Abstract
The nitrogen cycle is a biogeochemical cycle primarily associated with the microbial activity that occurs in various environments, including soil. Various genes related to the nitrogen cycle have been studied for different purposes by many researchers; therefore, the polymerase chain reaction (PCR) conditions and gene compositions differ among reports, making comparisons difficult. In this study, we compare the PCR methods to amplify 13 nitrogen cycle-related genes (amo (amoA and amoB), norB (cnorB and qnorB), hzs, napA, narG, nifH, nirK, nirS, nosZ, nrfA, and nxrA) in the soil samples collected from four land use types and selected a method with excellent applicability. However, the PCR method for five nitrogen cycle-related genes (amoC, hao, hzo, nirB, and nxrB) could not be presented. In addition, the nitrogen cycle-related genes from the four land use types (field, forest, bare land, and grassland) and the seasonally collected samples were analyzed and discussed. In the grassland samples, all the nitrogen cycle-related genes reviewed were amplified. These results vary from those of the field, forest, and bare land samples, and it was estimated that grassland, among the land use types, could play an important role in the nitrogen cycle in soil. However, an association between the seasons and the rainy season was not confirmed. Thus, this study may be used for future research in various fields related to the nitrogen cycle.
1. Introduction
The nitrogen (N) cycle is a biogeochemical cycle in which N is transformed into various chemical forms as it circulates in the Earth’s atmosphere, land, and oceans, particularly under the control of microbial activity [1,2]. Thus, the N cycle plays a crucial role in natural ecosystems and human existence in terms of supplying atmospheric N, limiting environmental pollution, preserving natural habitats, and ensuring the supply of food resources [1,3,4]. The major stages of the N cycle are elemental N (N2) fixation, nitrification, and denitrification. Other important but less studied stages include nitrous oxide (N2O) formation through the natural decomposition of hydroxylamine during nitrification, dissimilatory nitrite (NO2–) reduction to ammonium (NH4+) (DNRA) by bacteria, nitrate (NO3–) generation from the decomposition of organic N by microorganisms, nitrification of ammonia (NH3) by bacteria, reduction to N2, and anaerobic NH4+ oxidation or ANAMMOX [1,5]. The microorganisms involved in these processes are classified by their stage or function into N fixers, NH3-oxidizing, NO2−-oxidizing, and the denitrifiers, DNRA, and ANAMMOX. These microorganisms have genes for specific functional capabilities (Figure 1). Due to the difficulty of culturing N-cycle microorganisms, research, including diversity and monitoring studies, depends on non-culture-based methods [6,7,8]. However, the wide range of non-culture-based polymerase chain reaction (PCR) methods used in studies of N-cycle microorganisms [9,10,11,12,13] raises some issues. For instance, reported N cycle-related PCR methods are mainly used for research, such as non-culture-based diversity studies, and not for the development of optimal diagnostic methods. Therefore, comparative studies between reported PCR methods are insufficient. It is theoretically advantageous to have a long nucleotide sequence to identify microorganisms based on nucleotide sequences. Generally, 400–800 base pairs (bp) are suitable, and the comparisons should be made with a length of at least 300 bp [14]. Recently, next-generation sequencing has been applied in non-culture-based research on microorganism diversity, and various platforms are used depending on the number of samples, amplified sequence length, and the number of reads. In consideration of these factors, a sequence length of approximately 400 bp is suitable for diversity studies [15]. However, several studies overlook these factors and use short amplicons. The PCR primer sets used for amplification may not amplify all the related microbial genes, and these results are greatly affected by the inhibitors contained in the nucleic acids extracted from the sample. Therefore, the availability of PCR primer sets with high applicability may depend on the sample type, such as soil or water [16]. To address these problems, in this study, we attempted to identify the optimal PCR methods for studying N cycle-related genes in soil microorganisms and examined their applicability in various soil types.
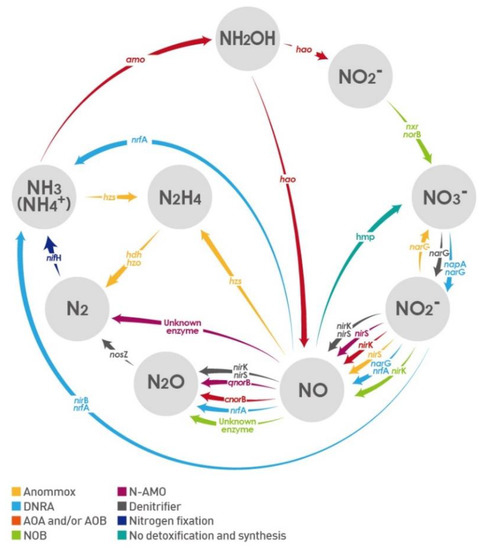
Figure 1.
The nitrogen cycle and related functional microorganism genes.
2. Materials and Methods
2.1. Collection of Positive Controls
For the denitrification microorganisms, six species of bacteria were selected as candidate positive controls: Achromobacter denitrificans KACC 12986, Aquitalea denitrificans KACC 12729, Comamonas denitrificans KACC 13430, Paracoccus denitrificans KACC 12251, Pseudomonas fluorescens KACC 10068, and Pseudomonas stutzeri KCTC 1066. The bacteria were collected and cultured using the standard method specified for each particular culture collection. After harvesting the cultured cells, chromosomal DNA was extracted using the FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH, USA). The candidate positive templates that can amplify the genes of other N-cycle microorganisms were obtained from a total of 25 surface soil samples. Four surface soil types (field, forest, bare land, and grassland) were collected for this study from sites near the Han River in Gyeoggi and Chungcheongbuk Provinces, South Korea, from April to December 2021. We selected two grassland sites and one site for each of the other three soil types (Table 1). A total of 25 samples were collected (five samplings at each site per season, including the rainy season in summer). The sampling method followed the Ministry of Environment National Institute of Environmental Research Notification No. 2017-22 soil pollution process test standard, and the sampling distances were adjusted according to the field conditions [17]; five locations per site were selected to ensure the representativeness of the samples. After removing a foreign substance layer that consisted of weeds and other organic matter, a soil sampler was used to collect the samples from a depth of 10–15 cm. The soil samples collected from the five locations were mixed well and packed into polyethylene bags and 50-mL conical tubes, which were placed in an ice box and transported to the laboratory. Total DNA was extracted from the surface soil samples using a FastDNA Spin Kit for Soil (MP Biomedicals). For each sample, nucleic acids were extracted in triplicate and combined. Chromosomal DNA from the bacterial cells and total DNA from the surface soils were quantified using a DS-11 spectrophotometer (DeNovix, Wilmington, VA, USA).

Table 1.
Information on sampling locations in South Korea. Five samples were collected per location.
ANAMMOX, anaerobic ammonium (NH4+) oxidation; AOA, ammonia (NH3)-oxidizing archaea; AOB, NH3-oxidizing bacteria; DNRA, dissimilatory nitrite (NO2–) reduction to NH4+; amo, ammonia monooxygenase; norB, nitric oxide reductase; hao, hydroxylamine oxidoreductase; hdh, hydrazine dehydrogenase; hzo, hydrazine oxidase; hzs, hydrazine synthase complex; hmp, flavohemoglobin; narG, nitrate (NO3–) reductase; napA, periplasmic NO3– reductase; nifH, N fixation and nitrogenase; nirB, NO2– reductase large subunit; nirK, NO2– reductase K; nirS, NO2– reductase S; nosZ, nitrous oxide reductase; nrf, NO2– reductase; nxr, NO2– oxidoreductase.
2.2. Comparison of PCR Methods for Nitrogen Cycle Microorganism Genes
A total of 67 PCR methods to amplify each of the 14 genes (18 detailed genes) related to the N cycle were targeted. The target genes were as follows: amo (amoA, amoB, and amoC), norB (cnorB and qnorB), hao, hzo, hzs, napA, narG, nifH, nirB, nirK, nirS, nosZ, nrfA, and nxr (nxrA and nxrB) (Figure 1). The numbers of the PCR primer sets for each gene were as follows: nine sets for the amo genes (six amoA, two amoB, and one amoC); seven sets for the norB genes (five cnorB and two qnorB); three sets for hao; two sets for hzo; five sets for hzs; four sets for napA; four sets for narG; two sets for nifH; three sets for nirB; six sets for nirK; six sets for nirS; seven sets for nosZ; five sets for nrfA; and three sets for the nxr genes (two nxrA and one nxrB) (Supplementary Table S1). To select a PCR primer set for each of the N cycle-related genes suitable for use in the surface soil samples, we collected candidate positive controls for the bacterial nucleic acid reactions (used only for the denitrification-related genes) and soil samples, followed by the first selection of a PCR primer set. In this process, each PCR method was reviewed in consideration of the product size, and its applicability was tested through sample monitoring (reaction level and non-specific amplification) of bacteria or the sample nucleic acids. Next, a secondary selection was performed by comparing the detection sensitivity of the primers. Finally, we improved the selected PCR method by performing a PCR analysis in a 20-μL reaction volume using the dry-type AccuPower HotStart PCR PreMix (Bioneer, Daejeon, South Korea), including 1 μL of 25 μL each of the forward and reverse PCR primers (2 μL in total), 17 μL of nucleic acid-free water, and 1 μL to serve as a template. The PCR conditions were established for each PCR primer set according to their corresponding references, and the PCR methods were improved by adjusting the annealing temperature. The PCR products were then subjected to electrophoresis using 2% agarose gel, and the results were checked under ultraviolet light.
3. Results
3.1. Securing the Positive Controls
The average nucleic acid concentrations were 169.0 ng/μL (the average A260/A280 ratio, 1.91) among the six bacteria and 122.0 ng/μL (A260/A280 ratio, 1.71) among 25 total DNAs. The denitrification-related genes (napA, narG, nirK, nirS, norB (cnorB and qnorB), and nosZ) were analyzed for the PCR levels and non-specific amplification using two types of nucleic acids (bacteria chromosomal DNA and total DNA from the surface soil samples). For the other N cycle-related genes (amo (amoA, amoB, and amoC), hao, hzo, hzs, nifH, nirB, nrfA, and nxr), we used total DNA extracted from the surface soil samples. The positive controls were secured for 13 of the detailed genes, excluding amoC, hao, hzo, nirB, and nxrB (Table 2 and Supplementary Figures S1–S14).

Table 2.
Description of selected PCR primer sets for functional gene monitoring related to the nitrogen cycle in surface soil.
3.2. PCR Method Selection for Each Gene
Each PCR reaction was analyzed using nine PCR methods (#1–9), which were expected to amplify amo (six amoA, two amoB, and one amoC). For each PCR method expected to amplify amoA and amoB, 10–13 and 6–8 amplicons among 25 surface soil DNAs were amplified to secure candidate positive controls. However, the PCR method that was expected to amplify amoC did not form an amplicon of a specific size. Among the six amoA PCR methods, weak amplification or multi-bands were formed in methods #3 and #5. PCR method #2 formed an excellent reaction; however, methods #1, #3, and #6 were selected due to their amplicon size (approximately 110 bp). Among the two amoB PCR methods, #1 showed weak amplification and multi-bands; therefore, #2 was selected (Supplementary Figure S1A). After comparing the detection sensitivity of PCR methods #1, #3, and #6 for amoA amplification, the detection sensitivity levels of 100–10−4 were analyzed from six surface soil DNAs. The average sensitivity values were −1.17, −1.50, and −1.67 for #1, #3, and #6, respectively. PCR method #2 for amoB showed a 10−1 detection sensitivity (Supplementary Figure S1B).
We analyzed seven PCR methods expected to amplify norB (five cnorB and two qnorB). Among these, PCR methods #1–4, to amplify cnorB, formed a band of a specific size in the bacterial nucleic acid, thereby securing a positive control. However, methods #1, #3, and #4 did not amplify the cnorB-specific nucleic acids from the samples. By contrast, method #2 amplified approximately 64% of the samples (16/25); therefore, only a short length (approximately 177 bp) was selected. PCR method #1, used to amplify qnorB, showed no reaction, whereas, in method #2, a specific band was formed from one bacterial and eight sample DNAs (Supplementary Figure S2).
Among the three and two PCR primer sets for hao and hzo, respectively, an amplification band of a specific size did not appear in the 25 surface soil total DNA samples (Supplementary Figures S3 and S4).
Among the five PCR methods to amplify hzs, methods #1–4 did not form specific bands from the sample nucleic acids. By contrast, the expected band sizes were observed for method #5 from six sample DNAs, yielding a detection sensitivity of 10−1 (Supplementary Figure S5).
A comparison of the four PCR methods for napA amplification demonstrated that method #3 did not show amplification in the bacteria and sample nucleic acids, and method #1 showed excellent performance but with an amplicon size of approximately 152 bp. PCR methods #2 and #4 were selected due to their detection sensitivity levels of 100–10−1 (average, −0.33) and 10−1–10−3 (average, −2.00) and their amplification of approximately 60% of the samples (15/25) by method #4 and approximately 52% of the samples (13/25) by method #4 (Supplementary Figure S6).
Among the four PCR methods expected to amplify narG, methods #2 and #3 did not show amplification in the bacteria or sample nucleic acids. Method #4 showed excellent performance but with an amplicon size of approximately 173 bp. By contrast, method #1 was expected to have an amplicon size of 524 bp, showed a response to four bacterial DNAs, and amplified approximately 56% of the samples (14/25), with a detection sensitivity of approximately 10−3 (Supplementary Figure S7).
Among the three PCR methods tested for nifH gene amplification, method #3 showed no amplification for the sample nucleic acid, and in method #1, only one band of a specific size was formed. However, method #2 showed sufficient amplification of 40% of the samples (10/25), with a detection sensitivity of 100 (Supplementary Figure S8).
Among the three PCR primer sets for nirB, a specific band did not appear from any of the 25 surface soil total DNA samples (Supplementary Figure S9).
Among the six PCR methods expected to amplify the nirK gene, one or two bacterial DNA samples showed amplification by PCR methods #1, #2, #3, and #6, and 6–16 specific bands were detected from the 25 samples by all the PCR methods. Among these, we selected methods #2 and #3, which showed relatively good sample reaction results (Supplementary Figure S10A) and amplified approximately 64% of the samples (16/25), and #3, which showed > 40% sample coverage (Supplementary Figures S10A,B).
Among the six PCR methods for nirS amplification, methods #1, #3, and #6 showed no amplification from the bacteria and sampled nucleic acids, and methods #4 and #5 had relatively small amplicon sizes (244 and 257 bp), with many multi-bands (Supplementary Figure S11). By contrast, method #2 produced an amplicon size of 425 nt, and specific amplifications were found from three bacteria and nine samples (Supplementary Figure S11A), with an average detection sensitivity of 10−1–10−2 (average, −1.33) (Supplementary Figure S11B).
Among seven nosZ PCR methods, method #7 did not react with the bacteria and sampled nucleic acids, and methods #1–3 were amplified from 4–6 bacterial DNA samples. PCR methods #1–6 showed a response to 10–12 of 25 samples (Supplementary Figure S12A). However, method #2 formed similar-sized multi-bands, method #3 showed weak amplification with multi-bands, and method #4 showed few sample reactions and multi-bands. Methods #1, #5, and #6 showed detection sensitivity levels of 10−2, 100, and 100, respectively. Method #1 produced an amplicon of 267 bp; this was smaller than those of methods #5 and #6, which amplified 300 and 279 bp, respectively. However, method #1 amplified four bacterial DNA and showed excellent detection sensitivity (10−3–10−4 in bacteria) and sample reaction (Supplementary Figure S12B).
Among the five PCR methods for nrfA gene amplification, methods #1–4 showed no amplification in the total DNA samples. Method #5 produced an amplicon size of 236 bp but formed a band of a specific size in approximately 48% of the samples (12/25), with a detection sensitivity of approximately 10−1 (Supplementary Figure S13).
Among the three PCR methods to amplify two nxrA genes and one nxrB gene, responses to six and ten samples were observed for the nxrA PCR methods #1 and #2, respectively, with a detection sensitivity of 10−1. According to the detection sensitivity results, we selected method #1, which also had wide sample coverage. In contrast, no reaction was observed among the methods expected to amplify nxrB (Supplementary Figure S14).
4. Discussion
In this study, we selected suitable PCR methods for the study of N cycle-related genes in soil microorganisms. Among 18 detailed genes related to the N cycle, we identified several PCR methods with excellent applicability for surface soil and gene diversity studies in all but five genes (amoC, hao, hzo, nirB, and nxrB). To the extent possible, we selected those PCR methods that amplified a base sequence of 400–700 nt (≥300 nt) to take full advantage of the abilities of a conventional PCR (e.g., follow-up research, such as microbiome gene diversity testing) [15,24,25]. However, we were only able to select the PCR methods that produced amplicons of <300 bp in length for four genes: cnorB (177 bp), hzs (200–300 bp), nosZ (267 bp), and nrfA (236 bp). The PCR methods selected in this study are expected to be useful for the study of N cycle-related microbial communities in various soil types.
We also analyzed the microorganism genes related to the N cycle among surface soil land-use types and five seasons (four conventional seasons and the rainy season). However, we were unable to identify suitable PCR methods for five genes: amoC, hao, hzo, nirB, and nxrB. Among 65 reactions (13 detailed N cycle-related genes in five replicate samples per point), we analyzed 16.9% of the field samples (11/65), 23.1% of the forest samples (15/65), 26.2% of the bare land samples (17/65), and 80.8% of the grassland samples (average, 52.5/65). Among the 25 reactions attempted for each detailed gene, we observed positive reactions for amoA (13), amoB (8), cnorB (16), qnorB (8), hzs (6), napA (15), narG (14), nifH (10), nirK (16), nirS (9), nosZ (11), nrfA (12), and nxrA (10). Among the 65 responses according to season, reactions were observed in 32.3% of the spring samples (21/65), 50.8% of the summer samples (33/65), 49.2% of the rainy season samples (32/65), 67.7% of the autumn samples (44/65), and 32.3% of the winter samples (21/65) (Table 3).

Table 3.
Surface soil monitoring results according to land use type and season. Sp, spring; Su, summer; R, rainy season in summer; A, autumn; W, winter; ●, positive; -, negative.
Among the field type samples, the amoA, cnorB, hzs, napA, nifH, nirK, nosZ, and nxrA genes were amplified and mainly appeared during summer and the rainy season. Among the forest samples, ten genes (amoA, cnorB, hzs, napA, narG, nifH, nirK, nosZ, nrfA, and nxrA) were amplified: cnorB, nirK, and nxrA mainly in the rainy season and autumn samples, amoA, hzs, nifH, nosZ, and nrfA only in the autumn samples, and napA and narG in the autumn and winter samples. The bare land samples showed similar results to the forest samples, except for the amplification of qnorB and nirS in the autumn samples, perhaps because the bare land soils were sampled at a short distance (1.6 km) from the forest sampling location. Among the four land use types examined in this study, a high percentage of N cycle-related genes were analyzed in the grassland samples, and 13 N cycle-related detailed genes were amplified (Table 3 and Supplementary Figure S15). Blaud et al. [26] reported that the abundance of N-cycle genes and the potential greenhouse gas fluxes depend mainly on the land use type and less on the soil aggregate size. In this study, the amplified genes were found to differ according to the soil land use type. Notably, the grassland microorganism genes related to the N cycle appeared in most samples regardless of the season, unlike those in the samples from the other three land use types. This phenomenon may be explained by the fact that grasslands have the highest level of biomass on Earth, and natural grasslands have the N balance of a managed grassland system, with carbon:N:organic and sulfur:organic phosphorus ratios maintained at nearly constant values [27,28,29]. Because grassland is an important land use type for the soil’s N cycle, it may be necessary to examine samples from more diverse land use types and more seasonal periods in future research. The 13 detailed genes used in this study showed 6–16 reactions per 25 samples (average, 11.4).
Among the samples used in this study, cnorB and nirK (64.0%, 16/25), napA (60.0%, 15/25), narG (56.0%, 14/25), and amoA (52.0%, 13/25) were detected at particularly high rates, whereas other genes were detected at rates of 24.0–48.0%. South Korea is located at 33–38° N, 126–132° E, and the sampling sites for this study were Gapyeong and Yangpyeong in Gyeonggi-do and Cheongju in Chungcheongbuk-do, which are mainly in the central region and have cold climates. These regions have four distinct seasons; the main characteristics of the climate are as follows. From early March to early May, warm, dry spring weather appears under the influence of mobile anticyclones and Yangtze air masses. From May to mid-August, hot, humid summer weather appears under the influence of the North Pacific air mass. From late August to late October, the autumn weather is clear and dry under the influence of high pressure from mainland China and the Yangtze air mass. From November, the temperature and humidity gradually decrease under the influence of the Siberian air mass. From December to February, cold, dry winter weather appears under the influence of the Siberian air mass [30]. In this study, N cycle-related genes were identified at higher rates in autumn, whereas these genes had similar levels in the summer and rainy season and lower levels in spring and winter. Jung et al. [31] reported a seasonal variation in the abundance of several N cycle-related genes in the forest soils from Bukhansan, South Korea. Among the six genes, nirS, nirK, and norB showed seasonal changes, whereas nifH, narG, and nosZ changed little over time; nirS and nirK were at low abundance in January and September but at high abundance in April and October, and norB was rare in November, January, and December but abundant in April, September, October, and July. In this study, N cycle-related gene amplification was rare in spring and summer in the forest soils of the regions sampled in this study but was common in autumn, and several genes were identified in the rainy season and winter. The nirS gene did not appear in all seasons, whereas nirK and cnorB were identified in autumn and the rainy season, nifH and nosZ appeared in autumn, and narG appeared in autumn and winter. These patterns may change depending on environmental factors, such as the season, as well as other factors, such as tree composition.
PCR methods were unavailable for the genes amoC, hao, hzo, nirB, and nxrB at the time of this study; therefore, our findings should be supplemented in future research. PCR amplification of the total DNA extracted from environmental samples, such as soil, can offer visual confirmation of unknown, non-specific amplification, as seen in this study (Supplementary Figure S15). However, because the target band can be purified by gel electrophoresis, this issue is not expected to have a significant effect on subsequent studies, such as gene diversity analysis. Quantitative PCR (qPCR) can be used to rapidly monitor specific genes in environmental samples, such as soil; however, because qPCR mainly amplifies nucleotide sequences of less than approximately 200 bp, there is a limit to gene diversity analyses using amplification products. Among the non-selective PCR methods reviewed in this study, amoA method #2 (110 bp), napA method #1 (152 bp), narG method #4 (173 bp), nirK method #5 (165 bp), nirS methods #4 and #5 (244 and 257 bp), and nosZ method #6 (279 bp) may be suitable for rapid monitoring.
Approximately 95% of N in its natural state is found in soil organic matter, which is a determinant of soil quality and an important component of the carbon cycle. The N concentration in soil organic matter largely determines the degree of mineralization and immobilization, as well as N-cycle processes, including N2 fixation, nitrification, and denitrification. Thus, soil is an important medium for research on all of these processes [32,33,34]. However, our understanding of the role of soil in these vital processes has remained poor due to its more complex structure compared with atmospheric and water systems. Surface soil has the highest concentration of microorganisms and rich organic matter among various kinds of soils, making it the main network and motive engine of nature for plant growth [35]. Surface soil has a direct and vital influence on human life, development, and survival by facilitating agriculture, environmental preservation, and ecological balance [36]. Despite the crucial importance of surface soil, research has been lacking on the uses of surface soil in typical temperate climate regions and in monitoring the seasonal processes that affect it. The N cycle is also closely related to soils for measuring the effects of changes in land use and management, food resources, nature conservation, and environmental restoration [37,38,39,40], and to the carbon cycle [32]. Therefore, the results of this study are expected to be useful for future research in various fields related to the N cycle.
5. Conclusions
In this study, PCR methods for 13 nitrogen cycle-related genes (amoA, amoB, cnorB, qnorB, hzs, napA, narG, nifH, nirK, nirS, nosZ, nrfA, and nxrA) that can be used for soil nitrogen cycle research were presented. In addition, the nitrogen cycle genes, according to the four soil land use types and seasons, were discussed as the selected method. However, the methods for the five genes (amoC, hao, hzo, nirB and nxrB) were not presented, and further studies are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122010314/s1. Figure S1: Comparison of polymerase chain reaction (PCR) methods for amo genes. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics, Daejeon, South Korea); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S2: Comparison of PCR methods for norB genes. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season in summer; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S3: Comparison of PCR methods for the hao gene. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter. Figure S4: Comparison of PCR methods for the hzo gene. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter. Figure S5: Comparison of PCR methods for the hzs gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S6: Comparison of PCR methods for the napA gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S7: Comparison of PCR methods for the narG gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S8: Comparison of PCR methods for the nifH gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S9: Comparison of PCR methods for the nirB gene. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter. Figure S10: Comparison of PCR methods for the nirK gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S11: Comparison of PCR methods for the nirS gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S12: Comparison of PCR methods for the nosZ gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S13: Comparison of PCR methods for the nrfA gene. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S14: Comparison of PCR methods for nxr genes. Panel A, positive control collection and first selection. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive. Panel B, sensitivity test. M, 100-bp DNA ladder marker (Enzynomics); 0–5, dilution rate; N, negative control. Figure S15: Monitoring nitrogen cycle genes according to surface soil land-cover type and season. M, 100-bp DNA ladder marker (Enzynomics); Sp, spring; Su, summer; R, rainy season; A, autumn; W, winter; •, positive.
Author Contributions
Conceptualization, J.-E.Y.; methodology, S.L., J.M. and H.K.; validation, J.J., J.M., H.L. and H.-R.K.; formal analysis, H.K. and S.L.; investigation, J.-Y.L. and H.-R.K.; resources, J.-Y.L.; data curation, S.L.; original manuscript preparation, S.L. and Y.-J.J.; manuscript review and editing, H.K., J.-E.Y., J.M. and Y.-J.J.; visualization, S.L., H.K., J.M. and J.-Y.L.; supervision, S.L., Y.-J.J., J.-E.Y. and H.K.; project administration, H.K. and J.-E.Y.; funding acquisition, H.K. and J.-E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Ministry of Environment through the strategic EcoSSSoil Project at the Korea Environmental Industry and Technology Institute (grant no. 2019002820004) and National Research Foundation of Korea (NRF), funded by the Ministry of Education (grant numbers 2019R1I1A2A01057002 and 2019R1A61A03033167).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Hirsch, P.R.; Mauchline, T.H. The importance of the microbial N cycle in soil for crop plant nutrition. Adv. Appl. 2015, 93, 45–71. [Google Scholar]
- Takai, K. The nitrogen cycle: A large, fast, and mystifying cycle. Microbes Environ. 2019, 34, 223–225. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, 94–98. [Google Scholar] [CrossRef]
- Zhang, X.; Ward, B.B.; Sigman, D.M. Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem. Rev. 2020, 120, 5308–5351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Wang, S.; Ye, F.; Zhu, G. Global distribution of anaerobic ammonia oxidation (Anammox) bacteria: Field surveys in wetland, dryland, groundwater aquifer and snow. Front. Microbiol. 2019, 10, 2583. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Kits, K.D.; Sedlacek, C.J.; Lebedeva, E.V.; Han, P.; Bulaev, A.; Pjevac, P.; Daebeler, A.; Romano, S.; Albertsen, M.; Stein, L.Y.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, H.; Shiratori, Y.; Isobe, K.; Otsuka, S.; Senoo, K. Predominant but previously-overlooked prokaryotic drivers of reductive nitrogen transformation in paddy soils, revealed by metatranscriptomics. Microbes Environ. 2017, 32, 180–183. [Google Scholar] [CrossRef]
- Luo, G.; Wang, T.; Li, K.; Li, L.; Zhang, J.; Guo, S.; Ling, N.; Shen, Q. Historical nitrogen deposition and straw addition facilitate the resistance of soil multifunctionality to drying—Wetting cycles. Appl. Environ. Microbiol. 2019, 85, e02251–18. [Google Scholar] [CrossRef]
- Konneke, M.; Bernhard, A.E.; de la Torre, J.R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Keeley, R.F.; Rodriguez-Gonzalez, L.; Class, U.S.F.G.; Briggs, G.E.; Frazier, V.E.; Mancera, P.A.; Manzer, H.S.; Ergas, S.J.; Scott, K.M. Degenerate PCR primers for assays to track steps of nitrogen metabolism by taxonomically diverse microorganisms in a variety of environments. J. Microbiol. Methods 2020, 175, 105990. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Comparison of PCR primers for analyzing denitrifying microorganisms in the hyporheic zone. Appl. Sci. 2020, 10, 4172. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Harhangi, H.R.; Zhu, B.; Jetten, M.S.; Yin, C.; Op den Camp, H.J. Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil. FEMS. Microbiol. Lett. 2012, 336, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kwon, J.; Kim, B.Y.; Kim, J.H. Development of an accurate and sensitive diagnostic system based on conventional PCR for detection of African swine fever virus in food waste. Indian J. Microbiol. 2022, 62, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.; Yang, J.E.; Ryu, H.S.; Moon, J.; Lee, J.Y.; Lee, H.J. Comparison of microbial gene diversity in grassland topsoil depending on soil quality. Appl. Sci. 2021, 11, 9569. [Google Scholar] [CrossRef]
- Bae, K.S.; Lee, S.; Lee, J.Y.; Kim, J.H.; Joo, Y.L.; Lee, S.H.; Chung, H.M.; You, K.A. Development of diagnostic systems for wide range and highly sensitive detection of two waterborne hepatitis viruses from groundwater using the conventional reverse-transcription nested PCR assay. J. Virol. Methods 2022, 299, 114344. [Google Scholar] [CrossRef]
- Ministry of the Environment. Soil Pollution Process Test Standard, and Sampling Distance Adjustment According to Field Conditions; No. 2017-22; National Institute of Environmental Research: Incheon, Korea, 2017.
- Ma, Y.; Zilles, J.L.; Kent, A.D. An evaluation of primers for detecting denitrifiers via their functional genes. Environ. Microbiol. 2019, 21, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Hernandez, R.J.; Valenzuela-Encinas, C.; Marsch, R.; Dendooven, L. Respiratory and dissimilatory nitrate-reducing communities from an extreme saline alkaline soil of the former lake Texcoco (Mexico). Extremophiles 2009, 13, 169–178. [Google Scholar] [CrossRef]
- Xu, S.; Feng, S.; Sun, H.; Wu, S.; Zhuang, G.; Deng, Y.; Bai, Z.; Jing, C.; Zhuang, X. Linking N2O emissions from biofertilizer-amended soil of tea plantations to the abundance and structure of N2O-reducing microbial communities. Environ. Sci. Technol. 2018, 52, 11338–11345. [Google Scholar] [CrossRef]
- Achouak, W.; Abrouk, D.; Guyonnet, J.; Barakat, M.; Ortet, P.; Simon, L.; Lerondelle, C.; Heulin, T.; Haichar, F.E.Z. Plant hosts control microbial denitrification activity. FEMS Microbiol. 2019, 95, fiz021. [Google Scholar] [CrossRef]
- Song, B.; Lisa, J.A.; Tobias, C.R. Linking DNRA community structure and activity in a shallow lagoonal estuarine system. Front. Microbiol. 2014, 5, 460. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ding, L.; Pan, Y.; Hu, H.; Ye, L.; Ren, H. Nitrogen loading effects on nitrification and denitrification with functional gene quantity/transcription analysis in biochar packed reactors at 5 °C. Sci. Rep. 2018, 8, 9844. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kwon, D.; Kim, C.; Lee, S. Analysis of bacterial diversity in water from the Han River water source protection area via a pyrosequencing assay. J. Environ. Health Sci. 2016, 42, 274–279. [Google Scholar] [CrossRef][Green Version]
- Shahi, S.K.; Zarei, K.; Guseva, N.V.; Mangalam, A.K. Microbiota analysis using two-step PCR and next-generation 16S rRNA gene sequencing. J. Vis. Exp. 2019, 152, 59980. [Google Scholar] [CrossRef]
- Blauda, A.; Zaan, B.V.D.; Menona, M.; Laircd, G.J.; Zhanga, D.; Huberc, P.; Schieferc, J.; Blumc, W.E.H.; Kitzlere, B.; Huanga, W.E.; et al. The abundance of nitrogen cycle genes and potential greenhouse gas fluxes depends on land use type and little on soil aggregate size. Appl. Soil Ecol. 2018, 125, 1–11. [Google Scholar] [CrossRef]
- Giraud, M.; Groh, J.; Gerke, H.H.; Bruggemann, N.; Vereecken, H.; Putz, T. Soil nitrogen dynamics in a managed temperate grassland under changed climatic conditions. Water 2021, 13, 931. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Z.; Huang, Y.; Sun, W.; Wei, Y.; Xiao, L.; Deng, X.; Zhu, J.; Li, T.; Zhang, W. Simulating the spatiotemporal variations in aboveground biomass in Inner Mongolian grasslands under environmental changes. Atmos. Chem. Phys. 2021, 21, 3059–3071. [Google Scholar] [CrossRef]
- Gong, H.; Li, Y.; Li, S. Effects of the interaction between biochar and nutrients on soil organic carbon sequestration in soda saline–alkali grassland: A review. Glob. Ecol. 2021, 26, e01449. [Google Scholar] [CrossRef]
- Climate of Korea. Available online: https://www.weather.go.kr/w/obs-climate/climate/korea-climate/korea-char.do# (accessed on 29 September 2022).
- Jung, J.; Yeom, J.; Han, J.; Kim, J.; Park, W. Seasonal changes in nitrogen-cycle gene abundances and in bacterial communities in acidic forest soils. J. Microbiol. 2012, 50, 365–373. [Google Scholar] [CrossRef]
- Nevins, C.J.; Strauss, S.L.; Inglett, P. An overview of key soil nitrogen cycling transformations. EDIS 2020, 2020, 1. [Google Scholar] [CrossRef]
- Walworth, J. Nitrogen in soil and the environment. CALS 2013, 1, AZ1591. [Google Scholar]
- Zehr, J.P.; Kudela, R.M. Nitrogen cycle of the open ocean: From genes to ecosystems. Ann. Rev. Mar. Sci. 2011, 3, 197–225. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, C.; Sun, B.; Liang, Y. Response of global farmland soil organic carbon to nitrogen application over time depends on soil type. Geoderma 2022, 406, 115542. [Google Scholar] [CrossRef]
- Colloff, M.; Wakelin, S.; Gomez, D.; Rogers, S.L. Detection of nitrogen cycle genes in soils for measuring the effects of changes in land use and management. Soil Biol. Biochem. 2008, 40, 1637–1645. [Google Scholar] [CrossRef]
- Moring, A.; Hooda, S.; Raghuram, N.; Adhya, T.K.; Ahmad, A.; Bandyopadhyay, S.K.; Barsby, T.; Beig, G.; Bentley, A.R.; Bhatia, A.; et al. Nitrogen challenges and opportunities for agricultural and environmental science in India. Front. Sustain. Food Syst. 2021, 5, 505347. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Langenfeld, N.J.; Kusuma, P.; Wallentine, T.; Criddle, C.S.; Seefeldt, L.C.; Bugbee, B. Optimizing nitrogen fixation and recycling for food production in regenerative life support systems. Front. Astron. Space Sci. 2021, 8, 699688. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).