Accumulation of Vitamin C in Yeast under Pulsed Electric Field (PEF) Conditions
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Reagents
2.2. Biomass Cultivation
2.3. Optimizing the Pulsed Electric Field Process for Vitamin C Accumulation in Yeast
2.4. Determination of Vitamin C
2.5. Determination of Biomass and Viability of the Cells
2.6. Assessment of the Impact of Yeast Storage on Content and Antioxidant Activity of Vitamin C
2.7. Data Processing
3. Results and Discussion
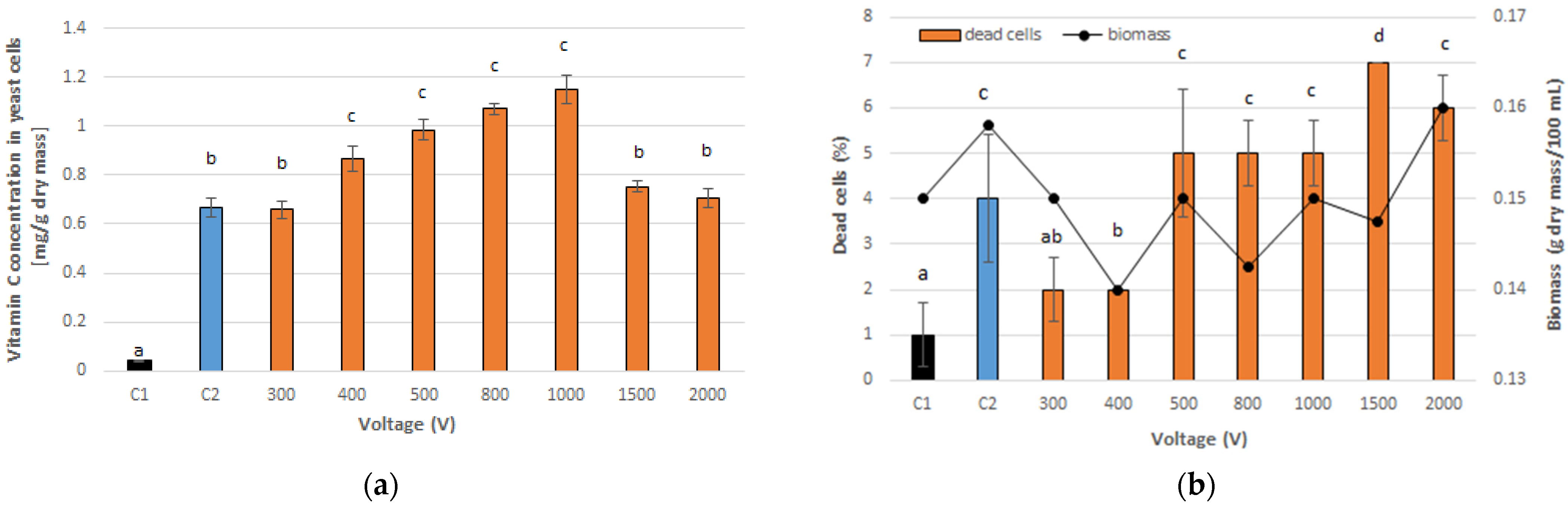
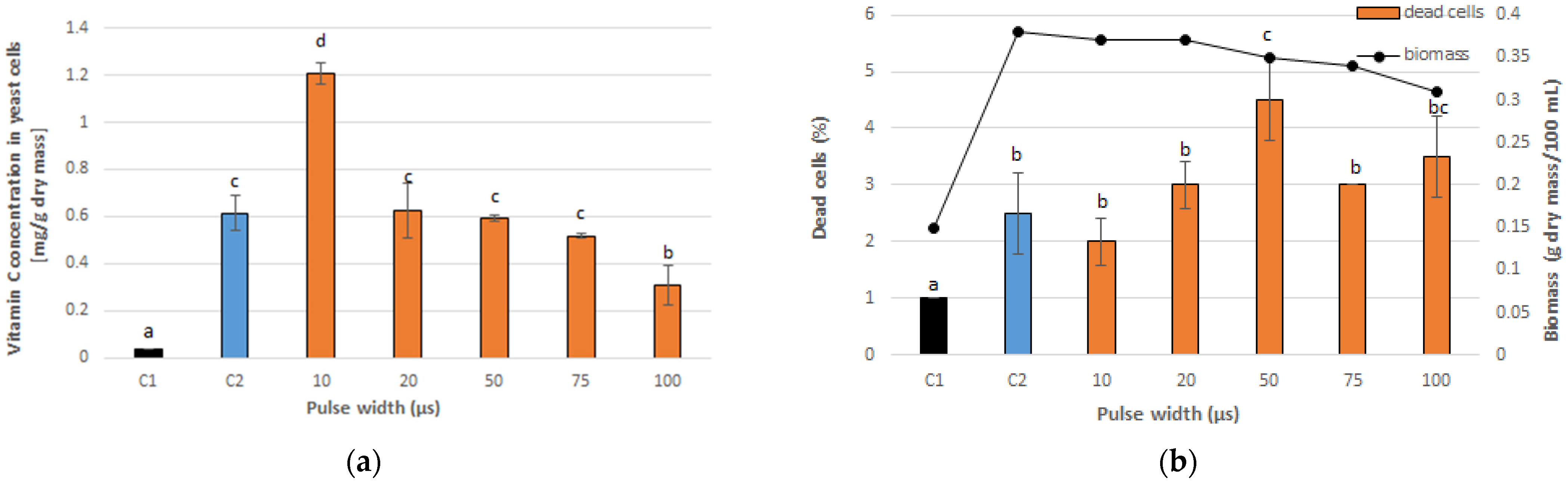
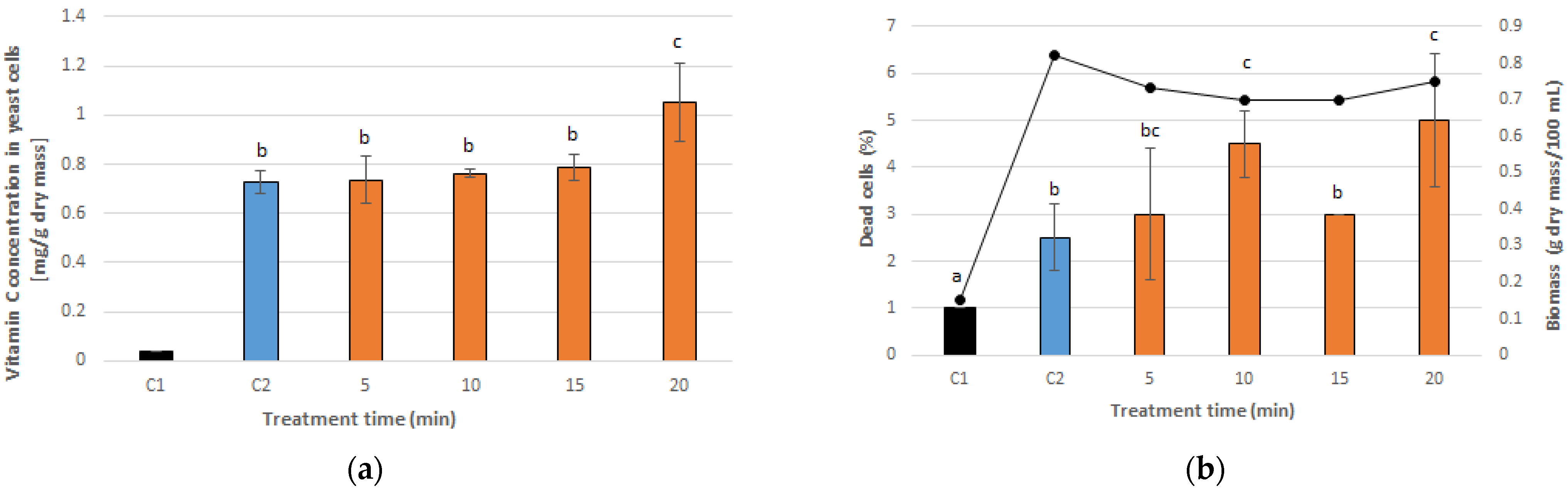
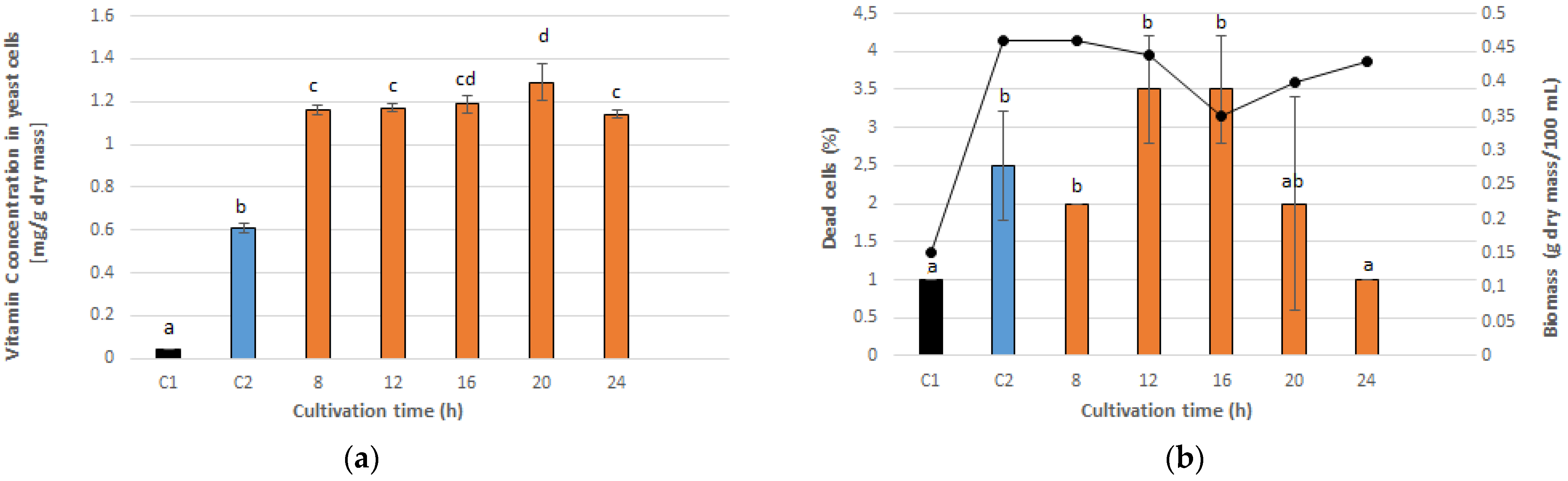
3.1. Optimal PEF Conditions for Vitamin C Accumulation in Yeast
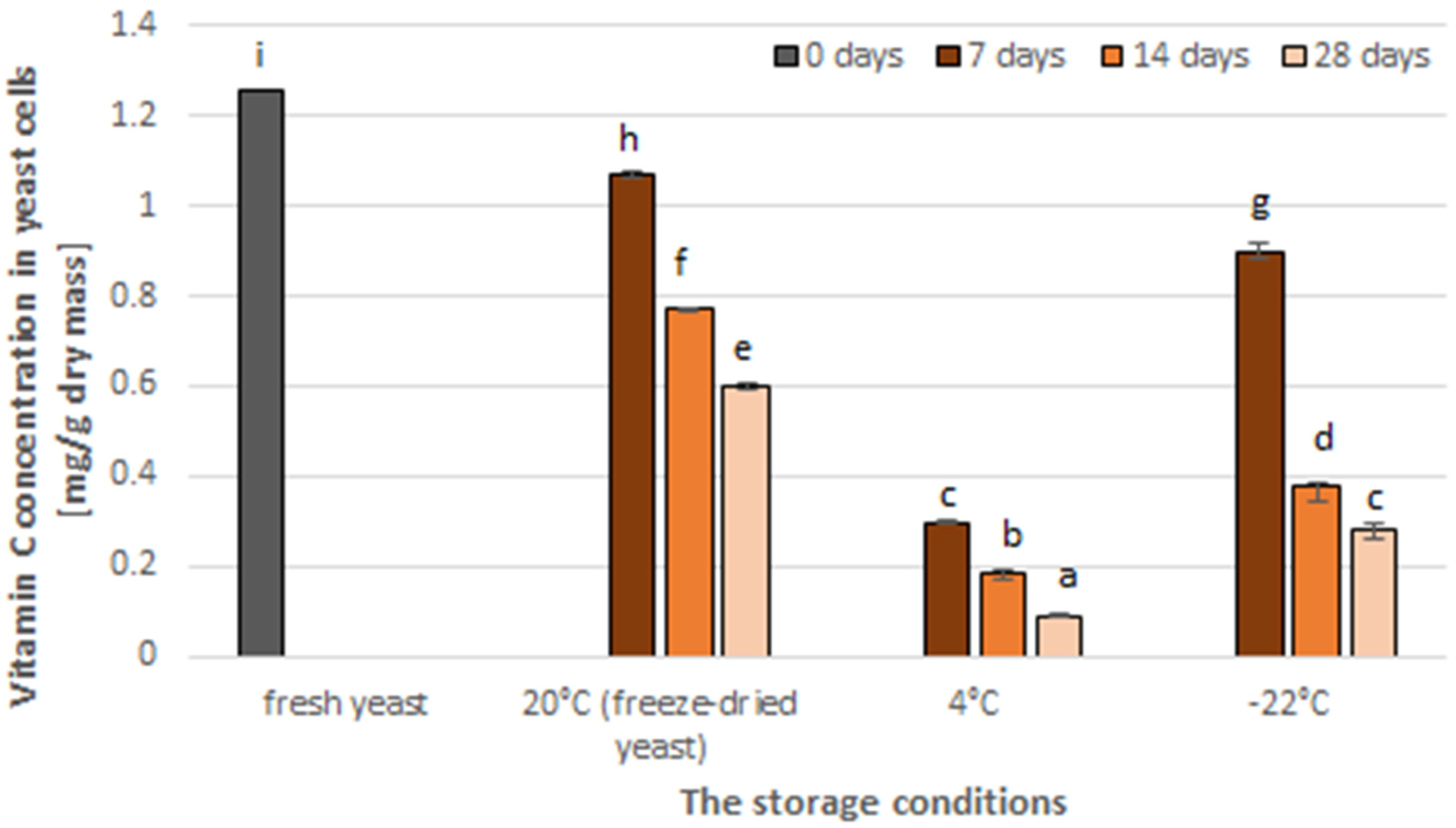
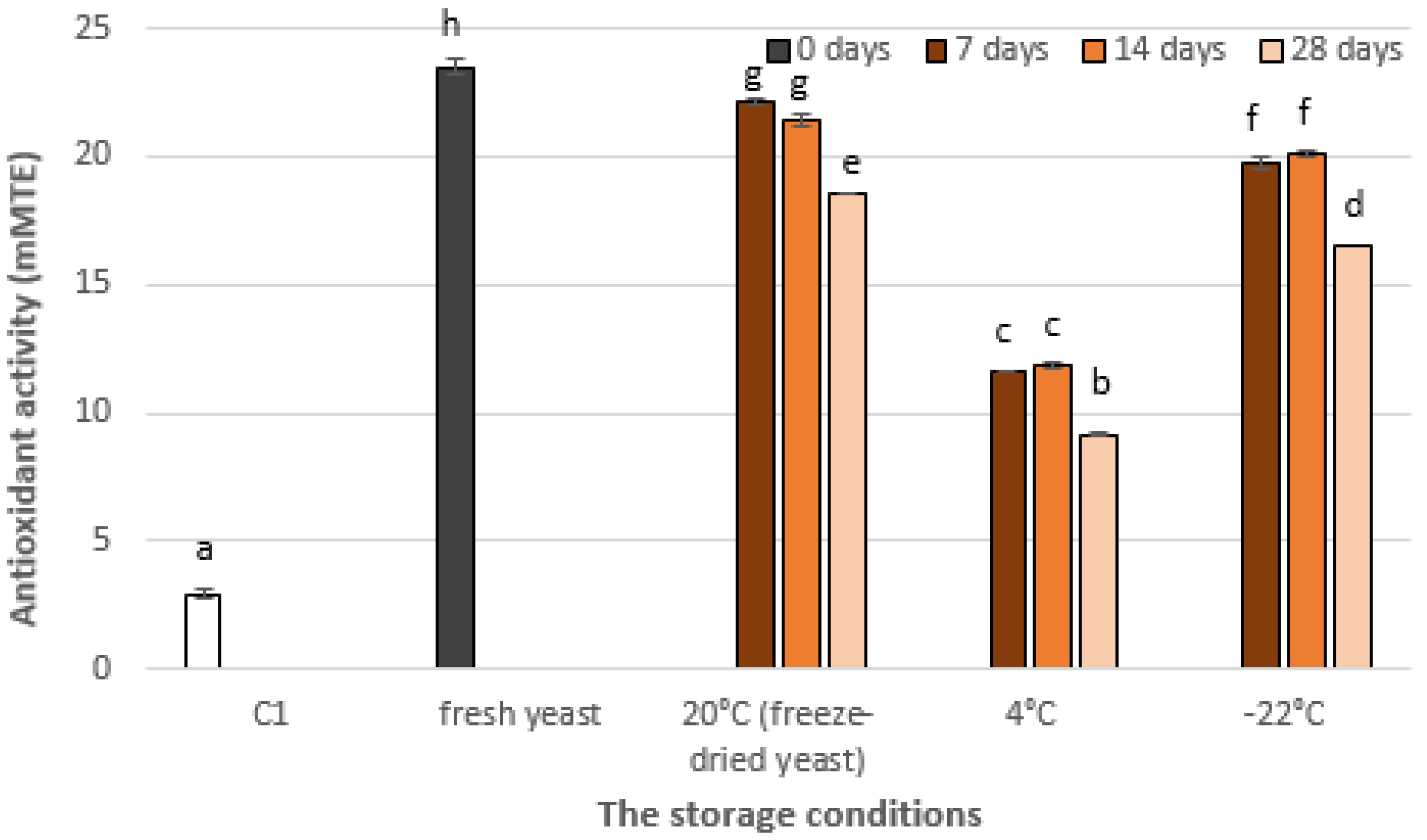
3.2. The Effect of Storage on the Vitamin C Content in Yeast Cells and Antioxidant Activity of Yeast Extract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [PubMed]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T.; Myint, P.K. Plasma vitamin C levels: Risk factors for defi-ciency and association with self-reported functional health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Wiemer, K.L.; Dary, O.; Keen, C.L.; King, J.C.; Miller, K.B.; Philbert, M.A.; Tarasuk, V.; Taylor, C.L.; Gaine, P.C.; et al. Fortification and Health: Challenges and Opportunities. Adv. Nutr. 2015, 6, 124–131. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Van Der Meer, R.; De Kruif, C.G. Microencapsulation: Its application in nutrition. Proc. Nutr. Soc. 2001, 60, 475–479. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.; Blaga, A.; Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. 2016, 289, 71–78. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Cho, Y.-S. Determination of Optimal Conditions for Zinc-Hyperaccumulation by Saccharomyces cerevisiae FF-10. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 227–233. [Google Scholar] [CrossRef]
- Pankiewicz, U.; Góral, M.; Kozłowicz, K.; Góral, D. Application of pulsed electric field in production of ice cream enriched with probiotic bacteria (L. rhamnosus B 442) containing intracellular calcium ions. J. Food Eng. 2020, 275, 109876. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Miklavčič, D.; Arczewska, M. Pulsed Electric Field (PEF) Enhances Iron Uptake by the Yeast Saccharomyces cerevisiae. Biomolecules 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nu-trition. J. Food Sci. Technol. 2020, 58, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomašević, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z.; Xiong, X.-Y. Effects of Pulsed Electric Fields (PEF) on Vitamin C and Its Antioxidant Properties. Int. J. Mol. Sci. 2015, 16, 24159–24173. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, U.; Jamroz, J. Effect of pulsed electric fields upon accumulation of magnesium in Saccharomyces cerevisiae. Eur. Food Res. Technol. 2010, 231, 663–668. [Google Scholar] [CrossRef]
- Mazurek, A.; Jamroz, J. Precision of dehydroascorbic acid quantitation with the use of the subtraction method—Validation of HPLC–DAD method for determination of total vitamin C in food. Food Chem. 2015, 173, 543–550. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast cells and yeast-based materials for microencapsulation. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobel, R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–281. [Google Scholar]
- Dadkhodazade, E.; Khanniri, E.; Khorshidian, N.; Hosseini, S.M.; Mortazavian, A.M.; Moghaddas Kia, E. Yeast cells for en-capsulation of bioactive compounds in food products: A review. Biotechnol. Prog. 2021, 37, e3138. [Google Scholar]
- Branduardi, P.; Fossati, T.; Sauer, M.; Pagani, R.; Mattanovich, D.; Porro, D. Biosynthesis of vitamin C by yeast leads to in-creased stress resistance. PLoS ONE 2007, 2, e1092. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, U.; Sujka, M.; Kowalski, R.; Mazurek, A.; Włodarczyk-Stasiak, M.; Jamroz, J. Effect of pulsed electric fields (PEF) on accumulation of selenium and zinc ions in Saccharomyces cerevisiae cells. Food Chem. 2017, 221, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Njoku, P.; Ayuk, A.; Okoye, C. Temperature Effects on Vitamin C Content in Citrus Fruits. Pak. J. Nutr. 2011, 10, 1168–1169. [Google Scholar] [CrossRef]
- Pankiewicz, U.; Jamroz, J. Effect of Pulsed Electric Fields upon Accumulation of Zinc in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2011, 21, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, U.; Sujka, M.; Włodarczyk-Stasiak, M.; Mazurek, A.; Jamroz, J. Effect of pulse electric fields (PEF) on accumulation of magnesium and zinc ions in Saccharomyces cerevisiae cells. Food Chem. 2014, 157, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Cserhalmi, Z.; Vidács, I.; Beczner, J.; Czukor, B. Inactivation of Saccharomyces cerevisiae and Bacillus cereus by pulsed electric fields technology. Innov. Food Sci. Emerg. Technol. 2002, 3, 41–45. [Google Scholar] [CrossRef]
- Aronsson, K.; Rönner, U.; Borch, E. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int. J. Food Microbiol. 2005, 99, 19–32. [Google Scholar] [CrossRef]
- Saldaña, G.; Álvarez, I.; Condón, S.; Raso, J. Microbiological aspects related to the feasibility of PEF technology for food pasteurization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1415–1426. [Google Scholar] [CrossRef]
- Simonis, P.; Kersulis, S.; Stankevich, V.; Kaseta, V.; Lastauskiene, E.; Stirke, A. Caspase dependent apoptosis induced in yeast cells by nanosecond pulsed electric fields. Bioelectrochemistry 2017, 115, 19–25. [Google Scholar] [CrossRef]
- Heinz, V.; Alvarez, I.; Angersbach, A.; Knorr, D. Preservation of liquid foods by high intensity pulsed electric fields—Basic concepts for process design. Trends Food Sci. Technol. 2001, 12, 103–111. [Google Scholar] [CrossRef]
- Ayhan, Z.; Yeom, H.W.; Zhang, Q.H.; Min, D.B. Flavor, Color, and Vitamin C Retention of Pulsed Electric Field Processed Orange Juice in Different Packaging Materials. J. Agric. Food Chem. 2001, 49, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of high-intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chem. 2009, 115, 1312–1319. [Google Scholar] [CrossRef]
- Abbas, S.; Da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic Acid: Microencapsulation Techniques and Trends—A Review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Sauer, M.; Branduardi, P.; Valli, M.; Porro, D. Production of l -Ascorbic Acid by Metabolically Engineered Saccharomyces cerevisiae and Zygosaccharomyces bailii. Appl. Environ. Microbiol. 2004, 70, 6086–6091. [Google Scholar] [CrossRef] [PubMed]
- Dadkhodazade, E.; Mohammadi, A.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Mirmoghtadaie, L.; Hosseini, S.M. Yeast Cell Microcapsules as a Novel Carrier for Cholecalciferol Encapsulation: Development, Characterization and Release Properties. Food Biophys. 2018, 13, 404–411. [Google Scholar] [CrossRef]
- Gregory, J.F. Vitamins. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA; London, UK, 2008. [Google Scholar]
- Dhakal, S.P.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef] [PubMed]
- Borrmann, D.; Pierucci, A.P.T.R.; Leite, S.G.F.; Leão, M.H.M.D.R. Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatised starch using spray-drying. Food Bioprod. Process. 2013, 91, 23–27. [Google Scholar] [CrossRef]
- Kirby, C.J.; Whittle, C.J.; Rigby, N.; Coxon, D.T.; Law, B.A. Stabilization of ascorbic acid by microencapsulation in liposomes. Int. J. Food Sci. Technol. 1991, 26, 437–449. [Google Scholar] [CrossRef]
- Diachkova, A.V.; Tikhonov, S.L.; Tikhonova, N.V. Prediction of persistence conditions for microencapsulated vitamin C. IOP Conf. Series Earth Environ. Sci. 2020, 421, 022069. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant properties and phenolic compounds of vitamin C- rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowosad, K.; Sujka, M.; Zielińska, E.; Pankiewicz, U. Accumulation of Vitamin C in Yeast under Pulsed Electric Field (PEF) Conditions. Appl. Sci. 2022, 12, 10206. https://doi.org/10.3390/app122010206
Nowosad K, Sujka M, Zielińska E, Pankiewicz U. Accumulation of Vitamin C in Yeast under Pulsed Electric Field (PEF) Conditions. Applied Sciences. 2022; 12(20):10206. https://doi.org/10.3390/app122010206
Chicago/Turabian StyleNowosad, Karolina, Monika Sujka, Ewelina Zielińska, and Urszula Pankiewicz. 2022. "Accumulation of Vitamin C in Yeast under Pulsed Electric Field (PEF) Conditions" Applied Sciences 12, no. 20: 10206. https://doi.org/10.3390/app122010206
APA StyleNowosad, K., Sujka, M., Zielińska, E., & Pankiewicz, U. (2022). Accumulation of Vitamin C in Yeast under Pulsed Electric Field (PEF) Conditions. Applied Sciences, 12(20), 10206. https://doi.org/10.3390/app122010206








