Influencing Factors of Steel States in Concrete Based on Electrochemical Impedance Spectroscopic Measurements
Abstract
1. Introduction
2. Background of EIS Corrosion Measurements
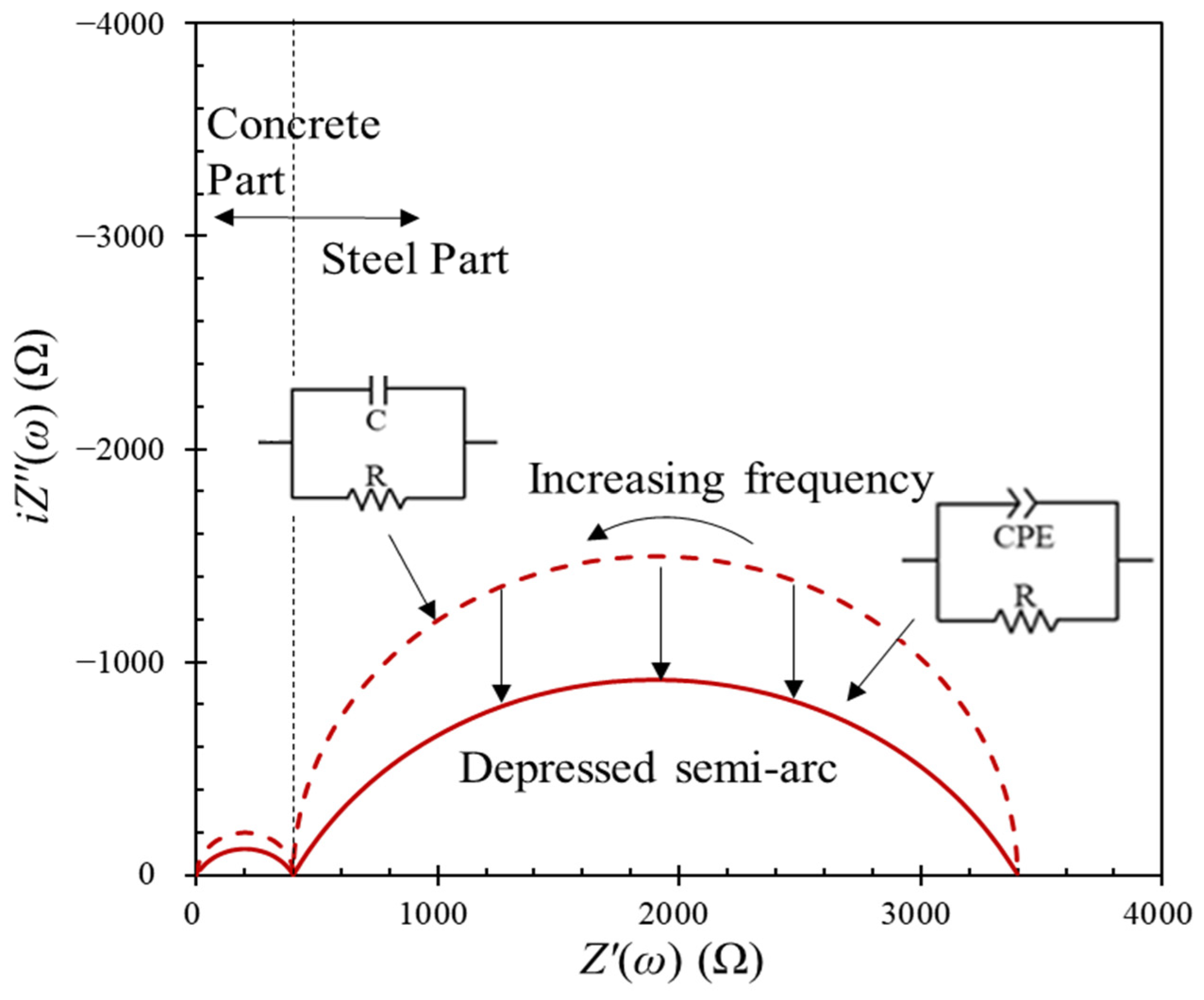
- Nyquist format: The real () and the fictitious ( parts are expressed on the x- and the y-axes, respectively. The impedance can be represented with the vector’s length ( and the angle between this vector and the x-axis. This representation cannot express the frequency directly.
- 2.
- Bode format: Both the modulus of the impedance and the phase shift on the y-axis are plotted with respect to the frequency on the x-axis. The Bode format explicitly shows frequency information, unlike the Nyquist format.
- 3.
- Reviews for Electrical Equivalent Circuits
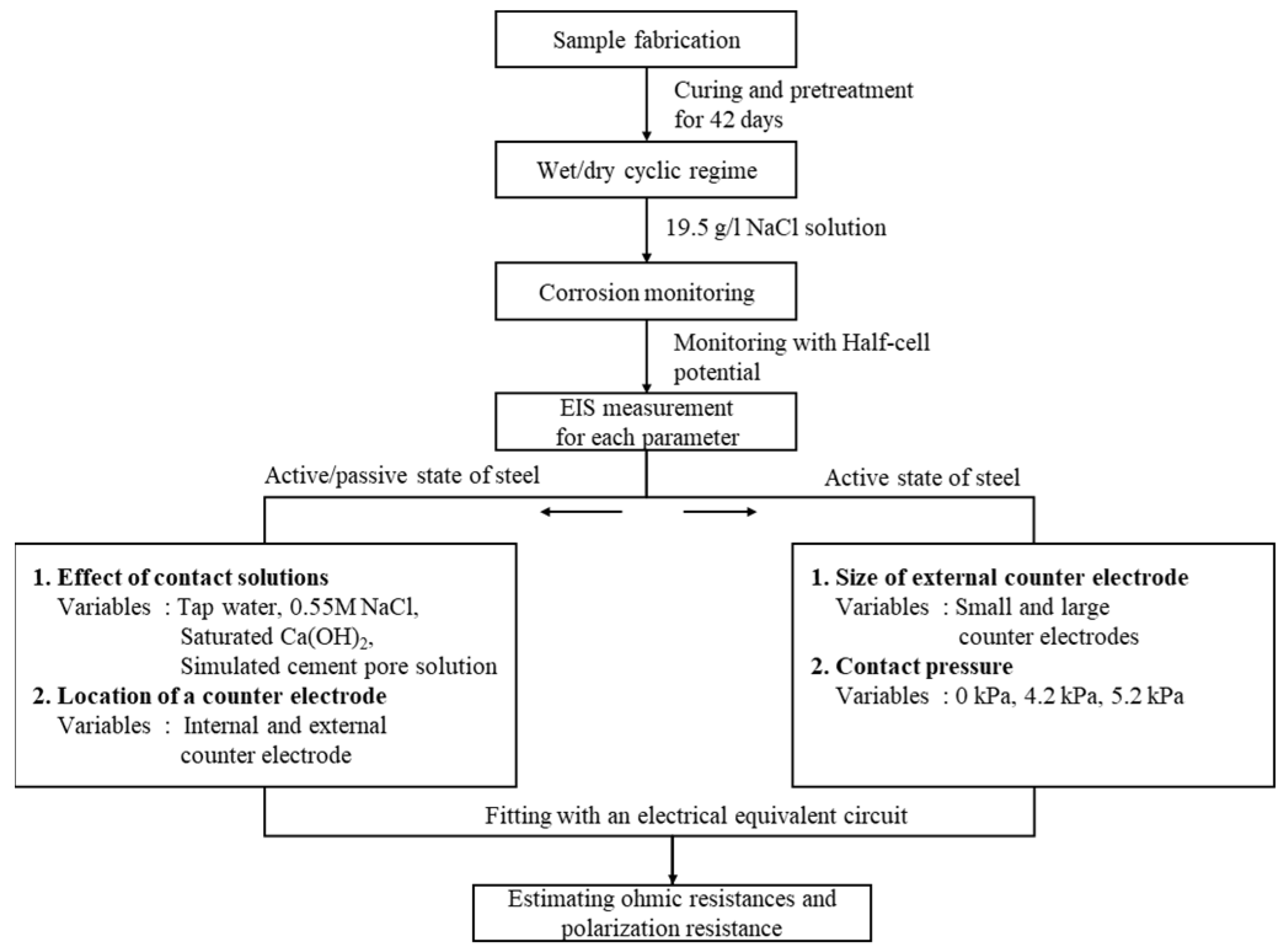
3. Experiments
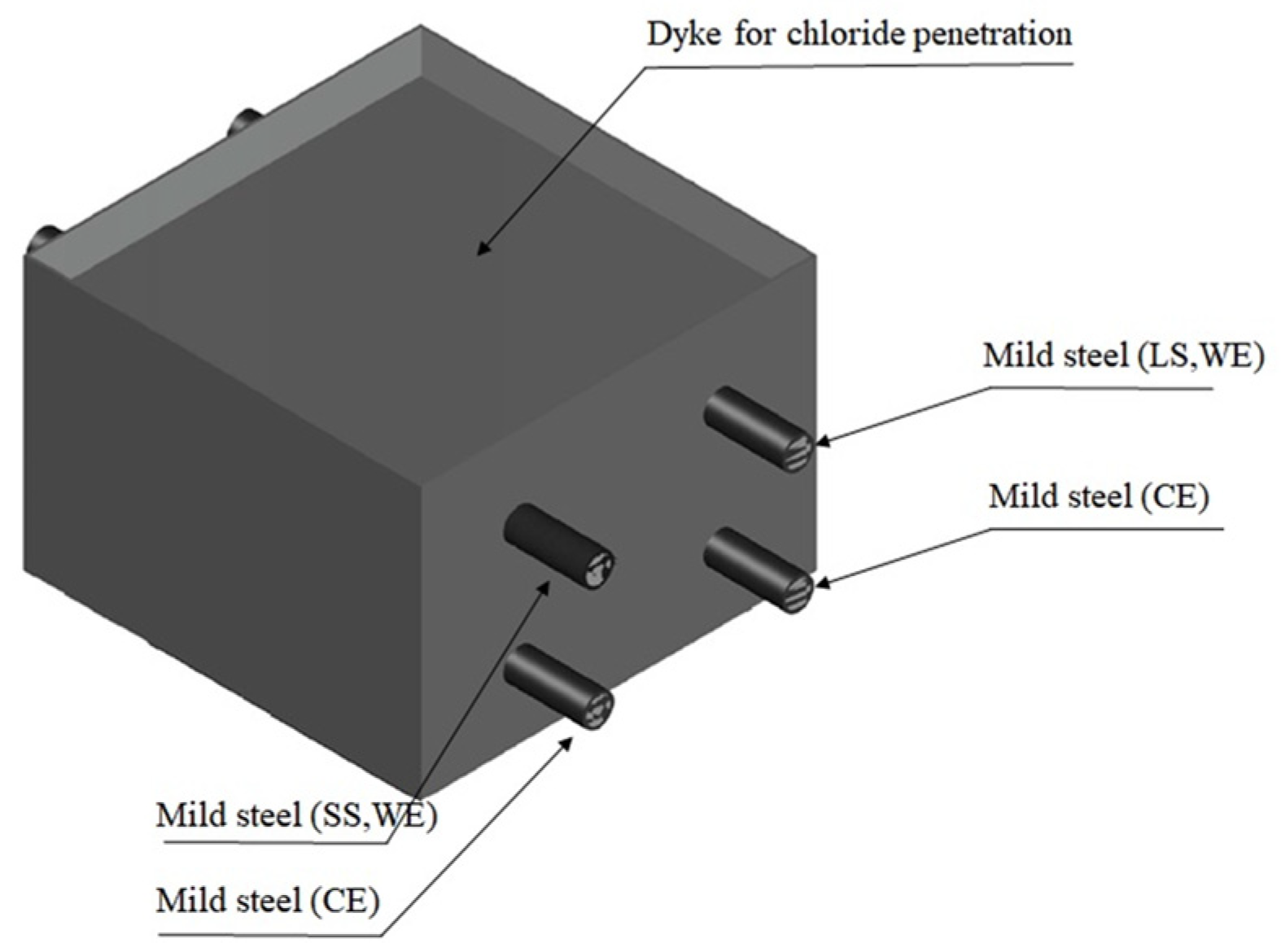
3.1. Materials and Sample Preparation
- Two steel bars were used (cover depth = 25 mm)One of them (length = 150 mm) had an exposed area equal to 75.40 cm2 and was denoted by the symbol LS (steel with long exposed area); the other accounted for an area equal to 50.27 cm2 and was denoted by the symbol SS (steel with small exposed area). The center of SS (length = 50 mm) was insulated to separate the corroded and non-corroded area locally by assuming that pit corrosion was formed by chloride.
- Two steel bars were used (cover depth = 100 mm); these were positioned in parallel with each working electrode (acting as counter electrodes).Both steel bars at this depth had the same exposed length (equal to 150 mm) and were used to monitor the chloride-induced corrosion behavior of steel. In addition, the steel located under the LS acted as a cathode so that the steel was always connected with the working electrode except for the measurement.
- To eliminate the effect on steel corrosion (in addition to the effect on the exposure area), the protruding ends of the steel specimens were also sealed with a heat-shrinkage band. Before the sealing of the protruding ends of steel, these ends were sandblasted to eliminate all types of blemishes, e.g., cement paste and corrosion products formed on steel during casting and curing.
3.2. Curing and Test Regime
- The effect of contact solution to confirm the existence of an interfacial effect.
- Mains tap water;
- A saturated solution of calcium hydroxide (as calcium hydration causes typical hydration products to leach out easily in concrete);
- Sodium chloride (0.55 M NaCl) solution (as the solution is the same as that used in the cyclic ponding regime);
- A simulated cement pore solution comprising 0.1 M sodium hydroxide (NaOH) and 0.3 M potassium hydroxide (KOH) (as the alkali oxides in the cement clinker are highly soluble).
- The effect of contact pressure to confirm the existence of an interfacial effect.
- No pressure was applied on the external counter electrode;
- A mass of 2 kg was placed on the center of the small counter electrode, or 4 kg (2 kg + 2 kg) were placed at the one-third and two-third loci of the long counter electrode to ensure uniform contact;
- A mass of 1 kg was placed at the one-third point, and a mass of 2 kg was placed at the two-third points of the large counter electrode to simulate workmanship-induced errors caused by the nonuniformly distributed pressure.
- The effect of counter electrode size to confirm the self-confinement of applied current to corroded steel.
- Use of stainless-steel plates (lengths = 200 mm and 100 mm);
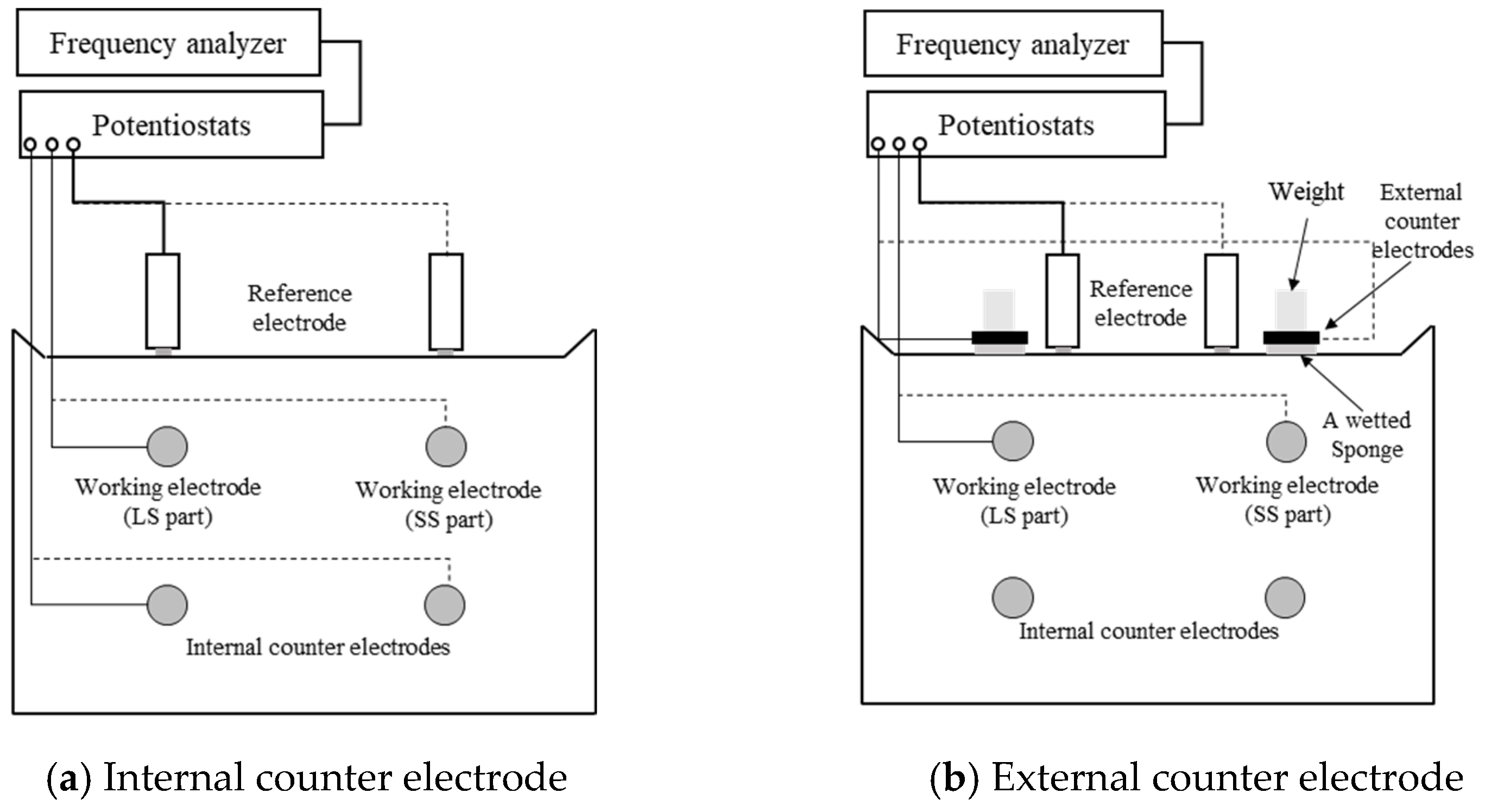
- The effect of the counter electrode’s position to confirm differences in high-frequency responses;
- Internal and external counter electrodes.
4. Results and Discussion
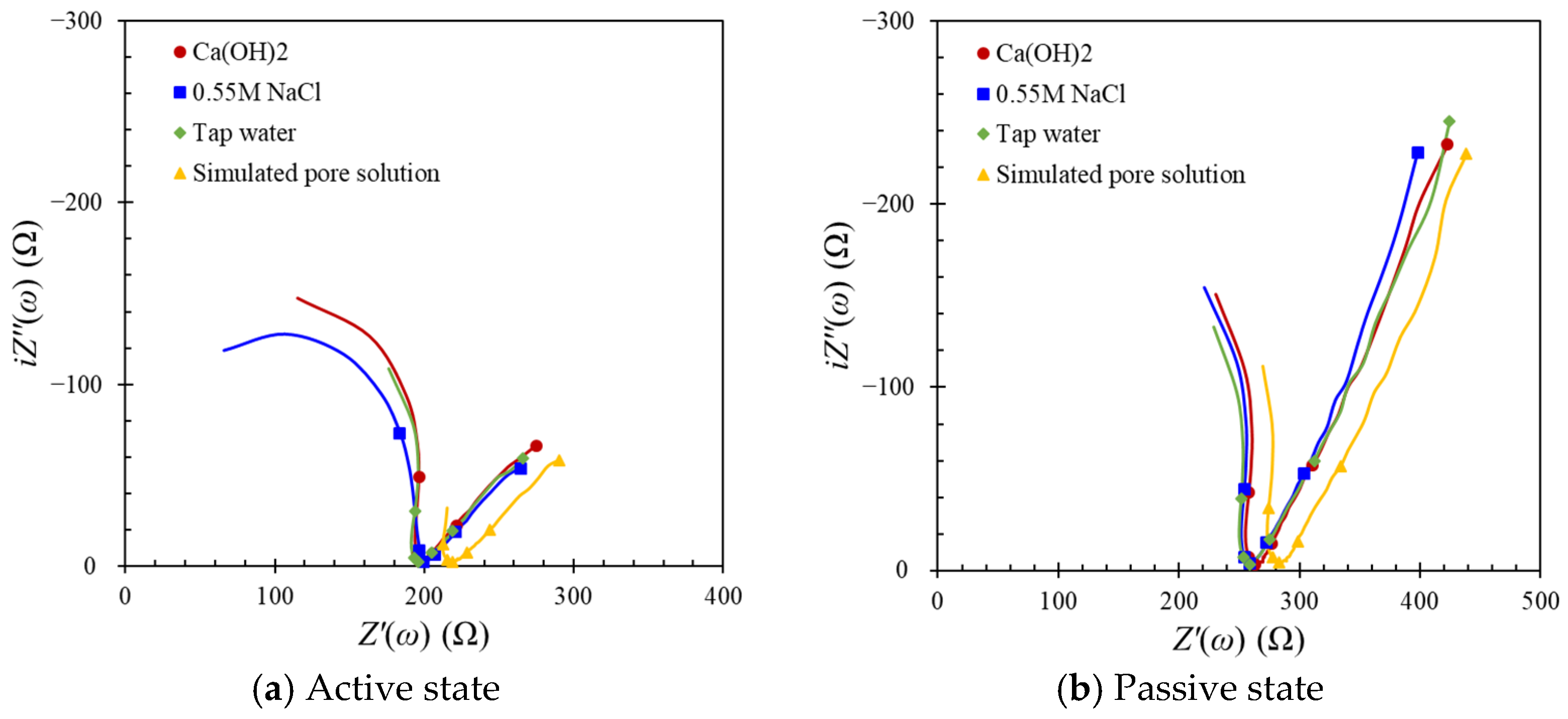
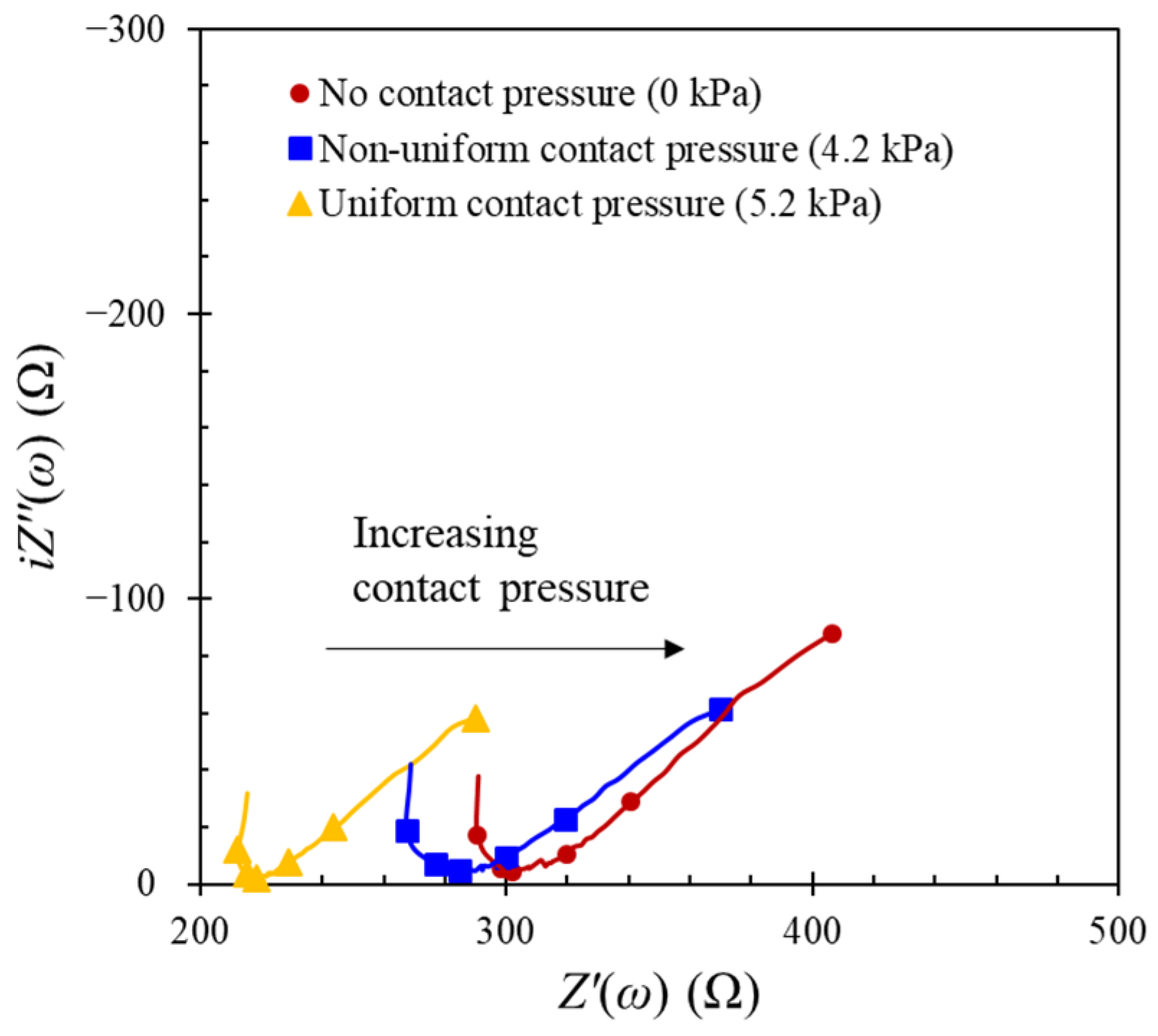
4.1. Influences on the Interfacial Effect between the Concrete Surface and the Counter Electrode
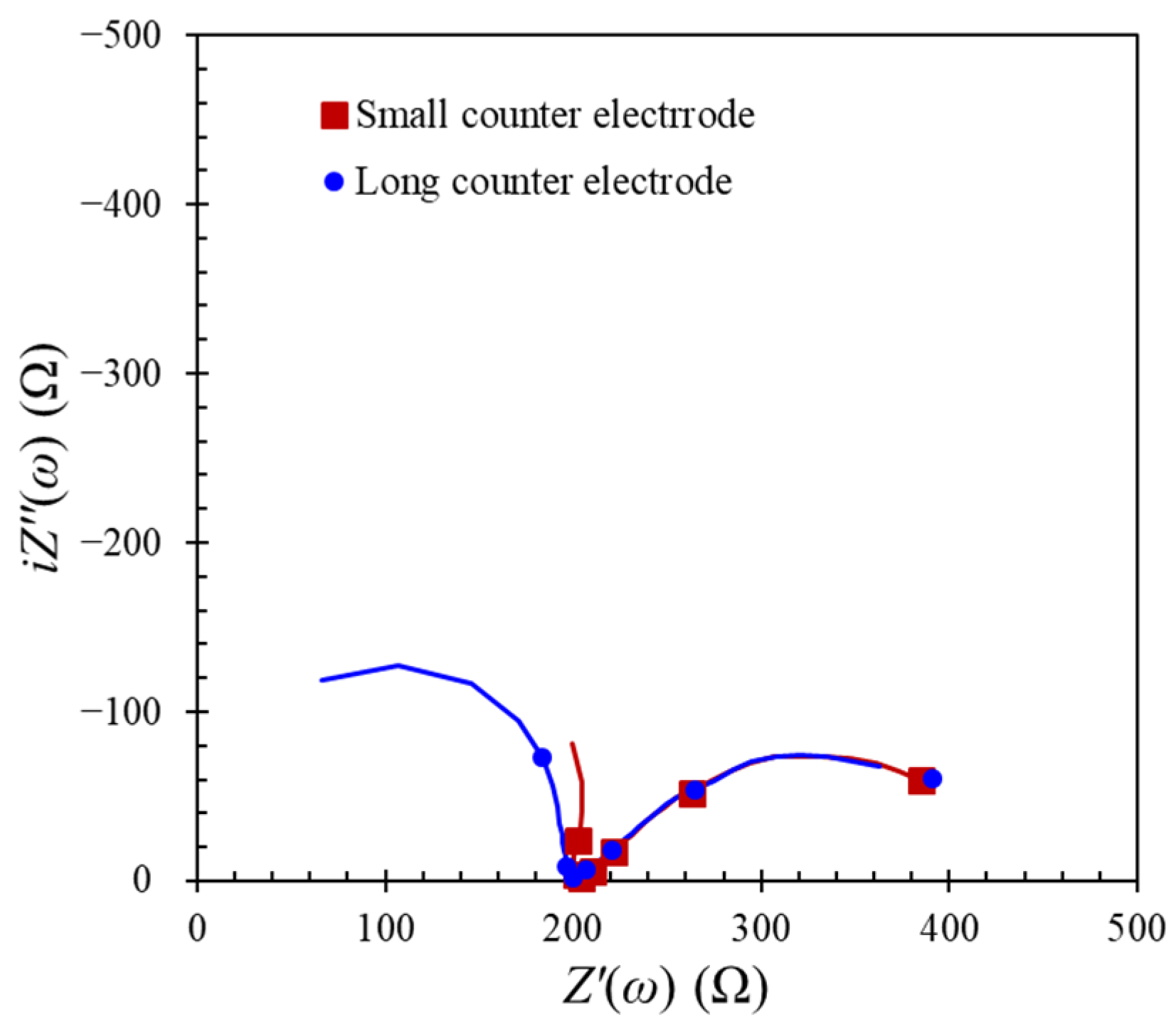
4.2. Influences on Electrode Positions
4.3. EIS Analysis
5. Conclusions
- The impedance response with the contact media was somewhat consistent except for a simulated pore solution. In other words, when corrosion measurements are carried out, the types of contact media are not important because the ohmic resistance included both interfacial effects and concrete resistance.
- The ohmic and polarization resistance obtained from EIS data can be used to estimate the corrosion rate. However, it is evident that additional studies in the polarized area are still required to evaluate corrosion rate accurately. Depending on the polarized area, the polarization and ohmic resistances are affected considerably.
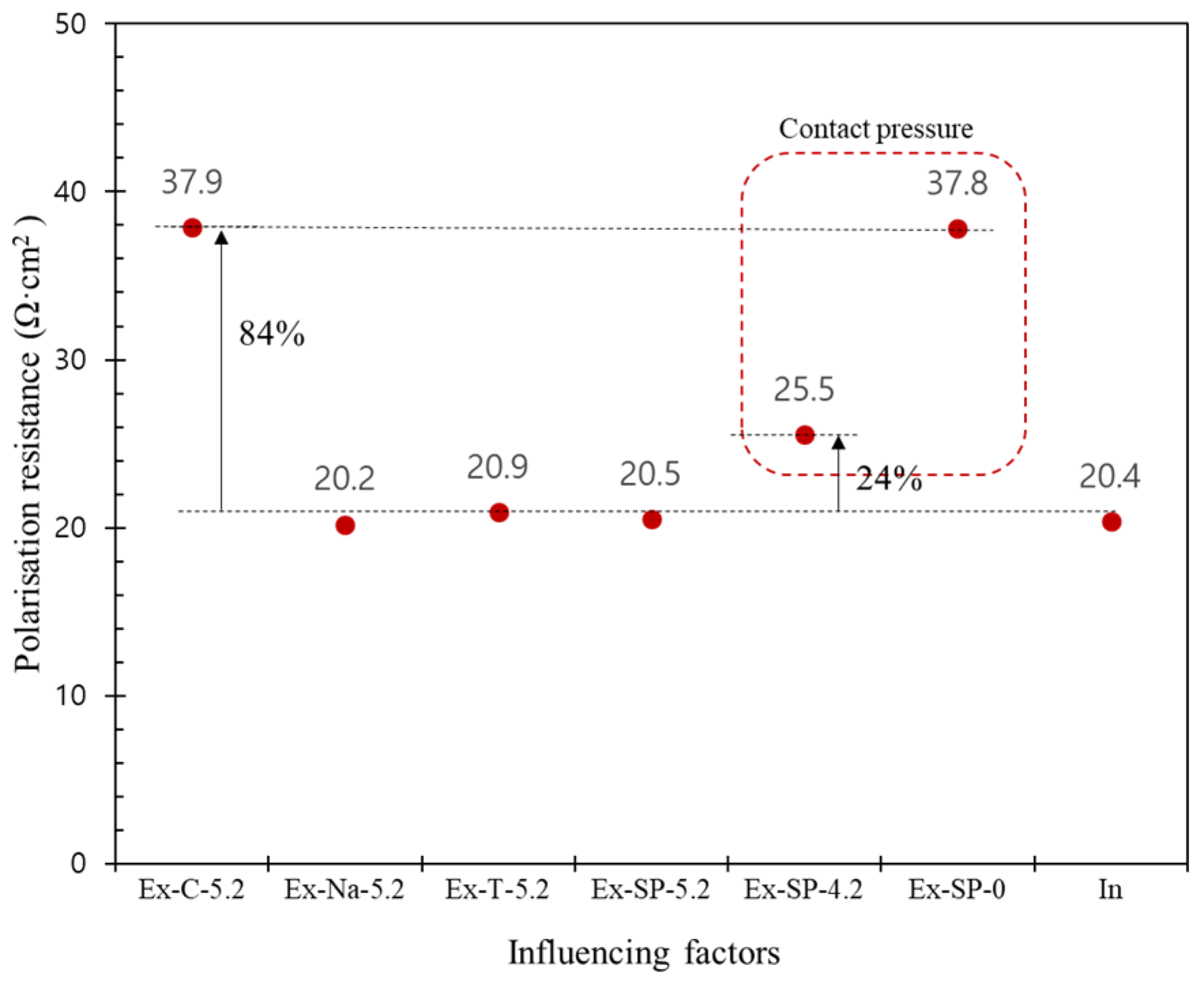
- From the simulated values, the influencing factors on the impedance response were ordered as contact pressure > location of counter electrode > contact media. However, it should be noted that the main factor was the estimation of the true polarized area required for the determination of the polarization resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taffesea, W.Z.; Sistonen, E. Service life prediction of repaired structures using concrete recasting method: State-of-the-art. Procedia Eng. 2013, 57, 1138–1144. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; CBI Betonginstitutet AB: Borås, Sweden, 1982; pp. 6–15. [Google Scholar]
- Austin, S.A.; Lyons, R.; Ing, M. Electrochemical behaviour of steel reinforced concrete during accelerated corrosion testing. Corrosion 2004, 60, 203–212. [Google Scholar] [CrossRef]
- Chang, C.W.; Tsai, C.A.; Shiau, Y.C. Inspection of steel bars corrosion in reinforced concrete structures by nondestructive ground penetrating radar. Appl. Sci. 2022, 12, 5567. [Google Scholar] [CrossRef]
- Feliu, V.; González, J.A.; Andrade, C.; Feliu, S. Equivalent circuit for modelling the steel-concrete interface. I. Experimental evidence and theoretical predictions. Corros. Sci. 1998, 40, 975–993. [Google Scholar] [CrossRef]
- Elsener, B. Macrocell corrosion of steel in concrete—Implications for corrosion monitoring. Cem. Concr. Compos. 2002, 24, 65–72. [Google Scholar] [CrossRef]
- Deus, J.M.; Díaz, B.; Freire, L.; Nóvoa, X.R. The electrochemical behaviour of steel rebars in concrete: An electrochemical impedance spectroscopy study of the effect of temperature. Electrochim. Acta 2014, 131, 106–115. [Google Scholar] [CrossRef]
- Bautista, A.; Paredes, E.C.; Velasco, F.; Alvarez, S.M. Corrugated stainless steels embedded in mortar for 9 years: Corrosion results of non-carbonated, chloride-contaminated samples. Constr. Build. Mater. 2015, 93, 350–359. [Google Scholar] [CrossRef]
- Polder, R.B.; Peelen, W.H.A. Characterisation of chloride transport and reinforcement corrosion in concrete under cyclic wetting and drying by electrical resistivity. Cem. Concr. Compos. 2002, 24, 427–435. [Google Scholar] [CrossRef]
- Sosa, M.; Pérez-López, T.; Reyes, J.; Corvo, F.; Camacho-Chab, R.; Quintana, P.; Aguilar, D. Influence of the marine environment on reinforced concrete degradation depending on exposure conditions. Int. J. Electrochem. Sci. 2011, 6, 6300–6318. [Google Scholar]
- Pereira, E.V.; Salta, M.M.; Fonseca, I.T.E. On the measurement of the polarisation resistance of reinforcing steel with embedded sensors: A comparative study. Mater. Corros. 2015, 65, 1029–1038. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Takenouti, H.; García-Jar No, J.J.; Vicente, F.; Alonso, C. A theoretical approach of impedance spectroscopy during the passivation of steel in alkaline media. Electrochim. Acta 2009, 54, 7222–7226. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Mcrae, G.A.; Gu, G.P. Electrochemical investigation of chloride-induced depassivation of black steel rebar under simulated service conditions. Corros. Sci. 2010, 52, 1649–1659. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Orazem, M.E. Impedance analysis of ASTM A416 tendon steel corrosion in alkaline simulated pore solutions. Corros. Sci. 2016, 104, 26–35. [Google Scholar] [CrossRef]
- Kot, P.; Muradov, M.; Gkantou, M.; Kamaris, G.S.; Hashim, K.; Yeboah, D. Recent advancements in non-destructive testing techniques for structural health monitoring. Appl. Sci. 2021, 11, 2750. [Google Scholar] [CrossRef]
- ASTM C876-15; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM: West Conshohocken, PA, USA, 2015.
- Andrade, C.; Soler, L.; Alonso, C.; Nbvoa, X.R.; Keddamt, M. The importance of geometrical consideration in the measurement of steel corrosion in concrete by means of AC impedance. Corros. Sci. 1995, 37, 2013–2023. [Google Scholar] [CrossRef]
- Videm, K. Electrochemical studies of steel in cement mortar containing chloride and micro-silica. Corros. Sci. 2007, 49, 1702–1717. [Google Scholar] [CrossRef]
- Montemor, M.; Simões, A.M.; Ferreira, M.G. Chloride-induced corrosion on reinforcing steel: From the fundamentals to the monitoring techniques. Cem. Concr. Compos. 2003, 25, 491–502. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy Theory, Experiment, and Application, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Stern, M.; Geary, A.L. Electrochemical polarization I. A theoretical analysis of the shape of polarization curve. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, J.-G.; Lee, K.-M. Corrosion behavior of steel bar embedded in fly ash concrete. Corros. Sci. 2006, 48, 1733–1745. [Google Scholar] [CrossRef]
- Pech-Canul, M.A.; Castro, P. Corrosion measurements of steel reinforcement in concrete exposed to a tropical marine atmosphere. Cem. Concr. Res. 2002, 32, 491–498. [Google Scholar] [CrossRef]
- Park, Z.-T.; Choi, Y.-S.; Kim, J.-G.; Chung, L. Development of a galvanic sensor system for detecting the corrosion damage of the steel embedded in concrete structure. Cem. Concr. Res. 2005, 35, 1814–1819. [Google Scholar] [CrossRef]
- Montemor, M.; Simões, A.M.; Salta, M. Effect of fly ash on concrete reinforcement corrosion studied by EIS. Cem. Concr. Compos. 2000, 22, 175–185. [Google Scholar] [CrossRef]
- Morozov, Y.; Castela, A.S.; Dias, A.P.S.; Montemor, M.F. Chloride-induced corrosion behavior of reinforcing steel in spent fluid cracking catalyst modified mortars. Cem. Concr. Res. 2013, 47, 1–7. [Google Scholar] [CrossRef]
- Dhouibi, L.; Triki, E.; Raharinaivo, A. The application of electrochemical impedance spectroscopy to determine the long-term effectiveness of corrosion inhibitors for steel in concrete. Cem. Concr. Compos. 2002, 24, 35–43. [Google Scholar] [CrossRef]
- Kranc, S.C.; Sagues, A.A. Computation of corrosion macrocell current distribution and electrochemical impedance of reinforcing steel in concrete. In Computer Modeling in Corrosion; Munn, R.S., Ed.; ASTM STP 1154; ASTM International: West Conshohocken, PA, USA, 1992; pp. 95–112. [Google Scholar]
- Kim, J. Monitoring Concrete Performance under Simulated and Natural Chloride Environments. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 2018. [Google Scholar]
- BS EN 197-1:2000; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. BSI: London, UK, 2000.
- Newlands, M.; Jones, R.; Kandasami, S.; Harrison, T. Sensitivity of electrode contact solutions and contact pressure in assessing electrical resistivity of concrete. Mater. Struct. 2007, 41, 621–632. [Google Scholar] [CrossRef]
- McCarter, W.J.; Taha, H.M.; Suryanto, B.; Starrs, G. Two-point concrete resistivity measurements: Interfacial phenomena at the electrode–concrete contact zone. Meas. Sci. Technol. 2015, 26, 085007. [Google Scholar] [CrossRef]
- Wang, R.; He, F.; Shi, C.; Zhang, D.; Chen, C.; Dai, L. AC impedance spectroscopy of cement—based materials: Measurement and interpretation. Cem. Concr. Compos. 2022, 131, 104591. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Nygaard, P.V.; Geiker, M.R.; Elsener, B. Corrosion rate of steel in concrete: Evaluation of confinement techniques for on-site corrosion rate measurements. Mater. Struct. 2009, 42, 1059–1076. [Google Scholar] [CrossRef]
- González, J.A.; Andrade, C.; Alonso, C.; Feliu, S. Comparison of rates of general corrosion and maximum pitting penetration on concrete embedded steel reinforcement. Cem. Concr. Res. 1995, 25, 257–264. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhões, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Saraswathy, V.; Song, H.-W.; Palaniswamy, N. Determination of diffusion coefficient of chloride in concrete using Warburg diffusion coefficient. Corros. Sci. 2009, 51, 1299–1307. [Google Scholar] [CrossRef]
- Nóvoa, X.R. Electrochemical aspects of the steel-concrete system. A review. J. Solid State Electrochem. 2016, 20, 2113–2125. [Google Scholar] [CrossRef]
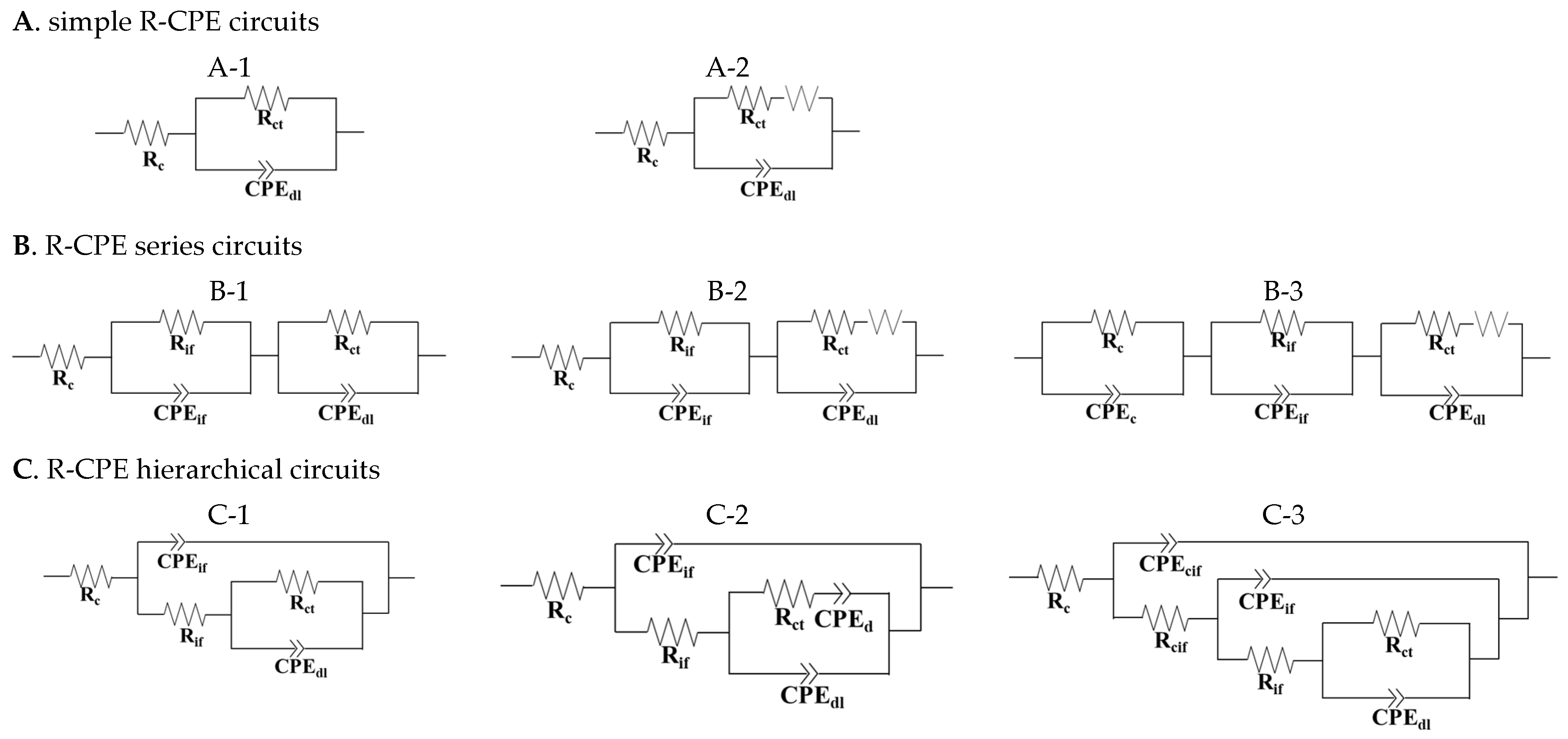



| Types in Figure 2 | Sample Type | Location | Exposure Condition | Counter Electrode Type | Steel Condition | Reference Electrode | Reference |
|---|---|---|---|---|---|---|---|
| A-1 | concrete | lab | immersion (3.5% NaCl) | graphite (Ex 1) | passive | SCE 3 | [22] |
| A-2 | concrete | field | atmospheric zone | elastomer (Ex) | passive/ active | activated titanium rod | [23] |
| mortar | lab | premixed (3% CaCl2) | N.I. 5 | passive/ active | N.I | [5] | |
| B-1 | concrete | lab | immersion (3.5% NaCl) | graphite (Ex) | active | SCE | [22] |
| concrete | lab | immersion (3.5% NaCl) | graphite (In 2) | passive/ active | CSE 4 | [24] | |
| B-2 | concrete | lab | partial or full immersion (3% NaCl) | steel (In) | passive/ active | SCE | [25] |
| B-3 | mortar | lab | wet/dry cycle (3% NaCl) | stainless steel (Ex) | passive/ active | SCE | [11] |
| C-1 | mortar | lab | immersion (sea water)/ premixed (0.1, 1.0 and 3.6% Cl−) | graphite (Ex) | passive/ active | SCE | [7] |
| mortar | lab | immersion (3.5% NaCl)/ premixed (3% CaCl2) | copper cylinder (Ex) | passive/ active | SCE | [8] | |
| C-2 | mortar | lab | partial immersion (3% NaCl) | titanium (Ex) | passive/ active | SCE | [26] |
| C-3 | concrete | lab | immersion (3% NaCl) | stainless steel (Ex) | passive/ active | SCE | [27] |
| Type of Cement | W/C 1 | Cement (kg/m3) | Aggregate (kg/m3) | Sand (kg/m3) | Compressive Strength (MPa) | |
|---|---|---|---|---|---|---|
| F28 2 | F180 2 | |||||
| CEM I 52.5N | 0.6 | 300 | 1100 | 707 | 38.34 (1.19) 3 | 46.99 (1.35) |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | |
|---|---|---|---|---|---|---|---|---|
| % by weight | 20.68 | 4.83 | 3.17 | 63.95 | 2.35 | 2.80 | 0.54 | 0.03 |
| Bulk Density (kg/m3) | Absorption (%) | Fineness Modulus | Specific Gravity | |
|---|---|---|---|---|
| Aggregate | 1450 | 1.02 | 6.04 | 2.63 |
| Sand | 1520 | 2.0 | 2.89 | 2.63 |
| (a) Passive State of Steel (SS) | ||||||||
| Sample Notation 1 | Ex2-C3-5.2 4 | Ex-Na 3-5.2 | Ex-SP 3-5.2 | Ex-T 3-5.2 | In 2 | |||
| Concrete response | RConc | 179.4 Ω | 169.2 Ω | 193.5 Ω | 154.7 Ω | 131.6 Ω | ||
| CPEConc (×10−12) | 7.0 Fs0.4 | 6.9 Fs0.41 | 7.4 Fs0.37 | 7.3 Fs0.41 | 20.6 Fs0.38 | |||
| RIF | 86.2 Ω | 89.8 Ω | 94.4 Ω | 104.2 Ω | 41.5 Ω | |||
| CPEIF (×10−6) | 9.3 Fs−0.53 | 2.8 Fs−0.45 | 25.5 Fs−0.62 | 2.96 Fs−0.48 | 1.9 Fs−0.33 | |||
| Steel response | RCP | 231.7 Ω | 180.4 Ω | 82.7 Ω | 161.9 Ω | 91.5 Ω | ||
| CPECP (×10−3) | 6.7 Fs −0.47 | 7.4 Fs−0.51 | 6.7 Fs−0.42 | 7.5 Fs−0.54 | 12.0 Fs−0.47 | |||
| RSteel | - | - | - | - | - | |||
| CPESteel (×10−3) | 7.7 Fs−0.18 | 7.5 Fs−0.17 | 6.1 Fs −0.26 | 6.3 Fs −0.24 | 7.0 Fs−0.19 | |||
| (b) Active State of Steel (LS) | ||||||||
| Sample Notation | Ex-C-5.2 | Ex-Na-5.2 | Ex-T-5.2 | Ex-SP-5.2 | Ex-SP-4.24 | Ex-SP-0 4 | In | |
| Concrete response | RConc | 119.3 Ω | 123.7 Ω | 120.2 Ω | 113.5 Ω | 157.8 Ω | 161 Ω | 133.4 Ω |
| CPEConc (×10−12) | 10.4 Fs0.43 | 7.03 Fs0.41 | 6.98 Fs0.43 | 4.47 Fs0.391 | 3.71 Fs0.37 | 7.25 Fs0.31 | 50.8 Fs0.30 | |
| RIF | 77.3 Ω | 79.9 Ω | 75.2 Ω | 105.1 Ω | 131.5 Ω | 138.5 Ω | 8.1 Ω | |
| CPEIF (×10−7) | 4.90 Fs−0.31 | 17.1 Fs−0.46 | 15.4 Fs−0.42 | 61.0 Fs−0.61 | 27.8 Fs−0.68 | 21.2 Fs−0.55 | 20.6 Fs−0.11 | |
| Steel response | RCP | 104.1 Ω | 28.87 Ω | 30.55 Ω | 73.01 Ω | 119.3 Ω | 54.53 Ω | 44.44 Ω |
| CPECP (×10−7) | 4.33 Fs−0.70 | 2.96 Fs−0.64 | 1.35 Fs−0.58 | 1.56 Fs−0.61 | 1.28 Fs−0.61 | 0.836 Fs−0.66 | 2.97 Fs−0.58 | |
| RSteel | 398.1 Ω | 239.3 Ω | 247.1 Ω | 199.5 Ω | 219.3 Ω | 447.4 Ω | 226.3 Ω | |
| CPESteel (×10−2) | 1.42 Fs−0.39 | 1.81 Fs−0.29 | 1.62 Fs−0.26 | 2.09 Fs−0.22 | 2.44 Fs−0.23 | 1.12 Fs−0.291 | 2.13 Fs−0.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, K.-T.; Kwon, T.H. Influencing Factors of Steel States in Concrete Based on Electrochemical Impedance Spectroscopic Measurements. Appl. Sci. 2022, 12, 12611. https://doi.org/10.3390/app122412611
Kim J, Park K-T, Kwon TH. Influencing Factors of Steel States in Concrete Based on Electrochemical Impedance Spectroscopic Measurements. Applied Sciences. 2022; 12(24):12611. https://doi.org/10.3390/app122412611
Chicago/Turabian StyleKim, Jaehwan, Ki-Tae Park, and Tae Ho Kwon. 2022. "Influencing Factors of Steel States in Concrete Based on Electrochemical Impedance Spectroscopic Measurements" Applied Sciences 12, no. 24: 12611. https://doi.org/10.3390/app122412611
APA StyleKim, J., Park, K.-T., & Kwon, T. H. (2022). Influencing Factors of Steel States in Concrete Based on Electrochemical Impedance Spectroscopic Measurements. Applied Sciences, 12(24), 12611. https://doi.org/10.3390/app122412611






