Parabolic Dish Collector as a New Approach for Biochar Production: An Evaluation Study
Abstract
1. Introduction
2. Materials and Methods
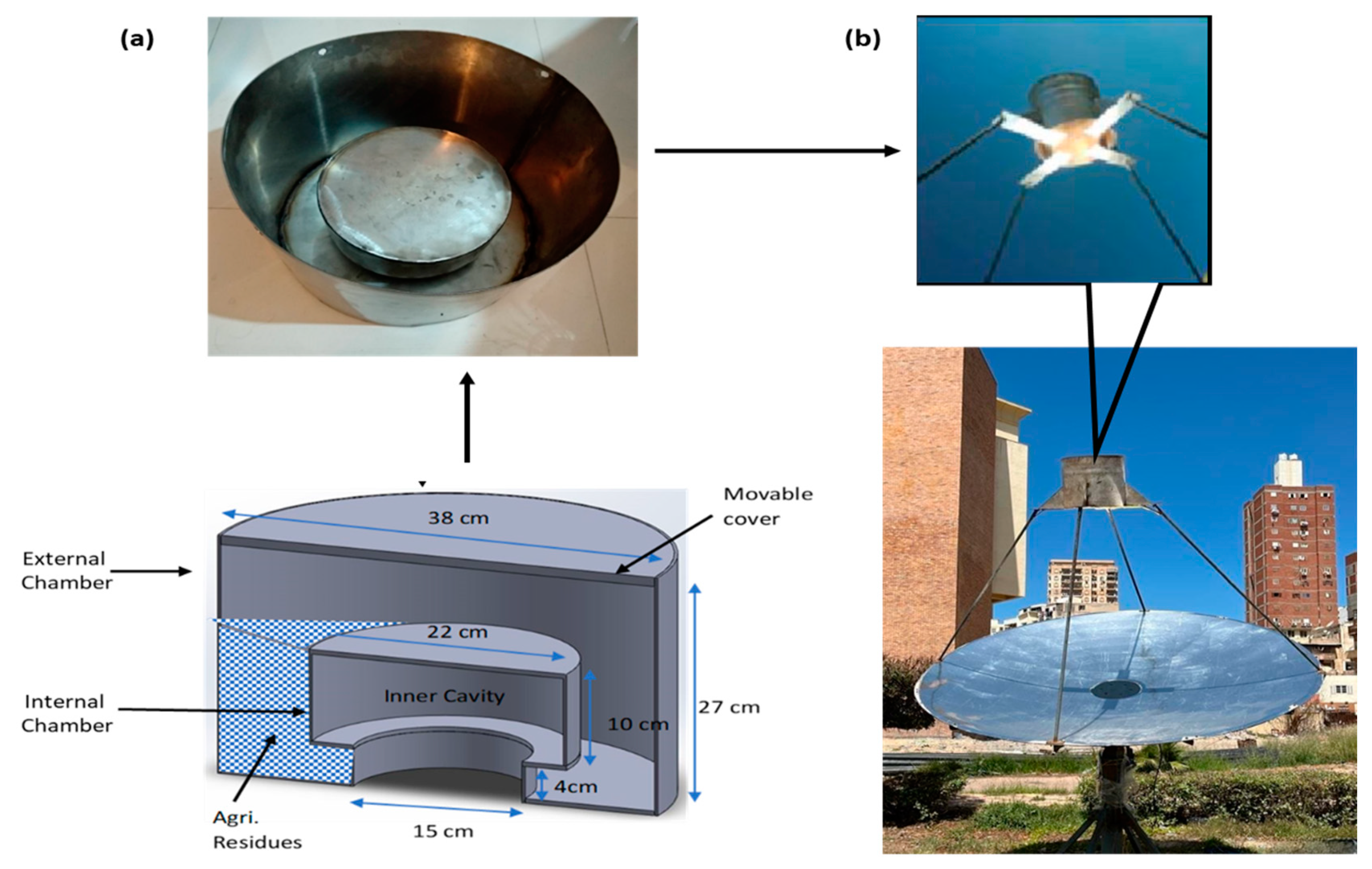
2.1. Solar Pyrolysis Chamber Design
2.2. Feedstock and Biochar Preparation
2.3. Sesame Feedstock and Produced Biochar Characterization
2.3.1. Ash Content and Elemental Analysis
2.3.2. Cation Exchange Capacity (CEC)
2.3.3. Zeta Potential
2.3.4. Infrared Spectroscopy (FTIR)
2.3.5. Scanning Electron Microscopy (SEM)
3. Results
3.1. Effect of Solar Pyrolysis Temperature on Biochar Production
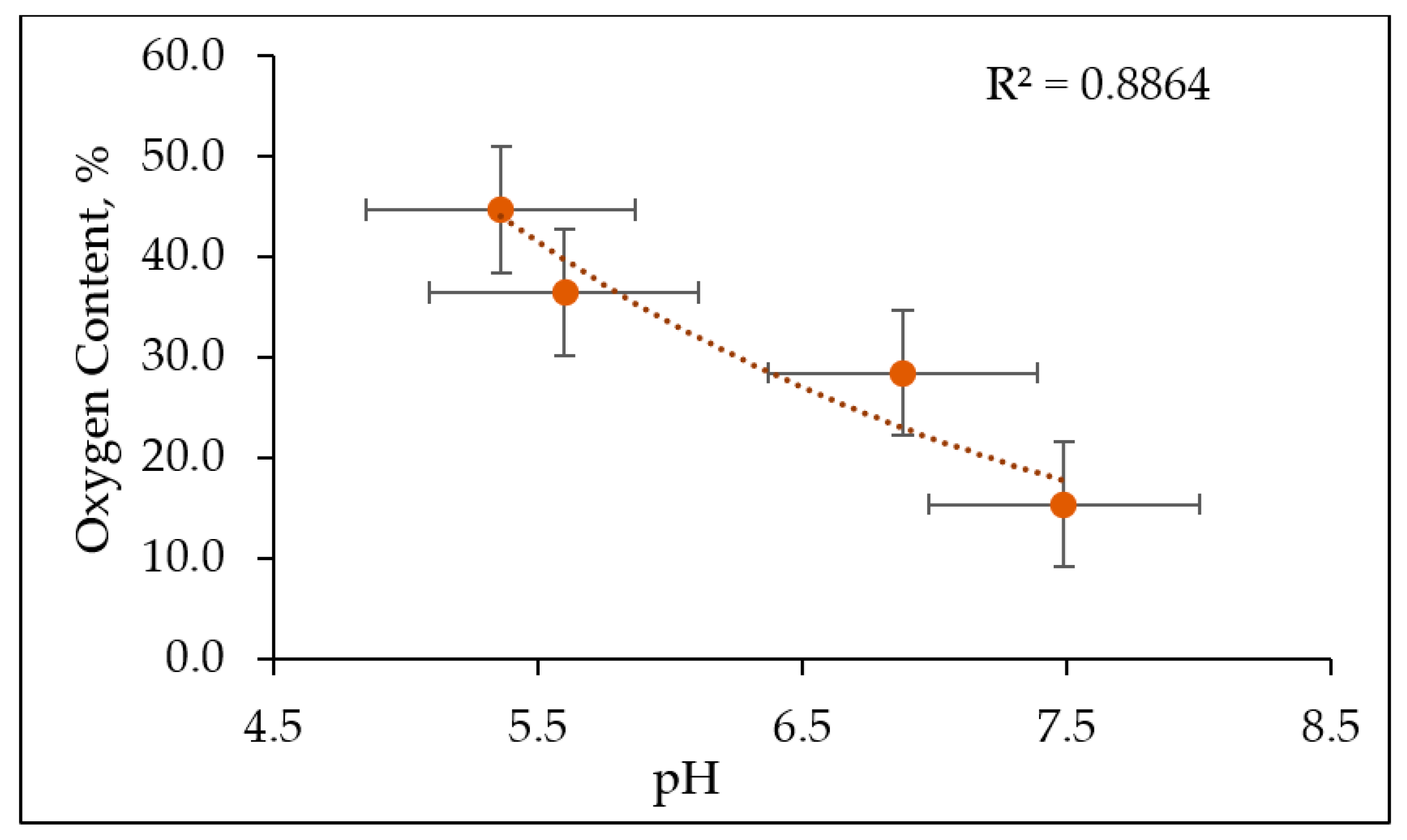
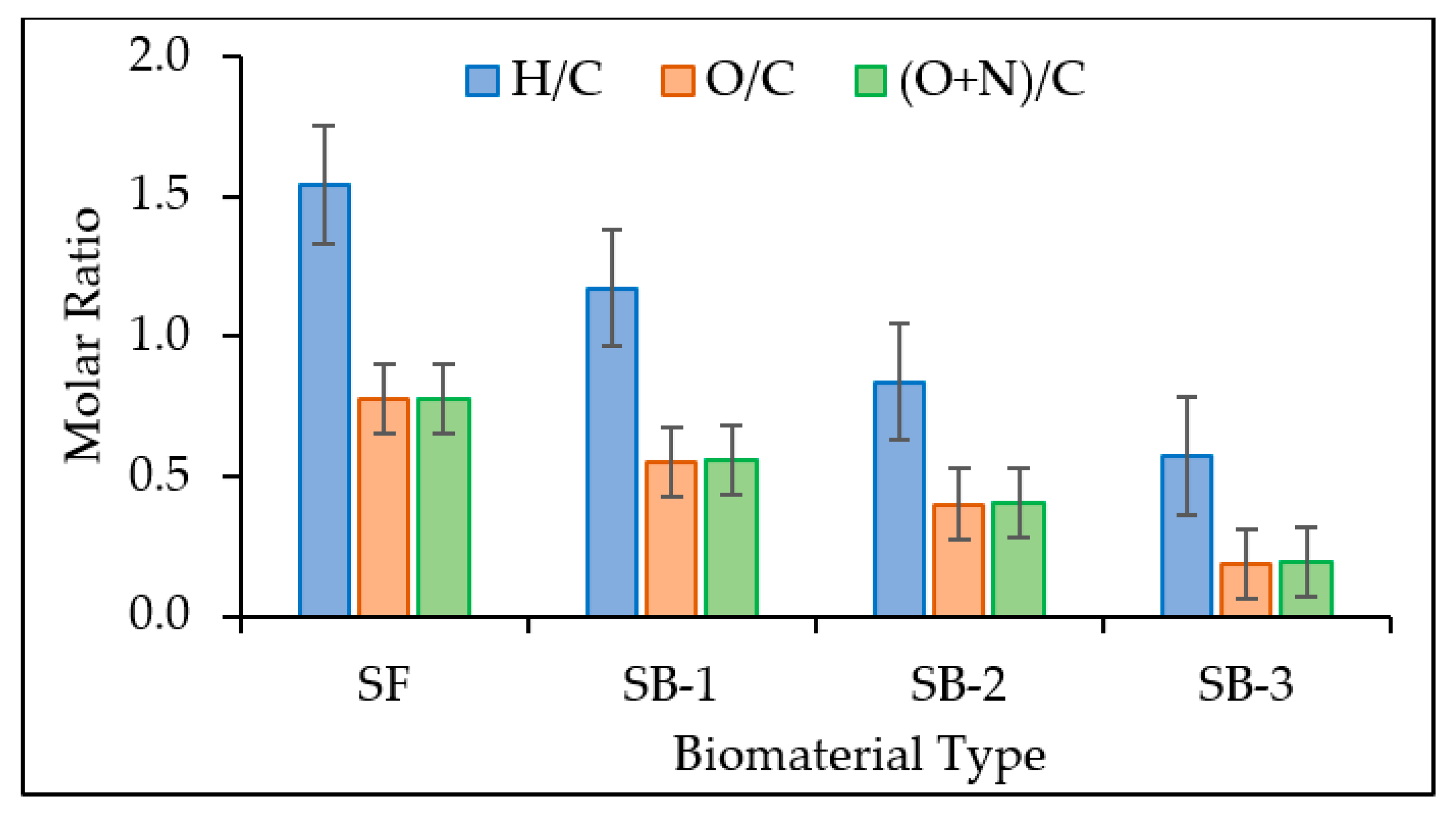
3.2. The Physicochemical Properties of Produced Biochar
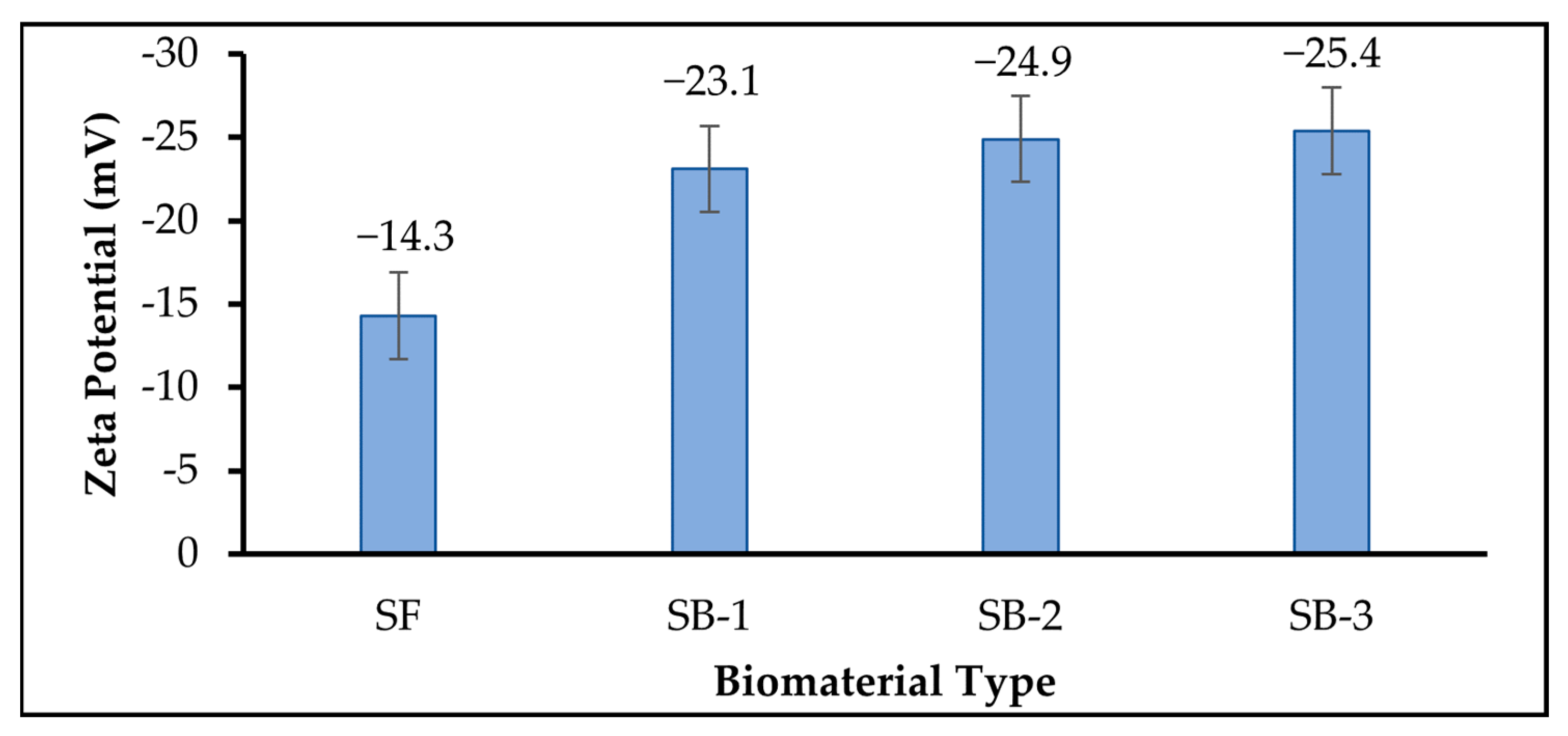
3.3. Zeta Potential
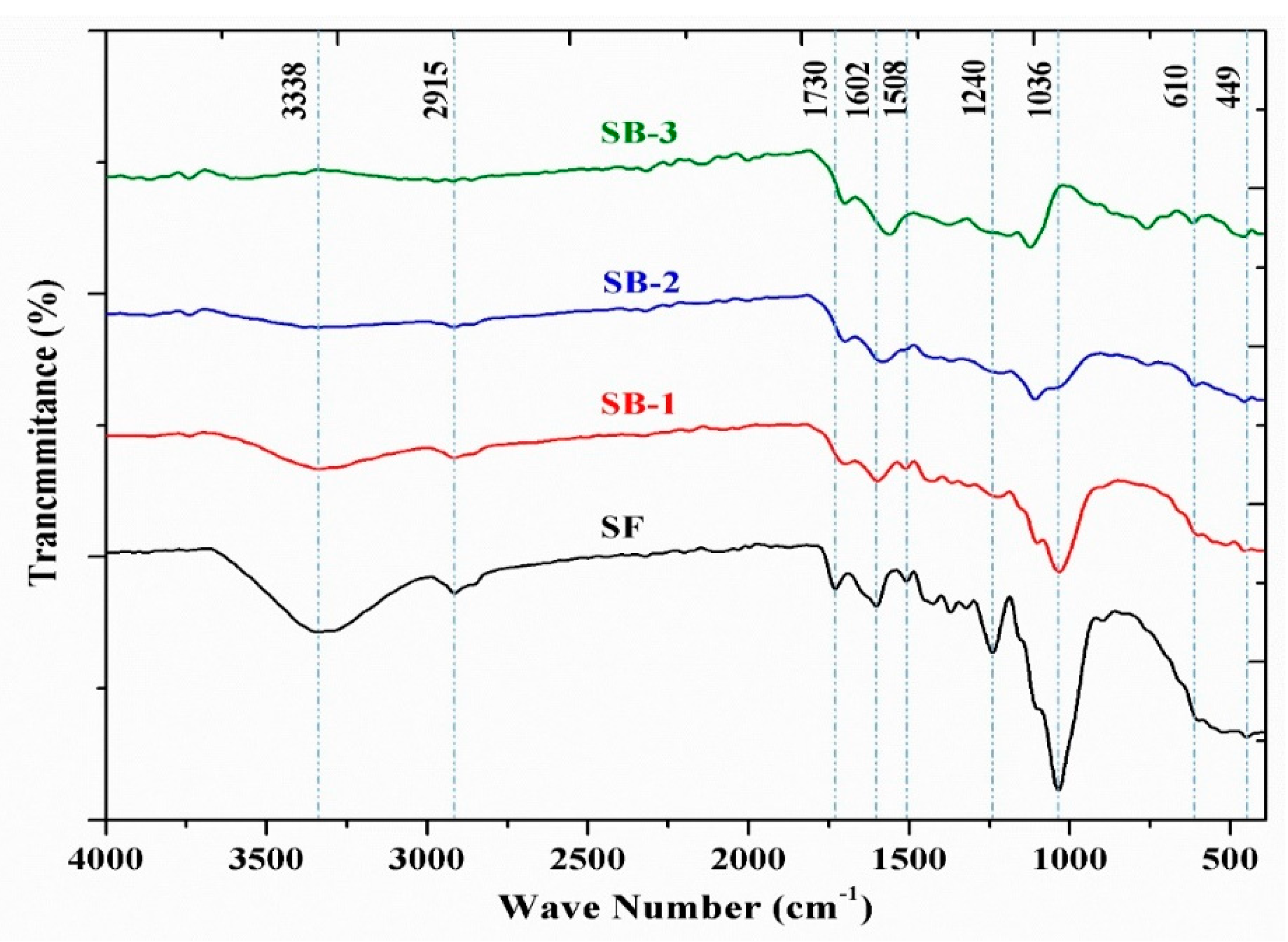
3.4. Fourier-Transform Infrared (FTIR)
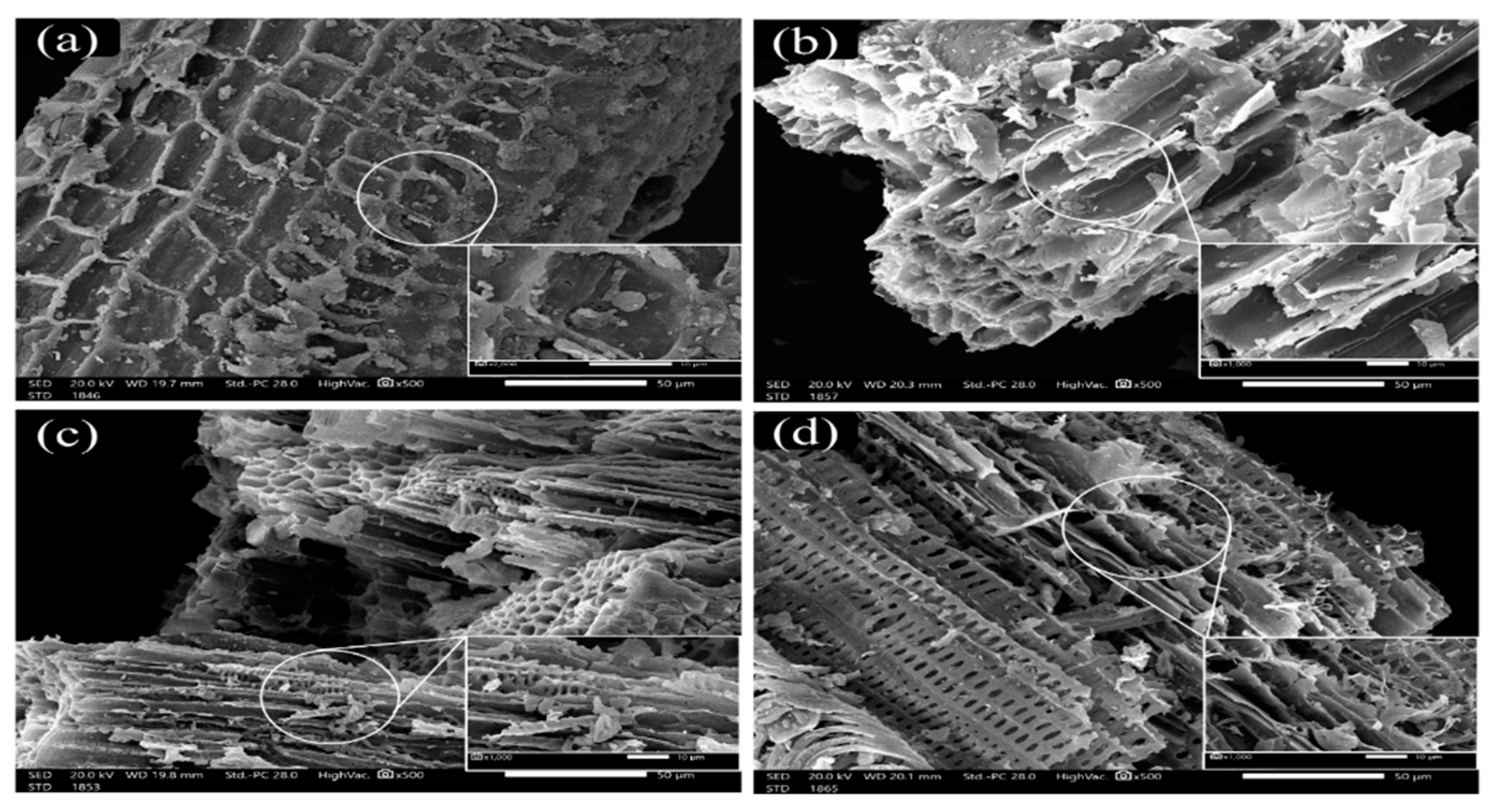
3.5. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Effect of Solar Pyrolysis Temperature on Biochar Production
4.2. Application of Biochar Produced by Parabolic Dish Collector
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 22, 137852. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Pecori, G.; Rosi, L.; Bettucci, L.; Fratini, E.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Biochar from lab-scale pyrolysis: Influence of feedstock and operational temperature. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The art, science and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Abbas, M.H.H.; Li, J. Biochar: The Black Diamond for Soil Sustainability, Contamination Control and Agricultural Production. In Engineering Applications of Biochar; Huang, W.-J., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Lehmann, J. Terra preta Nova–where to from here? In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Steiner, C., Prins, A.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 473–486. [Google Scholar]
- Sombroek, W.; Ruivo, M.L.; Fearnside, P.M.; Glaser, B.; Lehmann, J. Amazonian Dark Earths as carbon stores and sinks. In Amazonian Dark Earths: Origin, Properties, Management; Lehmann, J., Kern, D.C., Glaser, B., Woods, W.I., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 125–139. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M. Feedstock type, pyrolysis temperature and acid modification effects on physiochemical attributes of biochar and soil quality. Arab. J. Geosci. 2022, 15, 305. [Google Scholar] [CrossRef]
- Verma, M.; Godbout, S.; Brar, S.K.; Solomatnikova, O.; Lemay, S.P.; Larouche, J.P. Biofuels Production from Biomass by Thermochemical Conversion Technologies. Int. J. Chem. Eng. 2012, 2012, 542426. [Google Scholar] [CrossRef]
- El-Gamal, E.H.; Saleh, M.; Elsokkary, I.; Rashad, M.; Abd El-Latif, M.M. Comparison between Properties of Biochar Produced by Traditional and Controlled Pyrolysis. Alex. Sci. Exch. 2017, 38, 412–425. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Caputo, C.; Mašek, O. SPEAR (Solar Pyrolysis Energy Access Reactor): Theoretical Design and Evaluation of a Small-Scale Low-Cost Pyrolysis Unit for Implementation in Rural Communities. Energies 2021, 14, 2189. [Google Scholar] [CrossRef]
- Ndukwu, M.C.; Horsfall, I.T.; Ubouh, E.A.; Orji, F.N.; Ekop, I.E.; Ezejiofor, N.R. Review of solar-biomass pyrolysis systems: Focus on the configuration of thermal-solar systems and reactor orientation. J. King Saud Univ. Eng. Sci. 2021, 33, 413–423. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Thoai, D.N. Solar Thermal Application for Crop Residue Management. In Recent Advances in Thermofluids and Manufacturing Engineering; Kumar, A., Pal, A., Kachhwaha, S.S., Jain, P.K., Eds.; ICRAME 2020, Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; pp. 303–315. [Google Scholar] [CrossRef]
- Chintala, V.; Kumar, S.; Pandey, J.K.; Sharma, A.K.; Kumar, S. Solar thermal pyrolysis of non-edible seeds to biofuels and their feasibility assessment. Energ. Convers. Manag. 2017, 153, 482–492. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agron. Monograph no 9; ASA-SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of Low- Temperature Pyrolysis Conditionson Biochar for Agricultural Use. Am. Soc. Agric. Biol. Eng. (ACABE) 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, L.; Tan, Z.; Huang, Q. Effect mechanism of biochar’s zeta potential on farmland soil’s cadmium immobilization. Environ. Sci. Pollut. Res. 2019, 26, 19738–19748. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
- Gogoi, S.; Bhuyan, N.; Sut, D.; Narzari, R.; Gogoi, L.; Kataki, R. Agricultural Wastes as Feedstock for Thermo-ChemicalConversion: Products Distribution and Characterization. In Energy Recovery Processes from Wastes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–128. [Google Scholar] [CrossRef]
- Garcia-Jaramillo, M.; Cox, L.; Knicker, H.E.; Cornejo, J.; Spokas, K.A.; Hermosın, M.C. Characterization and selection of biochar for an efficient retention of tricyclazole in a flooded alluvial paddy soil. J. Hazard. Mater. 2015, 286, 581–588. [Google Scholar] [CrossRef]
- Cely, P.; Gasco, G.; Paz-Ferreiro, J.; Mendez, A. Agronomic properties of biochars from different manure wastes. J. Anal. Appl. Pyrolysis 2015, 111, 173–182. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Jewiarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Wan, J.; Lin, L.; Ayub, S.K.; Zhang, W.; Shen, G.; Hu, S.; Qian, X. Characterization and adsorption performance of biochars derived from three, key biomass constituents. Fuel 2020, 269, 117142. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science–Principles and Applications; Academic Press: London, UK, 1981. [Google Scholar]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Yang, W.; Shang, J.; Li, B.; Flury, M. Surface and colloid properties of biochar and implications for transport in porous media. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2484–2522. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Emran, M. Application of Biochar for Wastewater Treatment. In Biochar and Its Application in Bioremediation; Springer: Singapore, 2021; pp. 1–26. [Google Scholar]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar]
- Gao, T.; Gao, M.; Peng, J.; Li, N. Effects of different amount of biochar on nitrogen, phosphorus and potassium nutrients in soil. Mater. Sci. Eng. 2018, 394, 022043. [Google Scholar] [CrossRef]
- Salem, T.M.; Refaie, K.M.; Sherif, A.E.-H.E.-G.A.E.-L.; Eid, M.A.M. Biochar application in alkaline soil and its effect on soil and plant. Acta Agric. Slov. 2019, 114, 85. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]

| Parameter | Unit | SF 1 | SB-1 | SB-2 | SB-3 |
|---|---|---|---|---|---|
| Ash | % | 4.79 | 5.73 | 8.13 | 11.07 |
| Volatile Gases | % | 95.21 | 94.27 | 91.87 | 88.93 |
| pH | 5.36 | 5.60 | 6.88 | 7.49 | |
| EC | dS m−1 | 0.62 | 0.65 | 0.77 | 0.91 |
| CEC | cmol kg−1 | 100.48 | 59.59 | 57.61 | 51.42 |
| Total Elements | |||||
| N | % | 0.23 | 0.29 | 0.45 | 0.49 |
| C | % | 43.26 | 49.39 | 53.20 | 62.61 |
| H | % | 5.59 | 4.86 | 3.74 | 3.00 |
| S | % | 1.47 | 3.32 | 6.08 | 7.49 |
| O | % | 44.66 | 36.41 | 28.40 | 15.34 |
| P | % | 0.31 | 0.47 | 0.78 | 0.72 |
| Ca | % | 0.65 | 0.70 | 0.76 | 0.86 |
| Mg | % | 0.27 | 0.67 | 0.41 | 0.40 |
| K | % | 0.70 | 1.55 | 1.78 | 2.66 |
| Na | % | 0.41 | 0.36 | 0.48 | 0.55 |
| Cu | mg kg−1 | 7.78 | 9.37 | 9.52 | 12.59 |
| Fe | mg kg−1 | 146.20 | 193.30 | 209.30 | 341.00 |
| Mn | mg kg−1 | 7.09 | 10.32 | 11.94 | 16.93 |
| Zn | mg kg−1 | 33.68 | 83.27 | 105.60 | 97.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gamal, E.H.; Emran, M.; Elsamni, O.; Rashad, M.; Mokhiamar, O. Parabolic Dish Collector as a New Approach for Biochar Production: An Evaluation Study. Appl. Sci. 2022, 12, 12677. https://doi.org/10.3390/app122412677
El-Gamal EH, Emran M, Elsamni O, Rashad M, Mokhiamar O. Parabolic Dish Collector as a New Approach for Biochar Production: An Evaluation Study. Applied Sciences. 2022; 12(24):12677. https://doi.org/10.3390/app122412677
Chicago/Turabian StyleEl-Gamal, Eman H., Mohamed Emran, Osama Elsamni, Mohamed Rashad, and Ossama Mokhiamar. 2022. "Parabolic Dish Collector as a New Approach for Biochar Production: An Evaluation Study" Applied Sciences 12, no. 24: 12677. https://doi.org/10.3390/app122412677
APA StyleEl-Gamal, E. H., Emran, M., Elsamni, O., Rashad, M., & Mokhiamar, O. (2022). Parabolic Dish Collector as a New Approach for Biochar Production: An Evaluation Study. Applied Sciences, 12(24), 12677. https://doi.org/10.3390/app122412677







