Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
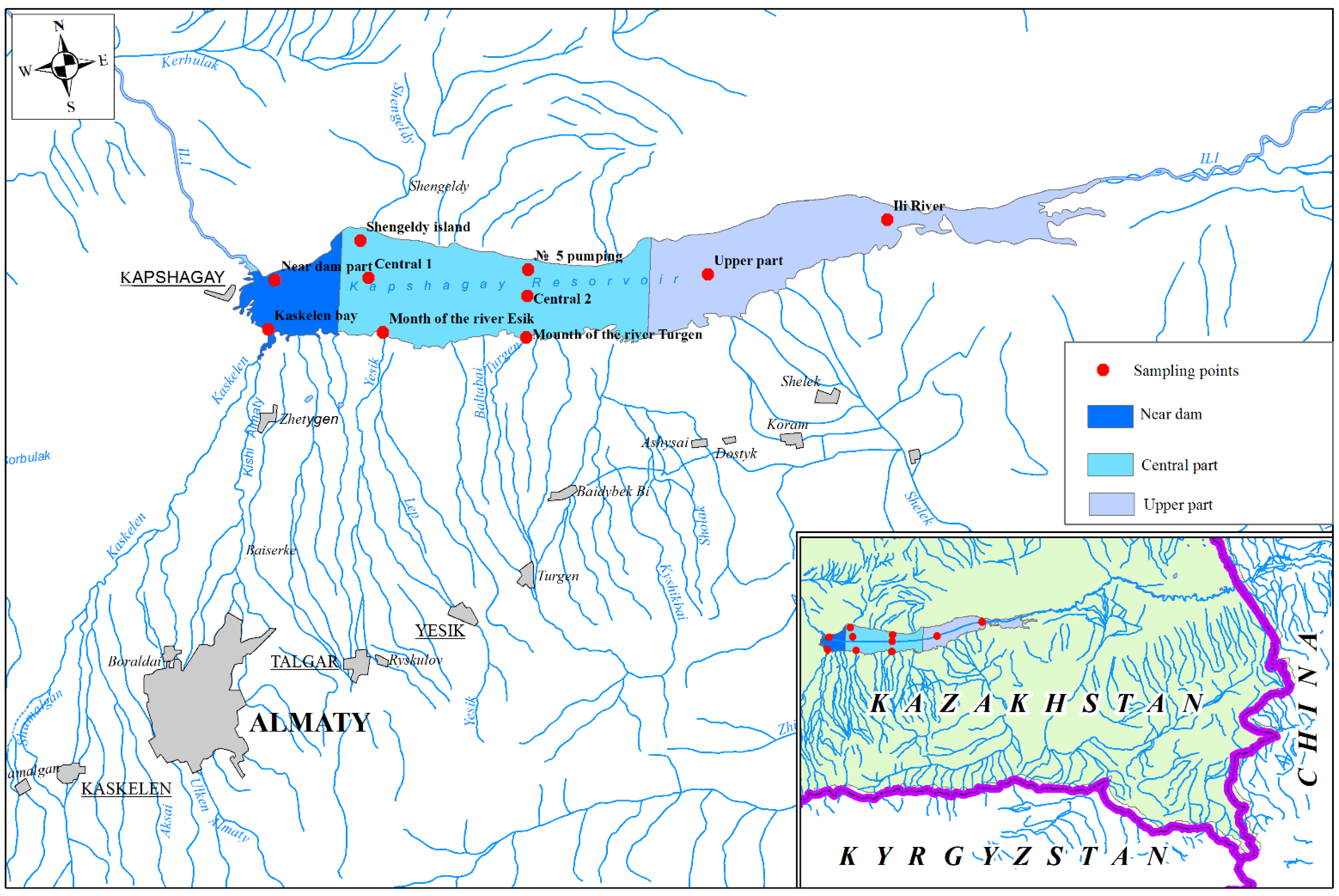
2.1. Research Object
2.2. Samples Selection
2.3. Sampling Methods
2.4. Spectrometric Analysis Methods of Atomic Absorption
2.5. Used Standard Samples
- −
- Composition of aqueous solution of copper ions (3K-1) SSS 7998-93 (CCu = 0.0125; 0.025; 0.05; 0.1 mg/L);
- −
- Composition of aqueous solution of zinc ions (4K-1) SSS 7837-2000 (CZn = 0.0125; 0.025; 0.05; 0.1 mg/L);
- −
- Composition of aqueous solution of lead ions (2K-1) SSS 7012-93 (CPb = 0.05; 0.1; 0.15; 0.2 mg/L);
- −
- Composition of aqueous solution of cadmium ions (1K-1) SSS 6690-93 (CCd = 0.0125; 0.025; 0.05; 0.1 mg/L).
3. Results
3.1. Concentration of Heavy Metals in Bottom Sediment
3.2. Assessment of the Bottom Sediment Pollution Level
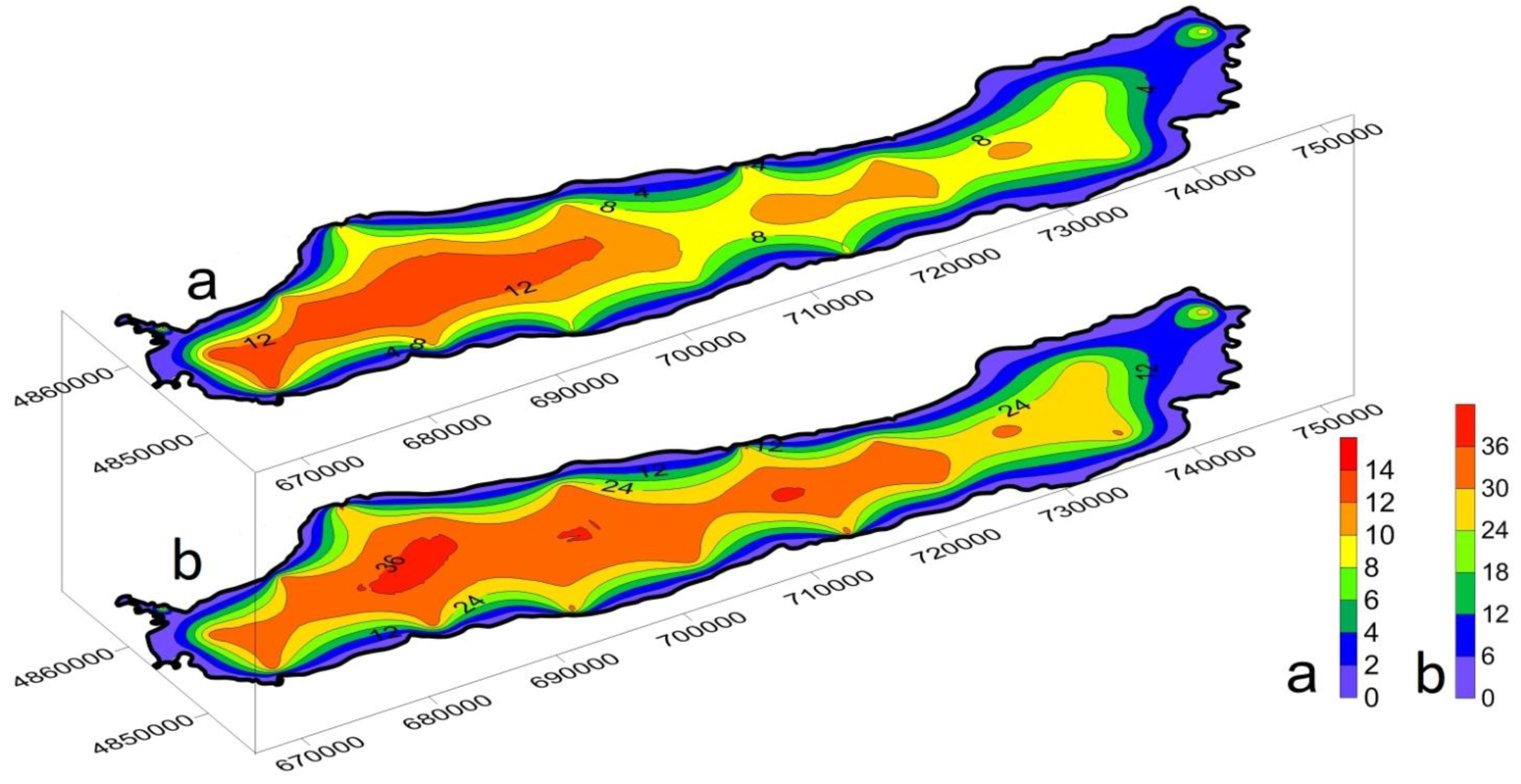
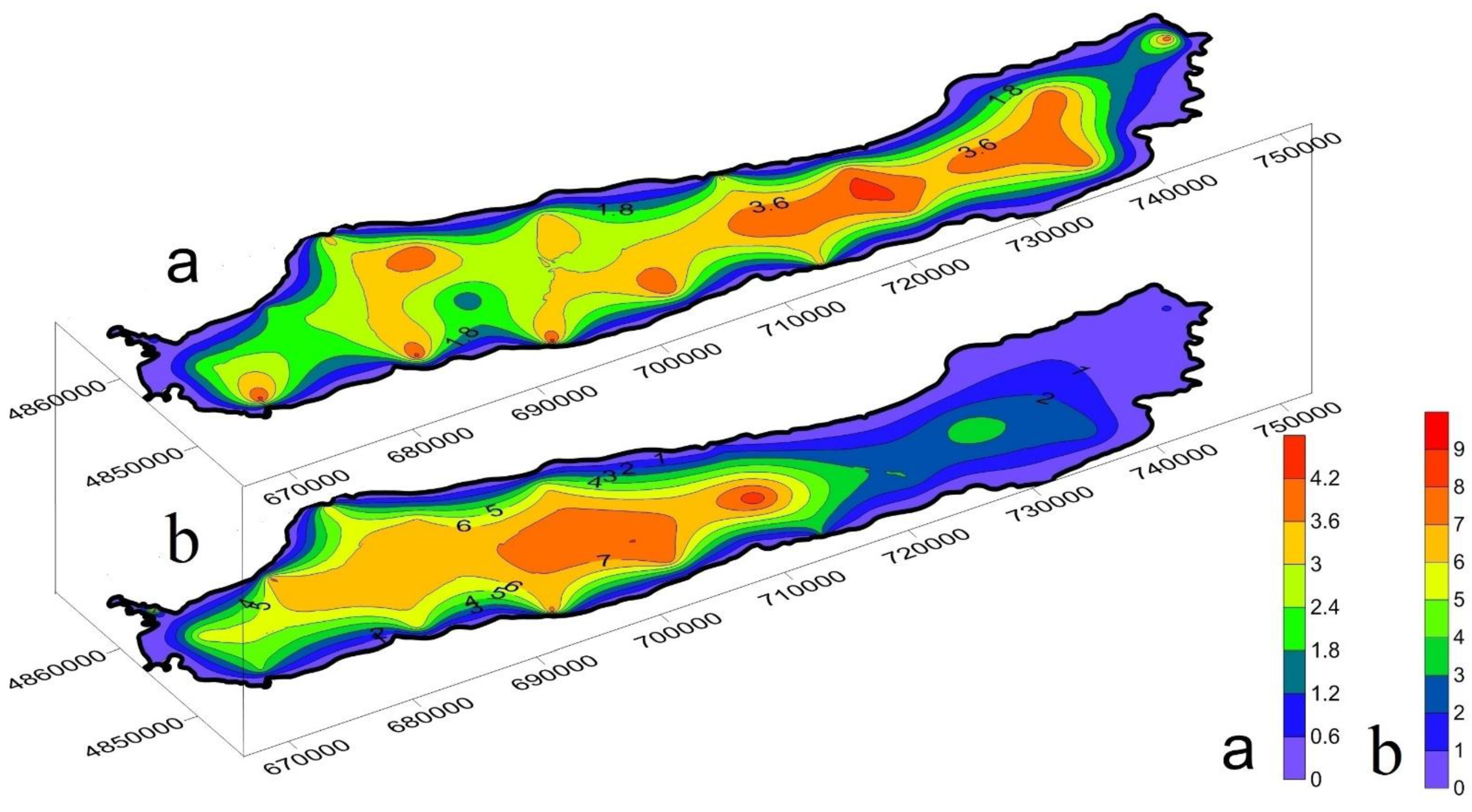
3.3. Spatial Distribution and Migration Activity of Heavy Metals in Bottom Sediment
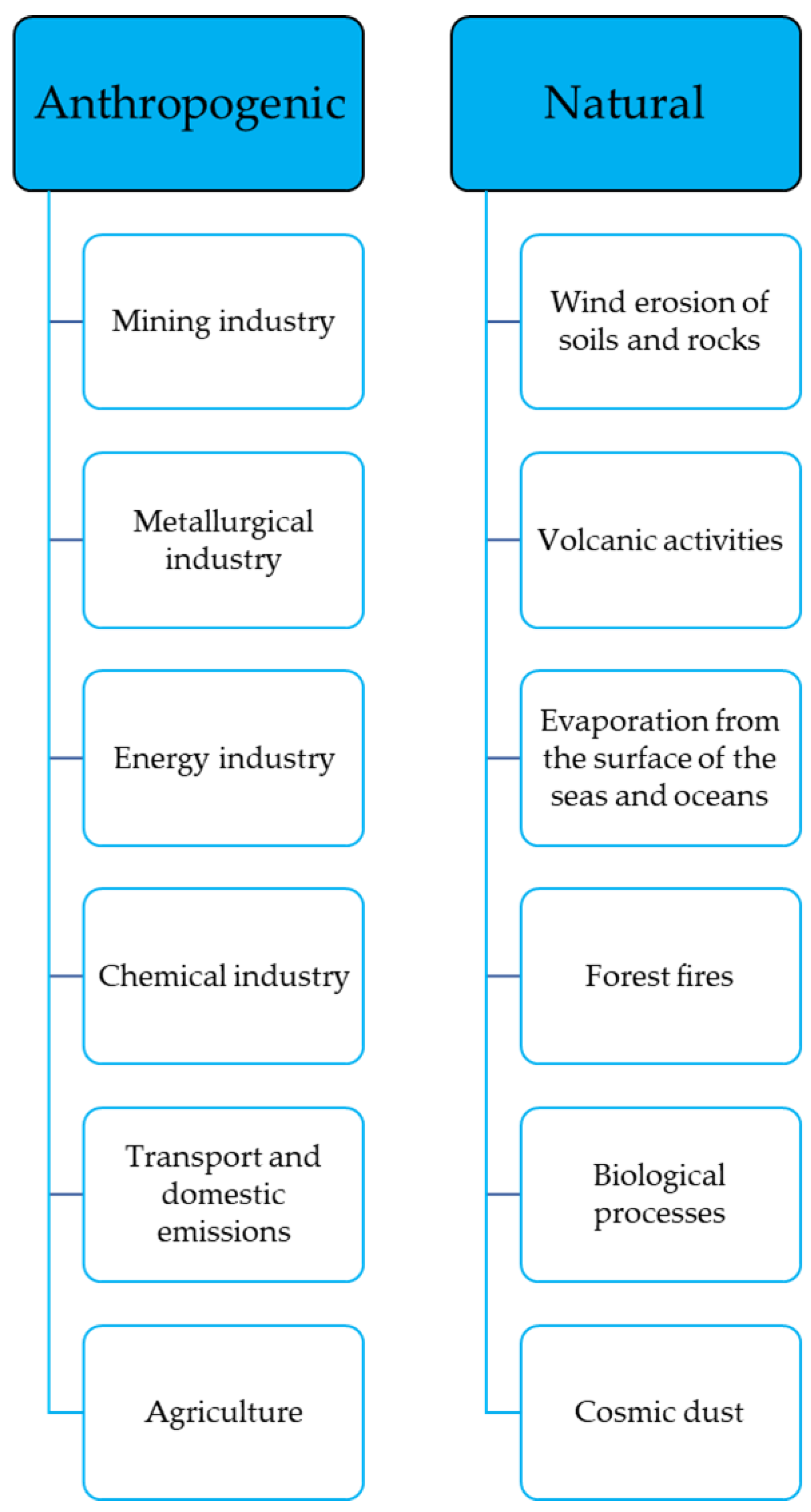
3.4. Sources of Anthropogenic Pollution
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahn, J.; Opp, C.; Evgrafova, A.; Groll, M.; Zitzer, N.; Laufenberg, G. Impact of dam draining on the mobility of heavy metals and arsenic in water and basin bottom sediments of three studied dams in Germany. Sci. Total Environ. 2018, 640–641, 1072–1081. [Google Scholar] [CrossRef]
- Yilmaz, F. The Comparison of Heavy Metal Concentrations (Cd, Cu, Mn, Pb, and Zn) in Tissues of Three Economically Important Fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) Inhabiting Kycegiz Lake-Mugla (Turkey). Turk. J. Sci. Technol. 2009, 4, 7–15. [Google Scholar]
- Hardaway, C.J.; Sneddon, J.; Sneddon, E.J.; Kiran, B.; Lambert, B.J.; McCray, T.C.; Bowser, D.Q.; Douvris, C. Study of selected metal concentrations in sediments by inductively coupled plasma-optical emission spectrometry from a metropolitan and more pristine bayou in Southwest Louisiana, United States. Microchem. J. 2016, 127, 213–219. [Google Scholar] [CrossRef]
- Abdel-Baki, A.S.; Dkhil, M.A.; Al-Quraishy, S. Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of, Wadi Hanifah, Saudi Arabia. Afr. J. Bio. Technol. 2011, 10, 2541–2547. [Google Scholar]
- Rendell, P.S.; Batley, G.E.; Cameron, A.J. Adsorption as a control of metal concentration in sediment extracts. Environ. Sci. Technol. 1980, 14, 314–318. [Google Scholar] [CrossRef]
- Hart, B.T. Uptake of trace metals by sediments and suspended particles: A review. Hydrobiologia. 1982, 91, 299–313. [Google Scholar] [CrossRef]
- Calmano, W.; Förstner, U. Chemical extraction of heavy metals in polluted river sediments in Central Europe. Sci. Total Environ. 1983, 28, 77–90. [Google Scholar] [CrossRef]
- Sharipova, O.A. Distribution of heavy metals in bottom sediments of Lake Balkhash depending on natural and anthropogenic factors. Bulletin. Tomsk State. Univ. 2015, 390, 225–230. [Google Scholar] [CrossRef]
- Toppalidis, V.; Harris, A.; Hardaway, C.J.; Benipal, G.; Douvris, C. Investigation of selected metals in soil samples exposed to agricultural and automobile activities in Macedonia, Greece using inductively coupled plasma-optical emission spectrometry. Microchem. J. 2017, 130, 213–220. [Google Scholar] [CrossRef]
- Benipal, G.; Harris, A.; Srirajatsayai, C.; Tate, A.; Topalis, V.; Eswani, Z.; Qureshi, M.; Hardaway, C.J.; Galiotos, J.; Douvris, C. Examination of Al, As, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, Sb, Se, V, and Zn in sediments collected around the downtown Houston, Texas area, using inductively coupled plasma-optical emission spectroscopy. Microcgemical J. 2017, 130, 255–265. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Role of nanomaterials in treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Mahmood, A.; Eqan, M.; Pervez, S.; Tabinda, A.B.; Yasar, A.; Brindhadevi, K.; Pugazhendhi, A. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ. 2020, 742, 140561. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Miras, J.J.; Roca-Perez, L.; Guzmán-Palomino, M.; Boluda, R.; Gil, C. Background levels and baseline values of available heavy metals in Mediterranean greenhouse soils (Spain). J. Geochem. Exp. 2011, 110, 186–192. [Google Scholar] [CrossRef]
- Petrosyan, V.; Pirumyan, G.; Perikhanyan, Y. Determination of heavy metal background concentration in bottom sediment and risk assessment of sediment pollution by heavy metals in the Hrazdan River (Armenia). Appl. Water Sci. 2019, 9, 102. [Google Scholar] [CrossRef]
- Beier, T.; Opp, C.; Hahn, J. Sink and source functions for metal(loid)s in sediments and soils oft wo water reservoirs of the Ore Mountains, Saxony, Germany. Appl. Sci. 2022, 12, 6354. [Google Scholar] [CrossRef]
- Sivalingam, P.; Al Salah, D.M.M.; Poté, J. Sediment Heavy Metal Contents, Ostracod Toxicity and Risk Assessment in Tropical Freshwater Lakes, Tamil Nadu, India. Soil Sediment Contam. 2021, 30, 231–252. [Google Scholar] [CrossRef]
- Hahn, J.; Bui, T.; Kessler, M.; Weber, C.J.; Beier, T.; Mildenberger, A.; Traub, M.; Opp, C. Catchment soil properties affect metal(oid) enrichment in reservoir sediments of German low mountain regions. Appl. Sci. 2022, 12, 2277. [Google Scholar] [CrossRef]
- Yin, H.; Gao, Y.; Fan, C. Distribution, sources and ecological risk assessment of heavy metals in surface sediments from Lake Taihu, China. Environ. Res. Lett. 2011, 6, 044012. [Google Scholar] [CrossRef]
- Ilyin, V.B.; Syso, A.I.; Baidina, N.L.; Konarbayeva, G.A.; Cherevko, A.S. Background amount of heavy metals in soils of the south of western Siberia. Soil Sci. 2003, 5, 550–556. (In Russian) [Google Scholar]
- Lalah, J.O.; Ochieng, E.Z.; Wandiga, S.O. Sources of heavy metal input into Winam Fulf, Kenya. Bull. Environ. Contam. Toxicol. 2008, 81, 277–284. [Google Scholar] [CrossRef]
- Iqbal, J.; Saleem, M.; Shah, M.H. Spatial distribution, environmental assessment and source identification of metals content in surface sediments of freshwater reservoir, Pakistan. Chem. Der Erde 2016, 76, 171–177. [Google Scholar] [CrossRef]
- Varol, M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, W.W.; Wang, S.H.; Zhang, B.; Hu, J.C. Initial identification of heavy metals contamination in Taihu Lake, a eutrophic lake in China. J. Environ. Sci. 2012, 24, 1539–1548. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Zeng, G.; Yuan, X.; Li, X.; Liang, J.; Wang, X.; Tang, X.; Bai, B. Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J. Geochem. Explor. 2013, 132, 75–83. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef]
- Loska, K.; Danuta, W. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Sinha, S.; Singh, V.K.; Murthy, R.C. Estimation of source of heavy metal contamination in sediments of Gomti river (India) using principal component analysis. Water Air Soil Pollut. 2005, 166, 321–341. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Hoque, M.F. Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ. Earth Sci. 2015, 73, 1837–1848. [Google Scholar] [CrossRef]
- Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Tarnawski, M.; Szara, M. The infuence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health 2019, 41, 2893–2910. [Google Scholar] [CrossRef]
- Dobrovolskiy, V.V. The role of humic acids in the formation of migration mass flows of heavy metals. Soil Sci. 2004, 1, 32–39. (In Russian) [Google Scholar]
- Bantan, R.A.; Al-Dubai, T.A.; Al-Zubieri, A.G. Geo-environmental assessment of heavy metals in the bottom sediments of the Southern Corniche of Jeddah, Saudi Arabia. Mar. Pollut. Bull. 2020, 161 Pt A, 111721. [Google Scholar] [CrossRef]
- Michalski, R.; Kostecki, M.; Kernert, J.; Pecyna, P. Time and spatial variability in concentrations of selected metals and their species in water and bottom sediments of Dzierżno Duże (Poland). J. Environ. Sci. Health 2019, 54, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Jingbo, C.; Xueshi, S.; Zhizhou, H.; Dejiang, F. Accumulation and transformation of heavy metals in surface sediments from the Yangtze River estuary to the East China Sea shelf. Environ. Pollut. 2019, 245, 111–121. [Google Scholar] [CrossRef]
- Dauvalter, V.A. Chalcophile elements (Hg, Cd, Pb, and As) in bottom sediments of water bodies of the white sea catchment area on the kola peninsula. Geochem. Int. 2006, 44, 205–208. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Wood, B.J. A simple model for chalcophile element partitioning between sulphide and silicate liquids with geochemical applications. Earth Planet. Sci. Lett. 2013, 383, 68–81. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Wood, B.J. The effects of composition and temperature on chalcophile and lithophile element partitioning into magmatic sulphides. Earth Planet. Sci. Lett. 2015, 424, 280–294. [Google Scholar] [CrossRef]
- Lee, C.-T.A.; Luffi, P.; Chin, E.J.; Bouchet, R.; Dasgupta, R.; Morton, D.M.; Le Roux, V.; Yin, Q.-Z.; Jin, D. Copper systematics in arc magmas and implications for crust-mantle differentiation. Science 2012, 336, 64–68. [Google Scholar] [CrossRef]
- Li, Y.; Audétat, A. Partitioning of V, Mn, Co, Ni, Cu, Zn, As, Mo, Ag, Sn, Sb, W, Au, Pb, and Bi between sulfide phases and hydrous basanite melt at upper mantle conditions. Earth Planet. Sci. Lett. 2012, 355–356, 327–340. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Luguet, A. Chalcophile and siderophile elements in mantle rocks: Trace elements controlled by trace minerals. Rev. Mineral. Geochem. 2016, 81, 441–488. [Google Scholar] [CrossRef]
- Luoma, S.N. Bioavailability of trace metals to aquatic organisms—A review. Sci. Total Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- Adams, W.; Blust, R.; Dwyer, R.; Mount, D.; Nordheim, E.; Rodriguez, P.H.; Spry, D. Bioavailability Assessment of Metals in Freshwater Environments: A Historical Review. Environ. Toxicol. Chem. 2020, 39, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Amirgaliev, N.A.; Askarova, M.; Opp, C.; Medeu, A.; Kulbekova, R.; Medeu, A.R. Water quality problems analysis and assessment oft he ecological security level oft he transboundary Ural-Caspian Basin of the Republic of Kazakhstan. Appl. Sci. 2022, 12, 2059. [Google Scholar] [CrossRef]
- Esbaugh, A.J.; Brix, K.V.; Mager, E.M.; De Schamphelaere, K.; Grosell, M. Multi-linear regression analysis, preliminary biotic ligand modeling, and cross species comparison of the effects of water chemistry on chronic lead toxicity in invertebrates. Comp. Biochem. Physiol. 2012, 155, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, D.; Monica R da Marques, C.; Baptista, D.; Forsin Buss, D. Metal bioavailability and toxicity in freshwaters. Environ. Chem. Lett. 2015, 13, 69–87. [Google Scholar] [CrossRef]
- Namieśnik, J.; Rabajczyk, A. The speciation and physicochemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 2010, 22, 1–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Dai, R.; Leszek, S.; Wang, X.; Xiao, L. Adsorption and migration of heavy metals between sediments and overlying water in the Xinhe River in central China. Water Sci. Technol. 2021, 84, 1257–1269. [Google Scholar] [CrossRef]
- Sokolov, A.A. Natural zones of Kazakhstan. In Agrochemical Characteristics of the Soils of the USSR; Kazakhstan and Chelyabinsk region: Nauka, Moscow, 1968; pp. 9–24. (In Russian) [Google Scholar]
- Durasov, A.M.; Tazabekov, T.T. Soils of Kazakhstan; Alma-Ata; Publishing House: “Kainar”: Karakol, Kyrgyzstan, 1981; 152p. (In Russian) [Google Scholar]
- Kurmangaliyev, A.B. Serozems. Characteristics of the soils of the Republic. In Agrochemical Characteristics of Soils of the USSR. Kazakhstan and Chelyabinsk Region; Publishing House Nauka: Moscow, Russia, 1968; pp. 82–99. (In Russian) [Google Scholar]
- Dostay, Z.D. Scientific and Applied Bases of Management of Hydroecological Condition of Balkhash Lake Basin. In Dissertation of Doctor of Geographical sciences: 25.00.27, 25.00.36; St. Petersburg State University: St. Petersburg, Russia, 1999; 305p. (In Russian) [Google Scholar]
- Dostay, Z.D.; Sarsenbayev, M.K.; Baimyrzayev, K.M. Water consumption in Ile river basin and measures to save Balkhash lake. In Modern Problems of Hydroecology of Inland Closed Basins of Central Asia: Materials of the International Scientific and Practical Conference; Publishing House: “Kaganat”: Almaty, Kazakhstan, 2003; pp. 87–93. (In Russian) [Google Scholar]
- Supervisors, T.A.A.; Dostay, Z.D. (Eds.) To Assess the Water Resources of Ili River, Taking into Account Climatic Changes and to Develop Principles for Their Protection and Common Use: Report on Scientific Research Works; MES RK, Institute of Geography: Almaty, Kazakhstan, 2002; State registration No 0100РК00308; 237p. (In Russian) [Google Scholar]
- Amirgaliyev, N.A.; Ismukhanova, L.T. The Level of Anthropogenic Pollution of the Kapshagay Water Reservoir, Republic of Kazakhstan. Water Res. Manag. Cent. Asia. Handb. Environ. Chem. 2020, 105, 143–162. [Google Scholar] [CrossRef]
- Amirgaliyev, N.A.; Dzhusupbekov, D.K.; Ismukhanova, L.T. Hydrochemical parameters and the level of anthropogenic impacts on the water quality of the Kapshagai reservoir. In Bull. of KGU (Kazakh-German University). Special Issue “Water Resources of Kazakhstan: Current Condition, Problems, Ways of Their Solution”; Kazakh-German University: Almaty, Kazakhstan, 2014; Volume 2, pp. 138–147. (In Russian) [Google Scholar]
- Ismukhanova, L.; Amirgaliev, N.; Bektursunov, K. Monitoring quality parameters of the transboundary outflow in the Ili River. In Proceedings of the IWA 6th European Conference of Young Specialists in the Water Sector “East + West”, Istanbul, Turkey, 28–30 May 2014; pp. 132–137. [Google Scholar]
- Amirgaliyev, N.A.; Madibekov, A.S.; Normatov, I.S. About the criteria of estimation of surface water quality of Kazakhstan on the basis of accounting of its natural features. News Natl. Acad. Sci. Repub. Kaz. Ser. Geol. Tech. Sci. 2019, 4, 188–198. [Google Scholar] [CrossRef]
- Amirgaliev, N.A.; Opp, C.; Askarova, M.; Kulbekova, R.A.; Ismukhanova, L.T. About ratio and values of the empirical coefficient of alkali metals (Na+ and K+) in surface waters of Kazakhstan on the example of the Ile river. News Natl. Acad. Sci. Repub. Kaz. Ser. Geol. Tech. Sci. 2020, 1, 6–13. [Google Scholar] [CrossRef]
- Cherednichenko, V.S.; Cherednichenko, A.V.; Zheksenbaeva, A.K.; Madibekov, A.S. Heavy metal deposition through precipitation in Kazakhstan. Heliyon 2021, 7, e05844. [Google Scholar] [CrossRef]
- Madibekov, A.; Kogutenko, L. The Issue of transporting pollutants with atmospheric precipitation. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kazan, Russian, 27–29 September 2017; Volume 107, p. 012064. [Google Scholar] [CrossRef]
- Dauvalter, V.A. Geoecology of Bottom Sediments of Lakes; Murmansk, Publishing House “Murmansk State Technical University”: Murmansk, Russia, 2012; 242p. (In Russian) [Google Scholar]
- GD (guiding document) 52.18.289-90; Methodical Instructions. Method for Measuring the Mass Fraction of Mobile Forms of Metals (Copper, Lead, Zinc, Nickel, Cadmium, Cobalt, Chromium, Manganese) in Soil Samples by Atomic Absorption Analysis. USSR State Committee for Hydrometeorology: Moscow, Russia, 1990; 36p. (In Russian)
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based quality guidelines for freshwater ecosystem. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Guchte, C. Ecological Risk Assessment of polluted sediments. Eur. Water Pollut. Control 1995, 5, 16–24. [Google Scholar]
- Methodical Recomendations 2609-82; Methodical Recomendations for Hygienic Substantiation of MPC of Chemicals in the Soil. USSR Ministry of Health: Moscow, Russia, 1982; 58p. (In Russian)
- Vinogradov, A.P. Average contents of chemical elements in the main types of effusive rocks of the earth’s crust. Geochemical 1962, 7, 555–571. (In Russian) [Google Scholar]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Et Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Geochemie; Sammlung Göschen; Verlag Walter de Gruyter: Berlin, Germany, 1967; Volume 1224–1224a/1224b, 220p. [Google Scholar]
- Clarke, F.W.; Washington, H.S. The Composition of the Earth’s Crust. U.S.; Department Interior Geological Survey: Reston, VA, USA, 1924; Volume 770, 518p. [Google Scholar]
- Sayet, Y.Y.; Revich, B.A.; Yanin, Y.P. Geochemistry of the Environment; Springer: Cham, Switzerland, 1990; 335p. (In Russian) [Google Scholar]
- Budnikov, G.K. Heavy metals in environmental monitoring of water systems. Sorovskiy Educ. J. 1998, 5, 23–29. (In Russian) [Google Scholar]
- Amirgaliyev, N.A.; Ismukhanova, L.T. Transboundary inflow of mineral salts and toxic compounds along Ile river. Quest. Geogr. Geoecology 2012, 1, 20–28. (In Russian) [Google Scholar]
- Alekin, O.A.; Brazhnikova, L.V. Runoff of Dissolved Substances from the Territory of the USSR; Publishing House Nauka: Moscow, Russia, 1964; 143p. (In Russian) [Google Scholar]
- Problems of Hydroecological Sustainability in the Basin of Lake Balkhash; Publishing House “Kaganat”: Almaty, Kazakhstan, 2003; 584p. (In Russian)
- Program for the Development of Small Towns in Almaty Region. Decree of the Government of the Republic of Kazakhstan dated November 16, 2018 No 767. 122p. Available online: https://primeminister.kz/assets/media/p180000076716-11-2018rus.pdf (accessed on 8 February 2019). (In Russian).
- Scheme for the Integrated Use and Protection of Water Resources in Ili River Basin Including Tributaries: Summary Note Volume III; Book 3; Committee for Water Resources of the Ministry of the Republic of Kazakhstan: Almaty, Kazakhstan; Production Cooperative “Kazgiprovodkhoz Institute”: Almaty, Kazakhstan, 2008; 78p. (In Russian)
- Gadde, R.R.; Laitinen, H.A. Studies of heavy metal adsorption by hydrous iron and manganese oxides. Anal. Chem. 1974, 46, 2022. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 1990; 339p, ISBN 978-94-017-8391-0. [Google Scholar]
- Yang, Q.; Yang, Z.; Zhang, Q.; Liu, X.; Zhuo, X.; Wu, T.; Wang, L.; Wei, X.; Ji, J. Ecological risk assessment of Cd and other heavy metals in soil-rice system in the karst areas with high geochemical background of Guangxi, China. Sci. China Earth Sci. 2021, 64, 1126–1139. [Google Scholar] [CrossRef]
- Wojtkowska, M. Migration and Forms of Metals in Bottom Sediments of Czerniakowskie Lake. Bull. Environ. Contam. Toxicol. 2013, 90, 165–169. [Google Scholar] [CrossRef]
- Kalembasa, D.; Pakuła, K. Fractions of zinc and copper in forest luvisols of the south Podlasie Lowland. Pol. J. Environ. Stud. 2006, 15, 98–103. [Google Scholar]
- Wojtkowska, M. Content of selected heavy metals in water and riverbed sediments of the Utrata river. Environ. Prot. Eng. 2011, 37, 57–64. [Google Scholar]
- Yao, Z.; Gao, P. Heavy metal research in lacustrine sediment: A review. Chin. J. Oceanol. Limnol. 2007, 25, 444–454. [Google Scholar] [CrossRef]
- Belzunce Segarra, M.J.; Szefer, P.; Wilson, M.J.; Bacon, J.; Bolalek, J. Chemical forms and distribution of heavy metals in core sediments from the Gdansk basin, Baltic Sea. Pol. J. Environ. Stud. 2007, 16, 505–515. [Google Scholar]
- Bakke, T.; Källqvist, T.; Ruus, A.; Breedveld, G.D.; Hylland, K. Development of sediment quality criteria in Norway. J. Soils Sediments 2010, 10, 172–178. [Google Scholar] [CrossRef]
- Song, J.; Duan, X.; Han, X.; Li, Y.; Li, Y.; He, D. The accumulation and redistribution of heavy metals in the water-level fluctuation zone of the Nuozhadu Reservoir, Upper Mekong. Catena 2019, 172, 335–344. [Google Scholar] [CrossRef]
- Hahn, J.; Opp, C.; Ganzenmüller, R.; Ewert, A.; Schneider, B.; Zitzer, N.; Laufenberg, G. Catchment soils as a factor of trace metal accumulation in sediments of the reservoir Klingenberg (eastern Ore Mountains, Germany). J. Environ. Sci. 2019, 86, 1–14. [Google Scholar] [CrossRef]
- Pandiyan, J.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Jagadheesan, R.; Krishnappa, K. An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 2021, 28, 1218–1225. [Google Scholar] [CrossRef]
- Smal, H.; Ligęza, S.; Wójcikowska-Kapusta, A.; Baran, S.; Urban, D.; Obroślak, R.; Pawłowski, A. Spatial distribution and risk assessment of heavy metals in bottom sediments of two small dam reservoirs (south-east Poland). Arch. Environ. Protect. 2015, 41, 67–80. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskuła, J.; Siepak, M. Heavy Metals in Bottom Sediments of Reservoirs in the Lowland Area of Western Poland: Concentrations, Distribution, Sources and Ecological Risk. Water 2019, 11, 56. [Google Scholar] [CrossRef]
- Steell, K.F.; Wagner, G.H. Trace metal relationships in bottom sediments of freshwater stream the Buffalo River. Ark. J. Sediment Petrol. 1975, 45, 310–319. [Google Scholar]
- Vasiliev, O.F.; Papina, T.S.; Pozdnjakov, S.R. Suspended sediment and associated mercury transport—The case study on the Katun River. In Proceedings of the 4th International Symposium on River Sedimentation, Beijing, China, 5–9 June 1989; pp. 155–162. [Google Scholar]
- Salomons, W. Biogeodynamics of Pollutants in Soils and Sediments; Salomons, W., Stigliani, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; 353p. [Google Scholar]
- Reshetnyak, O.S.; Zakrutkin, V.Y. Bottom sediments as a source of secondary pollution of river waters by metals. News of Universities. North Caucasian region. Nat. Sci. 2016, 4, 102–109. (In Russian) [Google Scholar] [CrossRef]
- Afanasiev, M.I. Background content of organochlorine pesticides in water bodies of the North-West. In Monitoring of Background Contamination of the Environment; Elsevier: Amsterdam, The Netherlands, 1987; pp. 51–55. (In Russian) [Google Scholar]
- Huang, B.W. Heavy metal concentrations in the common benthic fishes caught from the coastal waters of Eastern Taiwan. J. Food Drug Anal. 2003, 11, 324–330. [Google Scholar] [CrossRef]
- Nakhshina, Y.P. Heavy metals in the system "water-bottom sediments" of water bodies (review). Hydrobiol. J. 1985, 21, 80–90. (In Russian) [Google Scholar]
- Forstner, V. Metal concentration in Freshwater sediments—Natural effects. In Interaction between Sediments and Fresh Water, Proceedings of an International Symposium Held at Amsterdam, The Netherlands, 6–10 September 1976; Springer: The Hague, The Netherlands, 1977; pp. 94–103. [Google Scholar]
- Mansour, S.A.; Sidky, M.M. Ecotoxicological Studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem. 2002, 78, 15–22. [Google Scholar] [CrossRef]
- Obasohan, E.E. Bioaccumulation of chromium, copper, manganese, nickel and lead in a freshwater cichlid, hemichromis fasciatus from Ogba River in Benin City, Nigeria. Afr. J. Gen. Agric. 2008, 4, 141–152. [Google Scholar]
- Yang, R.; Yao, T.; Xu, B.; Jiang, G.; Xin, X. Accumulation features of organochlorine pesticides and heavy metalas in fish from high mountain lakes and Lbasa River in Tibetan plateau. Environ. Int. 2007, 33, 151–156. [Google Scholar] [CrossRef]
- Jones, H.J.; Swadling, K.M.; Tracery, S.R.; Macleod, C.K. Long term trends of Hg uptake in resident fish from polluted estuary. Mar. Pollut. Bull. 2013, 73, 263–272. [Google Scholar] [CrossRef]
- Muneer, J.; AlObaid, A.; Ullah, R.; Rehman, K.U.; Erinle, K.O. Appraisal of toxic metals in water, bottom sediments and fish of fresh water lake. J. King Saud Univ. Sci. 2022, 34, 101685. [Google Scholar] [CrossRef]
- Hassaan, M.H.; Al-Kahali, M.; Al-Edres, M. Heavy metal contamination in the white muscles of some commercial fish species from Al-Hodeidah-Red Sea coast of Yemen. J. Appl. Sci. 2007, 10, 79–96. [Google Scholar]

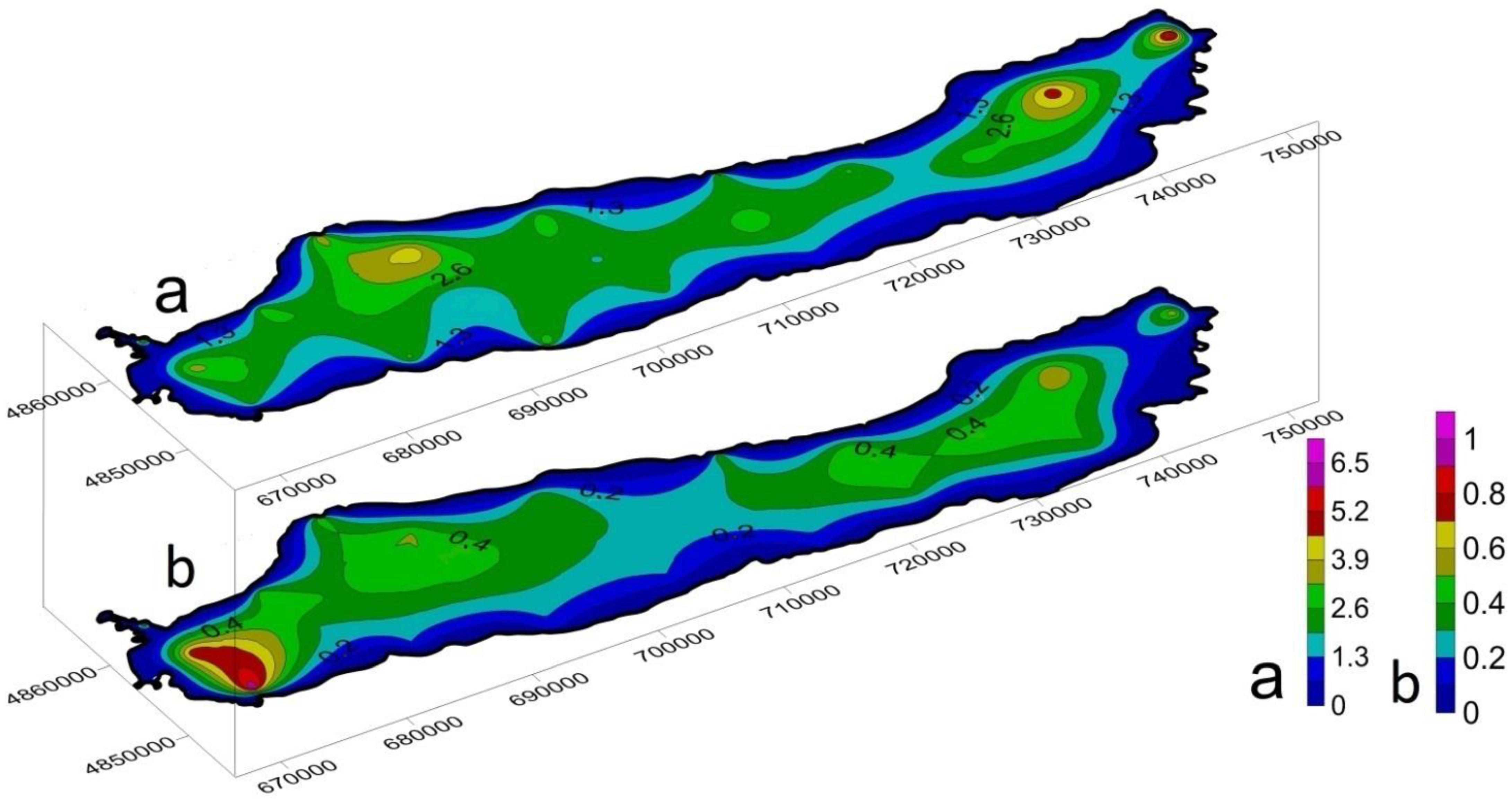

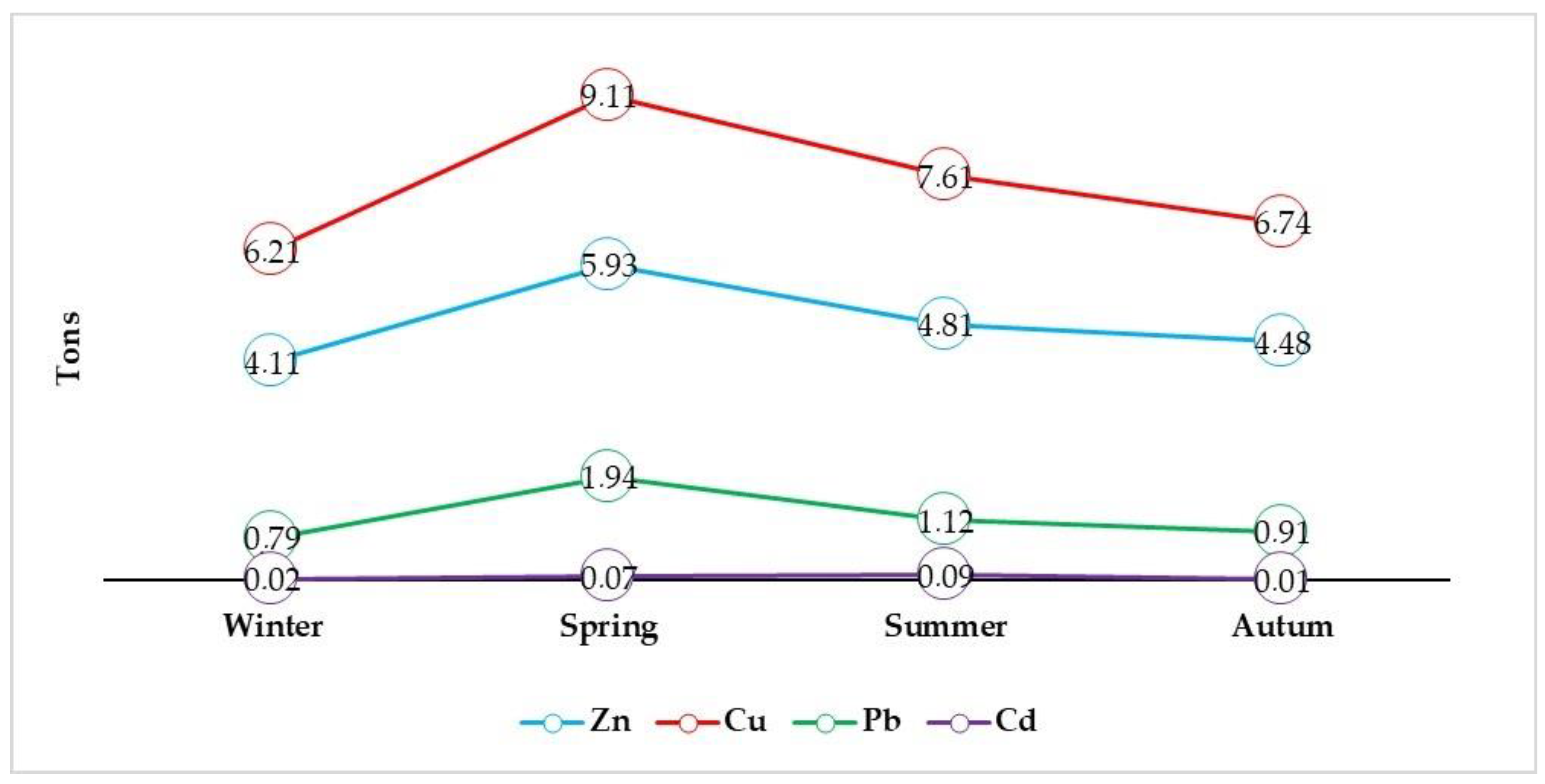
| Metals | mg/kg | |||
|---|---|---|---|---|
| Zn | Cu | Pb | Cd | |
| amplitude | 30.0–37.0 | 0.12–0.38 | 1.20–8.80 | 0.16–0.96 |
| average | 33.6 | 0.23 | 5.18 | 0.46 |
| Metals Content | Heavy Metals | |||
|---|---|---|---|---|
| Zn | Cu | Pb | Cd | |
| Kapshagay Reservoir bottom sediment | 33.6 | 0.23 | 5.18 | 0.46 |
| USA standards | 30 | 21 | 10 | 0.16 |
| Parameters | Zn | Cu | Pb | Cd |
|---|---|---|---|---|
| Background [65] | 83.0 | 47.0 | 16.0 | 0.13 |
| Concentration | 33.6 | 0.23 | 5.18 | 0.46 |
| Kc | 0.40 | 0.005 | 0.32 | 3.54 |
| Zc | 1.26 | |||
| Pollution Level | Zc | Sanitary and Toxicological Danger | Content of Toxic Elements in Water |
|---|---|---|---|
| Low | 10 | Acceptable | Mostly within background limits |
| Middle | 10–30 | Moderate | Many are higher relative to the background; some occasionally reach MPC |
| High | 30–100 | Dangerous | Many elements are above the background; some exceed MPC |
| Very high | 100–300 | Very dangerous | Many are many times higher than the background; some consistently exceed MPC |
| Extremely high | >300 | Extremely dangerous | Mostly many times higher than the background; many consistently exceed the MPC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismukhanova, L.; Choduraev, T.; Opp, C.; Madibekov, A. Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan. Appl. Sci. 2022, 12, 11474. https://doi.org/10.3390/app122211474
Ismukhanova L, Choduraev T, Opp C, Madibekov A. Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan. Applied Sciences. 2022; 12(22):11474. https://doi.org/10.3390/app122211474
Chicago/Turabian StyleIsmukhanova, Laura, Temirbek Choduraev, Christian Opp, and Azamat Madibekov. 2022. "Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan" Applied Sciences 12, no. 22: 11474. https://doi.org/10.3390/app122211474
APA StyleIsmukhanova, L., Choduraev, T., Opp, C., & Madibekov, A. (2022). Accumulation of Heavy Metals in Bottom Sediment and Their Migration in the Water Ecosystem of Kapshagay Reservoir in Kazakhstan. Applied Sciences, 12(22), 11474. https://doi.org/10.3390/app122211474








