Chao, W.Y.; Bridges, W.C., Jr. Relative toxicity of the components of the original formulation of Roundup® to five North American anurans. Ecotoxicol. Environ. Saf. 2012, 78, 128–133. [Google Scholar] [CrossRef]
- Koedooder, C.; Stock, W.; Willems, A.; Mangelinckx, S.; De Troch, M.; Vyverman, W.; Sabbe, K. Diatom-bacteria interactions modulate the composition and productivity of benthic diatom biofilms. Front. Microbiol. 2019, 10, 1255. [Google Scholar] [CrossRef]
- Jacobs, P.; Pitarch, J.; Kromkamp, J.C.; Philippart, C.J. Assessing biomass and primary production of microphytobenthos in depositional coastal systems using spectral information. PLoS ONE 2021, 16, e0246012. [Google Scholar] [CrossRef]
- Rode, D.K.H.; Singh, P.K.; Drescher, K. Multicellular and unicellular responses of microbial biofilms to stress. Biol. Chem. 2020, 401, 1365–1374. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- De Beer, D.; Srinivasan, R.; Stewart, P.S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 1994, 60, 4339–4344. [Google Scholar] [CrossRef]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274. [Google Scholar] [CrossRef]
- DePas William, H.; Syed Adnan, K.; Margarita, S.; Lee John, S.; David, W.; Vinay, S.; Györgyi, C.; Boles Blaise, R.; Chapman Matthew, R.; Nojiri, H. Biofilm formation protects Escherichia coli against killing by Caenorhabditis elegans and Myxococcus xanthus. Appl. Environ. Microbiol. 2014, 80, 7079–7087. [Google Scholar] [CrossRef]
- Wucher, B.R.; Elsayed, M.; Adelman, J.S.; Kadouri, D.E.; Nadell, C.D. Bacterial predation transforms the landscape and community assembly of biofilms. Curr. Biol. 2021, 31, 2643–2651.e3. [Google Scholar] [CrossRef]
- Chan, S.Y.; Liu, S.Y.; Seng, Z.; Chua, S.L. Biofilm matrix disrupts nematode motility and predatory behavior. ISME J. 2021, 15, 260–269. [Google Scholar] [CrossRef]
- Collins, S.M.; Kohler, T.J.; Thomas, S.A.; Fetzer, W.W.; Flecker, A.S. The importance of terrestrial subsidies in stream food webs varies along a stream size gradient. Oikos 2016, 125, 674–685. [Google Scholar] [CrossRef]
- Margulis, L. Words as battle cries: Symbiogenesis and the new field of endocytobiology. Bioscience 1990, 40, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.W.; Volkelt-Igoe, M.; Hawkins, S.J.; Jesus, B.; Thompson, R.C.; Doncaster, C.P. Past and present grazing boosts the photo-autotrophic biomass of biofilms. Mar. Ecol. Prog. Ser. 2010, 401, 101–111. [Google Scholar] [CrossRef]
- Collins, P.C.; Hunter, W.R.; Carlsson, J.; Carlsson, J. Fortuitous insights into the ecology of a recently charted deep-sea hydrothermal vent, using snails’ feet. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 163, 103358. [Google Scholar] [CrossRef]
- Rugenski, A.T.; Murria, C.; Whiles, M.R. Tadpoles enhance microbial activity and leaf decomposition in a neotropical headwater stream. Freshw. Biol. 2012, 57, 1904–1913. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Halvorson, H.M.; Kuehn, K.A.; Winebarger, M.; Hamid, A.; Waters, M.N. Filter-feeders have differential bottom-up impacts on green and brown food webs. Oecologia 2021, 195, 187–198. [Google Scholar] [CrossRef]
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539. [Google Scholar] [CrossRef]
- Chrzanowski, Ł.; Ławniczak, Ł.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef]
- Schmitz, K.S. Chapter 4: Life Science. In Physical Chemistry; Elsevier: Boston, MA, USA, 2018; pp. 755–832. [Google Scholar]
- Milovanovic, M.; Arsenijevic, A.; Milovanovic, J.; Kanjevac, T.; Arsenijevic, N. Chapter 14: Nanoparticles in Antiviral Therapy. In Antimicrobial Nanoarchitectonics; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 383–410. ISBN 0323527337. [Google Scholar]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the glycolipid biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Factories 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Grime, J.P. The CSR model of primary plant strategies—Origins, implications and tests. In Plant Evolutionary Biology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 371–393. [Google Scholar]
- Malik, A.A.; Martiny, J.B.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Elser, J.J.; Martiny, A.C.; Sterner, R.W.; Cotner, J.B. Editorial: Progress in ecological stoichiometry. Front. Microbiol. 2018, 9, 1957. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 July 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: New York, NY, USA, 2018. [Google Scholar]
- Wickham, H. Package ‘ggplot2′: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Volume 10, pp. 978–980. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis; R Package Version 0.9; 2022. [Google Scholar]
- Simões, M.; Simões, L.C.; Pereira, M.O.; Vieira, M.J. Sodium dodecyl sulfate allows the persistence and recovery of biofilms of Pseudomonas fluorescens formed under different hydrodynamic conditions. Biofouling 2008, 24, 35–44. [Google Scholar] [CrossRef][Green Version]
- Lin, J.; Wang, Z.; Zang, Y.; Zhang, D.; Xin, Q. Detection of respiration changes inside biofilms with microelectrodes during exposure to antibiotics. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2019, 54, 202–207. [Google Scholar] [CrossRef]
- Quinn, G.A.; Maloy, A.P.; Banat, M.M.; Banat, I.M. A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Curr. Microbiol. 2013, 67, 614–623. [Google Scholar] [CrossRef]
- Seiler, C.; Van Velzen, E.; Neu, T.R.; Gaedke, U.; Berendonk, T.U.; Weitere, M. Grazing resistance of bacterial biofilms: A matter of predators’ feeding trait. FEMS Microbiol. Ecol. 2017, 93, fix112. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Bordalo, A.A.; Wiebe, W.J. Intertidal biofilms on rocky substratum can play a major role in estuarine carbon and nutrient dynamics. Mar. Ecol. Prog. Ser. 2003, 258, 275–281. [Google Scholar] [CrossRef]
- Colls Lozano, M.; Timoner, X.; Font, C.; Sabater, S.; Acuña, V. Effects of duration, frequency, and severity of the non-flow period on stream biofilm metabolism. Ecosystems 2019, 22, 1393–1405. [Google Scholar] [CrossRef]
- Gill, S.P.; Hunter, W.R.; Coulson, L.E.; Banat, I.M.; Schelker, J. Synthetic and biological surfactant effects on freshwater biofilm community composition and metabolic activity. Appl. Microbiol. Biotechnol. 2022, 106, 6847–6859. [Google Scholar] [CrossRef]
- Thompson, R.C.; Norton, T.A.; Hawkins, S.J. Physical stress and biological control regulate the producer–consumer balance in intertidal biofilms. Ecology 2004, 85, 1372–1382. [Google Scholar] [CrossRef]
- Androuin, T.; Polerecky, L.; Decottignies, P.; Dubois, S.F.; Dupuy, C.; Hubas, C.; Jesus, B.; Le Gall, E.; Marzloff, M.P.; Carlier, A. Subtidal microphytobenthos: A secret garden stimulated by the engineer species Crepidula fornicata. Front. Mar. Sci. 2018, 5, 475. [Google Scholar] [CrossRef]
- Hillebrand, H.; Kahlert, M. Effect of grazing and nutrient supply on periphyton biomass and nutrient stoichiometry in habitats of different productivity. Limnol Oceanograph 2001, 46, 1881–1898. [Google Scholar] [CrossRef]
- Heisterkamp, I.M.; Schramm, A.; Larsen, L.H.; Svenningsen, N.B.; Lavik, G.; de Beer, D.; Stief, P. Shell biofilm-associated nitrous oxide production in marine molluscs: Processes, precursors and relative importance. Environ. Microbiol. 2013, 15, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Nickzad, A.; Déziel, É. The involvement of rhamnolipids in microbial cell adhesion and biofilm development—An approach for control. Lett. Appl. Microbiol. 2014, 58, 447–453. [Google Scholar] [CrossRef]
- Du, M.; Xu, D.; Trinh, X.; Liu, S.; Wang, M.; Zhang, Y.; Wu, J.; Zhou, Q.; Wu, Z. EPS solubilization treatment by applying the biosurfactant rhamnolipid to reduce clogging in constructed wetlands. Bioresour. Technol. 2016, 218, 833–841. [Google Scholar] [CrossRef]
- Russell, B.D.; Connell, S.D.; Findlay, H.S.; Tait, K.; Widdicombe, S.; Mieszkowska, N. Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Philos. Trans. R. Soc. 2013, 368, 20120438. [Google Scholar] [CrossRef]
- Calapez, A.R.; Elias, C.L.; Alves, A.; Almeida, S.F.P.; Brito, A.G.; Feio, M.J. Shifts in biofilms’ composition induced by flow stagnation, sewage contamination and grazing. Ecol. Indic. 2020, 111, 106006. [Google Scholar] [CrossRef]
- Muñoz, I.; Real, M.; Guasch, H.; Navarro, E.; Sabater, S. Effects of atrazine on periphyton under grazing pressure. Aquat. Toxicol. 2001, 55, 239–249. [Google Scholar] [CrossRef]
- Rosi, E.J.; Bechtold, H.A.; Snow, D.; Rojas, M.; Reisinger, A.J.; Kelly, J.J. Urban stream microbial communities show resistance to pharmaceutical exposure. Ecosphere 2018, 9, e02041. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, S.P.; Kregting, L.; Banat, I.M.; Arnscheidt, J.; Hunter, W.R. Rhamnolipids Mediate the Effects of a Gastropod Grazer in Regards to Carbon–Nitrogen Stoichiometry of Intertidal Microbial Biofilms. Appl. Sci. 2022, 12, 12729. https://doi.org/10.3390/app122412729
Gill SP, Kregting L, Banat IM, Arnscheidt J, Hunter WR. Rhamnolipids Mediate the Effects of a Gastropod Grazer in Regards to Carbon–Nitrogen Stoichiometry of Intertidal Microbial Biofilms. Applied Sciences. 2022; 12(24):12729. https://doi.org/10.3390/app122412729
Chicago/Turabian StyleGill, Stephanie P., Louise Kregting, Ibrahim M. Banat, Joerg Arnscheidt, and William R. Hunter. 2022. "Rhamnolipids Mediate the Effects of a Gastropod Grazer in Regards to Carbon–Nitrogen Stoichiometry of Intertidal Microbial Biofilms" Applied Sciences 12, no. 24: 12729. https://doi.org/10.3390/app122412729
APA StyleGill, S. P., Kregting, L., Banat, I. M., Arnscheidt, J., & Hunter, W. R. (2022). Rhamnolipids Mediate the Effects of a Gastropod Grazer in Regards to Carbon–Nitrogen Stoichiometry of Intertidal Microbial Biofilms. Applied Sciences, 12(24), 12729. https://doi.org/10.3390/app122412729







