Abstract
Research of new input raw materials for biogas plants is a very actual topic. There are only a very few studies dealing with the possibility of using silage prepared from the above-ground parts of the Jerusalem artichoke (Helianthus tuberosus L.) and maize (Zea mayse L.) for methane production. This study deals with the determination of methane production and methane content in biogas during the fermentation of maize silage with dissimilar additions of the biomass of the Jerusalem artichoke (JA). Except for the effect of the JA’s addition on the yield of methane, we also studied its potential influence on the inhibition of the process of anaerobic digestion and the bacterial and methanogenic archaeal composition of anaerobic digestate. There were five model silages prepared; two of them contained only maize or JAs, and the remaining three were mixtures of maize and JA silages (30%wt; 50%wt and 70%wt). The fermentation tests showed that the JA addition (from 30 to 70%wt) resulted in the production of biogas decreasing, on average, by 15%. Based on the performed metagenomic analysis, we cannot confirm an essential influence of JA biomass addition on the composition of the community of microorganisms during fermentation.
1. Introduction
Maize silage is a standard input raw material for biogas plants. The reason is clear, it exhibits an ideal nutritional composition of biomass and high yield of green matter, which also naturally relates to a high production of biogas [1]. On the other hand, the number of studies dealing with the negative impacts of maize growing on the environment is increasing [2]. The most significant negative impacts include water erosion and soil compaction. Maize is often sown in wide rows (32.5 and 75 cm) where the soil is not protected from water. In addition, the root system of maize is not as extensive as that of other crops (e.g., legumes), so the soil is not sufficiently rooted and soil compaction occurs. This manifests itself in a reduction of gravity pores, which are necessary for water infiltration into the soil and gas exchange in the soil [3]. Another negative effect on the soil environment is the increased use of N mineral fertilizers, which are required to produce sufficient biomass. The production of these fertilizers is environmentally damaging [4,5] and excessive N mineral fertilizer input into the soil negatively affects soil microbial communities and leads to environmental eutrophication [5,6]. The use of other biomass sources or their mixtures may have a lesser impact on the environment than the production of maize silage [7,8]. Stinner [9] informs, for example, that exactly the biogas production offers new challenges for using legumes, which could positively stimulate their inclusion into the rotation of crops. The use of a mixed culture with legumes in the process of biogas production is described in Kintl et al. [10]. Mixed cultures using Sida hermaphrodita and leguminous plants such as Melilotus albus MED. and others were tested with the aim to enhance sustainable biomass production on marginal soil [11]. Growing biomass on less fertile soils requires thorough proposal, establishment, and maintenance of appropriate cultivation systems [12].
In the search for alternative input biomasses for biogas plants, some factors affecting the process of biogas (or methane) production should be considered. For example, Herrmann et al. [13] state that a limiting factor in the production of methane was the content of lignin, while the production rate was influenced by the content of nitrogen-free extracts and neutral detergent fiber. When the white melilot is used in a mixed culture, the presence of coumarin can be perceived as an element affecting the process of methanogenesis [10,14]. According to Kadankova et al. [15], there is even a direct correlation between the coumarin and melilot contents in shreddings and hence in silage. Kintl et al. [16] stated that silage prepared from the mixed culture of maize and melilot led to a higher production of methane in variants with up to 20% of melilot biomass [17]. Microbiome of the biogas plant fermenter has to adapt to the presence of substances that may have a negative influence on methane production, e.g., coumarin [14]. In case that individual substrates are lacking, this can be eliminated by their mixture, which was demonstrated by Cortesi [18] in the combination of maize and red chicory. There were many authors looking for other substrates suitable for biogas production. Larsen et al. [19], for example, tested the potential of common ensiling of wheat straw and sugar beet leaves, Vitez et al. [20] tested the digestion of waste from vegetable production, Sukačová et al. [21] dealt with the fermentation of micro-algae enriched with lipids. Nevertheless, the choice of a mixture of substrates that would lead to the stability of the process of anaerobic fermentation is not simple as it requires experience and professional knowledge of the ongoing biological processes.
In recent years, problems connected with climate change and with the lack of non-renewable resources have led to the ever increasing use of biorefinery principles, i.e., procedures based on renewable resources of biomass in order to break free from dependence on fossil resources [22]. According to Johansson et al. [23], an ideal crop for the production of renewable energy is the Jerusalem artichoke (JA) (Helianthus tuberosus L.) thanks to its high biomass production and low agrotechnological requirements. It is a perennial herb with the C4 photosynthesis, with sturdy and massive stems that are sometimes forking at the base, which grows to a height of 2.5–3.0 m. According to various authors, the dry matter (DM) yield of above-ground parts of the Jerusalem artichoke ranges between 4 to 30 t/ha and from 4 to 15 t/ha of tubers in dependence on genotype, climatic conditions, soil type and stand age [13,23,24,25,26,27]. The crop is also characterized by good tolerance to frost and drought, strong resistance to pests and diseases and can withstand even less fertile soils [28]. The Jerusalem artichoke has been cultivated for ages thanks to edible tubers and medicinal properties [29,30]. However, there are some ways of its use that are tested. For example, Hay and Offer [31] evaluated the JA as an alternative forage crop. Moreover, with the developing bioenergetics, the JA appears as a crop potentially usable for anaerobic co-digestion (AcoD), both its above-ground biomass [32] and tubers [33].
A hypothesis was tested about the influence of the JA content in the maize silage on the change in the composition of the microbial community during the anaerobic fermentation of the silage in laboratory reactors. Further, hypotheses were tested about the influence of JA content in the maize silage on methane production and methane concentration in biogas.
2. Materials and Methods
2.1. Localization of Biomass Source
The plant biomass intended for biogas production was grown in the Vatín Research Station of Fodder Crops. The station is located in the Bohemian-Moravian Highlands (Česko-Moravská vrchovina) in the central part of the Czech Republic. The experimental station Vatín is located at an altitude of 540 m above sea level, its location can be seen in Figure 1. The area of the experimental station falls in the moderately warm climatic zone.
Figure 1.
Location of the Vatín Experimental Station; (1) position of the station in the Czech Republic; (2) village of Vatín; (3) rear of the station; (4) experimental plot.
In the experimental year 2019, DASA fertilizer was applied on 18 April 2019 at 300 kg/ha prior to sowing maize on 26 April 2019. DASA fertilizer (AGRO CS s.r.o., Czech Republic) consists of 26% N (1/3 NO3−-N and 2/3 NH4+-N) and 13% S (NH₄)₂SO₄). The application of other macronutrients (P, K, Mg and Ca) was not necessary because of the sufficient nutrient supply in the arable soil (Table 1). Selected seeds of maize (Zea mays L., FAO 270) were individually sown in 0.375 m wide rows using the Kinze 3500 seeder (Kinze Manufacturing, Williamsburg, IA, USA) “interplant system” at a rate 75,000 individuals/ha.

Table 1.
Arable soil characteristics from the experimental site (expressed as mean ± SD; for n = 9)—average plant-available nutrient content before application of fertilizers.
The plot with the naturally occurring stand of the Jerusalem artichoke (Helianthus tuberosus L.) was selected and marked on the same date as the sowing of maize. The above-ground biomass was then sampled to prepare shredding usable for the preparation of silages.
2.2. Production of Model Silage
The plant biomass was harvested manually at a stubble height of 18 cm and then shredded into 15–20 mm particles using the Deutz-Fahr MH 6505 chopper (Deutz-Fahr, Lauingen, Germany). The obtained shredding was used to produce model silages—five variants of experimental silage, each in triplicates. Two silage variants were made only from maize and JA monosubstrates. The other silage variants were prepared by mixing the maize shredding and the JA in different weight ratios (Table 2).

Table 2.
Overview of the model silages produced.
The procedure for preparing microsilage was the same in all variants. Eight kilograms of shredding was placed in three mini-silos (plastic vessel diameter 150 mm, height 1000 mm) together with inoculum (SiloSolve EF, Chr. Hansen, Hoersholm, Denmark) at 5 g + 3.5 L H2O/t. The prepared silage was compacted with a pneumatic press with a force of 6 kN/m2. Afterwards the mini silos were hermetically sealed and stored in the dark at a temperature of 28 °C ± 1 °C. Each vessel was equipped with a safety valve to exhaust excess gases. Silage compaction in the mini silos ranged from 150.2 to 173.9 kgDM/m3 (Table 2).
At the end of the incubation period (90 days), the mini silos were opened, and the collected samples were homogenized, frozen and transported to the laboratory for chemical analyses and fermentation tests. The methodology of the production of model silages is described in detail in Kintl et al. [10] and Kintl et al. [16].
2.3. Silage Characteristics
Frozen silage samples were defrosted at laboratory temperature before the fermentation test. The content of dry matter (DM) and volatile solids (VS) in the silage samples was determined gravimetrically by drying/annealing in the LMH 07/12 electric furnace (LAC, Czech Republic) at a temperature of 105 °C and 550 °C, respectively, according to ČSN EN 15934 [34] and ČSN EN 15935 [35] standards. N-substances were determined by the Kjeldahl method (ISO 20483:2013) [36]. Protein content was determined using the KjeltecTM 2300 analyzer (FOSS Analytical, Hillerød, Denmark) and then multiplied by the empirical factor 6.25. Fat content was determined gravimetrically using the water-cooled Soxhlet extractor by direct sample extraction with diethyl ether. Crude fiber (CF) content was determined using the two-stage hydrolysis by sulfuric acid and potassium hydroxide, and then the ash content was determined. The acid detergent fiber (ADF) content was determined using a solution containing concentrated sulfuric acid and acetyltrimethylamoniumbromide. Neutral detergent fiber (NDF) was determined using a solution of sodium laurylsulphate and ethylendiamintetra-acetic acid. Analyses were performed using the ANKOM 200 fiber analyzer (ANKOM Technology, Macedon, NY, USA). Acid detergent lignin (ADL) was determined according to ČSN EN ISO 13906 [37].
2.4. Fermentation Tests
Fermentation tests were made in laboratory conditions according to the modified standard VDI 4630:2016 and with respect to recommendation by Holliger et al. [38]. There were three systems with a total number of 24 batch fermenters used for the experiment. A simplified scheme of the laboratory system for the performance of fermentation tests is shown in Figure 2.
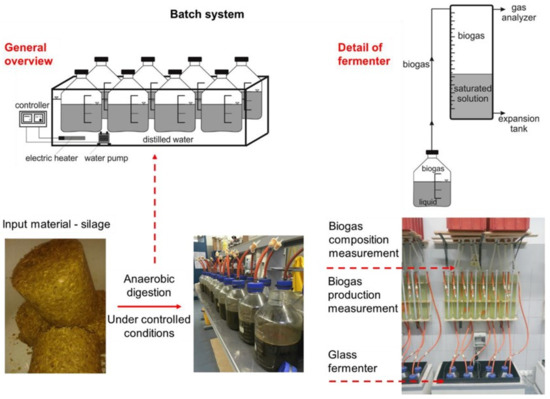
Figure 2.
Diagram of laboratory batch fermenters. Adapted with permission from [16].
A set of eight 5 L glass fermenters was placed in each of the three water baths. In the water baths, a temperature of 40 °C ± 0.2 °C was maintained by means of thermostat-controlled electric heating. On day 1 of the experiment, 3 kg of inoculum from an agricultural biogas plant (Table 3) were dosed to all fermenters. Two fermenters in each system were used as blank samples in which the endogenous biogas production of the inoculum was measured. For the remaining six fermenters, 50–53 g of fresh silage samples was dosed. The retention time of the substrate in the fermenter was 35 days, and the test was terminated when daily methane production during three consecutive days was <1% of the accumulated volume of methane. Anaerobic conditions prevailed in the fermenters throughout the experiment. Each fermenter was connected to a graduated measuring cylinder via a hose. The biogas produced pushed the salt-saturated solution from the glass cylinder into an expansion vessel. The production of biogas was recorded daily on the scale of the measuring cylinder. The biogas accumulated in the glass cylinder could be analyzed thanks to a collection point with a quick coupling, which all glass cylinders were equipped with. The composition of the biogas produced was analyzed using the Dräger X-am 8000 analyzer (Dräger, Lübeck, Germany). The resulting biogas production was converted to normal conditions (p = 101,325 Pa; T = 273.15 K) and expressed in Nm3 per kg of added volatile solids of the tested silage.

Table 3.
Inoculum characteristics and parameters of the test of methane production.
Theoretical methane yield was calculated as previously described in Kintl et al. [10].
2.5. DNA Isolation and 16S rRNA Sequencing
DNA was extracted from five digestate samples (M100, JA100, M30JA70, M50JA50 and M70JA30) by the QIAamp PowerFecal DNA Kit (Qiagen, Hilden, Germany), according to the instructions of the standard protocol provided by the manufacturer. After DNA isolation, amplification of genes coding for 16S rRNA was performed. For this reaction, following primers were used enabling the dual-index bar-coding method: 515F Forward primer 5′-AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3′ and 806R Reverse primer 5′-CAAGCAGAAGACGGCATACGAGATAGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3′ [39]. This primer set targeted the hyper-variable region V4 of 16S rRNA, allowing the detection of microbial taxa present in each sample [40]. PCR amplification was performed according to the Earth Microbiome Project protocol [41] in a reaction volume of 25 μL with 10 µL Platinum™ II Hot-Start PCR Master Mix (Thermo Fisher Scientific, USA), 200 nM primers and 2 μL isolated DNA. The PCR program consisted of a hot start at 94 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 45 s, primer annealing at 52 °C for 60 s with a 50% thermal ramp, and extension at 72 °C for 90 s. PCR was completed with a final extension at 72 °C for 10 min. After PCR, amplification products were purified using AMPure XP Beads (Beckman Coulter, Brea, CA, USA) according to the protocol provided by the manufacturer. Samples were normalized using Qubit 4 (Thermo Fisher Scientific, Waltham, MA, USA) and their quality was checked using Fragment Analyzer (Agilent Technologies, Santa Clara, CA, USA). The final library was subjected to NGS using the MiniSeq® MidOutput Reagent Kit (2 × 150 paired-end sequencing) and Illumina MiniSeq sequencer according to the manufacturer’s instructions (Illumina, San Diego, CA, USA).
Sequence Analysis
The raw sequences were processed by using the DADA2 package (version 1.16.) according to the DADA2 pipeline tutorial [42]. A set of Illumina sequenced paired-end fastq files was split (demultiplexed) into individual per-sample fastq files. Firstly, reads were filtered and trimmed (maximum of five ambiguous bases, expected error threshold of 5 and the last 20 bases truncated). Filtered reads were filtered, dereplicated, de-noised and merged following the standard workflow [43]. Chimaeras were removed and taxonomy was assigned using the RDP naive Bayesian classifier method [44] against the SILVA NR v138 database [45]. Overall, the end product is an amplicon sequence variant (ASV) table, a higher-resolution analogue of the traditional OTU table. ASVs were used because they record the number of how many times each exact amplicon sequence variant was observed in each sample instead of clustering similar sequences (into OTUs).
2.6. Statistical Analysis–Data Treatment
All experimental variants had three repetitions and investigated parameters were determined in triplicates as well. The data were statistically processed using the Statistica 12 software, (Dell Software, Round Rock, TX, USA). An exploratory data analysis was performed first, then the ANOVA and the post-hoc Tukey’s HSD test. All analyses were made at a significance level of p < 0.05. The plots were built by the Origin2021 software package (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
3.1. Silage Characteristics
The quality of silage prepared in the respective variants was evaluated based on the following parameters: total content of dry matter (DM), crude proteins (CP), starch, lipids, neutral detergent fibre (NDF), acid detergent fibre (ADF), crude fibre (CF) and acid detergent lignin (ADL). These parameters were determined at the end of the ensiling process, i.e., after the ensiling vessels were opened. The highest content of dry matter (DM) was recorded in the M100 silage (33.6%) and was decreased with the decreasing proportion of maize in the silage to 30.6% in JA100. The value is nearly identical with that mentioned by Ţîţei [46]: 30.5%. A similar trend was also exhibited by the statistically significant differences between the respective silage variants. According to Wang et al. [47], the content of DM is closely related to JA growth stages. The content of neutral detergent fiber (NDF) in the percent of DM ranged on average from 43.8% (M100) to 33.2% (JA100). Statistically significant differences were found among all five variants. This corresponds to the fact that according to NRC [48], the content of NDF in JA silages (43.9% of DM) is lower as compared with other silages, such as maize silage (45.0% of DM), barley silage (56.3%) and oat silage (60.6%). Nevertheless, some other authors declare mostly higher values in the JA, such as Papi et al. [49] 38.8% and Ţîţei [46] 49.6%. Ţîţei [46] also claims that a significant decrease in the content of NDF and increased content of hemicellulose were recorded during the process of JA ensiling, which had a positive influence on the yield of methane. Liu et al. [50] observed that the fiber content in mixed silages of maize and JA decreased with the increasing share of JA biomass in the mixture, while the degradability of NDF increased. A similar issue is being discussed in the fodder industry: the main source of fiber in fodders is the cell wall of plants, which consists of cellulose, hemicellulose and the non-saccharide component of lignin; the nutritional availability of all nutrients then depends on the composition and structure of fiber, which affect its degradability and digestibility for ruminants [26]. The content of NDF in maize is claimed to be 42.1% by Kintl et al. [16], 47.3% and 38.6% by Vítěz et al. [20] and 55.3% by Papi et al. [49]. The NDF content is also related to the date of harvest. The content of ADF ranged on average from 27.81% (JA) to 21.14% (M) and the content of CF ranged on average from 19.81% (JA) to 16.82% (M). According to NRC [48], the average ADF values are higher—31.7% in JA and 28.1% in M, Papi et al. [49] 30.3% in JA and 34.6% in M. Based on the research of nutritional and fiber composition of silages prepared from various crops in Germany, Herrmann et al. [13] inform that the JA silage contained 44.1% of NDF and 39.6% of ADF. In these parameters (Table 4), determined differences were not so conspicuous as in NDF and their trend was opposite—the contents of ADF and CF decreased with the increasing share of maize; statistically significant differences were recorded between the JA100, M30 + JA70 and M50 + JA50 groups of variants and the variant of maize monoculture (M100) (Table 4 and Table 5).

Table 4.
DM, NDF, ADF and CF contents in the prepared model silages.

Table 5.
Comparison of DM, CP, NDF, ADF and ADL contents in Jerusalem artichoke silages and several main fodder silages (% DM).
The highest and lowest contents of starch were recorded in the variants M100 (16.1%) and JA100 (9.2%), respectively. Statistically significant differences were found among all five variants. The content of CP in the percent of dry matter ranged on average from 9.3% (M100) to 5.1% (JA100). Statistically significant differences were found among all five variants. In contrast, according to NRC [48], Wang et al. [47] claim the CP content to be higher in JA (11.6% of DM) and lower in maize (8.8% of DM). Papi et al. [49] recorded 10.5% in JA and 8.3% in maize. Razmkhah et al. [51] arrived at a value of 10.3% in JA. The content of ADL was the highest in JA100 (8.1%) and decreased with the decreasing share of JA and with the increasing share of maize in the silage to 2.3% in M100. The differences were statistically significant. Loughrin et al. [52] suggested the use of the technology of micro-aeration in order to increase degradability of lignin during which facultatively aerobic bacteria are supported, and hence the production of necessary enzymes. Conversely, the content of lipids was the highest in M100 (4.4%) and the lowest in JA100 (2.9%), with differences being statistically significant too. Thus, the Jerusalem artichoke has a lower content of readily degradable substances (starch, CP and lipids) as compared with maize, and a higher content of substances that are more difficult for degradation (ADF, CF and ADL), which results in lower biogas yields [26] (Table 6).

Table 6.
Contents of starch, CP, ADL and lipids in the prepared model silages.
Triolo et al. [53] and Li et al. [54] inform that biomass with a high content of lignocellulose exhibits low biogas yield and biological degradability due to the low biological degradability of lignin. Ozbayram et al. [55] maintain that enhanced degradability of lignocellulose biomass for the production of methane can be supported by inoculation or bioaugmentation using the rumen microbiota.
3.2. Methane Yield
The cumulative production of methane and the concentration of methane in the biogas during the 35 days of the fermentation test are shown in Table 7 and Appendix A. The sample of maize silage (M100) exhibited the highest production of methane throughout the experiment. The measured final methane production was 0.352 ± 0.017 Nm3/kgVS. The results indicate that the increasing weight representation of the Jerusalem artichoke in the silage samples caused the decreasing production of methane as compared with the production of methane from the maize sample (M100).

Table 7.
Methane yield, theoretical methane yield and methane content in biogas after 35 days.
The production of methane from samples of silage prepared only from the Jerusalem artichoke (JA100) was 0.274 ± 0.013 Nm3/kgVS. This is by 22.1% less as compared with the sample of maize silage (M100). The sample of M70 + JA30 silage (70% maize silage + 30% Jerusalem artichoke) exhibited a lower methane production by 18.4% compared with the maize silage; the measured methane production in this sample was 0.287 ± 0.014 Nm3/kgVS. The addition of the Jerusalem artichoke to the silage samples did not influence the methane concentration in biogas (Table 7, Figure A1b). From day 15 of fermentation, the methane concentration in biogas ranged between 64–65%vol in all tested samples. The results of methane production during the fermentation of maize silage (0.352 ± 0.017 Nm3/kgVS) agree with results published by other authors [10,56]. The measured methane production from JA (0.274 ± 0.013 Nm3/kgVS) corresponds with the former studies of Gunnarson et al. [32], who measured the production of methane from the ensiled JA to be 0.265 and 0.281 Nm3/kgVS but claim the methane concentration in biogas ranging from 52–55%vol, which is a lower value than 65%vol reached by us. Zubr [57] measured the methane production in the JA silage to be 0.301 Nm3/kgVS, claiming a methane concentration of 68.4%vol, which are values higher than those recorded by us. Lehtomäki et al. [58] inform that the methane production from the JA silage was 0.37 ± 0.06 Nm3/kgVS; nevertheless, the retention time in the experiment was 150 days, and a methane production converted to a retention time of 30 days was 0.20 Nm3/kgVS. The lower production of methane from silages with the addition of JA can be caused by inappropriate representation of nutrients [59]. Silages with the addition of JA exhibited a higher share of difficult-to-degrade polysaccharides (lignin, cellulose), a higher share of residue after combustion, and a lower share of readily degradable substances (lipids and starch), which contradicts the assumption of Ţîţei and Coșman [60] that the best plants suitable for biogas production are those with a high content of degradable carbohydrates, lipids and proteins. On the other hand, JA silages feature a low content of NH3–N [47]. According to Hutňan et al. [61], the low content of nitrogen can affect the evenness of the fermentation process. An increased amount of nitrogen can be achieved, for example, by using silage prepared from mixed cultures [16], i.e., by involving plants from the family of Fabaceae in the process of AD. Contents of flavonoids, phenolic acids and sesquiterpenoids may inhibit the process too. According to Wang et al. [47], the production of methane could be affected by substances with antibacterial, anti-inflammatory and antioxidation functions that are contained in JA flowers and leaves.
3.3. Composition of Microorganisms
A metagenomic study of the effect of JA addition to maize silage on the bacterial and methanogenic archaeal composition of anaerobic digestate was performed. The analysis was made to assess the effect of bioactive components that are contained in the JA. The structure of the microbial community was analyzed by sequencing the PCR products of the V4 16S rRNA gene amplified by the 515F and 806R primer pairs in five samples. The sequencing run produced 2.13 Gbp of data in total (8,159,519 total reads) with an average error rate of 0.55% and 94.86% of bases passing >Q30 filter. From the five analyzed samples, rarefied to even sequencing depth (63,159 reads), 928 unique ASVs were inferred. Figure 3 shows the composition of the microbial community in different silage samples after 35 days of fermentation. No significant changes were observed in the microbial diversity of the samples.
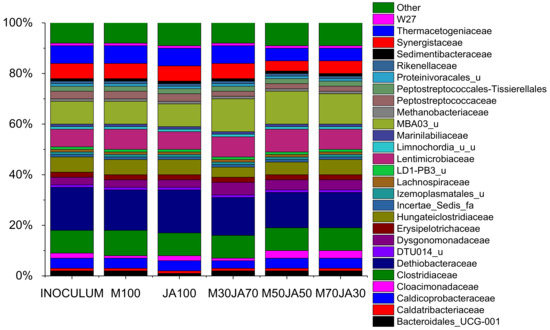
Figure 3.
Silage samples bacterial and archaeal 16S rRNA genes relative abundance at family level, based on the average ASV reads from triplicates. Sampled on Day 35, except inoculum, which was sampled on day 0.
Members of the Dethiobacteraceae family, which are very often described as syntrophic acetate-oxidizing bacteria [62], represented between 12–14% of all bacteria in our samples. Members of the Hungateiclostridiaceae family hydrolyse carbohydrates and cellulose to produce CO2, H2, acetate, lactate, formate and ethanol [63]. Members of this family represented between 11–13% in our samples. Members of Clostridiaceae family are very important hydrolytic bacteria degrading the lignocellulose biomass [63]. In our samples, the abundance of Clostridiaceae family ranged from 6% to 8%. Another abundant family was Lentimicrobiaceae, with the presence in samples between 7–9%. Members of this family are described as fermentative and hydrolytic organisms able to ferment a wide range of compounds into acetate, malate, propionate, formate, etc. [64]. A large fraction of reads affiliated with uncultured/uncultivated? bacteria of the 8at order to family level was determined. It represented 9–13% of all bacteria. These microorganisms are correlated with carbohydrate fermentation during anaerobic digestion [62]. Members of the Synergistaceae family ferment organic acids and amino acids (arginine, alanine, glutamic acid, leucine, cysteine), and they also reduce cysteine and elemental sulphur to H2S [63]. The members of Synergistaceae family represented 3–4% of all bacteria. Members of the Thermacetogeniaceae family belong to syntrophic acetate-oxidizing bacteria. The Caldicoprobacteraceae family is associated with the direct or indirect involvement in the protein hydrolysis [63] represented 4–6% of all bacteria. Members of the Dysgonomonadaceae family represented 2–3% of all bacteria in the samples. They utilize pyruvate and produce acetic acid and CO2 as fermentation products [63]. The hypothesis that the addition of JA affected the composition of microorganisms during the fermentation of the samples tested by us cannot be confirmed.
In all samples, Archaea represented 0.7–1% of the total. The most abundant Methanobacteriaceae family represented 89–95% of all Archaea. Figure 4 shows the major taxonomic subgroups within the phyla.
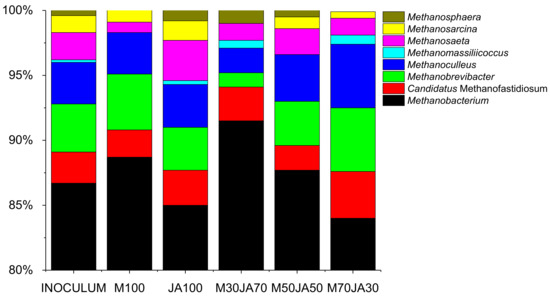
Figure 4.
Silage samples archaeal 16S rRNA genes relative abundance at the genus level, based on the average ASV reads from triplicates. Sampled on day 35, except the inoculum, which was sampled on day 0.
The archaeal community in all samples was dominated by Euryarchaeota (92–96% of total archaea), Halobacterota represented 3–8% of total archaea. Hydrogenotrophic Methanobacterium genus became dominant in all tested samples, reaching relative abundance levels > 84% of total archaea on Day 35. Other genera present in the samples were Candidatus_Methanofastidiosum (2–4% of total archaea), using methylated thiol reduction and hydrogenotrophic Methanobrevibacter and Methanoculleus represented 1–5% and 2–5% of total archaea. Acetoclastic Methanothrix (Methanosaeta) represented 1–3% of total archaea. As it can be seen from Figure 4, the addition of Jerusalem artichoke to the silage samples did not cause changes in the community of methanogens. The Methanobacterium genus remains the most abundant genus in all samples. If we compare the composition of JA100 and M100 samples, it can be seen that the representation of the Methanobacterium genus was lower in the fermenter with the addition of JA100. The sample with the addition of JA was also observed to have a higher share of acetoclastic Methanothrix (Methanosaeta) microorganisms, which can metabolize volatile fatty acids (VFA) produced during the degradation of JA [47]. Furthermore, representatives of the Methanosphaera genus were detected in the samples with JA using H2 to reduce the methyl group of methanol, which may relate to the degradation of JA biomass. Several samples with JA also contained representatives of hydrogenotrophic genus of Methanomassillicoccus spp., whose presence in biogas plants is common and which are typical organisms in the cattle rumen [65].
4. Conclusions
The above-ground part of the Jerusalem artichoke contains a range of valuable nutrients and bioactive substances, and it also reaches a high production of biomass. The shoot biomass of the JA is an ideal material for the production of silage; moreover, the biomass contains fructo-oligosaccharides that represent a substrate suitable for lactic acid bacteria during ensiling. This could be a certain advantage when the JA is ensiled together with other plants. Compared with maize, the Jerusalem artichoke has a lower content of readily degradable substances (starch, crude proteins and lipids) and a higher content of difficult-to-degrade substances (ADF, CF and ADL). Although the nutritional value of silage prepared from the above-ground part of JA is high, a lower production of methane from this biomass was recorded when compared with the maize silage. There is however a possibility to use a mixed JA and maize silage in the biogas plants. Our experiments did not confirm the hypothesis about the impact of the JA addition to silages on the composition of microbial community. The hypothesis about the effect of the JA addition to silages on the production of methane was confirmed. The samples with the addition of JA exhibited a significantly lower methane production as compared with the production of methane from silages prepared only from maize. The hypothesis about the impact of JA addition to silages on the concentration of methane in biogas was not confirmed. The fermentation of silages with the addition of JA had no influence on the methane concentration in biogas. When deciding on the Jerusalem artichoke as a co-substrate for biogas plants, it is necessary to carefully consider also agrotechnological, economic and ecological aspects, as well as an appropriate share of the Jerusalem artichoke in the input raw material.
Author Contributions
Conceptualization, A.K., T.V. and J.E.; methodology, T.V., J.L., D.N. and M.V.; validation, T.V., J.H. and T.H.; formal analysis, A.K.,T.V., J.H., M.B. and J.E.; investigation, A.K, T.V., I.H., D.N, J.L., M.V. and J.E.; resources, A.K. and T.V.; data curation, T.V., I.H. and J.E.; writing—original draft preparation, A.K., T.V., I.H., M.V., T.H., J.H. and J.E.; writing—review and editing, T.V. and J.E.; visualization, T.V., I.H. and J.E.; supervision, A.K., T.V. and J.E.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO1722.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
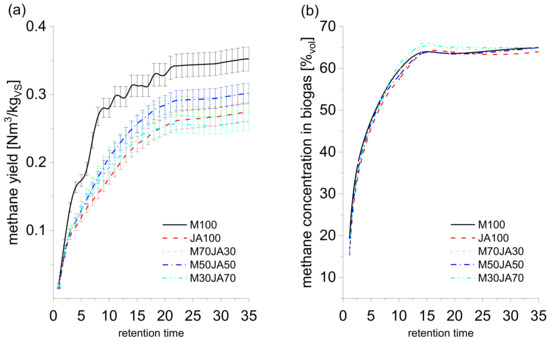
Figure A1.
Methane yield (a) and methane content in biogas (b) during 35 days of the experiment, n = 3, error bars represent ±SD.
References
- Lajdova, Z.; Lajda, J.; Kapusta, J.; Bielik, P. Consequences of maize cultivation intended for biogas production. Agric. Econ. Czech 2016, 62, 543–549. [Google Scholar]
- Herrmann, A. Biogas Production from Maize: Current State, Challenges and Prospects. 2. Agronomic and Environmental Aspects. Bioenergy Res. 2013, 6, 372–387. [Google Scholar] [CrossRef]
- Menšík, L.; Kincl, D.; Nerušil, P.; Srbek, J.; Hlisnikovský, L.; Smutný, V. Water erosion reduction using different soil tillage approaches for maize (Zea mays L.) in the Czech Republic. Land 2020, 9, 358. [Google Scholar] [CrossRef]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O.O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 8539. [Google Scholar] [CrossRef] [PubMed]
- Mezera, J.; Lukas, V.; Horniaček, I.; Smutný, V.; Elbl, J. Comparison of proximal and remote sensing for the diagnosis of crop status in site-specific crop management. Sensors 2021, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Moreno, F.; Lukas, V.; Neudert, L.; Dryšlová, T. Spatial interpretation of plant parameters in winter wheat. Precis. Agric. 2014, 15, 447–465. [Google Scholar] [CrossRef]
- García-Gen, S.; Rodríguez, J.; Lema, J.M. Optimisation of substrate blends in anaerobic co-digestion using adaptive linear programming. Bioresour. Technol. 2014, 173, 159–167. [Google Scholar] [CrossRef]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Stinner, P.W. The use of legumes as a biogas substrate—Potentials for saving energy and reducing greenhouse gas emissions through symbiotic nitrogen fixation. Energy Sustain. Soc. 2015, 5, 4. [Google Scholar] [CrossRef]
- Kintl, A.; Vítěz, T.; Elbl, J.; Vítězová, M.; Dokulilová, T.; Nedělník, J.; Skládanka, J.; Brtnický, M. Mixed Culture of Corn and White Lupine as an Alternative to Silage Made from Corn Monoculture Intended for Biogas Production. Bioenergy Res. 2019, 12, 694–702. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Temperton, V.M.; Harrison, L.; Jablonowski, N.D. Legume Intercropping With the Bioenergy Crop Sida hermaphrodita on Marginal Soil. Front. Plant Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Solinas, S.; Fazio, S.; Seddaiu, G.; Roggero, P.P.; Deligios, P.A.; Doro, L.; Ledda, L. Environmental consequences of the conversion from traditional to energy cropping systems in a mediterranean area. Eur. J. Agron. 2015, 70, 124–135. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Popp, D.; Schrader, S.; Kleinsteuber, S.; Harms, H.; Strauber, H. Biogas production from coumarin-rich plants--inhibition by coumarin and recovery by adaptation of the bacterial community. FEMS Microbiol. Ecol. 2015, 91, fiv103. [Google Scholar] [CrossRef] [PubMed]
- Kadankova, P.; Kintl, A.; Koukalova, V.; Kucerova, J.; Brtnicky, M. Coumarin content in silages made of mixed cropping biomass comprising maize and white sweet clover. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference SGEM2019, Sofia, Bulgaria, 30 June–6 July 2019. [Google Scholar]
- Kintl, A.; Elbl, J.; Vítěz, T.; Brtnický, M.; Skládanka, J.; Hammerschmiedt, T.; Vítězová, M. Possibilities of Using White Sweetclover Grown in Mixture with Maize for Biomethane Production. Agronomy 2020, 10, 1407. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Pyzik, A.; Poszytek, K.; Krawczyk, P.S.; Sobczak, A.; Lipinski, L.; Roubinek, O.; Palige, J.; Sklodowska, A.; Drewniak, L. Adaptation of Methanogenic Inocula to Anaerobic Digestion of Maize Silage. Front. Microbiol. 2017, 8, 1881. [Google Scholar] [CrossRef]
- Cortesi, A. Assessing the Synergistic Effects of Co-digestion of Maize Silage and Red Chicory Waste. Chem. Biochem. Eng. Q. 2018, 32, 383–390. [Google Scholar] [CrossRef]
- Larsen, S.U.; Hjort-Gregersen, K.; Vazifehkhoran, A.H.; Triolo, J.M. Co-ensiling of straw with sugar beet leaves increases the methane yield from straw. Bioresour. Technol. 2017, 245 Pt A, 106–115. [Google Scholar] [CrossRef]
- Vitez, T.; Dokulilova, T.; Vitezova, M.; Elbl, J.; Kintl, A.; Kynicky, J.; Hladky, J.; Brtnicky, M. The Digestion of Waste from Vegetables and Maize Processing. Waste Biomass Valorization 2020, 11, 2467–2473. [Google Scholar] [CrossRef]
- Sukačová, K.; Búzová, D.; Trávníček, P.; Červený, J.; Vítězová, M.; Vítěz, T. Optimization of microalgal growth and cultivation parameters for increasing bioenergy potential: Case study using the oleaginous microalga Chlorella pyrenoidosa Chick (IPPAS C2). Algal Res. 2019, 40, 101519. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Johansson, E.; Prade, T.; Angelidaki, I.; Svensson, S.E.; Newson, W.R.; Gunnarsson, I.B.; Hovmalm, H.P. Economically viable components from Jerusalem artichoke (Helianthus tuberosus L.) in a biorefinery concept. Int. J. Mol. Sci. 2015, 16, 8997–9016. [Google Scholar] [CrossRef]
- Shao, T.; Long, X.; Liu, Y.; Gao, X.; Liu, M.; Rengel, Z. Effect of industrial crop Jerusalem artichoke on the micro-ecological rhizosphere environment in saline soil. Appl. Soil Ecol. 2022, 166, 104080. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L., 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 496. [Google Scholar]
- Ţîţei, V.; Teleuţă, A.; Muntean, A. The perspective of cultivation and utilization of the species Silphium perfoliatum L. and Helianthus tuberosus L. in Moldova. Bulletin of the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Agriculture 2013, 70, 160–166. [Google Scholar]
- Heuzé, V.; Tran, G.; Chapoutot, P.; Bastianelli, D.; Lebas, F. Jerusalem artichoke (Helianthus tuberosus). Feedipedia, aprogramme by INRA. 2015. Available online: https://www.feedipedia.org/node/544 (accessed on 8 November 2022).
- Bogucka, B.; Jankowski, K.J. The effect of harvest strategy on the energy potential of Jerusalem artichoke. Ind. Crop. Prod. 2022, 177, 114473. [Google Scholar] [CrossRef]
- Baldini, M.; Danuso, F.; Monti, A.; Amaducci, M.T.; Stevanato, P.; Mastro, G. Chichory and Jerusalem artichoke productivity in different areas of Italy, in relation to water availability and time of harvest. Ital. J. Agron. Riv. Agron. 2006, 1, 291–307. [Google Scholar] [CrossRef]
- Szewczyk, A.; Zagaja, M.; Bryda, J.; Kosikowska, U.; Stępień-Pyśniak, D.; Winiarczyk, D.; Andres-Mach, M. Topinambur—New possibilities for use in a supplementation diet. AAEM 2019, 26, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.K.M.; Offer, R.W. Helianthus tuberosus as an alternative forage crop for cool maritime regions: A preliminary study of the yield and nutritional quality of shoot tissues from perennial stands. J. Sci. Food Agric. 1992, 60, 213–221. [Google Scholar] [CrossRef]
- Gunnarson, S.; Malmberg, A.; Mathisen, B.; Theander, O.; Thyselius, L.; Wünsche, U. Jerusalem artichoke (Helianthus tuberosus L.) for biogas production. Biomass 1985, 7, 85–97. [Google Scholar] [CrossRef]
- Emmerling, C.; Barton, J. Anaerobic co-digestion of topinambour (Helianthus tuberosus L.) and properties of the remaining biogas manure. Arch. Acker Pflanzenbau Bodenkd. 2007, 53, 683–690. [Google Scholar] [CrossRef]
- ČSN EN 15934; Sludge, Treated Biowaste, Soil and Waste—Calculation of Dry Matter Fraction After Determination of Dry Residue or Water Content. European Committee for Standardization: Brussel, Belgium, 2012.
- ČSN EN 15935; Sludge, Treated Biowaste, Soil and Waste—Determination of Loss on Ignition. European Committee for Standardization: Brussel, Belgium, 2012.
- ISO 20483:2013; Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method—Kjeldahl Method. European Committee for Standardization: Brussel, Belgium, 2013.
- ČSN EN ISO 13906; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. European Committee for Standardization: Brussel, Belgium, 2008.
- Hoflliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Pichler, M.; Coskun, Ö.K.; Ortega-Arbulú, A.S.; Conci, N.; Wörheide, G.; Vargas, S.; Orsi, W.D. A 16S rRNA gene sequencing and analysis protocol for the Illumina MiniSeq platform. MicrobiologyOpen 2018, 7, e00611. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2010, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Callahan, B.J.; Sankaran, K.; Fukuyama, J.A.; McMurdie, P.J.; Holmes, S.P. Bioconductor Workflow for Microbiome Data Analysis: From raw reads to community analyses. F1000Research 2016, 5, 1492. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Ţiţei, V. The biochemical composition and the feed value of green mass and silage from Cynara cardunculus and Helianthus tuberosus in the Republic of Moldova. In Scientific Papers. Series D. Animal Science; University of Agronomic Sciences & Veterinary Medicine Bucharest: Bucharest, Romania, 2020; pp. 122–127. [Google Scholar]
- Wang, Y.; Zhao, Y.; Xue, F.; Nan, X.; Wang, H.; Hua, D.; Liu, J.; Yang, L.; Jiang, L.; Xiong, B. Nutritional value, bioactivity, and application potential of Jerusalem artichoke (Helianthus tuberosus L.) as a neotype feed resource. Anim. Nutr. 2020, 6, 429–437. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle; National Research Council: Washington, DC, USA, 2001.
- Papi, N.; Kafilzadeh, F.; Fazaeli, H. Effects of incremental substitution of maize silage with Jerusalem artichoke silage on performance of fat-tailed lambs. Small Rumin. Res. 2017, 147, 56–62. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, X.F.; Wang, Y.J.; Huang, X.; Li, Q.; Liu, P. Study on mixed silage of corn straw and Jerusalem artichoke stalk as feed sources. China Dairy Cattle 2017, 12, 20–23. [Google Scholar]
- Razmkhah, M.; Rezaei, J.; Fazaeli, H. Use of Jerusalem artichoke tops silage to replace corn silage in sheep diet. Anim. Feed Sci. Technol. 2017, 228, 168–177. [Google Scholar] [CrossRef]
- Loughrin, J.; Antle, S.; Bryant, M.; Berry, Z.; Lovanh, N. Evaluation of Microaeration and Sound to Increase Biogas Production from Poultry Litter. Environments 2020, 7, 62. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Ince, O.; Ince, B.; Harms, H.; Kleinsteuber, S. Comparison of Rumen and Manure Microbiomes and Implications for the Inoculation of Anaerobic Digesters. Microorganisms 2018, 6, 15. [Google Scholar] [CrossRef]
- Fuksa, P.; Hakl, J.; Míchal, P.; Hrevušová, Z.; Šantrůček, J.; Tlustoš, P. Effect of silage maize plant density and plant parts on biogas production and composition. Biomass Bioenergy 2020, 142, 105770. [Google Scholar] [CrossRef]
- Zubr, J. Methanogenic fermentation of fresh and ensiled plant materials. Biomass 1986, 11, 159–171. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Viinikainen, T.A.; Rintala, J.A. Screening boreal energy crops and crop residues for methane biofuel production. Biomass Bioenergy 2008, 32, 541–550. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef]
- Ţîţei, V.; Coșman, S. Biochemical characteristics of the Asteraceae species silage and possible use as a feedstock for livestock and biogas production in Republic of Moldova. RJAS 2016, 48, 105–112. [Google Scholar]
- Hutňan, M.; Špalková, V.; Bodík, I.; Kolesárová, N.; Lazor, M. Biogas Production from Maize Grains and Maize Silage. Pol. J. Environ. Stud. 2010, 19, 323–329. [Google Scholar]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 2020, 8, 105. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.; Chen, C.; Liu, G.; Zhang, R. Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. 2020, 763, 143007. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Toyonaga, M.; Ohashi, A.; Tourlousse, D.M.; Matsuura, N.; Meng, X.-Y.; Tamaki, H.; Hanada, S.; Cruz, R.; Yamaguchi, T.; et al. Lentimicrobium saccharophilum gen. nov., sp. nov., a strictly anaerobic bacterium representing a new family in the phylum Bacteroidetes, and proposal of Lentimicrobiaceae fam. nov. Int. J. Syst. Evol. 2016, 66, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tamayo, R.; Popova, M.; Tillier, M.; Morgavi, D.P.; Morel, J.P.; Fonty, G.; Morel-Desrosiers, N. Hydrogenotrophic methanogens of the mammalian gut: Functionally similar, thermodynamically different—A modelling approach. PLoS ONE 2019, 14, e0226243. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).